Summary
In order to further define O2 store utilization during dives and understand the physiological basis of the aerobic dive limit (ADL, dive duration associated with the onset of post-dive blood lactate accumulation), emperor penguins (Aptenodytes forsteri) were equipped with either a blood partial pressure of oxygen (PO2) recorder or a blood sampler while they were diving at an isolated dive hole in the sea ice of McMurdo Sound, Antarctica. Arterial PO2 profiles (57 dives) revealed that (a) pre-dive PO2 was greater than that at rest, (b) PO2 transiently increased during descent and (c) post-dive PO2 reached that at rest in 1.92±1.89 min (N=53). Venous PO2 profiles (130 dives) revealed that (a) pre-dive venous PO2 was greater than that at rest prior to 61% of dives, (b) in 90% of dives venous PO2 transiently increased with a mean maximum PO2 of 53±18 mmHg and a mean increase in PO2 of 11±12 mmHg, (c) in 78% of dives, this peak venous PO2 occurred within the first 3 min, and (d) post-dive venous PO2 reached that at rest within 2.23±2.64 min (N=84). Arterial and venous PO2 values in blood samples collected 1–3 min into dives were greater than or near to the respective values at rest. Blood lactate concentration was less than 2 mmol l–1 as far as 10.5 min into dives, well beyond the known ADL of 5.6 min. Mean arterial and venous PN2 of samples collected at 20–37 m depth were 2.5 times those at the surface, both being 2.1±0.7 atmospheres absolute (ATA; N=3 each), and were not significantly different. These findings are consistent with the maintenance of gas exchange during dives (elevated arterial and venous PO2 and PN2 during dives), muscle ischemia during dives (elevated venous PO2, lack of lactate washout into blood during dives), and arterio-venous shunting of blood both during the surface period (venous PO2 greater than that at rest) and during dives (arterialized venous PO2 values during descent, equivalent arterial and venous PN2 values during dives). These three physiological processes contribute to the transfer of the large respiratory O2 store to the blood during the dive, isolation of muscle metabolism from the circulation during the dive, a decreased rate of blood O2 depletion during dives, and optimized loading of O2 stores both before and after dives. The lack of blood O2 depletion and blood lactate elevation during dives beyond the ADL suggests that active locomotory muscle is the site of tissue lactate accumulation that results in post-dive blood lactate elevation in dives beyond the ADL.
Keywords: aerobic dive limit, blood sampler, emperor penguin, hemoglobin, lactate, nitrogen, oxygen electrode, oxygen store, shunt
INTRODUCTION
The concept of an aerobic dive limit (ADL) is central to most eco-physiological models of foraging behavior in marine mammals and diving birds. Although routinely calculated (but rarely measured), the ADL has remained a physiological black box. Conceptually, it should be dependent on the rate, pattern and magnitude of O2 store depletion during dives. However, the management and utilization of O2 stores during diving may vary in different species or situations due to (a) differences in the magnitude and distribution of O2 stores and (b) differences in the intensity of physiological responses underlying the rate of O2 store depletion during a dive. The ADL has been defined as the dive duration associated with the onset of post-dive blood lactate accumulation, and it has been proposed that the lactate accumulation at the ADL is secondary to localized tissue O2 depletion and increased glycolysis, most probably in active but ischemic locomotory muscle (Kooyman and Ponganis, 1998). On the other hand, less-than-expected decreases in muscle O2 saturation during dives of Weddell seals (Leptonychotes weddellii) have led to the suggestion that muscle blood flow persists during dives (Guyton et al., 1995), and to the hypothesis that maintenance of muscle blood flow during dives allows for the matched parallel depletion of blood and muscle O2 stores (Davis and Kanatous, 1999). The primary difference between these two different ADL scenarios is dependent on the isolation of working muscle from the blood O2 store during a dive.
Therefore, in order to increase our understanding of O2 store management and the physiological basis of the ADL, we reviewed blood oxygen partial pressure (PO2) profiles and analyses of blood samples from diving emperor penguins (Aptenodytes forsteri) for findings indicative of blood flow patterns and the utilization of O2 stores during dives. Emperor penguins diving at an isolated dive hole are particularly appropriate for such investigations because the ADL has been determined by post-dive blood lactate measurements to be 5.6 min, and neither the respiratory nor the blood O2 stores are depleted at the ADL (Ponganis et al., 1997; Ponganis et al., 2007; Stockard et al., 2005). For dive durations beyond the 5.6 min ADL, post-dive blood lactate concentrations are elevated.
We had two primary hypotheses. First, we hypothesized that the maintenance of gas exchange with the large respiratory O2 store in emperor penguins contributes to significant O2 transfer from the respiratory system to the blood during dives. Second, we hypothesized that muscle is isolated from the blood O2 store during dives, i.e. muscle blood flow and blood-to-muscle O2 transfer stop during dives. In particular, we reasoned that blood PO2 profiles during dives should be a reflection of (a) pulmonary gas exchange, (b) a reduction in cardiac output secondary to the bradycardia of diving, and (c) changes in blood O2 extraction due to the reduction/redistribution of organ blood flow secondary to both the fall in cardiac output and peripheral vasoconstriction during dives.
MATERIALS AND METHODS
General approach, catheterizations and recorders
In the austral springs of 2001, 2003–2005 and 2007, non-breeding emperor penguins (Aptenodytes forsteri Gray, 20–30 kg in body mass) were captured on the sea ice of McMurdo Sound or at Terra Nova Bay near Cape Washington (74°36′, 165°24′). They were maintained at the corralled isolated dive hole of the Penguin Ranch in McMurdo Sound (77°41′, 165°59′) for 6 weeks as in past studies (Kooyman et al., 1992; Ponganis et al., 2001; Ponganis et al., 2004; Ponganis et al., 2003), and then released at the McMurdo Sound ice edge. Blood-sampling catheters or PO2 electrodes/thermistors (Licox C1.1 Revoxode; Integra LifeSciences, Plainsboro, NJ, USA; model 554, Yellow Springs Instruments, Yellow Springs, OH, USA) were inserted percutaneously into the aorta or vena cava of emperor penguins under general isoflurane anesthesia as described previously (Ponganis et al., 2007; Ponganis et al., 2001; Ponganis et al., 2004; Ponganis et al., 2003). In addition, in two birds in 2007, successful femoral artery catheterization was achieved with a 4.5 in, 20 g catheter (Arrow International, Reading, PA, USA); only a PO2 electrode was inserted through this small catheter. The birds were also equipped with a custom-made PO2/temperature recorder (UFI, Morro Bay, CA, USA) and an Mk9 time depth recorder (TDR, Wildlife Computers, Redmond, WA, USA) as previously described (Stockard et al., 2005). After overnight recovery from anesthesia, birds were allowed to dive at the isolated dive hole. After 1–2 days of diving and data collection, catheters, probes and recorders were removed under general anesthesia. Data from the recorders were downloaded to a personal computer and analyzed with Excel (Microsoft, Redmond, WA, USA), Origin (Origin Lab., Northampton, MA, USA) and SPSS (SPSS Inc., Chicago, IL, USA) software. As previously reviewed (Ponganis et al., 2007), all PO2 values were corrected to 38°C for construction of the PO2 profiles. In the two birds without thermistors in 2007, blood temperature was assumed to be 38°C.
