Summary
To examine the evolution of endurance-exercise behaviour, we have selectively bred four replicate lines of laboratory mice (Mus domesticus) for high voluntary wheel running (`high runner' or HR lines), while also maintaining four non-selected control (C) lines. By generation 16, HR mice ran ∼2.7-fold more than C mice, mainly by running faster (especially in females), a differential maintained through subsequent generations, suggesting an evolutionary limit of unknown origin. We hypothesized that HR mice would have higher glycogen levels before nightly running, show greater depletion of those depots during their more intense wheel running, and have increased glycogen synthase activity and GLUT-4 protein in skeletal muscle. We sampled females from generation 35 at three times (photophase 07:00 h–19:00 h) during days 5–6 of wheel access, as in the routine selection protocol: Group 1, day 5, 16:00 h–17:30 h, wheels blocked from 13:00 h; Group 2, day 6, 02:00 h–03:30 h (immediately after peak running); and Group 3, day 6, 07:00 h–08:30 h. An additional Group 4, sampled 16:00 h–17:30 h, never had wheels. HR individuals with the mini-muscle phenotype (50% reduced hindlimb muscle mass) were distinguished for statistical analyses comparing C, HR normal, and HR mini. HR mini ran more than HR normal, and at higher speeds, which might explain why they have been favored by the selective-breeding protocol. Plasma glucose was higher in Group 1 than in Group 4, indicating a training effect (phenotypic plasticity). Without wheels, no differences in gastrocnemius GLUT-4 were observed. After 5 days with wheels, all mice showed elevated GLUT-4, but HR normal and mini were 2.5-fold higher than C. At all times and irrespective of wheel access, HR mini showed approximately three-fold higher [glycogen] in gastrocnemius and altered glycogen synthase activity. HR mini also showed elevated glycogen in soleus when sampled during peak running. All mice showed some glycogen depletion during nightly wheel running, in muscles and/or liver, but the magnitude of this depletion was not large and hence does not seem to be limiting to the evolution of even-higher wheel running.
Keywords: adaptive plasticity, artificial selection, experimental evolution, glycogen, GLUT-4, phenotypic plasticity, selection limit, voluntary exercise
INTRODUCTION
In mammalian skeletal muscle, increases in exercise intensity are sustained by a concomitant increase in the proportion of glucose oxidation relative to fatty acids (Brooks, 1998; Hargreaves and Spriet, 2006). This increased reliance on carbohydrates is attributed to several physiological processes, including increased rates of glucose transport into muscle cells, increased activation of pyruvate dehydrogenase (PDH) and an enhanced recruitment of type 2B muscle fibers, partially mediated by increased activation of the sympathetic nervous system (Wasserman and Cherrington, 1991; Putnam et al., 1995; Brooks and Mercier, 1994). The relative increase in muscle glycolysis (breakdown of glucose to pyruvate) and glycogenolysis (breakdown of glycogen) with intensity of exercise occurs even in aerobically trained individuals, despite the biochemical and endocrine adjustments to spare glycogen use and enhance lipid oxidation during submaximal exercise [e.g. increased mitochondrial density that allows a given respiratory rate to be accomplished at a higher ATP:ADP ratio, decreased rates of glycogenolysis and glycolysis, augmented β-oxidation and decreased circulating blood lactate and catecholamine levels at given exercise power outputs (Brooks, 1998)].
In mammals, the maximum rate of fatty acid oxidation meets only approximately 50% of the rate of energy utilization required to support the maximum aerobically sustainable running speed (Newsholme, 1988). Exhaustion (inability to maintain a specified workload) at high-intensity aerobic exercise occurs when muscle glycogen stores are depleted. Therefore, the size of the pre-exercise glycogen stores has been recognized as one of the most important factors limiting the maintenance of moderate-to-high power output for extended periods of time (Hultman and Harris, 1988).
