Summary
The role of cocaine as an addictive drug of abuse in human society is hard to reconcile with its ecological role as a natural insecticide and plant-protective compound, preventing herbivory of coca plants (Erythroxylum spp.). This paradox is often explained by proposing a fundamental difference in mammalian and invertebrate responses to cocaine, but here we show effects of cocaine on honey bees (Apis mellifera L.) that parallel human responses. Forager honey bees perform symbolic dances to advertise the location and value of floral resources to their nest mates. Treatment with a low dose of cocaine increased the likelihood and rate of bees dancing after foraging but did not otherwise increase locomotor activity. This is consistent with cocaine causing forager bees to overestimate the value of the floral resources they collected. Further, cessation of chronic cocaine treatment caused a withdrawal-like response. These similarities likely occur because in both insects and mammals the biogenic amine neuromodulator systems disrupted by cocaine perform similar roles as modulators of reward and motor systems. Given these analogous responses to cocaine in insects and mammals, we propose an alternative solution to the paradox of cocaine reinforcement. Ecologically, cocaine is an effective plant defence compound via disruption of herbivore motor control but, because the neurochemical systems targeted by cocaine also modulate reward processing, the reinforcing properties of cocaine occur as a `side effect'.
Keywords: cocaine, Apis mellifera, reinforcement, dance language, drug abuse, dopamine, octopamine
INTRODUCTION
Cocaine is a neurotoxin that protects the coca plant (Erythroxylem spp.) from herbivory by critically disrupting insect motor control (Nathanson et al., 1993). In humans, cocaine is also highly toxic at medium to high doses but highly rewarding and reinforcing at low doses and potentially addictive (Kelley and Berridge, 2002; Sullivan et al., 2008). Why should a chemical that evolved to protect plants from herbivory be rewarding to humans and other mammals? This has been called the `paradox of drug reward' (Sullivan et al., 2008).
Solutions to this paradox often propose that cocaine evolved to deter insect and not mammalian herbivores and that fundamental differences exist in the responses of mammals (especially humans) to cocaine compared with those of arthropods (Nathanson et al., 1993). Seemingly in support of this view is the observation that while there is abundant evidence for cocaine disruption of insect motor systems (Hardie et al., 2007; McClung and Hirsh, 1997; Nathanson et al., 1993; Wolf and Heberlein, 2003), it has not previously been shown to be rewarding to insects (Wolf and Heberlein, 2003).
New data, however, have revealed many similarities in neurochemical systems and cocaine's mode of action between insects and mammals that call into question this solution to the paradox of drug reward. In both mammals (Kelley and Berridge, 2002; Wise, 2004) and insects (Roeder, 2005; Wolf and Heberlein, 2003), cocaine operates by blocking biogenic amine reuptake transporters (Corey et al., 1994; Gallant et al., 2003), thereby disrupting biogenic amine signalling (Bainton et al., 2000; McClung and Hirsh, 1999; Nathanson et al., 1993). In mammals, the biogenic amine systems disrupted by cocaine [principally dopamine (DA)] modulate both motor control and reward processing (Cenci, 2007; Uhl et al., 2002; Wise and Rompre, 1989; Wise, 2004). In insects, biogenic amines [principally DA and octopamine (OA)] also modulate motor control (Fussnecker et al., 2006; Hardie et al., 2007; Roeder et al., 2003), arousal (Adamo et al., 1995; Kume et al., 2005; Stevenson et al., 2005) and reward processing (Barron et al., 2007b; Hammer and Menzel, 1998; Schwaerzel et al., 2003; Unoki et al., 2005). Given these mechanistic similarities we revisited the question of whether cocaine could be rewarding to insects.
We used the dance language of the honey bee (Apis mellifera) as a natural bioassay to study the effect of cocaine on reward assessment. On return to the hive, forager honey bees may perform highly stereotyped movements (dances) to signal the location and value of floral resources to their nest mates (Seeley, 1995). The function of the dance is to advertise profitable resources to improve the foraging efficiency of the colony. For resources close to the hive, bees perform `round dances', and the likelihood and rate of round dancing are related to the value of the resources (Waddington, 1982). Resource value is influenced by several factors including the costs and benefits of the foraging trip and the nutritional needs of the colony (Seeley, 1995; Waddington, 1982). Bees use these factors to develop a gestalt estimate of the value of collected floral resources to the colony (Seeley, 1994; Seeley, 1995; Waddington, 1982). Dance thus provides a unique, natural and quantifiable assay for a forager bee's assessment of the value of collected floral resources. Here, we used the dance response of forager bees to sucrose and pollen feeders as an assay to assess the effects of cocaine on honey bee reward processing. We examined the effects of chronic and acute treatment with low doses of cocaine on honey bee behaviour and found honey bee responses that paralleled those of mammals.
