Summary
The release of insulin from pancreatic islets requires negative regulation to ensure low levels of insulin release under resting conditions, as well as positive regulation to facilitate robust responsiveness to conditions of elevated fuel or glucose. The first phase of release involves the plasma-membrane fusion of a small pool of granules, termed the readily releasable pool; these granules are already at the membrane under basal conditions, and discharge their cargo in response to nutrient and also non-nutrient secretagogues. By contrast, second-phase secretion is evoked exclusively by nutrients, and involves the mobilization of intracellular granules to t-SNARE sites at the plasma membrane to enable the distal docking and fusion steps of insulin exocytosis. Nearly 40 years ago, the actin cytoskeleton was first recognized as a key mediator of biphasic insulin release, and was originally presumed to act as a barrier to block granule docking at the cell periphery. More recently, however, the discovery of cycling GTPases that are involved in F-actin reorganization in the islet β-cell, combined with the availability of reagents that are more specific and tools with which to study the mechanisms that underlie granule movement, have contributed greatly to our understanding of the role of the cytoskeleton in regulating biphasic insulin secretion. Herein, we provide historical perspective and review recent progress that has been made towards integrating cytoskeletal reorganization and cycling of small Rho-, Rab- and Ras-family GTPases into our current models of stimulus-secretion coupling and second-phase insulin release.
Keywords: Biphasic insulin secretion, Small GTPases, SNARE protein, F-actin reorganization, Microtubule, Islet
Introduction
In response to elevated blood-glucose levels, a pancreatic-islet β-cell will release insulin in order to maintain glucose homeostasis. Dysfunction of this secretory response is considered to be one of the causal factors in the etiology of type 2 diabetes mellitus. More than 99% of the insulin that is secreted from the pancreatic β-cell is released via a regulated secretory pathway (Rhodes, 2000). Within each β-cell, insulin is contained in dense core granules. Regulated insulin-granule exocytosis ensues when extracellular glucose levels rise and glucose enters the β-cell via the cell-surface-localized glucose transporter GLUT2 (Fig. 1). Intracellular glucose is rapidly metabolized to yield a net increase in the ATP:ADP ratio (Kennedy et al., 1999; Malaisse and Sener, 1987), triggering closure of ATP-sensitive K+ channels (KATP) and cell depolarization (Cook and Hales, 1984; Meglasson and Matschinsky, 1986). Membrane depolarization induces the opening of voltage-gated Ca2+ channels to increase the intracellular Ca2+concentration [Ca2+]i (Rorsman et al., 2000; Safayhi et al., 1997; Satin and Cook, 1985). In response to this rise in [Ca2+]i, granules fuse with the plasma membrane in a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent process (Rorsman et al., 2000). This entire process is collectively referred to as `stimulus-secretion coupling' – the rapid exocytosis that occurs within the initial 5-10 minutes of stimulation is referred to as the first phase, and the subsequent, less robust but sustained release is referred to as second-phase secretion. The second phase can become quantitatively very important, given that it can be sustained for up to several hours if elevated blood-glucose levels persist (Curry et al., 1968; Grodsky, 2000; Henquin et al., 2006; Kahn, 2001).
Fig. 1.
The stimulus-secretion coupling pathway of glucose-dependent insulin exocytosis. Glucose enters the cells via the GLUT2 transporter (1) and undergoes glycolytic and mitochondrial metabolism (2), which ultimately has the effect of increasing the ATP:ADP ratio (3). An increased ATP:ADP ratio leads to the closure of ATP-sensitive KATP channels (4) and to membrane depolarization (5), which triggers the opening of voltage-dependent Ca2+ channels (VDCCs) (6). The resulting influx of Ca2+ (7) induces the fusion of insulin-containing granules with the plasma membrane and insulin release from the cell (8). PM, plasma membrane.
The biphasic release of insulin in response to glucose was first reported in the 1960s (Curry et al., 1968). Despite subsequent decades of research, the molecular mechanism(s) underlying the biphasic response of the β-cell remain unresolved. Initial models, which suggested that the two phases of insulin secretion resulted from intra-islet β-cell heterogeneity or from different populations of β-cells preferentially secreting during first- or second-phase secretion, have since been discarded in favor of the currently accepted `storage-limited model' (Henquin et al., 2002). In this model, it is proposed that biphasic secretion corresponds to the release of geographically or functionally distinct pools of insulin-containing granules (Cerasi et al., 1974; Daniel et al., 1999; Grodsky, 1972; O'Connor et al., 1980). The first phase results from the rapid fusion of granules that are pre-docked at the plasma membrane; these pre-docked granules reside in what is referred to as the `readily releasable pool' (RRP). Concurrently, granules that are deeper within the cell (referred to as the `storage-granule pool') are mobilized to traffic to the cell periphery to replenish the RRP at the cell surface (Barg et al., 2002; Renstrom et al., 1996). Methods including photorelease of caged Ca2+, repetitive stimulation and quantitative electron microscopy were used to estimate that a small pool (50-200) of the cell's >10,000 mature insulin-containing granules constitutes the RRP and accounts for first-phase insulin release (Barg et al., 2002; Straub et al., 2004). Replenishment of the RRP begins during the first phase with the mobilization, docking and priming of previously non-releasable granules; these support second-phase insulin secretion, which is calculated to occur at a rate of 5-40 granules per cell per minute (Barg et al., 2002). Although KCl and other non-nutrient secretagogues can evoke first-phase release, only fuel secretagogues such as glucose can yield a substantial and sustained second-phase insulin release (Gembal et al., 1992). Although several of the clonal β-cell model systems do appear to secrete in a biphasic manner, they generally fail to fully replicate the kinetics of biphasic insulin release that are exhibited by an islet (Hohmeier et al., 2000; Noda et al., 1996). Thus, there is a lack of connection between islet function and the data obtained from clonal β-cell lines; however, the use of these cell lines for molecular-mechanism-based studies is necessary because of the paucity of primary islet β-cells that are obtainable.
Whether or not the size of the RRP is kept constant remains a topic of investigation. For example, varying the stimulus intensity (of glucose and other stimuli) can induce dose-dependent changes in the amplitude of the first-phase release peak (Nesher and Cerasi, 2002). Variations in pre-stimulatory glucose concentration also affect the magnitude and pattern of subsequent glucose-induced biphasic insulin secretion (Henquin et al., 2006). These observations support the proposal that the pool size is not constant. Other signals, such as changes in granule properties or priming after docking, also impact insulin release (Barg et al., 2001; Daniel et al., 1999). Another possibility is that the release competence of docked granules rather than the actual number of docked granules determines the size of the RRP; such actions are linked to secretion-potentiating agents such as glucagon-like-peptide-1 (GLP-1) and cAMP-releasing agents (Huypens et al., 2000). Thus, the biphasic response might be the result of the sum of signals with differing dynamics at different levels of stimulation (Grodsky, 1972; Licko, 1973; Meissner and Atwater, 1976; Nesher and Cerasi, 1987; Nesher and Cerasi, 2002). Recent utilization of total internal reflection fluorescence microscopy (TIRFM; Box 1) has added new variables to this list, such as the preferential release of younger (more recently synthesized) granules for first-phase release and older granules for second-phase release by human islet β-cells (Michael et al., 2007). Although these data remain supportive of the storage-limited model, it has been proposed that first-phase secretion comprises the release not only of pre-docked granules, but also of newly recruited granules (Konstantinova et al., 2007; Ohara-Imaizumi et al., 2002; Ohara-Imaizumi et al., 2004). Because of potential methodological caveats that are associated with TIRFM, this revised model awaits further independent confirmation.
Box 1. Key techniques used to study biphasic insulin secretion
Islet perifusion
Isolated islets are placed into a flow culture system to assess insulin release continuously and non-invasively with high kinetic resolution. Perifused islets exhibit biphasic insulin secretion in response to a step increase in glucose, which mimics the dynamic response in vivo. Islets of very high quality are required for clear biphasic data, and controversy exists over whether mouse or rat islets fully recapitulate the biphasic pattern that is exhibited by human islets, and over which of the two organisms is a better model of biphasic secretion.
Capacitance measurement
When a granule fuses with the plasma membrane, its own membrane is integrated into the plasma membrane. This leads to an increase in membrane area, which can be monitored electrically as an increase in surface membrane capacitance. However, changes in capacitance represent the sum of endocytosis and exocytosis, and as such this technique is considered most relevant for measurement of the rapid first phase of insulin release. Capacitance studies are usually performed with dispersed β-cells rather than with cells within the context of an intact islet.
Total internal reflection fluorescence microscopy (TIRFM)
TIRFM is used to view the dynamics of granule docking and fusion in living β-cells. Cells that express a fluorescent marker protein of the insulin-containing granule are studied, and TIRFM selectively illuminates the fluorescent granules only when they are within 100 nm of the inner leaflet of the plasma membrane. Docked granules are visible in this region and, upon fusion with the plasma membrane, emit a flash or burst of fluorescence as the marker protein is released. Similar to capacitance studies, TIRFM is usually performed with isolated β-cells.
In the etiology of type 2 diabetes, the contribution of β-cell dysfunction, which results in aberrant insulin release, is well accepted. Impairment of the first phase of glucose-induced insulin secretion has long and repeatedly been recognized as an early sign of β-cell dysfunction in patients with type 2 diabetes (Cerasi, 1975; Gerich, 2002; Pfeifer et al., 1981). However, the second-phase insulin response is also impaired in patients with type 2 diabetes; the relatively `normal' second-phase response is already reduced when matched for the degree of glucose elevation (Kahn, 2001; Polonsky et al., 1996; Porte, 1991; Ward et al., 1984). Quantitatively, second-phase insulin secretion is important, because only ∼1% of the total number of granules present release insulin to constitute first-phase secretion, whereas second-phase insulin secretion occurs at a rate of 5-40 granules per cell per minute over a period of hours (Barg et al., 2002). In this Commentary, we focus on the advances that have been made in unveiling the mechanism(s) underlying biphasic insulin release. We place particular emphasis on studies that have been performed using the physiologically relevant primary islet (as opposed to clonal β-cells) as a model system in which to study the roles of the cytoskeleton, small GTPases and SNARE proteins in controlling the second phase of insulin release.
Role of the cytoskeleton in granule mobilization
Reorganization of filamentous actin
Early studies of isolated β-cells reported the presence of microfilamentous structures and found that they impacted stimulus-secretion coupling (Malaisse et al., 1971; Orci et al., 1972). Insulin-containing granules that were isolated from β-cells were found to co-sediment with filamentous actin (F-actin); the addition of Ca2+ reduced the extent of their sedimentation with F-actin (Howell and Tyhurst, 1979). Ultrastructural analysis indicated that F-actin was organized as a dense web beneath the plasma membrane; this web was hypothesized to impede access of insulin-containing granules to the cell periphery (Howell and Tyhurst, 1979; Orci et al., 1972; Snabes and Boyd, 1982; Somers et al., 1979; Wang et al., 1990). Consistent with this notion, numerous studies using F-actin-disrupting agents such as clostridial toxins and cytochalasins showed enhanced secretagogue-induced insulin secretion, suggesting that F-actin blocks granule movement (Li et al., 1994; Malaisse et al., 1975; van Obberghen et al., 1973). However, such agents were later recognized as having off-target effects, resulting in discrepant data when used in HIT-T15 β-cells as opposed to primary islets (Li et al., 1994). More recently, the more selective G-actin-binding agent latrunculin has been used to re-investigate the importance of F-actin in insulin release, and these experiments consistently resulted in the potentiation of stimulus-induced insulin release in multiple clonal β-cell lines, in rat and mouse islets, and most recently in human islets (Jewell et al., 2008a; Thurmond et al., 2003; Tomas et al., 2006). In all cases, latrunculin was found to ablate the cortical F-actin network and to permit granule redistribution to the cell periphery. Perifused rat islets treated with latrunculin show dramatic increases in insulin secretion over the course of both phases (Thurmond et al., 2003). Given the granule-mobilizing effect of F-actin depolymerization, it remains to be determined whether F-actin is actually required for first-phase secretion, or whether the enhancement of the first phase is instead an artifact of default granule flow to the cell periphery in the absence of the cortical F-actin barrier.