Few studies of mammals have focused on the evolution of substrate use to sustain exercise. A series of studies comparing dogs and goats found that, although they exhibit a similar pattern of increased carbohydrate reliance at increased relative exercise intensities (Roberts et al., 1996), dogs show 2.2-fold higher maximal oxygen consumption (V̇O2,max), 4-fold higher muscle glycogen concentration, 1.6-fold greater glucose flux rates from glycogen during exercise and can sustain aerobic exercise at high intensities for twice as long as goats (Vock et al., 1996).
As a complement to such comparative studies, we have used an experimental evolution approach (Swallow and Garland, 2005; Garland and Kelly, 2006; Garland and Rose, 2009). Four replicate lines of laboratory house mice (Mus domesticus) have been bred to increase voluntary wheel-running behavior (`high runner' or HR lines), while four non-selected lines serve as controls [C lines (Swallow et al., 1998)]. Wheel-running distance of the HR lines increased for approximately 16 generations until the HR line ran ∼2.7-fold more than C lines, and this differential has remained approximately constant since then, in spite of continued selection (Garland, 2003) (T. Garland, unpublished observations). Both sexes of HR mice exhibit elevated V̇O2,max during forced treadmill exercise (Rezende et al., 2005; Rezende et al., 2006a; Rezende et al., 2006c) as well as higher endurance capacity per se in a forced-exercise treadmill test (Meek et al., 2007). The increase in distance covered by HR lines has been achieved entirely by an increase in average running speed in females (Swallow et al., 1998; Koteja and Garland, 2001; Rezende et al., 2005) rather than in time spent running. HR females also run more intermittently, with shorter and more frequent exercise bouts [males were not studied (Girard et al., 2001)]. Such characteristics of the HR lines should impact the patterns of fuel use during sustained wheel running. Indeed, Dumke and colleagues found a higher rate of insulin-mediated glucose uptake in isolated extensor digitorum longus (EDL) muscles of HR-line males (females were not studied), suggesting an increased GLUT-4 abundance in these muscles (Dumke et al., 2001). Re-analysis of data presented by Houle-Leroy and colleagues (Houle-Leroy et al., 2003) by use of the SAS Procedure Mixed (as in the present study – see Materials and Methods) demonstrates that HR males (but not females) have significantly elevated carnitine palmitoyltransferase (indicative of capacity for fatty acid oxidation) in mixed hindlimb muscle in comparison with mice from the C lines (results not shown). Finally, HR mice have extremely low levels of body fat in comparison with both C mice and several other strains, including ones bred for low body fat (Nehrenberg et al., in review) (see also Swallow et al., 2001).
One remarkable finding of the selection experiment has been an increase in frequency of a small hind-limb muscle phenotype in two of the four HR lines, which illustrates the principle of multiple adaptive responses to uniform selection (Garland, 2003; Swallow et al., 2009). This `mini-muscle' phenotype results from an autosomal recessive allele (Garland et al., 2002; Hannon et al., 2008) that has been fine-mapped to a 2.6335-Mb interval on mouse chromosome MMU11 (Hartmann et al., 2008). Mice from generation 14 with this `mini-muscle' phenotype exhibited ∼2-fold higher mass-specific activities of mitochondrial enzymes, including cytochrome c oxidase and citrate synthase, in comparison with their counterparts with normally sized muscles, along with elevated hexokinase and carnitine palmitoyl transferase activities (Houle-Leroy et al., 2003; Guderley et al., 2008). On a whole-muscle basis, such enhanced mass-specific aerobic capacity of mini-muscles almost completely compensates for their reduced muscle mass (Houle-Leroy et al., 2003). In addition, mini-muscled individuals have an elevated whole-animal maximal oxygen consumption during forced exercise in hypoxia (Rezende et al., 2006a), which is supported by higher capillary:fiber ratio and higher capillary density in the medial gastrocnemius (Wong et al., in revision).