MATERIALS AND METHODS
Experiments were performed at The Australian National University Research School of Biological Sciences, Canberra. Bees used were the standard commercially available hybrid of various European races available in Australia and were reared according to standard bee keeping practices.
Pharmacological treatments
In these experiments, bees were treated with freebase cocaine, OA hydrochloride or mianserin hydrochloride by applying the compounds dissolved in 1 μl dimethylformamide (DMF) to the dorsal thorax of forager bees using a glass microcapillary while they fed at sucrose or pollen feeders. DMF is a solvent that can penetrate bee cuticle and act as a `vehicle' to allow administered compounds to pass into the haemocoel and reach the brain (Barron et al., 2007a).
Experiment 1: effects of cocaine and mianserin hydrochloride treatment on dance behaviour
Dances were observed using colonies of ∼8000 bees housed in a glass-walled observation hive. Individually paint-marked foragers were trained to a 1.5 mol l–1 sucrose feeder 10 m from the hive, and their round dances (Seeley, 1995; Waddington, 1982) were video recorded during a 50 min period. We compared the round dance behaviour of bees treated with a single acute treatment of 3 μg, 6 μg or 12 μg cocaine dissolved in 1 μl DMF. Control groups were sham-treated (simply touching the bee's back with an empty glass microcapillary) or treated with 1 μl DMF only. Inclusion of both sham- and DMF-treated control groups allowed us to determine whether DMF itself was affecting behaviour. Bees treated with 2 μg OA in 1 μl DMF were included as a positive control, since OA is known to modulate bee dance behaviour (Barron et al., 2007b). Dance observations began 20 min after treatment. During the observation period, the number of visits by each bee to the feeder was also recorded. Videos were later analysed to score how many feeder visits caused dances on return to the hive (dance likelihood) and the rate of dancing (number of dance circuits per minute).
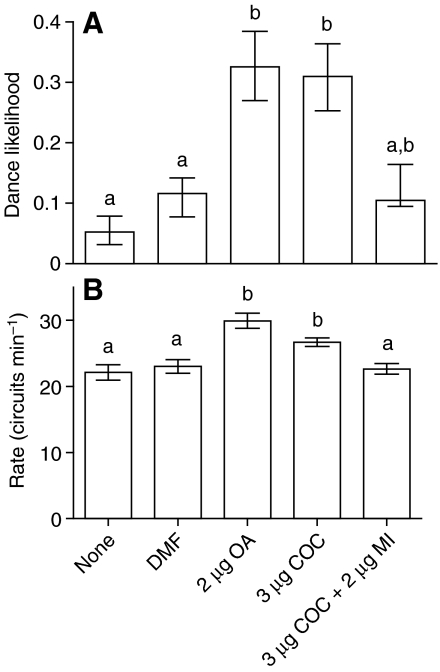
Mianserin hydrochloride is a biogenic amine antagonist (Degen et al., 2000; Roeder, 1999). To test whether cocaine influenced dance behaviour by affecting biogenic amine signalling, we examined whether mianserin treatment could reduce the effects of cocaine on dance behaviour. In a separate experiment using a different bee colony we compared dance behaviour in bees treated topically with 3 μg cocaine or 3 μg cocaine + 2 μg mianserin. We have shown previously that this dose of mianserin does not make bees sick or reduce dance performance on its own but is an effective antagonist of biogenic amine treatments (Barron et al., 2007b). Control groups were sham, DMF or OA treated.
Cocaine could affect dancing by stimulation of motor pathways or by changing forager sensitivity to the floral rewards they collected. Experiments 2 and 3 tested which of these alternatives is more likely. Cocaine is known to affect motor activity in Drosophila (McClung and Hirsh, 1997), and the honey bee dance is obviously a locomotor behaviour. Experiment 2 tested whether cocaine generally increased locomotor behaviour in honey bees by studying the effects of cocaine on activity in a simple locomotor assay. Experiment 3 tested whether cocaine increased responsiveness to sucrose in the proboscis extension response (PER) assay to examine whether cocaine affected responses to rewarding stimuli in a non-dance assay.
Experiment 2: effect of cocaine on locomotor behaviour
Forager bees were caught at a sucrose feeder and chilled briefly to immobility by ∼2 min exposure to –20°C in a domestic freezer. Individual bees were treated topically with 3 μg cocaine, or DMF as a control. Bees were placed individually in 9 cm-diameter Petri dishes. Each dish was placed over a simple radial grid and we recorded the number of times the bee walked across a line in the grid during a 5 min observation period. Each bee was observed twice at 30 and 60 min post-treatment. This assay has been used previously to detect activity differences in pharmacologically treated bees (Beggs et al., 2007).