All partial pressures are expressed, as measured, in mmHg (7.5 mmHg=1 kPa). Means are expressed ±s.d. Significance was assumed at P<0.05. All procedures were approved under a UCSD Animal Subjects Committee protocol and US Antarctic Treaty Permit.
Blood sampler
The custom-designed blood sampler (1.25 kg, 24 cm×8.5 cm diameter) collected one sample per dive and consisted of an underwater housing, a programmable, microprocessor-based control board (UFI), a pressure transducer (Model 96, IC Sensors, Milpitas, CA, USA), a blood access port, and two solenoid valves (2W13W-1NR-A1C4, Snap Tite, Erie, PA, USA) which each controlled access to a syringe, one for waste (>3 times dead space volume) and one for the blood sample (Fig. 1). Collection of blood relied upon the pressure difference between the ambient pressure at depth and that inside the housing. The catheter port inside the housing was connected to the solenoid valves via high pressure Teflon tubing and swedgelock fittings. The sampler was programmed to obtain the sample after detection of a specified depth, or to obtain the sample when a specified time interval had elapsed while the bird was beneath a specified `start-of-dive' depth threshold. The sampler was also programmed to delay activation for a minimum of 1 h after the sampler was mounted on a bird in order to avoid any effects secondary to restraint. The unit was retrieved by recapture/restraint of the bird when blood was visible in the external transparent stopcock connection between the catheter and blood sampler port (no. 91041 stopcock, Mallinckrodt, Glen Falls, NY, USA; 3.5 in extension tubing no. 53035, Medex, Dublin, OH, USA). Catheters for blood sampling were in the femoral artery, femoral vein or axillary vein. Lyophilized heparin (210-6, Sigma Aldrich, St Louis, MO, USA) was placed in the sample syringe (Becton Dickinson, Franklin Lakes, NJ, USA) to prevent clotting. Because the volume of the blood sample syringe was diluted by the dead space flush volume of its solenoid valve, analyses for blood gases, O2 content and lactate concentration were performed on samples collected anaerobically from the undiluted blood in the Teflon tubing in the sampler. As in past blood sampler studies (Falke et al., 1985), nitrogen content and, in some cases, lactate concentration determined from the sample syringe were corrected for dilution by comparison of hematocrit (determined by microcentrifugation) or hemoglobin (Hb) concentration in the sample syringe versus that in the tubing. Blood samples were analyzed within 120 min after collection of the sample due to continued diving activity and the time required to recapture the bird and dismantle the blood sampler.
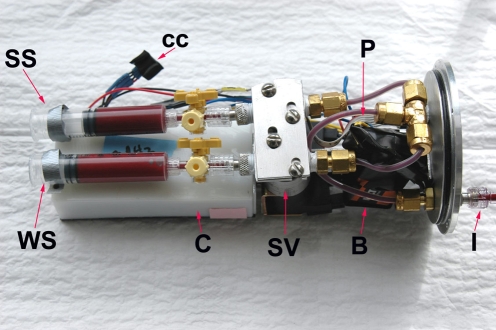
Fig. 1.
Overhead view of the blood sampler after removal from underwater housing. B, 9 V battery; C, circuit board housing; cc, computer communication port; I, inlet; P, cable from bulkhead-mounted pressure transducer; SS, sample syringe; SV, solenoid valve; WS, waste syringe.
Blood sample analyses
In addition to collection of blood samples from birds at rest (Ponganis et al., 2007) and from diving birds, occasional blood samples were also manually collected from birds under anesthesia and from restrained birds during surface intervals. Blood gas (PO2, PCO2 and pH) and lactate concentration analyses on these samples were conducted with a Series 200 i-STAT Portable Clinical Analyzer (CG4+ cartridge, Abbott Point of Care Inc., East Windsor, NJ, USA) at 37°C (Stockard et al., 2007). O2 content was determined with a Tucker chamber technique (Models SI 782 O2 meter and 1302 O2 electrode, Strathkelvin, Motherwell, UK) (Tucker, 1967). Blood N2 content was determined with the Van Slyke technique (Ponganis et al., 1999). The N2 solubility coefficient (1.44 ml N2 per 100 ml blood per atmosphere N2) was determined in blood tonometered with ambient air or 100% N2 at 38°C. Hb concentration was determined with a cyanmethemoglobin spectrophotometric technique (Stockard et al., 2007). Blood samples were analyzed within 10 min of collection. Blood gas, O2 content, lactate concentration and PN2 were stable for as long as 4 h at room temperature in the blood gas syringes (Model 4041, Sims Portex, Keene, NH, USA).
RESULTS
Behavior
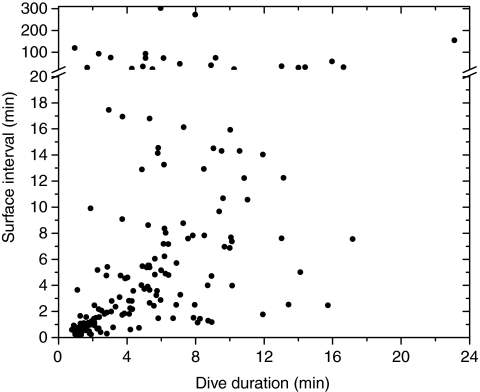
For birds equipped with the PO2 recorder, most dive durations and maximum depths have previously been described, and were similar to those in past studies at the isolated dive hole (Ponganis et al., 2007; Stockard et al., 2005). Additional arterial PO2 profiles obtained from two birds in 2007 increased the number to 57 dives in five birds; venous profiles were from 130 dives in nine birds. Mean dive duration of all 187 dives with PO2 recorders was 5.68±3.99 min; mean dive depth was 34±22 m. The relationship of surface interval to dive duration (Fig. 2) was similar to that previously reported for emperor penguins at sea (Kooyman and Kooyman, 1995; Wienecke et al., 2007).
Fig. 2.
The relationship of surface interval to dive duration of emperor penguins diving at the isolated dive hole.