In the present study, we tested the following hypotheses. First, HR lines have higher initial glycogen stores in comparison with C lines. Second, the elevated wheel running of HR lines causes depletion of their glycogen stores to a greater extent than in C lines. Third, HR lines have higher levels of the glucose transporter GLUT-4 in gastrocnemius muscle. Fourth, HR mini-muscle mice run faster and accrue more total revolutions on wheels, and have increased glycogen in muscle owing to their elevated hexokinase activity. The second hypothesis, if supported, would implicate a physiological factor that might be limiting to further evolutionary increases in wheel running. To test these hypotheses, females from HR and C lines had wheel access for 5–6 days when they were approximately 50 days old, simulating the normal selection protocol, and were then sacrificed at three different times of day: during the resting phase before nightly activity (Group 1); during the dark phase, just after the peak of voluntary wheel running (Group 2); and at the end of the dark phase of the light cycle (Group 3). Tissue samples from an additional group of females that never had access to wheels were also obtained (Group 4). We measured voluntary wheel running, GLUT-4 abundance and glycogen synthase activity in muscle samples, glycogen concentration in liver and muscle samples, and plasma glucose concentration.
MATERIALS AND METHODS
Selection experiment and study animals
The original progenitors of the selection experiment were laboratory house mice (Mus domesticus Schwarz and Schwarz 1943) of the outbred, genetically variable Hsd:ICR strain obtained from Harlan Sprague Dawley (Indianapolis, IN, USA). Four lines are selected for high voluntary wheel running (HR lines) and four are bred as controls, without regard to how much they run [C lines (Swallow et al., 1998; Garland, 2003)]. Females were studied here because they present greater voluntary wheel running and run at higher speeds than males in both C and HR lines (e.g. see Swallow et al., 1998; Koteja and Garland, 2001; Garland, 2003). Consequently, we reasoned that fuel-usage correlates of voluntary wheel running would be more apparent in females (but see Rezende et al., 2006c).
Experimental design
Females from generation 35 (N=204) were chosen at random during weaning (21 days of age), weighed, toe-clipped and housed in groups of four per cage. As in the routine propagation of the selection experiment, mice at approximately 50 days of age were individually housed in cages with access to wheels, with food and water ad libitum, for 5–6 days. A photocell counter attached to each wheel allowed computer monitoring of revolutions in one-minute intervals by means of customized software from San Diego Instruments (San Diego, CA, USA). Data were downloaded every 24 h and summarized as total revolutions per day, total number of 1-min intervals per day with at least one revolution, mean speed (total revolutions per number of active intervals) and maximum speed in any 1 min interval. The photophase was 07:00 h to 19:00 h Pacific Standard Time. Red lights were automatically turned on during the dark period.
Mice were sacrificed and tissues were harvested (immediately) at three different times following five days of wheel access (Table 1). These three times corresponded to different states in the circadian rhythm of voluntary wheel running of both HR and C females (Girard et al., 2001; Girard and Garland, 2002) [see also Malisch and colleagues (Malisch et al., 2008) on males]. Tissue sampling was restricted to periods of 1.5 h to reduce variation related to the circadian rhythm of glycogen concentration already described for several tissues from mice and rats, including muscles and liver (Pessacq and Gagliardino, 1975; Garetto and Armstrong, 1983; Escobar et al., 1998). Additionally, ovarian hormones have been demonstrated to influence both rodent voluntary wheel running (Asdell et al., 1962; Morgan and Pffaf, 2002; Ogawa et al., 2003) and the circadian rhythm of glycogen concentration, either accentuating or depressing these circadian fluctuations (Pessacq and Gagliardino, 1975; Palmer et al., 1979). Therefore, we scored the estrous cycle of each female by microscopic observations of vaginal smears collected at the moment of the sacrifice and following the five stages outlined by Rugh (Rugh, 1968) (see also Girard and Garland, 2002).
Table 1.