Experiment 3: effect of cocaine on sucrose responsiveness
Cocaine could influence dance behaviour by changing responsiveness to floral rewards. To explore this possibility we studied the effect of cocaine treatment on responsiveness to sucrose using the PER assay. Bees were harnessed in metal tubes following previously published methods (Si et al., 2004) and starved for 4 h. One hour prior to testing, bees were treated topically with 3μg cocaine in DMF. DMF- and sham-treated bees were control groups. 20μl drops of seven different sugar solutions (0.1%, 1%, 2.5%, 10%, 30%, 60% sucrose and honey) were touched briefly to the antennae of each bee in order of ascending sucrose concentration and ending with honey. Water was presented to bees between each sucrose presentation. The first concentration eliciting proboscis extension was the sucrose sensitivity index. An index of 1 meant a bee responded to 0.1% sucrose, while 7 meant a bee responded to honey only. Bees that responded to water more than twice were judged to have sensitised to antennal stimulation and were excluded from analyses comparing sucrose responsiveness across experimental groups.
Experiment 4: effect of cocaine on dances for pollen
To test whether cocaine affected dances for resources other than sucrose we examined the effects of cocaine on dances for pollen. Pollen is the bees' protein and lipid source. Unlike nectar it is not ingested by foragers: it is carried in `baskets' on the hind legs. In this experiment, individually marked bees collected freeze-dried pollen from a dish 10 m from the hive. Bees were treated topically with 3 μg cocaine or sham or DMF treatments. At the time of our experiment (May 2006; late Autumn in Canberra, Australia) dances for pollen were rare and short; therefore, for each experimental group we compared the proportion of bees that danced at least once for the pollen dish during a 50 min observation period beginning 20 min post-treatment.
Experiment 5: effect of chronic cocaine treatment and cocaine `withdrawal' on learning
Rats show disruptions in learning and memory on abrupt cessation of chronic cocaine exposure (Calu et al., 2007), which is considered a model for human cocaine withdrawal. To examine whether bees exhibit something similar to withdrawal, we tested the performance of bees in a two-odour discriminant learning task using PER (Si et al., 2004). Bees were reared from adult emergence in groups of 60 in cages in a humidified incubator at 32°C for 6 days. Bees were given chronic oral drug treatments by being fed excess 1.5 mol l–1 sucrose containing either 0.66 mmol l–1 cocaine hydrochloride (a non-toxic dose) or 10.54 mmol l–1 OA hydrochloride or plain sucrose as a control. Training occurred on Day 6.
For PER training, commercial lemon essence (4 μlml–1) in 1 mol l–1 sucrose solution was the rewarding stimulus, while natural vanilla essence (4 μlml–1) in saturated NaCl solution was the non-rewarding stimulus. Bees were given three training sessions at 10 min intervals. During each training session, bees were exposed to the odour of the rewarding stimulus for 5 s, following which one antenna was touched with the stimulus, leading to the extension of the proboscis and tasting of the sugar. This was repeated with the non-rewarding stimulus. The responses of bees to vanilla and lemon odour were tested 20 h after training, and the presence or absence of proboscis extension was noted (Si et al., 2004). A `correct' response was proboscis extension to lemon (sugar associated) but not vanilla (salt associated).
Bees were held in their harnesses during the 20 h between training and testing. During this period the cocaine-treated and OA-treated bees were assigned to either `withdrawal' or `chronic' treatment groups. The `withdrawal' bees were all fed 20 μl of plain 1.5 mol l–1 sucrose solution 1, 3 and 7 h after training, giving 20 h without oral cocaine or OA treatment immediately before testing. The `chronic-treated' bees were fed 20 μl cocaine- or OA-containing sucrose 1, 3 and 7 h after training so that drug treatments were consistent throughout the training and testing period. The sucrose control group was fed 20 μl plain sucrose in both chronic and withdrawal experimental conditions. By comparing learning performance relative to sucrose control bees across these two experimental conditions, we could assess the effect of chronic oral cocaine treatment on learning performance and also the effect of cessation of chronic cocaine treatment on learning.