Phalloidin staining of F-actin and the use of the F-actin-nucleating and -stabilizing agent jasplakinolide in combination with confocal microscopy have revealed that glucose induces F-actin remodeling in primary and MIN6 or βHC9 clonal β-cells (Nevins and Thurmond, 2003; Thurmond et al., 2003; Tomas et al., 2006). In combination with the finding that this occurred without alteration of the F-actin:G-actin ratio, these results were supportive of the concept of F-actin remodeling, as opposed to a general loss of cellular F-actin content, in response to glucose. Furthermore, non-nutrient secretagogues, such as KCl and non-metabolizable glucose analogs of D-glucose, were incapable of eliciting visible reorganization (Thurmond et al., 2003; Wilson et al., 2001). These data have led to the formulation of the current model, wherein nutrient stimulation induces F-actin reorganization as a means to initiate RRP replenishment during the first phase of insulin release. Because non-nutrient secretagogues cannot elicit effects on F-actin remodeling, it seems likely that nutrient- or glucose-induced F-actin remodeling is a key element in the mechanism(s) underlying granule mobilization through the F-actin cell web.
The coordination of F-actin remodeling and exocytosis in β-cells has been linked to F-actin-binding and -severing proteins such as gelsolin and scinderin. Specifically, a mutant line of MIN6 β-cells lacking gelsolin was shown to maintain a visibly intact cortical F-actin structure, although the cortical F-actin was refractory to reorganization in response to glucose stimulation (Tomas et al., 2006). The mutant β-cells lacking gelsolin also had significantly impaired glucose-stimulated insulin secretion in static incubation studies (Tomas et al., 2006). Scinderin has been proposed to induce cortical F-actin disassembly in mouse pancreatic β-cells (Bruun et al., 2000). However, in the absence of islet perifusion or TIRFM of primary β-cells in these studies (see Box 1 for methodological details), the importance of gelsolin or scinderin in insulin release cannot yet be attributed to a particular phase of secretion.
Glucose stimulation has also been shown to transiently disrupt the interaction of F-actin with t-SNARE proteins at the plasma membrane to facilitate insulin secretion (Thurmond et al., 2003). This interaction, which was observed in both MIN6 β-cells and primary rat β-cells, was resistant to alteration in response to KCl-induced secretion, and was disrupted by treatment of cells with latrunculin. More recently, we demonstrated that F-actin directly interacts with the first two N-terminal α-helical coiled-coil domains of the t-SNARE protein syntaxin-4 (Jewell et al., 2008a), which is the syntaxin isoform that is implicated in mediating fusion of granules to support second-phase insulin release (Spurlin and Thurmond, 2006). Selective competitive disruption of F-actin–syntaxin-4 binding correlated with enhanced glucose-stimulated insulin secretion, which was mediated by increased granule accumulation at the plasma membrane and increased syntaxin-4 accessibility (Jewell et al., 2008a). Taken together, these data indicate that a working model of glucose-induced F-actin reorganization (Fig. 2) can be added to schematics of the stimulus-secretion pathway, although mechanistic details – such as how glucose initiates disruption of the F-actin–syntaxin-4 association and how F-actin transiently re-associates with syntaxin – remain unclear.
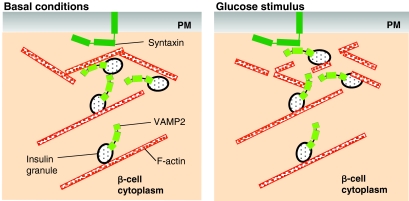
Fig. 2.
F-actin reorganization, granule mobilization and glucose-stimulated insulin secretion. Under basal conditions (left panel), F-actin not only functions as a barrier to block SNARE-complex formation, but also supplies transportation tracks for insulin-containing granules. Glucose stimulation (right panel) triggers transient F-actin reorganization to allow the granules access to the plasma membrane (PM) for subsequent docking, fusion (mediated by interactions between VAMP2 and syntaxins) and insulin release.
Before the more recent studies that showed that F-actin reorganization occurs in β-cells, it was recognized that actin filaments provide some of the motive force behind granule transport (Orci et al., 1972; Wang et al., 1990). Initially, the actin-associated motor protein myosin II was shown to be involved in insulin-granule transportation in HIT β-cells (Li et al., 1994). Pre-treatment of HIT β-cells with a myosin light chain kinase (MLCK) inhibitor resulted in significantly diminished insulin release, and MLCK was found to colocalize with the insulin-containing granules. Following this discovery, myosin light chain (MLC) was seen to co-fractionate with the insulin-containing granules and to undergo secretagogue-induced phosphorylation, which is suggestive of a role in modulating translocation of secretory granules (Yu et al., 2000). In RINm5F β-cells and rat pancreatic islets, myosin heavy chain (MHC) IIA colocalized to a large extent with F-actin (Wilson et al., 1999; Wilson et al., 2001). In addition to myosin II, myosin Va has been demonstrated to associate with insulin-containing granules (Waselle et al., 2003). More recently, RNAi-mediated depletion of myosin Va from INS-1 or MIN6 clonal β-cells was shown to inhibit granule recruitment to the cell periphery and to cause significant decreases in glucose-stimulated and depolarization-evoked insulin secretion (Ivarsson et al., 2005; Varadi et al., 2005).
Microtubule networks
Certain types of intracellular-organelle transport to the cell periphery, including the transport of insulin-containing granules, are thought to involve long-range movement of organelles by kinesins on microtubules, with subsequent handoff to vertebrate myosin Va for local delivery on actin tracks (Brown et al., 2004; Huang et al., 1999; Langford, 2002). The concept of secretagogue-induced microtubule-based shuttling of insulin-containing granules was first introduced in the 1970s, when numerous studies reported the requirement for microtubules in biphasic insulin secretion (Lacy et al., 1972; Malaisse et al., 1975; Malaisse et al., 1974; McDaniel et al., 1980; Montague et al., 1976). Early perifusion studies using microtubule-depolymerizing agents such as vinblastine and vincristine suggested that microtubules were required for both phases of secretion; however, studies using other microtubule-depolymerizing agents (colchicine and nocodazole, which are now the more commonly used drugs) suggested that the microtubule network is selectively important for the sustained second phase (Farshori and Goode, 1994). Glucose was also found to induce the formation of microtubules in pancreatic islets, such that the dynamic equilibrium between microtubule polymerization and depolymerization was deemed to be important for insulin-granule mobilization (Howell and Tyhurst, 1986). There is consensus among the studies mentioned above that microtubules in β-cells are important for the transfer of granules from the cell interior to the cortical actin filaments near the cell periphery, where they subsequently dock with SNARE proteins and release insulin, during second-phase secretion in particular (Fig. 3).
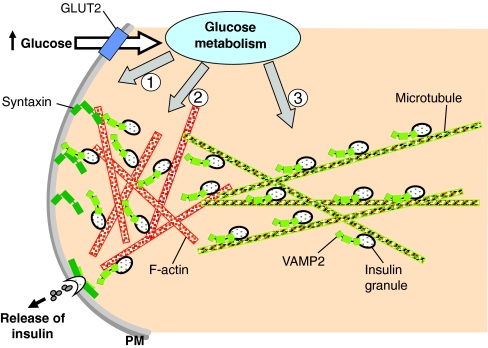
Fig. 3.
Granule recruitment supports the second phase of insulin secretion. When present at elevated levels, extracellular glucose enters the islet β-cell through the GLUT2 transporter and rapidly undergoes intracellular metabolism. Through the classic stimulus-secretion coupling pathway, the resulting increase in [Ca2+]i triggers the exocytosis of pre-docked granules in the RRP to give rise to the first phase of insulin secretion (1). Concurrently, the metabolic signal also induces F-actin reorganization (2) and recruits granules to the plasma membrane (PM) to support the sustained second phase of insulin secretion (3).
Kinesins are microtubule-dependent ATPases that transport granules and vesicles. By 1992, the first β-cell kinesin protein was identified (Balczon et al., 1992) and later the cDNA encoding the β-cell kinesin heavy chain (KHC) was cloned (Meng et al., 1997). By blocking KHC function using dominant-negative mutants or antisense oligonucleotides, multiple groups have shown the requirement for KHC in glucose-stimulated insulin secretion from clonal or primary mouse β-cells (Balczon et al., 1992; Donelan et al., 2002; Farshori and Goode, 1994; Meng et al., 1997; Varadi et al., 2002). KHC, a 138 kDa phosphoprotein, was shown to become rapidly dephosphorylated by protein phosphatase 2Bβ (PP2Bβ) as [Ca2+]i increased (Donelan et al., 2002). Because inhibitors of PP2Bβ reduced the magnitude of the second phase of insulin release, these data supported and expanded upon previous studies that implicated kinesin in microtubule-based transport of insulin-containing granules for refilling of the RRP. Granule movement was stimulated dose-dependently by ATP in permeabilized clonal cells, prompting the proposal that the glucose-induced increase in cytosolic ATP mediates the effects of glucose by enhancing kinesin activity (Varadi et al., 2002); this awaits confirmation in primary islets.
Small GTPases
Rho-family GTPases
Actin remodeling in several cell types is known to be regulated by the small Rho-family GTPases. This family of proteins is a subfamily of the Ras-related GTPase superfamily and contains more than ten members in mammals, including Rho, Rac and Cdc42 (Bishop and Hall, 2000). The Rho-family GTPases cycle between the GDP-bound (inactive) and GTP-bound (active) states through the coordinated actions of several cycling factors. In brief, guanine-nucleotide exchange factors (GEFs) catalyze the release of GDP to promote the formation of the GTP-bound and active GTPase, GTPase-activating proteins (GAPs) stimulate GTPase activity (which leads to inactivation), and guanine-nucleotide-dissociation inhibitors (GDIs) control the access of GTPases to GEFs and GAPs, as well as to membranes at which activated GTPase effectors reside (DerMardirossian and Bokoch, 2005). In the 1990s, it was discovered that Cdc42 and Rac1 in particular participated in insulin secretion and colocalized to the insulin-containing granule (Kowluru et al., 1996; Regazzi et al., 1992), as deduced through the use of a variety of toxins and inhibitors. From these early studies, it became clear that Cdc42 and Rac1 were particularly important for glucose-stimulated insulin secretion. Activation assays were later conducted using the highly selective p21-activated kinase protein-binding domain (PAK1-PBD) binding assay (which precipitates only the GTP-loaded form of Cdc42 or Rac1 from cell lysates), and both GTPases were found to become activated selectively in response to glucose, although they had distinct activation kinetics (Kowluru et al., 1997; Li et al., 2004; Nevins and Thurmond, 2003).