Dive durations and maximum depths of penguins equipped with the blood sampler ranged from 3 to 12.8 min and 28 to 55 m, respectively. These depths and durations were within the range of dive durations exhibited by birds without such a large recorder. However, as previously observed in emperor penguins equipped with and without a Crittercam camera (Ponganis et al., 2000), the increased drag of the larger unit probably resulted in dives of shorter duration. Nonetheless, dive durations as long as 12.8 min did occur in birds equipped with the sampler. Arterial blood samples were obtained 2.1, 2.8 and 5.2 min into dives of 4.4, 10.1 and 10.5 min duration in one bird. Venous samples were obtained 1.1, 1.7, 2.2, 2.3, 3.2 and 10.5 min into dives of 7.3, 7.8, 4.1, 3.0, 6.6 and 12.8 min duration in four birds. In general, the number of samples obtained was limited by (1) the design of the sampler (one sample per dive and size of the unit), (2) the time required for filling the syringes at these shallow depths (1 min per syringe), (3) our inability to predict the duration of a dive when trying to obtain samples late in a dive, and (4) a frequent lack of interest in diving that occurred after a bird was restrained and equipped with the sampler but which resolved itself after removal of the sampler.
PO2 profiles
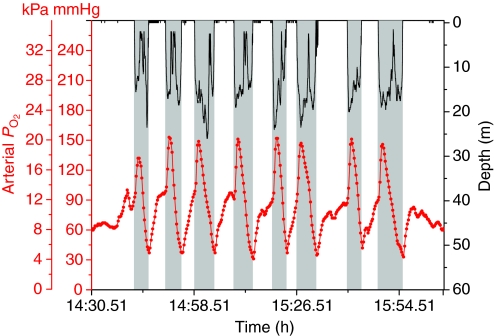
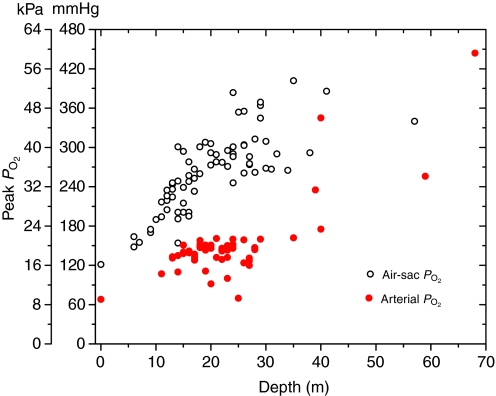
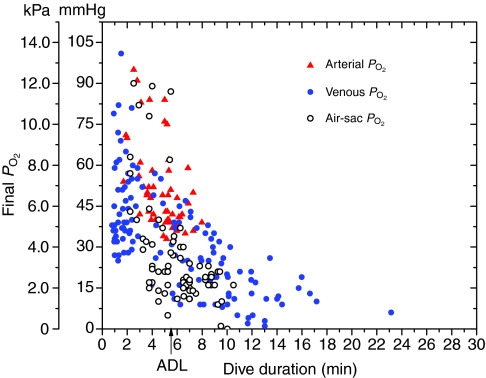
Arterial PO2 profiles during dives (see Fig. 3) revealed that (1) pre-dive PO2 was usually elevated above the previously reported level (68 mmHg) for emperor penguins at rest (Ponganis et al., 2007), (2) PO2 consistently increased during the early part of the dive, usually peaking at the end of the initial descent of the dive, (3) at similar depths (Fig. 4) these peak arterial PO2 values were distinctly less than the previously measured peak air-sac PO2 values (Stockard et al., 2005), (4) arterial PO2 declined as the dive progressed, resulting in final PO2 values similar to those previously reported for air-sac and venous PO2 (Fig. 5), and (6) prior to the next dive, post-dive arterial PO2 reached 68 mmHg within 1.92±1.89 min (N=53).
Fig. 3.
Arterial PO2 (red) and depth (black) profiles during dives of emperor penguin 10. Shaded area indicates diving.
Fig. 4.
The relationship of peak air-sac (Stockard et al., 2005) and arterial PO2 to the depths at which they occurred in emperor penguins diving at the isolated dive hole.
Fig. 5.
The relationship of the final PO2 of a dive to dive duration in the air sac (Stockard et al., 2005), aorta [this study and Ponganis et al. (Ponganis et al., 2007)] and vena cava (Ponganis et al., 2007). ADL, aerobic dive limit.
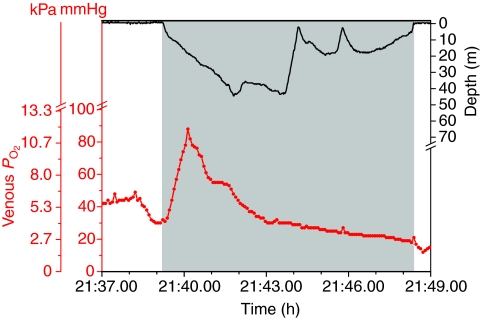
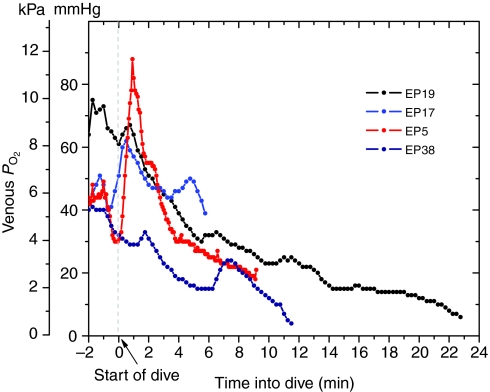
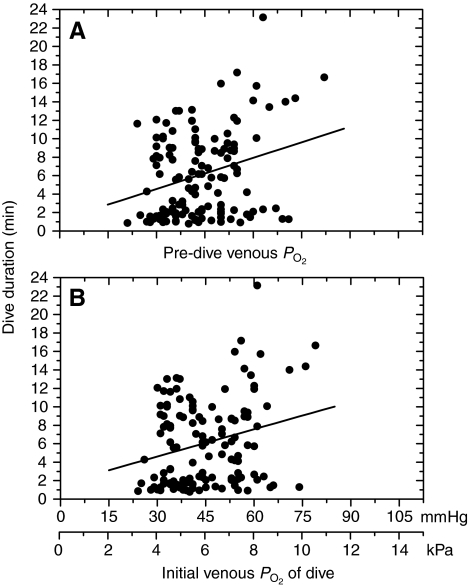
Venous PO2 profiles during dives (Figs 6 and 7) were remarkable for their variable patterns. During the pre-dive period, venous PO2 often fluctuated; it decreased prior to the start of the dive in 51% of dives (see Fig. 6 for an example). Pre-dive venous PO2 ranged from 21 to 82 mmHg (mean 44±12 mmHg), and initial dive PO2 ranged from 24 to 79 mmHg (mean 45±12 mmHg). In 61% of dives, pre-dive venous PO2 was greater than that (41 mmHg) of birds at rest (Ponganis et al., 2007). Dive duration was very weakly, but significantly, related to each of these variables (Fig. 8; pre-dive PO2: y=0.113x+1.18, R2=0.08, P<0.05; initial PO2: y=0.099x+1.18, R2=0.06, P<0.05). In 90% of dives, the venous PO2 increased transiently (within the first 3 min in 78% of dives) with a maximum PO2 of 53±18 mmHg (range 28–129 mmHg) and a mean increase in PO2 of 11±12 mmHg (range 2–76 mmHg). Small transient increases could occur later and more than once during a dive (see Fig. 7). Maximum venous PO2 during a dive was >68 mmHg in 15% of dives, and >41 mmHg in 71% of dives; again, these levels correspond to the arterial and venous PO2 values of emperor penguins at rest (Ponganis et al., 2007). Regression analysis revealed that dive duration was not significantly related to the maximum venous PO2 during a dive. During short dives, the increase in venous PO2 could result in final venous PO2 values that were greater than the start-of-dive venous PO2 [see figure 2 in Ponganis et al. (Ponganis et al., 2007)].