RESULTS
Experiment 1: effects of cocaine and mianserin hydrochloride treatment on dance behaviour
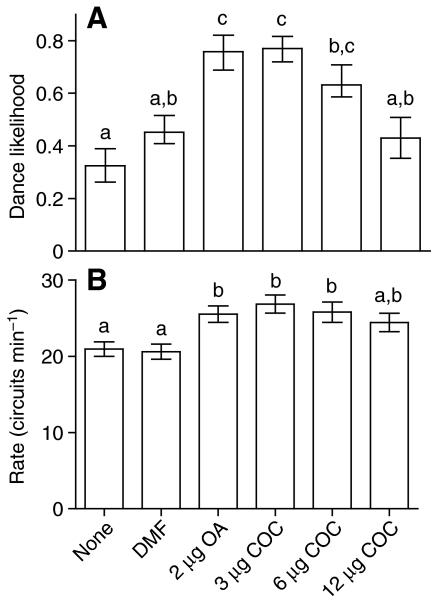
Cocaine caused a dose-dependent increase in both likelihood and rate of dancing (Fig. 1). This is consistent with how dance behaviour would be predicted to change if foragers were reporting resources of greater value. These effects of cocaine on dance behaviour are similar to those for OA (Fig. 1), which is known to modulate reward processing in insects (Barron et al., 2007b; Hammer and Menzel, 1998; Schwaerzel et al., 2003). The effects of cocaine on bee dance were eliminated by simultaneous treatment with the biogenic amine antagonist mianserin (Fig. 2), suggesting that cocaine modulated dance performance by interfering with biogenic amine signalling.
Fig. 1.
Effect of cocaine (COC) on the likelihood and rate of round dancing by bees returning from a 1.5 mol l–1 sucrose feeder. Bees were treated with one of three doses of cocaine or octopamine (OA) dissolved in DMF at the feeder. DMF- and sham-treated bees served as controls. N>34 bees per group. Differences between treatment groups were tested with Kruskal-Wallis tests, and comparisons between specific groups were performed using Dunn's post hoc tests. Groups that did not differ (Dunn's post hoc test, P>0.05) are marked by the same letter above the bars. (A) Likelihood of each bee dancing, calculated as the proportion of visits to the feeder that resulted in a dance during the 50-min observation period. Bars represent means and standard error calculated from arcsin-transformed values and consequently are not always symmetrical around the mean. (B) Number of dance circuits per minute. Bars represent means and standard error. Since our statistical comparisons used nonparametric methods, the standard error provides an indication of the distribution of the data but is less indicative of calculated significant differences between groups.
Fig. 2.
Effect of mianserin (MI) on the stimulation of dancing by cocaine (COC). Dance likelihood (A) and dance rate (B) of bees treated with MI and COC for a 1.5 mol l–1 sucrose feeder were compared with dances of bees treated with COC alone and DMF- and sham-treated controls (N>39 bees per group). Bar plots, analyses and statistical notation as in Fig. 1.
Cocaine-treated bees still foraged normally and effectively between dance bouts in the hive (mean ± s.e.m. number of foraging trips during 50 min observation period: untreated 11.42±0.43; DMF 12.36±0.31; 3 μg cocaine 10.86±0.62; Kruskal-Wallis test statistic=7.421, P=0.191). This result suggests that cocaine treatment within this dose range did not damage the ability to fly, locate the feeder and collect sucrose.
Cocaine-treated foragers only danced when they were interacting socially within the hive. Foragers treated with cocaine but held in small vials isolated from the hive never performed any movements resembling dances (N=240 bees, doses 3–50μg, each observed for 1.5 h). This indicates that cocaine stimulates dancing only in the appropriate social context of a forager returning resources to the colony.
Experiment 2: effect of cocaine on locomotor behaviour
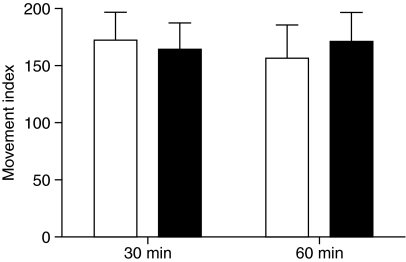
There was no sign of motor malfunction or locomotor hyperactivity in isolated bees treated with the 3 μg cocaine dose (Fig. 3). Since we observed no effect of cocaine on simple locomotor activity we argue it is unlikely that cocaine modulates dance behaviour via a general stimulation of motor pathways.
Fig. 3.
Effect of cocaine on locomotion. The movement index measured the amount that DMF- (white bars) and cocaine-treated bees (black bars) moved in a Petri dish during 5 min observation periods 30 and 60 min post-treatment. Bars show mean and standard error. N=20 for each group. Data were analysed with two-way ANOVA. There was no significant effect of drug (F=0.09, P=0.7600) or time (F=0.04, P=0.8418) and no significant interaction between these factors (F=0.83, P=0.3694).