Experimental groups of mice (photoperiod 07:00 h to 19:00 h)
| Group number | Time of sacrifice (h) | Condition | Wheel access |
|---|---|---|---|
| 1 | 16:00–17:30 | Typically resting; before the beginning of nightly running activity | Five days; wheel access blocked from 13:00 h |
| 2 | 02:00–03:30 | Immediately after the peak of nightly wheel-running activity [see fig. 1 in Girard and colleagues (Girard et al., 2001)] | Five days plus |
| 3 | 07:00–08:30 | Immediately after the dark phase, i.e. the normal activity period for both HR and C lines | Five days plus |
| 4 | 16:00–17:30 | Typically resting; before the beginning of nightly running activity | Never had wheel access |
Seven females from each of the four C lines and two of the HR lines were assigned to each of the three time groups. For the other two HR lines (lab-designated 3 and 6) that exhibit the mini-muscle phenotype, we sampled 13 females for each of the three experimental groups. During this study and during sampling of mice from generations 36–38 for Syme and colleagues, it was discovered that line 3 had gone to fixation for the mini-muscle allele (Syme et al., 2005). Thus, in the present study, as in Syme and colleagues, line 3 contained only mini-muscle individuals, whereas line 6 contained both normal and mini-muscle mice (Syme et al., 2005). Finally, an additional group of females (Group 4) from generation 37 (N=45) that never had wheel access was sampled to determine whether any differences between HR and C lines in the first three groups could be a short-term effect of the difference in voluntary wheel running. Except for wheel access, Group 4 animals were subjected to the same procedures as applied to Group 1.
Tissue harvesting
Mice were rapidly taken from cages, weighed and sacrificed by decapitation to avoid effects of pharmaceuticals. Trunk blood was collected in a weighing boat containing heparin, then transferred to micro-centrifuge tubes. After centrifugation at 8000 g for 10 min at 4°C, aliquots of plasma were frozen at –80°C. Soleus and gastrocnemius muscles were excised from the left leg, livers were dissected, and all tissues were weighed, wrapped in aluminium foil, dropped into liquid nitrogen and stored at –80°C. Gastrocnemius from the contralateral limb were also dissected and frozen for GLUT-4 assays. Vaginal smears were taken to determine estrous cycle stage.
GLUT-4 abundance
The gastrocnemius from left legs were weighed and then homogenized on ice using a glass homogenizer in an ice-cold lysis buffer (1:20 wt:vol.) containing 135 mmol l–1 NaCl, 1 mmol l–1 MgCl2, 2.7 mmol l–1 KCl, 20 mmol l–1 Tris (pH 8.0), 0.5 mmol l–1 sodium vanadate, 10 mmol l–1 sodium fluoride, 0.2 mmol l–1 phenylmethylsulfonyl fluoride, 1% Triton-X, 10% glycerol and 10 μgml–1 leupeptin (Singh et al., 2003). The homogenate was then placed in a Micromax RF microcentrifuge (IEC, Needham Heights, MA, USA) and centrifuged at 16,800 g for 10 min at 4°C. The supernatant was extracted and quantified for protein by the Bradford method (Bradford, 1976) using a Benchmark microplate reader (BioRad, Richmond, CA, USA) at a 1:50 dilution. Thirty-five microliters of sample lysate was diluted 1:1 with Laemmli sample buffer (Laemmli, 1970). Seventy micrograms of protein for GLUT-4 were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) run under reducing conditions on a 12.5% resolving gel on a MiniProtean 3 dual slab cell (BioRad) and transferred to polyvinylidene diflouride (PVDF) membranes using a semidry transfer unit. The membranes were blocked in 5% non-fat dry milk (NFDM) and incubated with αGLUT-4 (donated by Samuel W. Cushman, NIDDK, Bethesda, MD, USA) followed by incubation with goat anti-rabbit IgG conjugated to HRP (sc-2004, SCBT). Antibody binding was visualized using enhanced chemiluminescence (ECL) in accordance to the manufacturer's instructions (West Femto, Pierce Chemical Company, Rockford, IL, USA). The images were captured using a CCD camera in a ChemiDoc system (BioRad) and then saved to a Macintosh G4 computer. Bands were then quantified using Quantity One analysis software (BioRad). Using the freehand contour tool in the Quantity One analysis software, the densities of the bands were determined and expressed as a percentage of a muscle sample standard run on each gel.