Cdc42 becomes rapidly activated within 3 minutes of glucose stimulation (Nevins and Thurmond, 2003), whereas Rac1 activation is not apparent until 15-20 minutes after stimulation (Li et al., 2004); the activated forms of each protein are localized to the plasma-membrane fraction of the cell, as is typical for cycling GTPases (Wang et al., 2007). Cdc42 was found to cycle back to the GDP-bound form within 2 further minutes of glucose stimulation (∼5 minutes of glucose stimulation in total), concomitant with an increase in glucosylation of Cdc42, a modification that is known to inactivate this protein (Just et al., 1995; Nevins and Thurmond, 2003). Temporally, this correlated with the visualization of F-actin depolymerization at 5 minutes. Moreover, expression of the constitutively active form of Cdc42 (Q61L) inhibited glucose-stimulated insulin secretion but did not affect K+-stimulated secretion, suggesting that glucose-stimulated insulin secretion requires that Cdc42 cycles to the GDP-bound state (Nevins and Thurmond, 2003). Importantly, blockage of cycling using the Q61L mutant correlated with the formation of cortical F-actin that was resistant to reorganization in response to glucose stimulation. This was distinctly different from the response to constitutively active Cdc42 in PC12 neuroendocrine cells (Gasman et al., 2004), and demonstrates the selective responsiveness of Cdc42 to glucose in the β-cell. This fits well with the concept that the β-cell uses its cytoskeleton differently from neuronal cells to regulate exocytotic events, which is consistent with the intrinsic differences between the patterns of release of neurotransmitters and insulin.
Cdc42 was recently demonstrated to be selectively required for the second phase of glucose-stimulated insulin release from isolated mouse islets and MIN6 β-cells (Wang et al., 2007) as well as from human islets (Z.W. and D.C.T., unpublished), as determined by transduction of islets with adenoviral Cdc42 shRNA. Cdc42 depletion also ablated the glucose-induced activation of the Cdc42-GTP-binding and effector protein PAK1. The activation of PAK1 in response to glucose occurred shortly after the activation of Cdc42 in MIN6 cells, at ∼5 minutes of glucose stimulation (Wang et al., 2007). Furthermore, depletion of either Cdc42 or PAK1 from MIN6 β-cells resulted in loss of glucose-induced Rac1 activation at 15 minutes, indicating that Cdc42 functioned as the proximal signal in a cascade flowing through PAK1 to Rac1. Consistent with this, islets that were isolated from PAK1–/– mice have severely diminished second-phase insulin release in response to glucose stimulation (Z.W. and D.C.T., unpublished). Rac1–/–-knockout mice were also recently reported to exhibit reduced glucose-stimulated insulin release in static culture studies, with normal insulin secretion in response to KCl (Asahara et al., 2008). Both PAK1–/– and Rac1–/– mouse models also exhibited impaired glucose tolerance in vivo, supporting the concept that defects in second-phase insulin release can have consequences for whole-body glucose homeostasis.
In 2004, Rac1 cycling was shown to be important for glucose-stimulated insulin release, which was ablated in INS-1 β-cells expressing a dominant-negative form of Rac1 (Li et al., 2004). One year later, the same group identified RhoGDI as the cytosolic GDI protein for Rac1 in β-cells and islets (Kowluru and Veluthakal, 2005). A GEF protein, Tiam1, was later found to reside in the cytosolic compartment of β-cells and to display characteristic GEF activity for Rac1 (Veluthakal et al., 2009). NSC23766, a specific inhibitor of Tiam1-mediated activation of Rac1, markedly attenuated glucose-induced but not KCl-induced insulin secretion. Similar to Cdc42, Rac1 is found on the granule, although no cycling proteins that are specific for Rac1 have yet been identified in that compartment. The right-hand arm of the flowchart in Fig. 4 depicts the steps mediated by Cdc42 and Rac1 in the signaling pathway that is proposed to underlie glucose-induced F-actin remodeling in the islet β-cell.
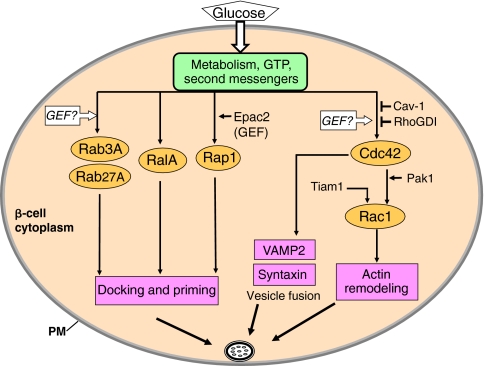
Fig. 4.
Roles of small GTPases and their cycling factors in glucose-stimulated insulin secretion. The small Rho-family GTPases Cdc42 and Rac1 are implicated in a common pathway that regulates glucose-induced actin remodeling. Cdc42 has also been implicated in directing granule targeting to t-SNARE sites through its ability to associate directly with VAMP2. The Rab GTPases Rab3A and Rab27A, as well as the Ras-family GTPases RalA and Rap1, have been proposed to function in insulin-granule docking and/or priming. PM, plasma membrane.
The role of Cdc42 in insulin secretion is not, however, restricted to its effects upon F-actin remodeling. In 2002, Cdc42 was first implicated in the targeting of insulin-containing granules to the SNARE protein syntaxin 1A (Daniel et al., 2002). Then, in 2005, subcellular-fractionation analyses revealed that complexes of Cdc42 with the SNARE VAMP2 (see next section for details) formed on the insulin-containing granule and moved to the plasma membrane in response to glucose; this also corresponded temporally with the glucose-induced activation of Cdc42 (Nevins and Thurmond, 2005). Further in vitro binding analyses showed that VAMP2 bound directly to Cdc42 and that a heterotrimeric Cdc42–VAMP2–syntaxin-1A could also be formed. The caveolar marker protein caveolin-1 (Cav-1) was later found to be a component of the Cdc42-VAMP2 complex, and was shown to interact directly with Cdc42 through a conserved motif within the Ras-binding domain of Cav-1 (Nevins and Thurmond, 2006). Cav-1 was characterized as a novel GDI for the granule pool of Cdc42 in the β-cell, because it fulfils several parameters of classical GDI function – Cav-1 dissociates from the Cdc42-VAMP2 complex upon the glucose-induced activation of Cdc42, and depletion of Cav-1 from isolated islets and MIN6 β-cells results in elevated basal-level secretion and in ablation of the glucose-stimulated increase. Thus, we have speculated that, through its interaction with the Cdc42-VAMP2-bound insulin-granule complex, Cav-1 might contribute to the specific targeting of granules to `active sites' of exocytosis that are organized by caveolae (Nevins and Thurmond, 2006).
The Rab family
The Rab GTPase protein family comprises more than 60 members (Grosshans et al., 2006; Izumi, 2007). Among these, Rab3A and Rab27A have been shown to associate with insulin-containing granules of pancreatic β-cells (Regazzi et al., 1996; Yi et al., 2002). Rab3A–/– mice were found to be glucose-intolerant, which could be accounted for by a significant loss in glucose-stimulated insulin release during both phases of secretion in islets that were isolated from these mice (Yaekura et al., 2003). Similarly, Rab27A depletion from INS-1 cells decreased exocytosis that was triggered by a cocktail containing glucose, forskolin and isobutylmethylxanthine (IBMX) (Waselle et al., 2003), and a line of mice with a naturally occurring mutation in Rab27A (known as ashen mice) exhibit defective glucose-stimulated insulin secretion (Kasai et al., 2005). A series of capacitance measurements in β-cells from Rab3A–/– and ashen mice revealed differences in the requirement for each Rab in the process of insulin exocytosis, whereby Rab3A was deemed to be crucial for initial RRP size and Rab27a was characterized as rate-limiting in RRP refilling (Merrins and Stuenkel, 2008). As it remains a possibility that the Rab27 mutation of ashen mice is not the only mutation or alteration in this line, studies of Rab27 depletion and/or ablation will be required to ascertain its precise role(s) in biphasic secretion.
Two Rab27 effector proteins, Slac2-c (also known as MyRIP) and granuphilin, have been found to localize to insulin-containing granules (Wang et al., 1999; Waselle et al., 2003). Attenuation of Slac2-c in INS-1 β-cells resulted in impaired insulin exocytosis in response to a secretagogue-cocktail stimulus (Waselle et al., 2003). By contrast, islets isolated from granuphilin-null mice showed enhanced insulin release during each phase and the mice showed increased whole-body glucose tolerance (Gomi et al., 2005). Unexpectedly, fewer than normal insulin-containing granules were docked at the plasma membrane in β-cells from the granuphilin-null islets. Although these data are seemingly counter-intuitive, granuphilin binds to the syntaxin-1A–Munc18a complex (Kasai et al., 2008; Tomas et al., 2008), which is considered to be fusion-incompetent (see the following section for details on the docking and fusion machinery); granuphilin-null β-cells contained fewer syntaxin-1A–Munc18a complexes and an increased amount of fusion-competent syntaxin 1A that could be used for rapid fusion of granules (Gomi et al., 2005). Thus, the working model suggests that granuphilin is important for the docking of insulin-containing granules, and simultaneously imposes a fusion constraint on them through an interaction with the syntaxin-1A fusion machinery (Gomi et al., 2005). The left arm of the flowchart in Fig. 4, which has been drawn on the basis of currently available data, shows that Rab3A and Rab27A have roles in granule docking and/or priming.
The Ras-like family
Two members of the Ras-like GTPase family, Rap1 and RalA, have been implicated in β-cell insulin release. Rap1 was the first to be identified in β-cells, where its stimulus-induced carboxylmethylation was found to correlate closely with increased insulin secretion (Leiser et al., 1995). The GEF protein identified for Rap1 is Epac2 (exchange protein directly activated by cAMP) (Bos, 2003; Leech et al., 2000; Zwartkruis and Bos, 1999), which mediates Rap1 activation by cAMP in β-cells. Activation of cAMP signaling potentiates both phases of glucose-induced fusion events, and a recent study using islets from Epac2–/– mice showed that Epac2 was selectively important for cAMP-potentiated fusion events in the first phase of insulin secretion (Shibasaki et al., 2007). The effects of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), which potentiate glucose-stimulated insulin release, are also thought to be mediated through the Rap1 pathway (Ehses et al., 2001; Ehses et al., 2002; Kwan and Gaisano, 2005). For example, GLP-1 rescues the first-phase defect in patients with type 2 diabetes, and this is mediated in part through cAMP-Epac2 signaling (Kwan et al., 2007). More recently, RalA was discovered in β-cells, in which it was found to localize to the plasma membrane and cytosol but to be excluded from secretory granules (Lopez et al., 2008). Depletion of RalA from INS-1 β-cells resulted in severe inhibition of depolarization-induced insulin exocytosis as determined by membrane capacitance, including a reduction in the size of the RRP and a decrease in the subsequent mobilization and exocytosis of the reserve pool of granules. In other cell types, RalA is known to mediate GTP-dependent exocytosis through interaction with the exocyst complex, so the exocyst complex has been implicated as a facilitator of insulin-granule tethering and docking (Lopez et al., 2008). Thus, similar to the Rab proteins, Rap1 and RalA appear to function in the docking and/or priming steps of granule mobilization to the plasma membrane, as depicted in the central pathway of the flowchart in Fig. 4.