Experiment 3: effect of cocaine on sucrose responsiveness
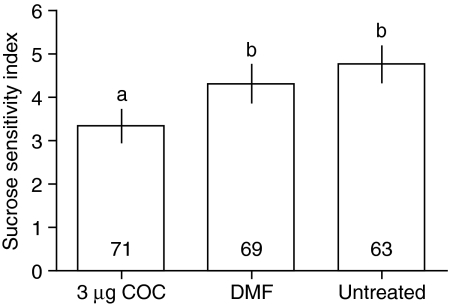
Cocaine increased responsiveness to sucrose in the PER assay (Fig. 4) but did not increase the number of bees that sensitised to water presentation (number of bees responding to two or more of seven water presentations: 3 μg cocaine 9/80, DMF 11/80, untreated 17/80; χ2=3.323, d.f.=2, P=0.189). This suggests that cocaine specifically increased responsiveness to sucrose reward rather than a general sensitisation of reflex motor responses to antennal stimulation in this assay.
Fig. 4.
Effect of cocaine (COC) on sucrose responsiveness. 20 μl drops of seven different sucrose solutions ranging in concentration from 0.1% sucrose to 60% sucrose were presented to the antennae of harnessed bees in the laboratory. The first sugar solution (1–7) eliciting proboscis extension was the sucrose sensitivity index; therefore a lower value indicates higher sucrose sensitivity. Bars show means and 95% confidence intervals. Differences between treatment groups were tested with Kruskal-Wallis tests (median sucrose sensitivity index: 3 μg cocaine, 3; DMF, 4; untreated, 5. Kruskal-Wallis statistic 24.17, d.f.=2, P<0.001) and comparisons between specific groups were performed using Dunn's post hoc tests. Bars that do not differ significantly (Dunns post hoc test, P>0.05) are marked by the same letter above the bar. Sample size shown in bars.
Experiment 4: effect of cocaine on dances for pollen
Cocaine also increased the proportion of bees that performed at least one dance on return from a pollen feeder (3μg cocaine 45.6%; DMF-treated 27.3%; untreated 22.2%; N>45 per group; χ2=6.867, d.f.=2, P=0.032). This indicates that cocaine modulated responses to collected resources other than ingested sucrose.
Experiment 5: effect of chronic cocaine treatment and cocaine `withdrawal' on learning
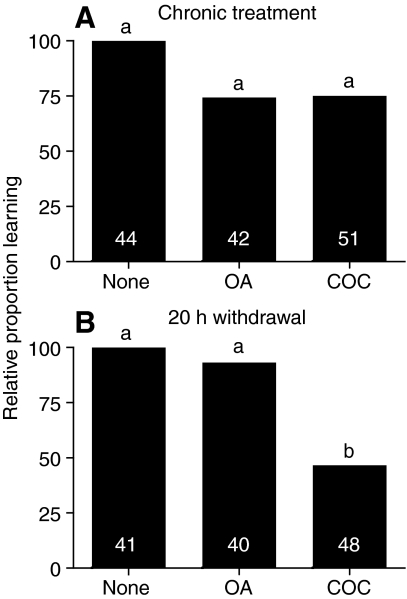
Learning performance in cocaine-treated bees did not differ from that in untreated or OA-treated control groups if drug treatments were maintained during the 20 h between training and testing (`chronic treatment'; Fig. 5A). If cocaine and OA treatment ceased during the interval between training and testing (giving the OA and cocaine groups 20 h without drug exposure), the cocaine-treated group performed only half as well as the control or OA-treated groups (`withdrawal treatment'; Fig. 5B). This demonstrates a significant learning deficit that manifests on cessation of prolonged cocaine exposure, suggestive of a withdrawal-like phenomenon in bees.
Fig. 5.
Effect of cocaine (COC) withdrawal on learning. Relative proportion of bees responding correctly in a proboscis extension response (PER) learning assay 20 h after three trials of training. Prior to training, bees were orally treated for 6 days with 10.54 mmol l–1 octopamine (OA) or 0.66 mmol l–1 cocaine (COC) in ad libitum 1.5 mol l–1 sucrose solution, or plain 1.5 mol l–1 sucrose. (A) Chronic treatment: bees continued to receive treatment during the 20 h interval between training and testing. (B) Withdrawal: treatment withheld during the 20 h interval between training and testing. In each panel the proportion of bees responding correctly is normalized with respect to the untreated control group. Sample size is shown in bars. Groups that did not differ at the 5% confidence interval share the same letter above the bar (pair-wise Fisher's exact tests with Bonferroni correction).
DISCUSSION
In summary, cocaine treatment caused a dose-dependent increase in the likelihood and rate of dancing. Dances for sucrose and pollen were both affected by cocaine, and these effects were eliminated by treatment with the biogenic amine antagonist mianserin. Cocaine also increased sucrose responsiveness in the PER assay, and bees performed poorly in a learning task following `withdrawal' of chronic cocaine treatment but not with continued chronic cocaine treatment. Cocaine treatment at the level that stimulated dancing did not stimulate general locomotion.