Fig. 6.
Venous PO2 and depth profiles during a 9.2 min dive of emperor penguin 5. Shaded area indicates dive. Venous PO2 declined both before and after this dive. The peak PO2 during the dive was 90 mmHg; it occurred during descent at less than 2 min into the dive.
Fig. 7.
Comparison of venous PO2 in four emperor penguins (EP5, 17, 19, 38) with dives of different durations. Decreases in venous PO2 occurred prior to diving. Transient elevations in venous PO2 are evident at various points in each dive; the initial elevations occurred within the first 2–3 min of these dives.
Fig. 8.
The relationship of dive duration to pre-dive venous PO2 and initial venous PO2. (A) Pre-dive PO2: y=0.113x+1.18, R2=0.08, P<0.05; (B) initial PO2: y=0.099x+1.18, R2=0.06, P<0.05.
After 84 dives, post-dive venous PO2 reached 41 mmHg prior to the next dive; this required 2.23±2.64 min. After a 23.1 min dive, venous PO2 reached this value in less than 2 min. Regression analysis revealed that the relationship of prior dive duration to the time for venous PO2 to recover to 41 mmHg was not significant. The relationship of surface interval to the time to reach a venous PO2 of 41 mmHg was also not significant.
In the post-dive period, venous PO2 initially continued to decline below the final dive value after 48% of dives. The time for post-dive venous PO2 to return to 41 mmHg was not significantly longer than that required for arterial PO2 to return to 68 mmHg, but both intravascular return times were significantly longer than the 0.92±0.44 min [N=73, data from Stockard et al. (Stockard et al., 2005)] required for air-sac PO2 to return to the level of birds at rest (ANOVA, F=9.308, P<0.05; Tukey HSD post-hoc analysis, P<0.05).
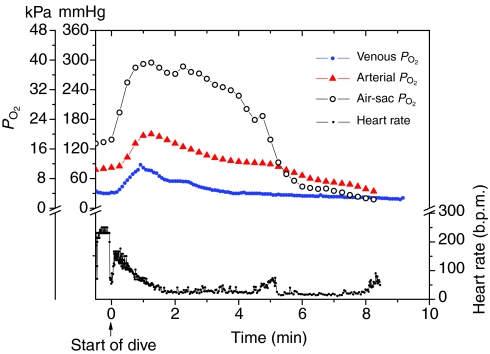
Fig. 9 represents a comparison of typical air-sac, arterial and venous PO2 profiles and a heart rate profile of different birds for shallow (<50 m) dives of approximately 8 min duration. The data are from this and prior studies (Meir et al., 2008; Stockard et al., 2005).
Fig. 9.
Air-sac PO2, arterial PO2, venous PO2 and heart rate profiles during shallow (<50 m), approximately 8 min dives of four emperor penguins. Elevations in air-sac, arterial and venous PO2 values and in heart rate all occur within the first 2 min of the dives. Air sac, bird 7 (Stockard et al., 2005); arterial, bird 10; venous, bird 5; heart rate, bird 37 (Meir et al., 2008). b.p.m., beats per min.
Blood analyses in penguins at rest and during non-diving periods
Arterial and venous N2 partial pressures (PN2) in emperor penguins at rest were 0.86±0.04 atmospheres absolute (ATA; N=3) and 0.85±0.06 ATA (N=9), respectively. Blood gas (PO2, PCO2, pH), O2 content and lactate concentrations of these birds at rest have previously been reported (Ponganis et al., 2007).
Venous PO2 and PCO2 from the brachial vein (wing) of a restrained penguin during a post-dive surface interval were 81 and 38 mmHg, respectively. During anesthesia of one bird on 100% O2, near-simultaneous sampling of the brachial artery and brachial vein yielded PO2 values of 247 and 260 mmHg, respectively. Hemoglobin concentrations of three penguins during anesthesia and at rest were 16.5±0.6 and 16.4±0.8 g dl–1, respectively.
Blood analyses in diving penguins
Blood gas (PO2, PCO2, pH) and O2 content were measured in two arterial and three venous samples obtained during dives (Table 1). It is notable that arterial and venous PO2 values at between 1 and 3 min into the dives were above or near the respective values of penguins at rest. Blood lactate concentration was less than 2.0 mmol l–1 as far as 10.5 min into a dive (Fig. 10). Mean arterial PN2 at depths of 25–32 m at 2.1–5.2 min into a dive was elevated at 2.1±0.7 ATA (N=3). Venous PN2 at 20–37 m depth at 1.7–3.2 min into a dive was 2.1±0.7 ATA (N=4). These arterial and venous PN2 values were not significantly different (Student's two sample t-test).
Table 1.