It is evident that activation of GTPases in response to glucose is important, but how the GTPases are specifically activated remains unclear. Several studies have suggested that an increase in GTP levels, triggered by glucose metabolism, has an important role in insulin release (Metz et al., 1993; Metz et al., 1992) because glucose stimulates a threefold increase in the de novo synthesis of GTP, which is required (and can be rate-limiting) for insulin release. Moreover, selective depletion of GTP (with ATP levels remaining stable) completely abolished the glucose-induced augmentation of insulin release, which is often equated with the nutrient-specific second-phase insulin release. By sharp contrast, insulin release that was induced by KCl was relatively unaffected by GTP depletion, which indicates that nutrient-induced augmentation is distinguished by its GTP-dependence (Komatsu et al., 1998).
SNARE proteins and their accessory factors
The first images of the fusion of insulin-containing granules with the plasma membrane during the process of insulin release were published in 1957 (Lacy and Davies, 1957; Lacy, 1961; Orci et al., 1973). However, it was not until 1993 that the molecular basis for vesicle fusion and secretion, termed `vesicle exocytosis', was elucidated by the discovery of SNARE proteins in neuronal cell types (Sollner et al., 1993a; Sollner et al., 1993b). Vesicle exocytosis entails the pairing at the target membrane of a vesicle-associated membrane protein (VAMP), a v-SNARE, with a binary cognate receptor complex that comprises SNAP25 or SNAP23 and a syntaxin protein (both of which are t-SNAREs) (Calakos et al., 1994; Chapman et al., 1994; Fasshauer et al., 1997; Hayashi et al., 1994; Hayashi et al., 1995; Kee et al., 1995; Poirier et al., 1998) to form the heterotrimeric SNARE core complex. On the basis of these studies, investigators from the β-cell field discovered that SNARE core complexes were also responsible for regulated secretion of insulin from islet β-cells in response to increased blood glucose (Daniel et al., 1999; Jacobsson et al., 1994; Kiraly-Borri et al., 1996; Nakamichi and Nagamatsu, 1999; Regazzi et al., 1995; Sadoul et al., 1995; Wheeler et al., 1996; Yang et al., 1999). Numerous recent reports link type 2 diabetes with single nucleotide polymorphisms (SNPs) and with alterations in the abundance of SNARE proteins (Ostenson et al., 2006; Romeo et al., 2008; Tsunoda et al., 2001). Moreover, several animal models of insulin resistance and diabetes lack normal levels of these proteins (Chan et al., 1999; Nagamatsu et al., 1999), and experimental induction of diabetes using streptozotocin results in loss of expression of SNARE proteins (Yechoor et al., 2004). Table 1 lists the SNARE proteins and SNARE accessory proteins that are implicated in β-cell insulin secretion, and selected SNAREs and accessory proteins are discussed in more detail below.
Table 1.
SNARE proteins and SNARE accessory factors implicated in biphasic insulin secretion
| Proteins | Phase implicated | Reference(s) |
|---|---|---|
| SNARE proteins | ||
| t-SNAREs | ||
| Syntaxin 1 | First | (Ohara-Imaizumi et al., 2007) |
| Syntaxin 2 | N.D. | (Jacobsson et al., 1994; Kang et al., 2002) |
| Syntaxin 3 | N.D. | (Kang et al., 2002) |
| Syntaxin 4 | First and second | (Spurlin and Thurmond, 2006) |
| SNAP-23 | N.D. | (Sadoul et al., 1997) |
| SNAP-25 | N.D. | (Sadoul et al., 1995) |
| v-SNAREs | ||
| VAMP2 | N.D. | (Jacobsson et al., 1994; Regazzi et al., 1995) |
| VAMP3 | N.D. | (Jacobsson et al., 1994; Regazzi et al., 1995) |
| Accessory factors | ||
| Syntaxin-1 accessory factors | ||
| Munc18a | N.D. | (Tomas et al., 2008; Zhang et al., 2000) |
| Munc13 | First and second | (Kwan et al., 2007) |
| Tomosyn | N.D. | (Cheviet et al., 2006; Zhang et al., 2006) |
| Granuphilin | First and second | (Gomi et al., 2005) |
| Munc18b | N.D. | (Zhang et al., 2000) |
| Doc2β | N.D. | (Verhage et al., 1997) |
| Syntaxin-4 accessory factors | ||
| Munc18c | Second phase | (Oh and Thurmond, 2009; Wheeler et al., 1996) |
| WNK1 | N.D. | (Oh et al., 2007) |
| Doc2β | N.D. | (Ke et al., 2007) |
| Synip | N.D. | (Saito et al., 2003) |
N.D., not determined
The syntaxin family
In the 1990s, toxin-mediated cleavage of the SNARE syntaxin 1 was shown to inhibit 95% of KCl-induced insulin secretion, but only 25% of glucose-induced secretion, from cultured and primary islet β-cells (Land et al., 1997; Yang et al., 1999). However, similar cleavage experiments revealed the absolute necessity for the v-SNARE VAMP2 in glucose-stimulated insulin release from islets (Lang, 1999; Regazzi et al., 1995; Sadoul et al., 1995). Taken together, these data suggested that the syntaxin-1-based SNARE complexes are responsible for KCl-induced secretion, but that glucose-stimulated secretion probably involves a second VAMP2-dependent SNARE complex that does not include syntaxin 1. It is now known that pancreatic β-cells contain three additional plasma-membrane-localized syntaxin isoforms (syntaxins 2, 3 and 4), which suggests that additional SNARE complexes participate in the regulation of insulin secretion (Jacobsson et al., 1994; Lang, 1999; Sadoul et al., 1997; Wheeler et al., 1996). In support of the early toxin studies, more recent perifusion studies using syntaxin 1A–/– mouse islets show impaired first-phase secretion (correlated with a reduction in pre-docked granules), and normal second-phase secretion (Ohara-Imaizumi et al., 2007). Concurrently, perifusion studies of islets from syntaxin 4+/– mice revealed impairments in both phases of glucose-stimulated insulin release (Spurlin and Thurmond, 2006), and islets isolated from transgenic mice overexpressing syntaxin 4 in pancreatic β-cells showed enhancements of both phases of secretion (Spurlin and Thurmond, 2006). Additional studies that implicate syntaxin-4 accessory factors (detailed below) in glucose-stimulated insulin secretion further substantiate its role in this process (Ke et al., 2007; Saito et al., 2003). The finding that numerous syntaxin isoforms are involved in glucose-stimulated insulin release echoes data from other cell systems that utilize multiple syntaxin isoforms, in which partitioning of SNARE interactions and localization of syntaxins to particular plasma-membrane sub-compartments has been implicated in differential modes of vesicle targeting and fusion (Low et al., 2006). In one example in β-cells, there is evidence that syntaxin 4, but not syntaxin 1A, can directly associate with F-actin (Jewell et al., 2008a).
Munc18 and Munc13 proteins – SNARE accessory factors
Concurrent with the discovery of SNARE proteins came evidence from yeast genetics that indicated a requirement for the Sec1 accessory protein in the regulation of SNARE-core-complex assembly (Ferro-Novick and Jahn, 1994). This led to the later discovery of the mammalian Munc18 secretory proteins, which are now collectively referred to as the SM (Sec1/Munc18) protein family (Hata et al., 1993; Pevsner et al., 1994). Islet β-cells contain all three Munc18 isoforms, which localize both to the plasma membrane and to the cytosolic compartment. Munc18a expression is restricted to neurons and islet β-cells, whereas Munc18b and Munc18c are more ubiquitously expressed. The Munc18 proteins show binding specificity for syntaxin isoforms, such that Munc18a and Munc18b associate with syntaxin isoforms 1-3, whereas only Munc18c can bind to syntaxin 4 (Garcia et al., 1995; Halachmi and Lev, 1996; Tellam et al., 1997; Tellam et al., 1995).
Munc18a
In 2000, Munc18a was shown to associate with syntaxin 1A in rat islet β-cells and INS-1 β-cells (Zhang et al., 2000). Through its interaction with syntaxin 1A, Munc18a has been shown to inhibit SNARE-complex assembly under unstimulated conditions or when it is overexpressed in β-cells; it has been proposed that this is because Munc18a can sequester syntaxin 1A in a `closed conformation' that is not favorable for interaction with other v- or t-SNARE proteins (Dong et al., 2007). However, as is the case for most known SM proteins, depletion of Munc18a from MIN6 β-cells resulted in reduced insulin exocytosis (Tomas et al., 2008). Moreover, the study by Tomas and colleagues localized the defect to aberrant insulin-granule docking at the plasma membrane – specifically, loss of coupling between granules and plasma-membrane syntaxin 1A, which, the authors show, occurs through a novel interaction with the granule protein granuphilin (Tomas et al., 2008). Syntaxin 1A can also associate with the exocytosis-inhibiting protein tomosyn and the Munc accessory factor double C2-like domain-containing protein β (Doc2β), although the function of these interactions in biphasic insulin release is untested (Cheviet et al., 2006; Zhang et al., 2006). Because Munc18a associates with the syntaxin-1A isoform, which is required for first-phase secretion only, it is hypothesized that Munc18a will also be required specifically for this phase, although this remains to be tested in islets.
Munc18c
Munc18c is expressed in primary mouse islets and clonal MIN6 β-cells, and has been shown to bind to syntaxin 4 in these cells (Oh et al., 2005; Spurlin and Thurmond, 2006; Wheeler et al., 1996). Islets isolated from Munc18c+/– mice have a 50% reduction in glucose-stimulated insulin secretion (Oh et al., 2005), and very recent data show that this is exclusively due to the ablation of second-phase release (Oh and Thurmond, 2009). Moreover, RNAi-mediated depletion and antibody immuno-depletion of endogenous Munc18c from MIN6 β-cells results in significant loss of glucose-stimulated but not KCl-stimulated insulin secretion, supporting the hypothesis that Munc18c is required for second-phase secretion. Munc18c has been shown to undergo glucose-induced tyrosine phosphorylation at Y219 in MIN6 β-cells, which reduces its association with syntaxin 4 and simultaneously increases its binding to Doc2β (Jewell et al., 2008b; Ke et al., 2007; Oh and Thurmond, 2006). Overexpression of Doc2β results in enhanced glucose-stimulated insulin release, a glucose-stimulated reduction of syntaxin-4–Munc18c binding and a coordinate increase in syntaxin-4-VAMP2 association; these findings indicate a positive role for Doc2β in granule docking and/or fusion (Ke et al., 2007). Interestingly, the associations of Doc2β with Munc18a and Munc18c are mediated through distinct calcium/phospholipid-binding (C2) domains of Doc2β (Ke et al., 2007). A second Munc18c-binding factor, WNK1 [`with no lysine (K) 1'], was found to associate with Munc18c at the plasma membrane (as syntaxin 4 and Doc2β do), but it also bound to Munc18c in the cytosolic compartment (Oh et al., 2007). The WNK1-Munc18c interaction was not that of the typical kinase-substrate as Munc18c failed to act as a substrate for WNK1, although the complex was determined to be important for syntaxin-4-mediated insulin exocytosis (Oh et al., 2007). Further studies of depletion and ablation of WNK1 and Doc2β in islets will be necessary to determine whether their effects on insulin release are through Munc18c and are selectively required for the second phase of insulin release.