The behavioural function of the dance is to advertise profitable resources to nest mates, and the effects of cocaine on dance behaviour are consistent with cocaine increasing responsiveness to floral rewards. Hence, we argue that our experiments present the first evidence for cocaine modulating reward processing systems in an insect brain. However, cocaine is known to modulate motor systems in insects (McClung and Hirsh, 1997; Nathanson et al., 1993; Wolf and Heberlein, 2003), and, in Drosophila, treatment with medium to high cocaine doses can sometimes release looping locomotor stereotypies (McClung and Hirsh, 1997). Therefore, an alternative interpretation of our findings could be that cocaine affected dance behaviour by stimulating motor and not reward pathways in the insect brain.
We do not believe the dance response observed in bees is a simple stereotypy or hyperkinesia for the following reasons. First, forager bees do not automatically dance on return to the hive; the decision of whether or not to dance and how to dance depends on the relative profitability of their foraging trip (Seeley, 1995). Cocaine increased the likelihood of an individual bee dancing (Fig. 1) but did not make bees dance every time, demonstrating that cocaine did not simply release dance behaviour in every treated bee. Second, within the cocaine dose range that was most effective in stimulating dancing, treated bees continued to forage normally. Their foraging rate was not hyperactive and they did not show any gross motor deficits that interfered with flight or navigation. This argues against cocaine releasing an abnormal motor stereotypy of the kind observed in Drosophila. Third, cocaine-treated bees only danced when in the appropriate social environment of the dance floor, indicating that the expression of dance behaviour in cocaine-treated bees remained sensitive to the colony social environment. This would not be predicted for a motor stereotypy. Fourth, in a locomotion assay (Fig. 3), we observed no evidence for general motor hyperactivity in bees treated with the cocaine dose most effective in stimulating dancing.
In a PER assay, cocaine increased sucrose responsiveness (Fig. 4) but did not increase sensitisation to water, implying that cocaine modulated behavioural responses to stimulation by sucrose but did not increase the general motor reactivity of the proboscis reflex to antennal stimulation. These data support our interpretation that cocaine affected dance behaviour by modulation of brain reward systems. Cocaine increased dances for pollen also, indicating that cocaine increased dance responses to general floral rewards and that the effects of cocaine on dance cannot be explained solely by cocaine modulation of peripheral sucrose sensitivity.
While we certainly do not rule out the possibility of cocaine affecting motor systems in honey bees [as it does in other insects (Nathanson et al., 1993) and mammals (Antoniou et al., 1998)], we argue that the sum of our results cannot be explained by cocaine stimulation of motor pathways alone at the doses used here. Rather we favour the interpretation that our results demonstrate cocaine stimulation of pathways for reward assessment and processing.
As a further parallel between honey bee and mammalian responses to cocaine, we observed a withdrawal-like response in honey bees following cessation of chronic cocaine treatment (Fig. 5). Cocaine withdrawal has been reported in rodents, which show disruptions in learning and memory (Calu et al., 2007), but this has not been shown previously in insects. In honey bees, we observed poor performance in a learning task that manifested only on cessation of chronic oral cocaine treatment and not with continued chronic cocaine treatment. Hence, our result is unlikely to be due to cocaine making bees sick. Whether the poor performance we observed was due to deficits in learning, recall or attention remains to be explored.
The effects of cocaine on dance behaviour were eliminated by mianserin treatment (Fig. 2). Since mianserin is an antagonist of biogenic amine receptors, this result implies that cocaine influences dance by interaction with biogenic amine pathways, consistent with the known mode of action of cocaine in mammals (Kelley and Berridge, 2002; Wise and Rompre, 1989; Wise, 2004) and insects (Nathanson et al., 1993; Wolf and Heberlein, 2003). In mammals, cocaine blocks biogenic amine reuptake transporters, with highest affinity for the dopamine reuptake transporter (Kelley and Berridge, 2002). Cocaine-sensitive dopamine and serotonin transporters have been cloned from Drosophila (Corey et al., 1994; Demchyshyn et al., 1994; Porzgen et al., 2001), and cocaine sensitivity in flies is modulated by manipulation of dopamine and serotonin levels (Bainton et al., 2000; Li et al., 2000). Cocaine-sensitive dopamine transporters have also been cloned from Trichoplusia ni (Gallant et al., 2003).