Arterial and venous blood gas and O2 content during dives of emperor penguins
| Penguin | PCO2 Site | PO2 pH | O2 content (mmHg) | Time into dive (mmHg) | Sample depth (ml O2 dl–1) | Dive duration (min) | Maximum depth (m) | (min) | (m) |
|---|---|---|---|---|---|---|---|---|---|
| 21 | A | 7.35 | 52 | 89 | 24.3 | 2.1 | 31-21 | 3.3 | 31 |
| 21 | A | 7.42 | 44 | 72 | 20.8 | 2.8 | 32-27 | 10.1 | 36 |
| 29 | V | 7.47 | 48 | 58 | 17.1 | 1.1 | 32 | 7.3 | 41 |
| 31 | V | 7.37 | 62 | 39 | 12.1 | 2.2 | 20-36 | 4.1 | 36 |
| 40 | V | 7.39 | 52 | 36 | 12.4 | 3.2 | 30 | 6.6 | 44 |
A, artery; V, vein. For comparison, mean arterial and venous pH, PCO2, PO2, and O2 content of emperor penguins at rest (Ponganis et al., 2007) were 7.50 and 7.50, 42 and 49 mmHg, 68 and 41 mmHg, and 22.5 and 17.4 ml O2 dl–1, respectively. In birds 31 and 40, while they were at rest, venous PCO2 values were 55 and 46 mmHg, and PO2 values were 30 and 41 mmHg, respectively. All catheters were inserted in femoral vessels except for bird 31, in which the catheter was inserted into the axillary vein
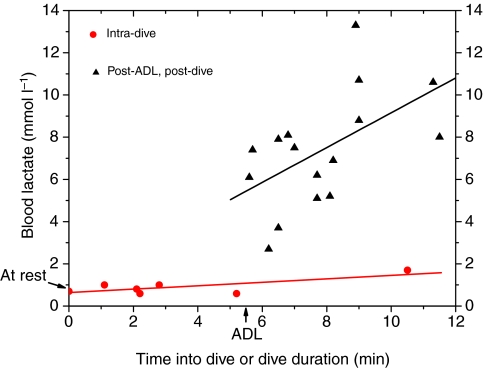
Fig. 10.
Blood lactate concentrations as a function of time into dive or dive duration. Intradive blood lactate concentration was obtained using the blood sampler; post-dive lactate concentrations were measured previously (Ponganis et al., 1997).
Hb concentration during dives of the same three penguins sampled for Hb under anesthesia and at rest was 16.3±0.7 g dl–1. The Hb concentrations during anesthesia, at rest and during dives were not significantly different (ANOVA, F=5.14, P=0.94).
DISCUSSION
Maintenance of gas exchange during dives
The initial transient increases in arterial PO2 profiles during dives reflected the compression hyperoxia previously observed in the air sacs of diving emperor penguins and confirmed the maintenance of pulmonary gas exchange and transfer of O2 from the respiratory store into the blood O2 store during the dive. As a result, during some dives, final arterial PO2 values were greater than the mean arterial PO2 of 68 mmHg of birds at rest (Fig. 5) and, in other dives, equivalent to or even greater than start-of-dive PO2 values (Ponganis et al., 2007). Similarly, final venous PO2 values of some dives, especially short-duration dives, were not only greater than the mean venous value of penguins at rest but also greater than start-of-dive PO2 (Ponganis et al., 2007).
Evidence for the maintenance of gas exchange during dives was also provided by the elevations of arterial/venous PO2 and PN2 recorded in the blood sampler data. In Table 1, arterial and venous PO2 values at 1–3 min into dives were greater than or near the corresponding values of penguins at rest (Ponganis et al., 2007). In addition, during dives, mean arterial PN2 rose to 2.1 ATA at depths of 25–32 m; this is again indicative of continued gas exchange during these shallow dives. During simulated dives, similar increases have been recorded in arterial PN2 in Adelie penguins (Pygoscelis adeliae) and in venous PN2 in king penguins (Aptenodytes patagonicus) (Kooyman et al., 1973; Ponganis et al., 1999).
The arterial PO2 profiles and PN2 values also provide insight into possible mechanisms affecting gas transfer in the lungs. Although the mean PN2 of 2.1 ATA at 25–32 m depth is approximately twice the arterial value of penguins at rest, it is notable that the calculated air-sac PN2 at these depths is 2.8–3.4 ATA, assuming an air-sac fraction of 0.8. The actual air-sac PN2 is probably even higher since the O2 fraction will decrease secondary to O2 consumption (Kooyman et al., 1973). Thus, there appears to be an air sac-to-arterial difference not just for PO2 (Fig. 4) but also for PN2. These differences may be due to ventilation–perfusion mismatch in the lung during the dive and/or due to the thickened parabronchial capillary blood-to-air barrier (Powell, 2000; Welsch and Aschauer, 1986). It is also notable that a large air sac-to-arterial difference in PO2 has been observed in emperor penguins at rest (Ponganis et al., 2007). The typical increases in arterial PO2 prior to the start of diving activity (see Fig. 3) suggest that the tachycardia and hyperventilation of the pre-dive period (Kooyman et al., 1971; Kooyman et al., 1992; Meir et al., 2008) improve ventilation–perfusion matching and account for these changes in PO2 during the pre-dive period.
Despite air sac-to-arterial differences in PO2 and PN2, the arterial PO2 profiles and blood gas analyses obtained in this study all support the concept that pulmonary gas exchange is maintained during these shallow dives of emperor penguins with net transfer of O2 from the respiratory O2 store to the blood (Stockard et al., 2005).
Blood flow patterns
Implications for muscle blood flow during dives
Venous PO2 profiles and intradive blood lactate concentration were first examined for evidence regarding muscle blood flow patterns during diving. We had hypothesized that a lack of muscle perfusion during dives would prevent muscle O2 extraction from blood and also prevent the washout of lactate from muscle during dives longer than the ADL. In particular, despite the fact that buoyancy, stroke frequency and, therefore, muscle workload were highest during the initial descent period (Sato et al., 2002; van Dam et al., 2002), we expected the decline in venous PO2 during the initial descent to be slow because of the hypothesized lack of muscle perfusion.
Although venous PO2 profiles were variable in pattern and in overall rate of decline of PO2, 71% of dives actually had an increase in venous PO2 during the initial descent period (Figs 6 and 7). In 15% of dives, venous PO2 increased to values that were greater than the mean arterial PO2 of birds at rest. The PO2 of venous blood from which muscle has extracted O2 should decline, not rise. Such increases in venous PO2 during the initial descent period are thus not consistent with perfusion of active locomotory muscle. This hypothesis is further supported by dives in which final venous PO2 values were greater than start-of-dive venous PO2 values (see range of final PO2 values for short-duration dives in Fig. 5) (see also Ponganis et al., 2007). In addition, blood lactate concentrations remained less than 2 mmol l–1 even as far as 10.5 min into a dive, well beyond the ADL (Fig. 10). Together, all these findings support a classical model of bradycardia and peripheral vasoconstriction in diving emperor penguins, in which muscle is isolated from the circulation during the dive, and lactate washout occurs during the eupneic period when muscle perfusion is re-established (Scholander, 1940; Scholander et al., 1942). This postulated lack of muscle perfusion during dives is also consistent with the lack of a relationship between stroke frequency and heart rate during dives of emperor penguins (Meir et al., 2008) and with increases in pectoral muscle temperature during dives of both emperor penguins and king penguins (Ponganis et al., 2003; Schmidt et al., 2007).