Munc13-1
Munc13-1 has been characterized as a priming factor in insulin-granule exocytosis, in that it increases the size and release probability of the RRP in β-cells (Kwan et al., 2006). Islets from Munc13-1+/– mice exhibited impaired first- and second-phase insulin secretion (Kwan et al., 2007). Munc13-1 protein contains a diacylglycerol (DAG)-binding domain, and this DAG-binding function is implicated in second-phase secretion (Kang et al., 2006). Furthermore, overexpression of Munc13-1 has been demonstrated to facilitate the release of granules from β-cells (Sheu et al., 2003). Similar to Munc18a, Munc13-1 can bind to syntaxin 1A and is believed to facilitate the transition of `closed' syntaxin 1A to its `open' form to facilitate association into SNARE core complexes. Interestingly, Munc13-1 has also been shown to interact with Doc2β [although this is via sites that are distinct from those that interact with the Munc18 isoforms (Orita et al., 1997; Verhage et al., 1997)] and, as such, might indirectly impact Munc18c function in second-phase secretion.
Conclusions and perspectives
Since the discovery decades ago that cortical F-actin and microtubule networks were present in β-cells, it has been widely accepted that these components regulated the partitioning and the mobility of insulin-containing granules. However, not until the past decade have we had the molecular tools, the mouse models and, most recently, the powerful microscopic devices with which to determine the molecular mechanisms that underlie this phenomenon. These recent advances have shown that F-actin does not simply act in a passive manner as a barrier between syntaxin-based fusion sites at the plasma membrane and the granules, but functions more as an active participant in corralling granules between pools and towards SNARE sites. F-actin is well suited to this task, as insulin release and RRP refilling must occur across the expanses of the β-cell, yet must be coordinated to occur simultaneously to achieve the precisely timed biphasic release of insulin. Because biphasic release is elicited by nutrients, with glucose being the most common nutrient used experimentally, glucose must be able to transmit signals to coordinate these simultaneous events.
The F-actin-remodeling small Rho-family GTPases Cdc42 and Rac1 have been shown to be intimately involved in selectively mediating the second phase of insulin release in response to nutrient signals, although the many cycling factors that must be required for these GTPases remain largely unidentified. Similarly, several Rab- and Ras-family GTPases are known to be involved in granule docking and/or priming but their roles in biphasic secretion in islets remain unconfirmed. This is a common lack among the proteins discussed in this Commentary, which cannot be integrated into the stimulus-secretion coupling models of second-phase insulin release until they are appropriately assessed in islets. Whether the mouse or rat islet best replicates the second-phase release pattern of human islets is also an issue (Berglund, 1980; Henquin et al., 2006), as is whether the islets should be studied after time in culture or fresh after isolation. The appropriateness of capacitance measurements, TIRFM, islet perifusion and in vivo pancreatic perfusion as reliable methodologies to study second-phase insulin secretion also remains to be fully resolved (see Box 1). It becomes particularly important for TIRFM and capacitance methods to be adapted for studies of β-cells within the context of the islet as opposed to isolated cells, as results gained might differ from and challenge existing models (Gopel et al., 2004). Resolution of these issues will certainly accelerate our progress towards gaining a detailed molecular understanding of events that underlie second-phase secretion.
We thank Michael Kalwat for careful reading of this manuscript. This work was supported by NIH grants DK-076614 and DK-067912 to D.C.T. Deposited in PMC for release after 12 months.
References
- Asahara, S., Kido, Y., Shigeyama, T., Matsuda, T., Takeda, A., Inoue, T., Shibutani, Y., Koyanagi, M., Uchida, T. and Kasuga, M. (2008). Rac1 regulates glucose-induced insulin secretion through modulation of cytoskeleton organization in beta cells. Diabetes 57, Suppl. 1, A55. [Google Scholar]
- Balczon, R., Overstreet, K. A., Zinkowski, R. P., Haynes, A. and Appel, M. (1992). The identification, purification, and characterization of a pancreatic beta-cell form of the microtubule adenosine triphosphatase kinesin. Endocrinology 131, 331-336. [DOI] [PubMed] [Google Scholar]
- Barg, S., Huang, P., Eliasson, L., Nelson, D. J., Obermuller, S., Rorsman, P., Thevenod, F. and Renstrom, E. (2001). Priming of insulin granules for exocytosis by granular Cl(-) uptake and acidification. J. Cell Sci. 114, 2145-2154. [DOI] [PubMed] [Google Scholar]
- Barg, S., Eliasson, L., Renstrom, E. and Rorsman, P. (2002). A subset of 50 secretory granules in close contact with L-type Ca(2+) channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes 51, S74-S82. [DOI] [PubMed] [Google Scholar]
- Berglund, O. (1980). Different dynamics of insulin secretion in the perfused pancreas of mouse and rat. Acta Endocrinol. 93, 54-60. [DOI] [PubMed] [Google Scholar]
- Bishop, A. L. and Hall, A. (2000). Rho GTPases and their effector proteins. Biochem. J. 348, 241-255. [PMC free article] [PubMed] [Google Scholar]
- Bos, J. L. (2003). Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell. Biol. 4, 733-738. [DOI] [PubMed] [Google Scholar]
- Brown, J. R., Stafford, P. and Langford, G. M. (2004). Short-range axonal/dendritic transport by myosin-V: a model for vesicle delivery to the synapse. J. Neurobiol. 58, 175-188. [DOI] [PubMed] [Google Scholar]
- Bruun, T. Z., Hoy, M. and Gromada, J. (2000). Scinderin-derived actin-binding peptides inhibit Ca(2+)- and GTPgammaS-dependent exocytosis in mouse pancreatic beta-cells. Eur. J. Pharmacol. 403, 221-224. [DOI] [PubMed] [Google Scholar]
- Calakos, N., Bennett, M. K. and Peterson, K. E. (1994). Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science 263, 1146-1149. [DOI] [PubMed] [Google Scholar]
- Cerasi, E. (1975). Mechanisms of glucose stimulated insulin secretion in health and in diabetes: some re-evaluations and proposals. Diabetologia 11, 1-13. [DOI] [PubMed] [Google Scholar]
- Cerasi, E., Fick, G. and Rudemo, M. (1974). A mathematical model for the glucose induced insulin release in man. Eur. J. Clin. Invest. 4, 267-278. [DOI] [PubMed] [Google Scholar]
- Chan, C. B., MacPhail, R. M., Sheu, L., Wheeler, M. B. and Gaisano, H. Y. (1999). Beta-cell hypertrophy in fa/fa rats is associated with basal glucose hypersensitivity and reduced SNARE protein expression. Diabetes 48, 997-1005. [DOI] [PubMed] [Google Scholar]
- Chapman, E., An, S., Barton, N. and Jahn, R. (1994). SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J. Biol. Chem. 269, 27427-27432. [PubMed] [Google Scholar]
- Cheviet, S., Bezzi, P., Ivarsson, R., Renstrom, E., Viertl, D., Kasas, S., Catsicas, S. and Regazzi, R. (2006). Tomosyn-1 is involved in a post-docking event required for pancreatic beta-cell exocytosis. J. Cell Sci. 119, 2912-2920. [DOI] [PubMed] [Google Scholar]
- Cook, D. L. and Hales, C. N. (1984). Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311, 271-273. [DOI] [PubMed] [Google Scholar]
- Curry, D. L., Bennett, L. L. and Grodsky, G. M. (1968). Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology 83, 572-584. [DOI] [PubMed] [Google Scholar]
- Daniel, S., Noda, M., Straub, S. G., Sharp, G. W., Komatsu, M., Schermerhorn, T. and Aizawa, T. (1999). Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes 48, 1686-1690. [DOI] [PubMed] [Google Scholar]
- Daniel, S., Noda, M., Cerione, R. A. and Sharp, G. W. (2002). A link between Cdc42 and syntaxin is involved in mastoparan-stimulated insulin release. Biochemistry 41, 9663-9671. [DOI] [PubMed] [Google Scholar]
- DerMardirossian, C. and Bokoch, G. M. (2005). GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356-363. [DOI] [PubMed] [Google Scholar]
- Donelan, M. J., Morfini, G., Julyan, R., Sommers, S., Hays, L., Briaud, I., Kajio, H., Easom, R. A., Molkentin, J. D., Brady, S. T. et al. (2002). Ca2+-dependent dephosphorylation of kinesin heavy chain on {beta}-granules in pancreatic {beta}-cells: implications for regulated {beta}-granule transport and insulin exocytosis. J. Biol. Chem. 26, 26. [DOI] [PubMed] [Google Scholar]
- Dong, Y., Wan, Q., Yang, X., Bai, L. and Xu, P. (2007). Interaction of Munc18 and Syntaxin in the regulation of insulin secretion. Biochem. Biophys. Res. Commun. 360, 609-614. [DOI] [PubMed] [Google Scholar]
- Ehses, J. A., Lee, S. S., Pederson, R. A. and McIntosh, C. H. (2001). A new pathway for glucose-dependent insulinotropic polypeptide (GIP) receptor signaling: evidence for the involvement of phospholipase A2 in GIP-stimulated insulin secretion. J. Biol. Chem. 276, 23667-23673. [DOI] [PubMed] [Google Scholar]
- Ehses, J. A., Pelech, S. L., Pederson, R. A. and McIntosh, C. H. (2002). Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J. Biol. Chem. 277, 37088-37097. [DOI] [PubMed] [Google Scholar]
- Farshori, P. Q. and Goode, D. (1994). Effects of the microtubule depolymerizing and stabilizing agents Nocodazole and taxol on glucose-induced insulin secretion from hamster islet tumor (HIT) cells. J. Submicrosc. Cytol. Pathol. 26, 137-146. [PubMed] [Google Scholar]
- Fasshauer, D., Otto, H., Eliason, W. K., Jahn, R. and Brunger, A. T. (1997). Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem. 272, 28036-28041. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick, S. and Jahn, R. (1994). Vesicle fusion from yeast to man. Nature 370, 191-193. [DOI] [PubMed] [Google Scholar]
- Garcia, E. P., McPherson, P. S., Chilcote, T. J., Takei, K. and De Camilli, P. (1995). rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J. Cell Biol. 129, 105-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasman, S., Chasserot-Golaz, S., Malacombe, M., Way, M. and Bader, M. F. (2004). Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol. Biol. Cell 15, 520-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembal, M., Gilon, P. and Henquin, J. C. (1992). Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J. Clin. Invest. 89, 1288-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich, J. E. (2002). Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 51, S117-S121. [DOI] [PubMed] [Google Scholar]
- Gomi, H., Mizutani, S., Kasai, K., Itohara, S. and Izumi, T. (2005). Granuphilin molecularly docks insulin granules to the fusion machinery. J. Cell Biol. 171, 99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopel, S., Zhang, Q., Eliasson, L., Ma, X. S., Galvanovskis, J., Kanno, T., Salehi, A. and Rorsman, P. (2004). Capacitance measurements of exocytosis in mouse pancreatic alpha-, beta- and delta-cells within intact islets of Langerhans. J. Physiol. 556, 711-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky, G. M. (1972). A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J. Clin. Invest. 51, 2047-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky, G. M. (2000). Kinetics of insulin secretion: underlying metabolic events. In Diabetes Mellitus: A Fundamental and Clinical Text (ed. D. LeRoith, S. Taylor and J. Olefsky). Philadelphia, PA: Lippincott Williams and Wilkins.