Presently, it is unclear how cocaine acts to influence dance behaviour and reward processing in the honey bee. Dance behaviour has been shown to be influenced by pharmacological manipulation of OA levels (Barron et al., 2007b), and OA has also been shown to modulate reward learning in honey bees (Hammer and Menzel, 1998). One plausible scenario is that cocaine interferes with bee reward processing by disrupting OA signalling. While this interpretation is consistent with known behavioural effects of OA in honey bees, presently we do not know which biogenic amine systems in bees are most sensitive to cocaine. Four putative biogenic amine transporters have been identified from the honey bee genome based on sequence similarity to the Drosophila dopamine transporter DAT; however, none of these genes has been functionally characterised and their sensitivity to cocaine is also unknown. Some insects (Drosophila) have transporters for DA and serotonin only whereas others (Trichopusia ni) have distinct OA, DA and serotonin transporters (Gallant et al., 2003; Malutan et al., 2002). Until the honey bee biogenic amine transporters have been characterised we will not know what complement of transporters the bee possesses and which are most sensitive to cocaine.
In both mammals and bees, the biogenic amines function as modulators of reward processing and motor control. In mammals, both motor control and reward processing are regulated by DA (Kelley and Berridge, 2002; Wise and Rompre, 1989; Wise, 2004). In bees, both DA and OA have been implicated in motor control systems and reward processing (Barron et al., 2007b; Beggs et al., 2007; Hammer and Menzel, 1998). Since the behavioural functions of the biogenic amine systems disrupted by cocaine are similar between insects and mammals, our findings imply a parsimonious explanation for the paradox of cocaine reward. Cocaine is a potent plant defence because it causes catastrophic failure of insect motor control by disrupting biogenic amine signalling (Nathanson et al., 1993). But because biogenic amine systems regulating motor function also modulate reward processing it is almost unavoidable that cocaine impacts reward systems. Despite its reinforcing properties, cocaine remains an effective plant defence because the concentrations naturally occurring in coca leaves are such that herbivorous insects very rapidly ingest a toxic dose (Nathanson et al., 1993). From an evolutionary perspective, the reinforcing properties of cocaine can be considered a `side effect' resulting from cocaine targeting neurochemical systems regulating multiple aspects of behaviour.
We thank Luke Roberts and Marianne Peso for assistance with locomotor assays, and members of the Robinson lab for comments and feedback on the manuscript. This work was supported by a National Institutes of Health Cutting Edge Biological Research Award DA-019864 to G.E.R. Deposited in PMC for release after 12 months.
References
- Adamo, S. A., Linn, C. E. and Hoy, R. R. (1995). The role of neurohormonal octopamine during `fight or flight' behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 198, 1691-1700. [DOI] [PubMed] [Google Scholar]
- Antoniou, K., Kafetzopoulos, E., Papadopoulou-Daifoti, Z., Hyphantis, T. and Marselos, M. (1998). D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci. Biobehav. Rev. 23, 189-196. [DOI] [PubMed] [Google Scholar]
- Bainton, R. J., Tsai, L. T. Y., Singh, C. M., Moore, M. S., Neckameyer, W. S. and Heberlein, U. (2000). Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 10, 187-194. [DOI] [PubMed] [Google Scholar]
- Barron, A. B., Maleszka, J., Vander Meer, R. K., Robinson, G. E. and Maleszka, R. (2007a). Comparing the efficiency of injection, feeding and topical application methods for pharmacological treatment of honey bees. J. Insect Physiol. 53, 187-194. [DOI] [PubMed] [Google Scholar]
- Barron, A. B., Maleszka, R., Vander Meer, R. K. and Robinson, G. E. (2007b). Octopamine modulates honey bee dance behavior. Proc. Natl. Acad. Sci. USA 104, 1703-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs, K. T., Glendining, K. A., Marechal, N. M., Vergoz, V., Nakamura, I., Slessor, K. N. and Mercer, A. R. (2007). Queen pheromone modulates brain dopamine function in worker honey bees. Proc. Natl. Acad. Sci. USA 104, 2460-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu, D. J., Stalnaker, T. A., Franz, T. M., Singh, T., Shaham, Y. and Schoenbaum, G. (2007). Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn. Mem. 14, 325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci, M. A. (2007). Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 30, 236-243. [DOI] [PubMed] [Google Scholar]