Implications for muscle blood flow before and after dives
Increased muscle perfusion and blood O2 extraction during both the pre-dive period and the post-dive period were suggested by decreases in venous PO2 during these times (see Fig. 6 as an example). Although venous PO2 may decrease due to variety of factors, including O2 consumption by tissues other than muscle, we suggest that these declines in venous PO2 and previously documented pre-dive and post-dive tachycardias (Froget et al., 2004; Kooyman et al., 1992; Meir et al., 2008) support the concept of increased muscle perfusion and muscle O2 extraction in both the pre-dive and post-dive period. Such pre-dive muscle hyperemia has also been postulated in king penguins because of decreases in muscle temperature prior to dives (Schmidt et al., 2006). If this hypothesis is correct, the declines in pre-dive venous PO2 imply that muscle with high myoglobin content is not always fully saturated with O2 during some rest periods and interdive intervals. This last suggestion is supported by the wide range of pre-submersion muscle O2 contents in the early research of Scholander and colleagues (Scholander, 1940; Scholander et al., 1942). During the post-dive period, muscle reperfusion is, of course, expected. Venous PO2 may not always decline during the post-dive period, however, because, even after muscle O2 extraction from re-oxygenated arterial blood, the resulting venous PO2 may still be greater than the end-of-dive venous PO2.
Implications for post-dive gas exchange
The return time of post-dive venous PO2 (2.3 min) to the mean value of penguins at rest was slightly longer than the 1.9 min value for arterial PO2, but these times did not differ significantly. This was contrary to our hypothesis that O2 uptake/consumption by muscle and other organs during the surface period would slow the replenishment of the venous blood O2 store relative to the arterial rate. As expected, the return time (0.9 min) for air-sac PO2 to reach the level at rest was significantly less than either of the blood values (ANOVA). Overall, the post-dive increases in air-sac, arterial and venous PO2 values during the surface interval were rapid and consistent with prior reports of (1) a decrease in the post-dive tachycardia and resumption of a respiratory sinus arrhythmia within 2 min (Meir et al., 2008) and (2) a rapid decline in post-dive respiratory rate to levels of birds at rest in 3 min (Kooyman et al., 1971). These observations all suggest that the blood O2 store is replenished and most CO2 is exhaled within 3 min after a dive. The relationship of surface interval to the time for post-dive venous PO2 to reach 41 mmHg was not significant. A surface interval could be as much as 30 times longer than the time for venous PO2 to return to a resting level. Although the time course of muscle re-oxygenation during the surface interval remains to be investigated, other factors such as sated appetites, food digestion, metabolic processing, social interactions and visits to the dive hole by Weddell seals probably also contribute to the duration of the surface interval of birds diving at the isolated dive hole.
Implications for arterio-venous shunts
The existence and function of arterio-venous shunts are often documented by elevations in venous PO2 or O2 content above control values or average values of animals at rest. Pre-dive venous PO2 was greater than that of penguins at rest prior to 61% of dives. Arterio-venous anastamoses, which have been described anatomically in the extremities of birds (Arad et al., 1989; Thomas and Fordyce, 2007), may contribute to these elevations during surface periods. In addition, high blood flow through the vasculature of an extremity such as the wing may act as an effective shunt given the relatively low O2 requirements of the bones, tendons and ligaments of the wing. Such flow through the wings and feet during the surface tachycardia is consistent with the re-warming previously recorded in the extremities during the surface period (Ponganis et al., 2003). In addition, this suggestion is supported by a brachial vein (in the wing) blood sample obtained during a surface interval. The PO2 (81 mmHg) and PCO2 (38 mmHg) not only were characteristic of arterial blood but, as would be expected during hyperventilation, also were above (PO2) and below (PCO2) the respective arterial values of penguins at rest. The similarity of the brachial vein PO2 of 260 mmHg to that in the brachial artery during anesthesia on 100% O2 was also consistent with arterio-venous shunting through the wing. Indeed, this mechanism probably contributes to the high venous PO2 values reported during avian anesthesia (Jaensch et al., 2002). Therefore, we propose that arterio-venous shunting through the extremities during the post-dive period contributes to elevated pre-dive venous PO2 values as well as to the rapid recovery of post-dive venous PO2.
Another site of arterio-venous shunting may be the brood patch vasculature. It has already been suggested that increased perfusion accounts for rewarming of the brood patch during surface periods of king penguins at sea (Schmidt et al., 2006). Since the brood patch contains arterio-venous anastamoses (Midtgard et al., 1985) and is not a site of high metabolic activity, increased flow in brood patch vessels could also contribute to elevations in venous PO2.
Although the relationship of dive duration to pre-dive or initial venous PO2 was weak (R2<0.1, Fig. 8), the potential significance of arterio-venous shunting during the surface period is exemplified by the pre-dive venous PO2 of 63 mmHg prior to a 23.1 min dive, the longest dive ever reported for an emperor penguin. The Hb saturation, blood oxygen content and total blood O2 store at that PO2 should be considerably greater than the respective values at the mean venous PO2 of 41 mmHg of birds at rest. Examination of the relationship of venous Hb saturation to dive duration awaits determination of the emperor penguin O2–Hb dissociation curve.
The most remarkable and unexpected increases in venous PO2 occurred in 78% of dives during early descent. In 15% of all dives, the peak venous PO2 values were greater than the arterial value of birds at rest. Values could be as high as 90 mmHg (Fig. 6). Such high PO2 values, which reflect arterial blood values and fully saturated Hb, suggest the existence of a significant arterio-venous shunt in addition to muscle ischemia during the dive. The elevated venous PN2 values collected during the early parts of dives were also not significantly different from arterial values in the same depth range. This suggests minimal tissue uptake of N2 and is again consistent with the existence of an arterio-venous shunt during the dive.
We propose that the blood flow through an arterio-venous shunt could be supported by the transient increase in heart rate that occurs during the early descent period of dives by penguins (Froget et al., 2004; Green et al., 2003; Meir et al., 2008). Therefore, as illustrated in Fig. 9, we hypothesize that a primary function of the transient increase in heart rate early in the dive is to enhance the transport of O2 from the air sac and lungs into the arterial system and then, through a still-open arterio-venous shunt, into the venous system. This essentially represents a mechanism by which the size of the venous O2 reservoir can be increased during the dive by transport of O2 from the large respiratory O2 store of the penguin into the blood. As such large increases in venous PO2 were not always seen, the magnitude of such a shunt may vary in different dives. Indeed, the variability in venous PO2 profile patterns during dives suggests that the vascular response may be quite plastic and adaptive to different conditions.