- Grosshans, B. L., Ortiz, D. and Novick, P. (2006). Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA 103, 11821-11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi, N. and Lev, Z. (1996). The Sec1 family: a novel family of proteins involved in synaptic transmission and general secretion. J. Neurochem. 66, 889-897. [DOI] [PubMed] [Google Scholar]
- Hata, Y., Slaughter, C. A. and Sudhof, T. C. (1993). Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 366, 347-351. [DOI] [PubMed] [Google Scholar]
- Hayashi, T., McMahon, H., Yamasaki, S., Binz, T., Hata, Y., Sudhof, T. C. and Niemann, H. (1994). Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 13, 5051-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, T., Yamasaki, S., Nauenburg, S., Binz, T. and Niemann, H. (1995). Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J. 14, 2317-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin, J. C., Ishiyama, N., Nenquin, M., Ravier, M. A. and Jonas, J. C. (2002). Signals and pools underlying biphasic insulin secretion. Diabetes 51, S60-S67. [DOI] [PubMed] [Google Scholar]
- Henquin, J. C., Nenquin, M., Stiernet, P. and Ahren, B. (2006). In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: pattern and role of cytoplasmic Ca2+ and amplification signals in beta-cells. Diabetes 55, 441-451. [DOI] [PubMed] [Google Scholar]
- Hohmeier, H. E., Mulder, H., Chen, G., Henkel-Rieger, R., Prentki, M. and Newgard, C. B. (2000). Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424-430. [DOI] [PubMed] [Google Scholar]
- Howell, S. L. and Tyhurst, M. (1979). Interaction between insulin-storage granules and F-actin in vitro. Biochem. J. 178, 367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, S. L. and Tyhurst, M. (1986). The cytoskeleton and insulin secretion. Diabetes Metab. Rev. 2, 107-123. [DOI] [PubMed] [Google Scholar]
- Huang, J. D., Brady, S. T., Richards, B. W., Stenolen, D., Resau, J. H., Copeland, N. G. and Jenkins, N. A. (1999). Direct interaction of microtubule- and actin-based transport motors. Nature 397, 267-270. [DOI] [PubMed] [Google Scholar]
- Huypens, P., Ling, Z., Pipeleers, D. and Schuit, F. (2000). Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43, 1012-1019. [DOI] [PubMed] [Google Scholar]
- Ivarsson, R., Jing, X., Waselle, L., Regazzi, R. and Renstrom, E. (2005). Myosin 5a controls insulin granule recruitment during late-phase secretion. Traffic 6, 1027-1035. [DOI] [PubMed] [Google Scholar]
- Izumi, T. (2007). Physiological roles of Rab27 effectors in regulated exocytosis. Endocr. J. 54, 649-657. [DOI] [PubMed] [Google Scholar]
- Jacobsson, G., Bean, A. J., Scheller, R. H., Juntti-Berggren, L., Deeney, J. T., Berggren, P. O. and Meister, B. (1994). Identification of synaptic proteins and their isoform mRNAs in compartments of pancreatic endocrine cells. Proc. Natl. Acad. Sci. USA 91, 12487-12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell, J. L., Luo, W., Oh, E., Wang, Z. and Thurmond, D. C. (2008a). Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4. J. Biol. Chem. 283, 10716-10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell, J. L., Oh, E., Bennett, S. M., Meroueh, S. O. and Thurmond, D. C. (2008b). The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2beta. J. Biol. Chem. 283, 21734-21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just, I., Selzer, J., Wilm, M., von Eichel-Streiber, C., Mann, M. and Aktories, K. (1995). Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500-503. [DOI] [PubMed] [Google Scholar]
- Kahn, S. E. (2001). Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J. Clin. Endocrinol. Metab. 86, 4047-4058. [DOI] [PubMed] [Google Scholar]
- Kang, L., He, Z., Xu, P., Fan, J., Betz, A., Brose, N. and Xu, T. (2006). Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab. 3, 463-468. [DOI] [PubMed] [Google Scholar]
- Kang, Y., Huang, X., Pasyk, E. A., Ji, J., Holz, G. G., Wheeler, M. B., Tsushima, R. G. and Gaisano, H. Y. (2002). Syntaxin-3 and syntaxin-1A inhibit L-type calcium channel activity, insulin biosynthesis and exocytosis in beta-cell lines. Diabetologia 45, 231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai, K., Ohara-Imaizumi, M., Takahashi, N., Mizutani, S., Zhao, S., Kikuta, T., Kasai, H., Nagamatsu, S., Gomi, H. and Izumi, T. (2005). Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J. Clin. Invest. 115, 388-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai, K., Fujita, T., Gomi, H. and Izumi, T. (2008). Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic 9, 1191-1203. [DOI] [PubMed] [Google Scholar]
- Ke, B., Oh, E. and Thurmond, D. C. (2007). Doc2beta is a novel Munc18c-interacting partner and positive effector of syntaxin 4-mediated exocytosis. J. Biol. Chem. 282, 21786-21797. [DOI] [PubMed] [Google Scholar]
- Kee, Y., Lin, R. C., Hsu, S. C. and Scheller, R. H. (1995). Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron 14, 991-998. [DOI] [PubMed] [Google Scholar]
- Kennedy, H. J., Pouli, A. E., Ainscow, E. K., Jouaville, L. S., Rizzuto, R. and Rutter, G. A. (1999). Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J. Biol. Chem. 274, 13281-13291. [DOI] [PubMed] [Google Scholar]
- Kiraly-Borri, C. E., Morgan, A., Burgoyne, R. D., Weller, U., Wollheim, C. B. and Lang, J. (1996). Soluble N-ethylmaleimide-sensitive-factor attachment protein and N-ethylmaleimide-insensitive factors are required for Ca2+-stimulated exocytosis of insulin. Biochem. J. 314, 199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu, M., Noda, M. and Sharp, G. W. (1998). Nutrient augmentation of Ca2+-dependent and Ca2+-independent pathways in stimulus-coupling to insulin secretion can be distinguished by their guanosine triphosphate requirements: studies on rat pancreatic islets. Endocrinology 139, 1172-1183. [DOI] [PubMed] [Google Scholar]
- Konstantinova, I., Nikolova, G., Ohara-Imaizumi, M., Meda, P., Kucera, T., Zarbalis, K., Wurst, W., Nagamatsu, S. and Lammert, E. (2007). EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 129, 359-370. [DOI] [PubMed] [Google Scholar]
- Kowluru, A. and Veluthakal, R. (2005). Rho guanosine diphosphate-dissociation inhibitor plays a negative modulatory role in glucose-stimulated insulin secretion. Diabetes 54, 3523-3529. [DOI] [PubMed] [Google Scholar]
- Kowluru, A., Seavey, S. E., Li, G., Sorenson, R. L., Weinhaus, A. J., Nesher, R., Rabaglia, M. E., Vadakekalam, J. and Metz, S. A. (1996). Glucose- and GTP-dependent stimulation of the carboxyl methylation of CDC42 in rodent and human pancreatic islets and pure beta cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J. Clin. Invest. 98, 540-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru, A., Li, G., Rabaglia, M. E., Segu, V. B., Hofmann, F., Aktories, K. and Metz, S. A. (1997). Evidence for differential roles of the Rho subfamily of GTP-binding proteins in glucose- and calcium-induced insulin secretion from pancreatic beta cells. Biochem. Pharmacol. 54, 1097-1108. [DOI] [PubMed] [Google Scholar]
- Kwan, E. P. and Gaisano, H. Y. (2005). Glucagon-like peptide 1 regulates sequential and compound exocytosis in pancreatic islet beta-cells. Diabetes 54, 2734-2743. [DOI] [PubMed] [Google Scholar]
- Kwan, E. P., Xie, L., Sheu, L., Nolan, C. J., Prentki, M., Betz, A., Brose, N. and Gaisano, H. Y. (2006). Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes 55, 1421-1429. [DOI] [PubMed] [Google Scholar]
- Kwan, E. P., Xie, L., Sheu, L., Ohtsuka, T. and Gaisano, H. Y. (2007). Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes 56, 2579-2588. [DOI] [PubMed] [Google Scholar]
- Lacy, P. E. (1961). Electron microscopy of the beta cell of the pancreas. Am. J. Med. 31, 851. [DOI] [PubMed] [Google Scholar]
- Lacy, P. E. and Davies, J. (1957). Preliminary studies on the demonstration of insulin in the islets by the fluorescent antibody technic. Diabetes 6, 354. [DOI] [PubMed] [Google Scholar]
- Lacy, P. E., Walker, M. M. and Fink, C. J. (1972). Perifusion of isolated rat islets in vitro. Participation of the microtubular system in the biphasic release of insulin. Diabetes 21, 987-998. [DOI] [PubMed] [Google Scholar]
- Land, J., Zhang, H., Vaidyanathan, V. V., Sadoul, K., Niemann, H. and Wollheim, C. B. (1997). Transient expression of botulinum neurotoxin C1 light chain differentially inhibits calcium and glucose induced insulin secretion in clonal beta-cells. FEBS Lett. 419, 13-17. [DOI] [PubMed] [Google Scholar]
- Lang, J. (1999). Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur. J. Biochem. 259, 3-17. [DOI] [PubMed] [Google Scholar]
- Langford, G. M. (2002). Myosin-V, a versatile motor for short-range vesicle transport. Traffic 3, 859-865. [DOI] [PubMed] [Google Scholar]
- Leech, C. A., Holz, G. G., Chepurny, O. and Habener, J. F. (2000). Expression of cAMP-regulated guanine nucleotide exchange factors in pancreatic beta-cells. Biochem. Biophys. Res. Commun. 278, 44-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser, M., Efrat, S. and Fleischer, N. (1995). Evidence that Rap1 carboxylmethylation is involved in regulated insulin secretion. Endocrinology 136, 2521-2530. [DOI] [PubMed] [Google Scholar]
- Li, G., Rungger-Brandle, E., Just, I., Jonas, J. C., Aktories, K. and Wollheim, C. B. (1994). Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Mol. Biol. Cell 5, 1199-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Luo, R., Kowluru, A. and Li, G. (2004). Novel regulation by Rac1 of glucose- and forskolin-induced insulin secretion in INS-1 beta-cells. Am. J. Physiol. Endocrinol. Metab. 286, E818-E827. [DOI] [PubMed] [Google Scholar]
- Licko, V. (1973). Threshold secretory mechanism: a model of derivative element in biological control. Bull. Math. Biol. 35, 51-58. [PubMed] [Google Scholar]
- Lopez, J. A., Kwan, E. P., Xie, L., He, Y., James, D. E. and Gaisano, H. Y. (2008). The RalA GTPase is a central regulator of insulin exocytosis from pancreatic islet beta cells. J. Biol. Chem. 283, 17939-17945. [DOI] [PubMed] [Google Scholar]