- Corey, J. L., Quick, M. W., Davidson, N., Lester, H. A. and Guastella, J. (1994). A cocaine-sensitive Drosophila serotonin transporter – cloning, expression, and electrophysiological characterization. Proc. Natl. Acad. Sci. USA 91, 1188-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen, J., Gewecke, M. and Roeder, T. (2000). Octopamine receptors in the honey bee and locust nervous system: pharmacological similarities between homologous receptors of distantly related species. Br. J. Pharmacol. 130, 587-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchyshyn, L. L., Pristupa, Z. B., Sugamori, K. S., Barker, E. L., Blakely, R. D., Wolfgang, W. J., Forte, M. A. and Niznik, H. B. (1994). Cloning, expression, and localization of a chloride-facilitated, cocaine-sensitive serotonin transporter from Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 91, 5158-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussnecker, B. L., Smith, B. H. and Mustard, J. A. (2006). Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera). J. Insect Physiol. 52, 1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant, P., Malutan, T., McLean, H., Verellen, L., Caveney, S. and Donly, C. (2003). Functionally distinct dopamine and octopamine transporters in the CNS of the cabbage looper moth. Eur. J. Biochem. 270, 664-674. [DOI] [PubMed] [Google Scholar]
- Hammer, M. and Menzel, R. (1998). Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5, 146-156. [PMC free article] [PubMed] [Google Scholar]
- Hardie, S. L., Zhang, J. X. and Hirsh, J. (2007). Trace amines differentially regulate adult locomotor activity, cocaine sensitivity, and female fertility in Drosophila melanogaster. Dev. Neurobiol. 67, 1396-1405. [DOI] [PubMed] [Google Scholar]
- Kelley, A. E. and Berridge, K. C. (2002). The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 22, 3306-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume, K., Kume, S., Park, S. K., Hirsh, J. and Jackson, R. F. (2005). Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25, 7377-7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Chaney, S., Forte, M. and Hirsh, J. (2000). Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr. Biol. 10, 211-214. [DOI] [PubMed] [Google Scholar]
- Malutan, T., McLean, H., Caveney, S. and Donly, C. (2002). A high-affinity octopamine transporter cloned from the central nervous system of cabbage looper Trichoplusia ni. Insect Biochem. Mol. Biol. 32, 343-357. [DOI] [PubMed] [Google Scholar]
- McClung, C. and Hirsh, J. (1997). Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr. Biol. 8, 109-112. [DOI] [PubMed] [Google Scholar]
- McClung, C. and Hirsh, J. (1999). The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr. Biol. 9, 853-860. [DOI] [PubMed] [Google Scholar]
- Nathanson, J. A., Hunnicutt, E. J., Kantham, L. and Scavone, C. (1993). Cocaine as a naturally occurring insecticide. Proc. Natl. Acad. Sci. USA 90, 9645-9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzgen, P., Park, S. K., Hirsh, J., Sonders, M. S. and Amara, S. G. (2001). The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: A primordial carrier for catecholamines. Mol. Pharmacol. 59, 83-95. [DOI] [PubMed] [Google Scholar]
- Roeder, T. (1999). Octopamine in invertebrates. Prog. Neurobiol. 59, 533-561. [DOI] [PubMed] [Google Scholar]
- Roeder, T. (2005). Tyramine and octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 50, 447-477. [DOI] [PubMed] [Google Scholar]
- Roeder, T., Seifert, M., Kahler, C. and Gewecke, M. (2003). Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch. Insect Biochem. Physiol. 54, 1-13. [DOI] [PubMed] [Google Scholar]
- Schwaerzel, M., Monasterioti, M., Scholz, H., Friggi-Grelin, F., Birman, S. and Heisenberg, M. (2003). Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, T. D. (1994). Honey-bee foragers as sensory units of their colonies. Behav. Ecol. Sociobiol. 34, 51-62. [Google Scholar]
- Seeley, T. D. (1995). The Wisdom of the Hive. Cambridge: Harvard University Press.
- Si, A., Helliwell, P. and Maleszka, R. (2004). Effects of NMDA antagonists on olfactory learning and memory in the honey bee (Apis mellifera). Pharmacol. Biochem. Behav. 77, 191-187. [DOI] [PubMed] [Google Scholar]
- Stevenson, P. A., Dyakonova, V., Rillich, J. and Schildberger, K. (2005). Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 25, 1431-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, R. J., Hagen, E. H. and Hammerstein, P. (2008). Revealing the paradox of drug reward in human evolution. Proc. Roy. Soc. Lond. B 275, 1231-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl, G. R., Hall, F. S. and Sora, I. (2002). Cocaine, reward, movement and monoamine transporters. Mol. Psychiatry 7, 21-26. [DOI] [PubMed] [Google Scholar]
- Unoki, S., Matsumoto, Y. and Mizunami, M. (2005). Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur. J. Neurosci. 22, 1409-1416. [DOI] [PubMed] [Google Scholar]
- Waddington, K. D. (1982). Honey bee foraging profitability and round dance correlates. J. Comp. Physiol. 148, 297-301. [Google Scholar]
- Wise, R. A. (2004). Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483-494. [DOI] [PubMed] [Google Scholar]
- Wise, R. and Rompre, P. (1989). Dopamine and reward. Annu. Rev. Psychol. 40, 191-225. [DOI] [PubMed] [Google Scholar]
- Wolf, F. W. and Heberlein, U. (2003). Invertebrate models of drug abuse. J. Neurobiol. 54, 161-178. [DOI] [PubMed] [Google Scholar]