Glycogen and glucose concentration
The whole left soleus, the proximal-half of the left gastrocnemius and a piece of the main lobe of liver were used to measure glycogen concentration by a modification of the Passoneau and Lauderdale procedure (Passoneeau and Lauderdale, 1974). Frozen muscle and liver samples were weighed and dissolved 1:20 (wt:vol.) in 1 M KOH at 70°C for 30 min. One hundred microliters of the dissolved homogenate were removed and neutralized with 250 μl of 0.3 mol sodium acetate buffer (pH 4.8) and 10 μl of 50% glacial acetic acid. Two hundred microliters of 2 mol HCl were added to the homogenate, and the reaction mixture was then incubated for 2 h at 100°C. Following the incubation, the reaction mixture was neutralized with 2 mol NaOH and samples were analyzed by measuring glucosyl units by the Trinder reaction (Catalog #1530-500, Thermo Electron Corporation, Waltham, MA, USA). The same colorimetric kit was used to measure plasma glucose concentration.
Glycogen synthase activity
Glycogen synthase activity in gastrocnemius (exclusive of soleus and plantaris) was determined as described previously (Thomas et al., 1968; Thorburn et al., 1990; Yaspelkis et al., 2004). Frozen muscle samples were homogenized on ice with 10 volumes of extraction buffer (2 mmol l–1 EDTA, 2 mmol l–1 1,4-dithiolthreitol, 20 mmol l–1 NaF, 50 mmol l–1 KH2PO4, and 50 mmol l–1 K2HPO4, pH 7.4). Following centrifugation (10,000 g for 30 min), 100 μl of the supernatant was added to 400 μl of synthase buffer (20 mmol l–1 EDTA, 25 mmol l–1 NaF, 50 mmol l–1 Tris-HCl, pH 7.8). Glycogen synthase was determined in this diluted extract by measuring the incorporation of radioactive [14C]glucose from uridine 5′-diphosphate [14C]glucose (UDPG; Amersham Bioscience, GE Healthcare, Piscataway, NJ, USA), into glycogen. 30μl of the diluted extract was added to 60 μl of reaction mixture [1111 Bq UDPG, 1% glycogen, 0.3 mmoll–1 UDPG and either 0, 5 mmoll–1 or 10 mmoll–1 glucose-6-phosphate (G6P)], which was then incubated at 30°C for 30 min. To terminate the reaction, 75μl of the sample was transferred onto filter paper, which was washed for 30 min in 66% ethanol, decanted, washed again for 30 min in 66% ethanol and dried. 14C was then counted in a scintillation counter (Beckman Coulter, Fullerton, CA, USA). Glycogen synthase was measured in triplicate for each mouse but in duplicate for mini-muscles owing to the smaller amount of tissue available (see Introduction); means were used for statistical analyses.
Glycogen synthase activity, expressed as nanomoles of [14C]UDPG incorporated into glycogen per minute per milligram of muscle extract protein, is presented as total activity, as an activity ratio, and as a fractional velocity, as described by Kochan and colleagues (Kochan et al., 1979). Total activity is at 10 mmol G6P, after subtracting background activity – that is, with 0 mmol G6P. The activity ratio monitors dephosphorylation and represents the proportion of the enzyme activity observed in the absence of G6P (I form) to that observed with a saturating concentration of G6P (10 mmol l–1). Fractional velocity (Kochan et al., 1979), which represents the sensitivity of the enzyme to activation by G6P, was calculated by dividing the activity observed with a submaximal concentration of G6P (5 mmol l–1) by that with a saturating concentration of G6P (10 mmol l–1) after subtraction of background activity (with 0 mmol l–1 G6P).