- Low, S. H., Vasanji, A., Nanduri, J., He, M., Sharma, N., Koo, M., Drazba, J. and Weimbs, T. (2006). Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol. Biol. Cell 17, 977-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse, W. J. and Sener, A. (1987). Glucose-induced changes in cytosolic ATP content in pancreatic islets. Biochim. Biophys. Acta 927, 190-195. [DOI] [PubMed] [Google Scholar]
- Malaisse, W. J., Malaisse-Lagae, F., Walker, M. O. and Lacy, P. E. (1971). The stimulus-secretion coupling of glucose-induced insulin release. V. The participation of a microtubular-microfilamentous system. Diabetes 20, 257-265. [DOI] [PubMed] [Google Scholar]
- Malaisse, W. J., Van Obberghen, E., Devis, G., Somers, G. and Ravazzola, M. (1974). Dynamics of insulin release and microtubular-microfilamentous system. V. A model for the phasic release of insulin. Eur. J. Clin. Invest. 4, 313-318. [DOI] [PubMed] [Google Scholar]
- Malaisse, W. J., Malaisse-Lagae, F., Van Obberghen, E., Somers, G., Devis, G., Ravazzola, M. and Orci, L. (1975). Role of microtubules in the phasic pattern of insulin release. Ann. NY Acad. Sci. 253, 630-652. [DOI] [PubMed] [Google Scholar]
- McDaniel, M. L., Bry, C. G., Homer, R. W., Fink, C. J., Ban, D. and Lacy, P. E. (1980). Temporal changes in islet polymerized and depolymerized tubulin during biphasic insulin release. Metabolism 29, 762-766. [DOI] [PubMed] [Google Scholar]
- Meglasson, M. D. and Matschinsky, F. M. (1986). Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab. Rev. 2, 163-214. [DOI] [PubMed] [Google Scholar]
- Meissner, H. P. and Atwater, I. J. (1976). The kinetics of electrical activity of beta cells in response to a `square wave' stimulation with glucose or glibenclamide. Horm. Metab. Res. 8, 11-16. [DOI] [PubMed] [Google Scholar]
- Meng, Y. X., Wilson, G. W., Avery, M. C., Varden, C. H. and Balczon, R. (1997). Suppression of the expression of a pancreatic beta-cell form of the kinesin heavy chain by antisense oligonucleotides inhibits insulin secretion from primary cultures of mouse beta-cells. Endocrinology 138, 1979-1987. [DOI] [PubMed] [Google Scholar]
- Merrins, M. J. and Stuenkel, E. L. (2008). Kinetics of Rab27a-dependent actions on vesicle docking and priming in pancreatic beta-cells. J. Physiol. 586, 5367-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz, S. A., Rabaglia, M. E. and Pintar, T. J. (1992). Selective inhibitors of GTP synthesis impede exocytotic insulin release from intact rat islets. J. Biol. Chem. 267, 12517-12527. [PubMed] [Google Scholar]
- Metz, S. A., Meredith, M., Rabaglia, M. E. and Kowluru, A. (1993). Small elevations of glucose concentration redirect and amplify the synthesis of guanosine 5′-triphosphate in rat islets. J. Clin. Invest. 92, 872-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael, D. J., Xiong, W., Geng, X., Drain, P. and Chow, R. H. (2007). Human insulin vesicle dynamics during pulsatile secretion. Diabetes 56, 1277-1288. [DOI] [PubMed] [Google Scholar]
- Montague, W., Howell, S. L. and Green, I. C. (1976). Insulin release and the microtubular system of the islets of Langerhans: effects of insulin secretagogues on microtubule subunit pool size. Horm. Metab. Res. 8, 166-169. [DOI] [PubMed] [Google Scholar]
- Nagamatsu, S., Nakamichi, Y., Yamamura, C., Matsushima, S., Watanabe, T., Ozawa, S., Furukawa, H. and Ishida, H. (1999). Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes 48, 2367-2373. [DOI] [PubMed] [Google Scholar]
- Nakamichi, Y. and Nagamatsu, S. (1999). Alpha-SNAP functions in insulin exocytosis from mature, but not immature secretory granules in pancreatic beta cells. Biochem. Biophys. Res. Commun. 260, 127-132. [DOI] [PubMed] [Google Scholar]
- Nesher, R. and Cerasi, E. (1987). Biphasic insulin release as the expression of combined inhibitory and potentiating effects of glucose. Endocrinology 121, 1017-1024. [DOI] [PubMed] [Google Scholar]
- Nesher, R. and Cerasi, E. (2002). Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes 51, S53-S59. [DOI] [PubMed] [Google Scholar]
- Nevins, A. K. and Thurmond, D. C. (2003). Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am. J. Physiol. Cell Physiol. 285, C698-C710. [DOI] [PubMed] [Google Scholar]
- Nevins, A. K. and Thurmond, D. C. (2005). A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J. Biol. Chem. 280, 1944-1952. [DOI] [PubMed] [Google Scholar]
- Nevins, A. K. and Thurmond, D. C. (2006). Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J. Biol. Chem. 281, 18961-18972. [DOI] [PubMed] [Google Scholar]
- Noda, M., Komatsu, M. and Sharp, G. W. (1996). The betaHC-9 pancreatic beta-cell line preserves the characteristics of progenitor mouse islets. Diabetes 45, 1766-1773. [DOI] [PubMed] [Google Scholar]
- O'Connor, M. D., Landahl, H. and Grodsky, G. M. (1980). Comparison of storage- and signal-limited models of pancreatic insulin secretion. Am. J. Physiol. 238, R378-R389. [DOI] [PubMed] [Google Scholar]
- Oh, E. and Thurmond, D. C. (2006). The stimulus-induced tyrosine phosphorylation of Munc18c facilitates vesicle exocytosis. J. Biol. Chem. 281, 17624-17634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, E. and Thurmond, D. C. (2009). Munc18c depletion selectively impairs the sustained phase of insulin release. Diabetes (in press). [DOI] [PMC free article] [PubMed]
- Oh, E., Spurlin, B. A., Pessin, J. E. and Thurmond, D. C. (2005). Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes 54, 638-647. [DOI] [PubMed] [Google Scholar]
- Oh, E., Heise, C. J., English, J. M., Cobb, M. H. and Thurmond, D. C. (2007). WNK1 is a novel regulator of Munc18c-syntaxin 4 complex formation in soluble NSF attachment protein receptor (SNARE)-mediated vesicle exocytosis. J. Biol. Chem. 282, 32613-32622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi, M., Nakamichi, Y., Tanaka, T., Ishida, H. and Nagamatsu, S. (2002). Imaging exocytosis of single insulin secretory granules with evanescent wave microscopy: distinct behavior of granule motion in biphasic insulin release. J. Biol. Chem. 277, 3805-3808. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi, M., Nishiwaki, C., Kikuta, T., Nagai, S., Nakamichi, Y. and Nagamatsu, S. (2004). TIRF imaging of docking and fusion of single insulin granule motion in primary rat pancre pub 2001 Dec 21.atic beta-cells: different behaviour of granule motion between normal and Goto-Kakizaki diabetic rat beta-cells. Biochem. J. 381, 13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi, M., Fujiwara, T., Nakamichi, Y., Okamura, T., Akimoto, Y., Kawai, J., Matsushima, S., Kawakami, H., Watanabe, T., Akagawa, K. et al. (2007). Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J. Cell Biol. 177, 695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci, L., Gabbay, K. H. and Malaisse, W. J. (1972). Pancreatic beta-cell web: its possible role in insulin secretion. Science 175, 1128-1130. [DOI] [PubMed] [Google Scholar]
- Orci, L., Amherdt, M., Malaisse-Lagae, F., Rouiller, C. and Renold, A. E. (1973). Insulin release by emiocytosis: demonstration with freeze-etching technique. Science 179, 82-84. [DOI] [PubMed] [Google Scholar]
- Orita, S., Naito, A., Sakaguchi, G., Maeda, M., Igarashi, H., Sasaki, T. and Takai, Y. (1997). Physical and functional interactions of Doc2 and Munc13 in Ca2+-dependent exocytotic machinery. J. Biol. Chem. 272, 16081-16084. [DOI] [PubMed] [Google Scholar]
- Ostenson, C. G., Gaisano, H., Sheu, L., Tibell, A. and Bartfai, T. (2006). Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes 55, 435-440. [DOI] [PubMed] [Google Scholar]
- Pevsner, J., Hsu, S. C., Braun, J. E., Calakos, N., Ting, A. E., Bennett, M. K. and Scheller, R. H. (1994). Specificity and regulation of a synaptic vesicle docking complex. Neuron 13, 353-361. [DOI] [PubMed] [Google Scholar]
- Pfeifer, M. A., Halter, J. B. and Porte, D., Jr (1981). Insulin secretion in diabetes mellitus. Am. J. Med. 70, 579-588. [DOI] [PubMed] [Google Scholar]
- Poirier, M. A., Hao, J. C., Malkus, P. N., Chan, C., Moore, M. F., King, D. S. and Bennett, M. K. (1998). Protease resistance of syntaxin·SNAP-25·VAMP complexes: implications for assembly and structure. J. Biol. Chem. 273, 11370-11377. [DOI] [PubMed] [Google Scholar]
- Polonsky, K. S., Sturis, J. and Bell, G. I. (1996). Seminars in medicine of the Beth Israel Hospital, Boston: non-insulin-dependent diabetes mellitus-a genetically programmed failure of the beta cell to compensate for insulin resistance. N. Engl. J. Med. 334, 777-783. [DOI] [PubMed] [Google Scholar]
- Porte, D., Jr (1991). Banting lecture 1990: Beta-cells in type II diabetes mellitus. Diabetes 40, 166-180. [DOI] [PubMed] [Google Scholar]
- Regazzi, R., Kikuchi, A., Takai, Y. and Wollheim, C. B. (1992). The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J. Biol. Chem. 267, 17512-17519. [PubMed] [Google Scholar]
- Regazzi, R., Wollheim, C. B., Lang, J., Theler, J. M., Rossetto, O., Montecucco, C., Sadoul, K., Weller, U., Palmer, M. and Thorens, B. (1995). VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J. 14, 2723-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi, R., Ravazzola, M., Iezzi, M., Lang, J., Zahraoui, A., Andereggen, E., Morel, P., Takai, Y. and Wollheim, C. B. (1996). Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J. Cell Sci. 109, 2265-2273. [DOI] [PubMed] [Google Scholar]
- Renstrom, E., Eliasson, L., Bokvist, K. and Rorsman, P. (1996). Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. J. Physiol. 494, 41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes, C. J. (2000). Processing of the insulin molecule. In Diabetes Mellitus: A Fundamental and Clinical Text (ed. T. LeRoith, S. I. Taylor and J. M. Olefsky), pp. 20-38. Philadelphia, PA: Lippincott Williams and Wilkins.