However, the anatomical basis of such an intradive arterio-venous shunt remains to be determined. Based on observations that bleeding from a nicked wing continued during the first 2.5 min of a dive of an emperor penguin (Kooyman et al., 1971), it is possible that blood flow through the wings contributes to these elevations in venous PO2 during dives. If so, the countercurrent heat exchangers of the extremities (Arad et al., 1989; Thomas and Fordyce, 2007) are extremely efficient, given the preservation of core temperature and the significant cooling of both the wings and feet during dives (Ponganis et al., 2001; Ponganis et al., 2004; Ponganis et al., 2003). Conceivably, shunt flow may also occur in arterio-venous anastamoses described in the proximal regions of the extremities of birds (Arad et al., 1989). In addition, blood flow through the brood patch region could contribute to an arterio-venous shunt during a dive. It has already been suggested that such flow contributes to paradoxical increases in brood patch temperature during dives of king penguins (Schmidt et al., 2006). Another mechanism of arterio-venous shunting may involve the muscle vascular bed because avian muscle has been considered to have `luxuriant', non-nutrient blood flow that contributes to higher than expected PO2 in the venous effluent of muscle (Folkow et al., 1966; Grubb, 1981). In summary, there may be several anatomical sites for the hypothesized arterio-venous shunt during dives.
It should also be noted that it is unlikely that contraction of a large spleen and expulsion of arterialized blood into the venous system contribute to such elevations in venous PO2 during dives of emperor penguins. First, we have never observed a large spleen in any of our many dissections of emperor penguin carcasses. Second, Hb concentration in three birds did not decrease during anesthesia or increase during diving in this study. Such changes would be expected if a large spleen dilated during anesthesia or contracted during diving activity as in seals (Hurford et al., 1996; Ponganis et al., 1993; Ponganis et al., 1992; Qvist et al., 1986). Third, blood introduced from the spleen should have a PN2 near that at the surface and should delay the rise in venous PN2 at depth, thus increasing, not decreasing, the arterial-to-venous difference in PN2.
CONCLUSIONS
Blood PO2 profiles and analyses of blood samples during dives of emperor penguins are consistent with (a) maintenance of gas exchange between the air sacs, lungs and blood during dives, (b) muscle ischemia during dives, (c) muscle hyperemia both before and after dives, and (d) utilization of arterio-venous shunts during the surface period and during the dive. These physiological processes allow (1) utilization of the large respiratory O2 store of penguins, (2) regulated depletion of the blood O2 store, (3) isolation of the respiratory and blood O2 stores from working muscle, and (4) optimized loading of O2 stores both before and after dives. The proposed muscle ischemia and the incomplete depletion of the respiratory and blood O2 stores at the ADL suggest that muscle O2 depletion and subsequent lactate accumulation represent the primary source of the post-dive increase in lactate at the ADL. The potential existence of an arterio-venous shunt during a dive was unexpected. Such increased flow through an arterio-venous shunt could be supported by the transient increases in heart rate previously reported during the early descent periods of dives.
This work was supported by NSF grants 98-144794, 02-29638 and 05-38594. J.U.M. was supported by an NDSEG fellowship, and Los Angeles ARCS fellowship provided by Ed and Nadine Carson. C.L.W. was supported by a UC Regents fellowship and a NIH Training Program in Marine Biotechnology fellowship. We thank R. van Dam, D. H. and C. Levenson, J. Heil, M. Tulis, C. Champagne, Y. Habara, K. Sato, J. St Leger, T. Zenteno-Savin and E. Stockard for assistance in the field, H. Hanish and M. Loughry of UFI for PO2 recorder design and construction, McMurdo Station personnel for outstanding support, and G. L. Kooyman for his support and manuscript review. Deposited in PMC for release after 12 months.
References
- Arad, Z., Midtgard, U. and Bernstein, M. H. (1989). Thermoregulation in turkey vultures: vascular anatomy, arteriovenous heat exchange, and behavior. Condor 91, 505-514. [Google Scholar]
- Davis, R. W. and Kanatous, S. B. (1999). Convective oxygen transport and tissue oxygen consumption in Weddell seals during aerobic dives. J. Exp. Biol. 202, 1091-1113. [DOI] [PubMed] [Google Scholar]
- Falke, K. J., Hill, R. D., Qvist, J., Schneider, R. C., Guppy, M., Liggins, G. C., Hochachka, P. W., Elliott, R. E. and Zapol, W. M. (1985). Seal lungs collapse during free diving: evidence from arterial nitrogen tensions. Science 229, 556-558. [DOI] [PubMed] [Google Scholar]
- Folkow, B., Fuxe, K. and Sonnenschein, R. R. (1966). Responses of skeletal musculature and it vasculature during “diving” in the duck: peculiarities of the adrenergic vasoconstrictor innervation. Acta Physiol. Scand. 67, 327-342. [DOI] [PubMed] [Google Scholar]
- Froget, G., Butler, P. J., Woakes, A. J., Fahlman, A., Kuntz, G., Le Maho, Y. and Handrich, Y. (2004). Heart rate and energetics of free-ranging king penguins (Aptenodytes forsteri). J. Exp. Biol. 207, 3917-3926. [DOI] [PubMed] [Google Scholar]
- Green, J. A., Butler, P. J., Woakes, A. J. and Boyd, I. L. (2003). Energetics of diving in macaroni penguins. J. Exp. Biol. 206, 43-57. [DOI] [PubMed] [Google Scholar]
- Grubb, B. R. (1981). Blood flow and oxygen consumption in avian skeletal muscle during hypoxia. J. App. Physiol. 50, 450-455. [DOI] [PubMed] [Google Scholar]
- Guyton, G. P., Stanek, K. S., Schneider, R. C., Hochachka, P. W., Hurford, W. E., Zapol, D. G. and Zapol, W. M. (1995). Myoglobin-saturation in free-diving Weddell seals. J. App. Physiol. 79, 1148-1155. [DOI] [PubMed] [Google Scholar]
- Hurford, W. E., Hochachka, P. W., Schneider, R. C., Guyton, G. P., Stanek, K. S., Zapol, D. G., Liggins, G. C. and Zapol, W. M. (1996). Splenic contraction, catecholamine release, and blood volume redistribution during diving in the Weddell seal. J. App. Physiol. 80, 298-306. [DOI] [PubMed] [Google Scholar]