Statistical analyses
We used SAS Procedure Mixed (1996, SAS Institute, Cary, NC, USA) version 8 to perform nested analyses of covariance (ANCOVAs) by REML estimation, where line type (HR vs C) was included as a fixed main effect, and replicate lines were nested within line type as a random effect. An additional factor classified individuals according to their gastrocnemius mass as mini (individuals with the mini-muscle phenotype) or normal (individuals with the `normal' phenotype). (Note that no individuals with the mini-muscle phenotype were found in the C lines.) Age was included as a covariate in all analyses. Mice from Groups 1, 2 and 3 were combined for analyses of wheel running from days 1 to 5 of wheel access because treatments were identical to that point. Wheel running of Groups 2 and 3 on day 6 was analyzed separately because the former had access to wheels only until 02:00 h, whereas the latter had access until 07:00 h. Time of removal from the wheel was used as a covariate, and estrous stage was also incorporated as an additional factor for groups 2 and 3 on the sixth day of wheel running.
Complementary statistical approaches were used to analyze the abundance of GLUT-4 and the glycogen synthase activity in gastrocnemius, plasma glucose, and glycogen in gastrocnemius, soleus and liver. Nested ANCOVAs were performed for each group and tissue per time, including line type, mini-muscle phenotype and estrous cycle as factors and age and time of capture as covariates. Analyses for the groups of mice with wheel access also included the number of revolutions until the time of capture as a covariate (for the glycogen synthase activity in Group 1, the number of revolutions was not included in the analyses). Additionally, to understand the relationship of glucose and glycogen concentration with time of measurement, analyses including all mice from groups 1, 2 and 3 were performed. These analyses included group, line type, mini-muscle phenotype and estrous cycle as factors and age as a covariate, as well as the interaction of group with both line type and mini-muscle phenotype. Finally, to investigate the possibility of short-term training effects of the 5–6 days of wheel access on GLUT-4, glycogen synthase activity, glucose, and glycogen concentration, we performed a nested ANCOVA comparing Groups 1 and 4. These two groups were both sacrificed at 16:00 h, but only Group 1 ever had wheel access. For this analysis, we included group, line type, mini-muscle phenotype and estrous cycle as factors and age as a covariate, as well as the interaction of group with both line type and mini-muscle phenotype.
One female from Group 2 and one from Group 3 had abnormally low revolutions on days 5 and 6 of wheel access and were excluded from analyses. Additional outliers were detected following Cook and Weisberg (Cook and Weisberg, 1999) and eliminated when appropriate. Statistical significance was judged at P≤0.05. Our main hypotheses (see end of Introduction) were directional and thus warrant one-tailed tests. However, we are making several comparisons of different traits measured on the same individual sets of mice (i.e. within one of the four experimental groups), and corrections for type I errors might be necessary. Exactly how to control for type I error due to multiple comparisons is controversial in the literature, especially when several of the comparisons are presented only for completeness and several factors and covariates are primarily nuisance variables (e.g. Middleton et al., 2008). Therefore, for simplicity, we present two-tailed P values throughout.
RESULTS
Wheel running
HR normal mice ran more than three-fold more revolutions per day than C mice on all days, and HR mini ran even more, with values 6–35% above those of HR normal (Tables S1–S5 in supplementary material). HR mice ran significantly more minutes per day than C (37–62% increase; Table S5 in supplementary material), and also at higher average speeds (HR mini also ran significantly faster than HR normal; Table S5 in supplementary material).