- Romeo, S., Sentinelli, F., Cavallo, M. G., Leonetti, F., Fallarino, M., Mariotti, S. and Baroni, M. G. (2008). Search for genetic variants of the SYNTAXIN 1A (STX1A) gene: the -352 A>T variant in the STX1A promoter associates with impaired glucose metabolism in an Italian obese population. Int. J. Obes. 32, 413-420. [DOI] [PubMed] [Google Scholar]
- Rorsman, P., Eliasson, L., Renstrom, E., Gromada, J., Barg, S. and Gopel, S. (2000). The cell physiology of biphasic insulin secretion. News Physiol. Sci. 15, 72-77. [DOI] [PubMed] [Google Scholar]
- Sadoul, K., Lang, J., Montecucco, C., Weller, U., Regazzi, R., Catsicas, S., Wollheim, C. B. and Halban, P. A. (1995). SNAP-25 is expressed in islets of Langerhans and is involved in insulin release. J. Cell Biol. 128, 1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul, K., Berger, A., Niemann, H., Weller, U., Roche, P. A., Klip, A., Trimble, W. S., Regazzi, R., Catsicas, S. and Halban, P. A. (1997). SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J. Biol. Chem. 272, 33023-33027. [DOI] [PubMed] [Google Scholar]
- Safayhi, H., Haase, H., Kramer, U., Bihlmayer, A., Roenfeldt, M., Ammon, H. P., Froschmayr, M., Cassidy, T. N., Morano, I., Ahlijanian, M. K. et al. (1997). L-type calcium channels in insulin-secreting cells: biochemical characterization and phosphorylation in RINm5F cells. Mol. Endocrinol. 11, 619-629. [DOI] [PubMed] [Google Scholar]
- Saito, T., Okada, S., Yamada, E., Ohshima, K., Shimizu, H., Shimomura, K., Sato, M., Pessin, J. E. and Mori, M. (2003). Syntaxin 4 and Synip (syntaxin 4 interacting protein) regulate insulin secretion in the pancreatic beta HC-9 cell. J. Biol. Chem. 278, 36718-36725. [DOI] [PubMed] [Google Scholar]
- Satin, L. S. and Cook, D. L. (1985). Voltage-gated Ca2+ current in pancreatic B-cells. Pflugers Arch. 404, 385-387. [DOI] [PubMed] [Google Scholar]
- Sheu, L., Pasyk, E. A., Ji, J., Huang, X., Gao, X., Varoqueaux, F., Brose, N. and Gaisano, H. Y. (2003). Regulation of insulin exocytosis by Munc13-1. J. Biol. Chem. 278, 27556-27563. [DOI] [PubMed] [Google Scholar]
- Shibasaki, T., Takahashi, H., Miki, T., Sunaga, Y., Matsumura, K., Yamanaka, M., Zhang, C., Tamamoto, A., Satoh, T., Miyazaki, J. et al. (2007). Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc. Natl. Acad. Sci. USA 104, 19333-19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snabes, M. C. and Boyd, A. E. (1982). Increased filamentous actin in islets of Langerhans from fasted hamsters. Biochem. Biophys. Res. Commun. 104, 207-211. [DOI] [PubMed] [Google Scholar]
- Sollner, T., Bennett, M. K., Whiteheart, S. W., Scheller, R. H. and Rothman, J. E. (1993a). A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75, 409-418. [DOI] [PubMed] [Google Scholar]
- Sollner, T., Whiteheart, S. W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P. and Rothman, J. E. (1993b). SNAP receptors implicated in vesicle targeting and fusion [see comments]. Nature 362, 318-324. [DOI] [PubMed] [Google Scholar]
- Somers, G., Blondel, B., Orci, L. and Malaisse, W. J. (1979). Motile events in pancreatic endocrine cells. Endocrinology 104, 255-264. [DOI] [PubMed] [Google Scholar]
- Spurlin, B. A. and Thurmond, D. C. (2006). Syntaxin 4 facilitates biphasic glucose-stimulated insulin secretion from pancreatic {beta}-cells. Mol. Endocrinol. 20, 183-193. [DOI] [PubMed] [Google Scholar]
- Straub, S. G., Shanmugam, G. and Sharp, G. W. (2004). Stimulation of insulin release by glucose is associated with an increase in the number of docked granules in the beta-cells of rat pancreatic islets. Diabetes 53, 3179-3183. [DOI] [PubMed] [Google Scholar]
- Tellam, J. T., McIntosh, S. and James, D. E. (1995). Molecular identification of two novel Munc-18 isoforms expressed in non-neuronal tissues. J. Biol. Chem. 270, 5857-5863. [DOI] [PubMed] [Google Scholar]
- Tellam, J. T., Macaulay, S. L., McIntosh, S., Hewish, D. R., Ward, C. W. and James, D. E. (1997). Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J. Biol. Chem. 272, 6179-6186. [DOI] [PubMed] [Google Scholar]
- Thurmond, D. C., Gonelle-Gispert, C., Furukawa, M., Halban, P. A. and Pessin, J. E. (2003). Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) Complex. Mol. Endocrinol. 17, 732-742. [DOI] [PubMed] [Google Scholar]
- Tomas, A., Yermen, B., Min, L., Pessin, J. E. and Halban, P. A. (2006). Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. J. Cell Sci. 119, 2156-2167. [DOI] [PubMed] [Google Scholar]
- Tomas, A., Meda, P., Regazzi, R., Pessin, J. E. and Halban, P. A. (2008). Munc 18-1 and granuphilin collaborate during insulin granule exocytosis. Traffic 9, 813-832. [DOI] [PubMed] [Google Scholar]
- Tsunoda, K., Sanke, T., Nakagawa, T., Furuta, H. and Nanjo, K. (2001). Single nucleotide polymorphism (D68D, T to C) in the syntaxin 1A gene correlates to age at onset and insulin requirement in Type II diabetic patients. Diabetologia 44, 2092-2097. [DOI] [PubMed] [Google Scholar]
- van Obberghen, E., Somers, G., Devis, G., Vaughan, G. D., Malaisse-Lagae, F., Orci, L. and Malaisse, W. J. (1973). Dynamics of insulin release and microtubular-microfilamentous system. I. Effect of cytochalasin B. J. Clin. Invest. 52, 1041-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi, A., Ainscow, E. K., Allan, V. J. and Rutter, G. A. (2002). Involvement of conventional kinesin in glucose-stimulated secretory granule movements and exocytosis in clonal pancreatic beta-cells. J. Cell Sci. 115, 4177-4189. [DOI] [PubMed] [Google Scholar]
- Varadi, A., Tsuboi, T. and Rutter, G. A. (2005). Myosin Va transports dense core secretory vesicles in pancreatic MIN6 beta-cells. Mol. Biol. Cell 16, 2670-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthakal, R., Madathilparambil, S. V., McDonald, P., Olson, L. K. and Kowluru, A. (2009). Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem. Pharmacol. 77, 101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage, M., de Vries, K. J., Roshol, H., Burbach, J. P., Gispen, W. H. and Sudhof, T. C. (1997). DOC2 proteins in rat brain: complementary distribution and proposed function as vesicular adapter proteins in early stages of secretion. Neuron 18, 453-461. [DOI] [PubMed] [Google Scholar]
- Wang, J., Takeuchi, T., Yokota, H. and Izumi, T. (1999). Novel rabphilin-3-like protein associates with insulin-containing granules in pancreatic beta cells. J. Biol. Chem. 274, 28542-28548. [DOI] [PubMed] [Google Scholar]
- Wang, J. L., Easom, R. A., Hughes, J. H. and McDaniel, M. L. (1990). Evidence for a role of microfilaments in insulin release from purified beta-cells. Biochem. Biophys. Res. Commun. 171, 424-430. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Oh, E. and Thurmond, D. C. (2007). Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J. Biol. Chem. 282, 9536-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, W. K., Bolgiano, D. C., McKnight, B., Halter, J. B. and Porte, D., Jr (1984). Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J. Clin. Invest. 74, 1318-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselle, L., Coppola, T., Fukuda, M., Iezzi, M., El-Amraoui, A., Petit, C. and Regazzi, R. (2003). Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Mol. Biol. Cell 14, 4103-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, M. B., Sheu, L., Ghai, M., Bouquillon, A., Grondin, G., Weller, U., Beaudoin, A. R., Bennett, M. K., Trimble, W. S. and Gaisano, H. Y. (1996). Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology 137, 1340-1348. [DOI] [PubMed] [Google Scholar]
- Wilson, J. R., Biden, T. J. and Ludowyke, R. I. (1999). Increases in phosphorylation of the myosin II heavy chain, but not regulatory light chains, correlate with insulin secretion in rat pancreatic islets and RINm5F cells. Diabetes 48, 2383-2389. [DOI] [PubMed] [Google Scholar]
- Wilson, J. R., Ludowyke, R. I. and Biden, T. J. (2001). A redistribution of actin and myosin IIA accompanies Ca(2+)-dependent insulin secretion. FEBS Lett. 492, 101-106. [DOI] [PubMed] [Google Scholar]
- Yaekura, K., Julyan, R., Wicksteed, B. L., Hays, L. B., Alarcon, C., Sommers, S., Poitout, V., Baskin, D. G., Wang, Y., Philipson, L. H. et al. (2003). Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J. Biol. Chem. 278, 9715-9721. [DOI] [PubMed] [Google Scholar]
- Yang, S. N., Larsson, O., Branstrom, R., Bertorello, A. M., Leibiger, B., Leibiger, I. B., Moede, T., Kohler, M., Meister, B. and Berggren, P. O. (1999). Syntaxin 1 interacts with the L(D) subtype of voltage-gated Ca(2+) channels in pancreatic beta cells. Proc. Natl. Acad. Sci. USA 96, 10164-10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechoor, V. K., Patti, M. E., Ueki, K., Laustsen, P. G., Saccone, R., Rauniyar, R. and Kahn, C. R. (2004). Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc. Natl. Acad. Sci. USA 101, 16525-16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, Z., Yokota, H., Torii, S., Aoki, T., Hosaka, M., Zhao, S., Takata, K., Takeuchi, T. and Izumi, T. (2002). The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol. Cell. Biol. 22, 1858-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, W., Niwa, T., Fukasawa, T., Hidaka, H., Senda, T., Sasaki, Y. and Niki, I. (2000). Synergism of protein kinase A, protein kinase C, and myosin light-chain kinase in the secretory cascade of the pancreatic beta-cell. Diabetes 49, 945-952. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Efanov, A., Yang, S. N., Fried, G., Kolare, S., Brown, H., Zaitsev, S., Berggren, P. O. and Meister, B. (2000). Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta-cell. J. Biol. Chem. 275, 41521-41527. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Lilja, L., Mandic, S. A., Gromada, J., Smidt, K., Janson, J., Takai, Y., Bark, C., Berggren, P. O. and Meister, B. (2006). Tomosyn is expressed in beta-cells and negatively regulates insulin exocytosis. Diabetes 55, 574-581. [DOI] [PubMed] [Google Scholar]
- Zwartkruis, F. J. and Bos, J. L. (1999). Ras and Rap1: two highly related small GTPases with distinct function. Exp. Cell Res. 253, 157-165. [DOI] [PubMed] [Google Scholar]