- Jaensch, S. M., Cullen, L. and Raidal, S. R. (2002). Air sac functional anatomy of the sulphur-crested cockatoo (Cacatua galerita) during isoflurane anesthesia. J. Avian Med. Surg. 16, 2-9. [Google Scholar]
- Kooyman, G. L. and Kooyman, T. G. (1995). Diving behavior of emperor penguins nurturing chicks at Coulman Island, Antarctica. Condor 97, 536-549. [Google Scholar]
- Kooyman, G. L. and Ponganis, P. J. (1998). The physiological basis of diving to depth: birds and mammals. Ann. Rev. Physiol. 60, 19-32. [DOI] [PubMed] [Google Scholar]
- Kooyman, G. L., Drabek, C. M., Elsner, R. and Campbell, W. B. (1971). Diving behavior of the emperor penguin, Aptenodytes forsteri. Auk 88, 775-795. [Google Scholar]
- Kooyman, G. L., Schroeder, J. P., Greene, D. G. and Smith, V. A. (1973). Gas exchange in penguins during simulated dives to 30 and 68 m. Am. J. Physiol. 225, 1467-1471. [DOI] [PubMed] [Google Scholar]
- Kooyman, G. L., Ponganis, P. J., Castellini, M. A., Ponganis, E. P., Ponganis, K. V., Thorson, P. H., Eckert, S. A. and LeMaho, Y. (1992). Heart rates and swim speeds of emperor penguins diving under sea ice. J. Exp. Biol. 165, 161-180. [DOI] [PubMed] [Google Scholar]
- Meir, J. U., Stockard, T. K., Williams, C. L., Ponganis, K. V. and Ponganis, P. J. (2008). Heart rate regulation and extreme bradycardia in diving emperor penguins. J. Exp. Biol. 211, 1169-1179. [DOI] [PubMed] [Google Scholar]
- Midtgard, U., Sejrsen, P. and Johansen, K. (1985). Blood flow in the brood patch of Bantam hens: evidence of cold vasodilatation. J. Comp. Physiol. B 155, 703-709. [Google Scholar]
- Ponganis, P. J., Kooyman, G. L., Sartoris, D. and Jobsis, P. F. (1992). Pinniped splenic volumes. Am. J. Physiol. 262, R322-R325. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J., Kooyman, G. L. and Castellini, M. A. (1993). Determinants of the aerobic dive limit of Weddell seals: analysis of diving metabolic rates, postdive end tidal PO2's, and blood and muscle oxygen stores. Physiol. Zool. 66, 732-749. [Google Scholar]
- Ponganis, P. J., Kooyman, G. L., Starke, L. N., Kooyman, C. A. and Kooyman, T. G. (1997). Post-dive blood lactate concentrations in emperor penguins, Aptenodytes forsteri. J. Exp. Biol. 200, 1623-1626. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J., Kooyman, G. L., Van Dam, R. and Le Maho, Y. (1999). Physiological responses of king penguins during simulated diving to 136m depth. J. Exp. Biol. 202, 2819-2822. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J., Van Dam, R. P., Marshall, G., Knower, T. and Levenson, D. H. (2000). Sub-ice foraging behavior of emperor penguins. J. Exp. Biol. 203, 3275-3278. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J., Van Dam, R. P., Knower, T. and Levenson, D. H. (2001). Temperature regulation in emperor penguins foraging under sea ice. Comp. Biochem. Physiol. 129A, 811-820. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J., Van Dam, R. P., Levenson, D. H., Knower, T., Ponganis, K. V. and Marshall, G. (2003). Regional heterothermy and conservation of core temperature in emperor penguins diving under sea ice. Comp. Biochem. Physiol. 135A, 477-487. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J., van Dam, R. P., Knower, T., Levenson, D. H. and Ponganis, K. V. (2004). Deep dives and aortic temperatures of emperor penguins: new directions for bio-logging at the isolated dive hole. Mem. Natl Inst. Polar Res. Spec. Issue 58, 155-161. [Google Scholar]
- Ponganis, P. J., Stockard, T. K., Meir, J. U., Williams, C. L., Ponganis, K. V., van Dam, R. P. and Howard, R. (2007). Returning on empty: extreme blood O2 depletion underlies dive capacity of emperor penguins. J. Exp. Biol. 210, 4279-4285. [DOI] [PubMed] [Google Scholar]
- Powell, F. L. (2000). Respiration. In Sturkie's Avian Physiology (ed. G. C. Whittow), pp. 233-264. San Diego: Academic Press.
- Qvist, J., Hill, R. D., Schneider, R. C., Falke, K. J., Liggins, G. C., Guppy, M., Elliott, R. L., Hochachka, P. W. and Zapol, W. M. (1986). Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. J. App. Physiol. 61, 1560-1569. [DOI] [PubMed] [Google Scholar]
- Sato, K., Naito, Y., Kato, A., Niizuma, Y., Watanuki, Y., Charassin, J. B., Bost, C.-A., Handrich, Y. and Le Maho, Y. (2002). Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J. Exp. Biol. 205, 1189-1197. [DOI] [PubMed] [Google Scholar]
- Schmidt, A., Alard, F. and Handrich, Y. (2006). Changes in body temperature in king penguins at sea: the result of fine adjustments in peripheral heat loss. Am. J. Physiol. 291, R608-R618. [DOI] [PubMed] [Google Scholar]
- Scholander, P. F. (1940). Experimental investigations on the respiratory function in diving mammals and birds. Hvalradets skrifter 22, 1-131. [Google Scholar]
- Scholander, P. F., Irving, L. and Grinnell, S. W. (1942). Aerobic and anaerobic changes in seal muscle during diving. J. Biol. Chem. 142, 431-440. [Google Scholar]
- Stockard, T. K., Heil, J., Meir, J. U., Sato, K., Ponganis, K. V. and Ponganis, P. J. (2005). Air sac PO2 and oxygen depletion during dives of emperor penguins. J. Exp. Biol. 208, 2973-2981. [DOI] [PubMed] [Google Scholar]
- Stockard, T. K., Levenson, D. H., Berg, L., Fransioli, J. R., Baranov, E. A. and Ponganis, P. J. (2007). Blood oxygen depletion during rest-associated apneas of northern elephant seals (Mirounga angustirostris). J. Exp. Biol. 210, 2607-2617. [DOI] [PubMed] [Google Scholar]
- Thomas, D. B. and Fordyce, R. E. (2007). The heterothermic loophole exploited by penguins. Aust. J. Zool. 55, 317-321. [Google Scholar]
- Tucker, V. A. (1967). Method for oxygen content and dissociation curves on microliter blood samples. J. App. Physiol. 23, 410-414. [DOI] [PubMed] [Google Scholar]
- van Dam, R. P., Ponganis, P. J., Ponganis, K. V., Levenson, D. H. and Marshall, G. (2002). Stroke frequencies of emperor penguins diving under sea ice. J. Exp. Biol. 205, 3769-3774. [DOI] [PubMed] [Google Scholar]
- Welsch, U. and Aschauer, B. (1986). Ultrastructural observations on the lung of the emperor penguin (Aptenodytes forsteri). Cell Tissue Res. 2443, 137-144. [Google Scholar]
- Wienecke, B., Robertson, G., Kirkwood, R. and Lawton, K. (2007). Extreme dives by free-ranging emperor penguins. Polar Biol. 30, 133-142. [Google Scholar]