Gastrocnemius GLUT-4
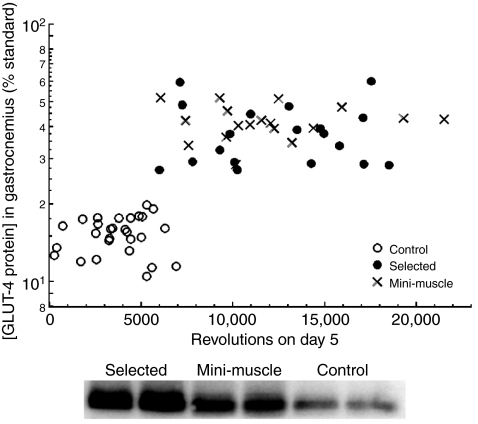
In mice that never had wheel access (Group 4), GLUT-4 abundance did not significantly differ among C, HR normal and HR mini individuals (Table 2). Wheel access for five days caused an increase in GLUT-4 concentration for all mice (Table 2), but the magnitude of the increase was much greater for HR normal (3.15-fold) and HR mini (4.46-fold) than for C mice (1.80-fold), as indicated by a highly significant wheel access × line type interaction in the combined analyses of Groups 1 and 4 (Table 3, P=0.0011). On average, HR mice had approximately 2.4 times higher GLUT-4 abundance than C lines after five days of wheel access, and, within the HR or C groups, GLUT-4 abundance was unrelated to the amount of wheel running performed by individual mice on the day before sacrifice (Fig. 1, Table 2). Results (not shown) were similar when the amount of running on days 1, 2, 3 or 4 was used as a covariate instead of running on day 5.
Table 2.
Plasma glucose, glycogen in gastrocnemius, soleus and liver, and GLUT-in gastrocnemius, of control (C) and high runner (HR) lines of house mice
| Trait | Group | Transform | N | Control | HR normal | HR mini | Line type | Mini muscle | Estrous cycle | Age | Time | Revolutions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma glucose | 1 | ^1.5 | 57 | 10.72 | 10.40 | 10.43 | F1,6=0.16 | F1,41=0.00 | F4,41=0.72 | F1,41=2.02 | F1,41=0.48 | F1,41=0.19 |
| P=0.7068 | P=0.9663 | P=0.5822 | P=0.1632 | P=0.4926 | P=0.6620 [–] | |||||||
| 2 | ^1.8 | 65 | 8.84 | 9.56 | 9.37 | F1,6=2.40 | F1,49=0.38 | F4,49=0.81 | F1,49=16.23 | F1,49=4.60 | F1,49=6.53 | |
| P=0.1725 | P=0.5424 | P=0.5223 | P=0.0002 | P=0.0370 | P=0.0138 [–] | |||||||
| 3 | ^1.8 | 61 | 8.81 | 8.91 | 9.45 | F1,6=0.04 | F1,45=1.79 | F4,45=0.27 | F1,45=0.94 | F1,45=4.25 | F1,45=7.60 | |
| P=0.8465 | P=0.1873 | P=0.8987 | P=0.3369 | P=0.0451 | P=0.0084 [–] | |||||||
| 4 | ^0.5 | 44 | 9.87 | 9.51 | 10.19 | F1,6=0.74 | F1,29=1.44 | F4,29=5.85 | F1,29=2.03 | F1,29=0.01 | ||
| P=0.4224 | P=0.2406 | P=0.0014 | P=0.1651 | P=0.9296 | ||||||||
| Glycogen gastrocnemius | 1 | ^0.3 | 65 | 16.96 | 12.38 | 37.52 | F1,6=1.70 | F1,49=35.03 | F4,49=1.32 | F1,49=0.23 | F1,49=0.11 | F1,49=2.89 |
| P=0.2396 | P<0.0001 | P=0.2774 | P=0.6346 | P=0.7450 | P=0.0954 [+] | |||||||
| 2 | ^0.3 | 67 | 16.46 | 16.65 | 42.48 | F1,6=0.00 | F1,51=40.74 | F4,51=2.23 | F1,51=0.59 | F1,51=4.17 | F1,51=0.03 | |
| P=0.9593 | P<0.0001 | P=0.0785 | P=0.4468 | P=0.0463 | P=0.8739 [–] | |||||||
| 3 | ^0.5 | 65 | 19.54 | 23.28 | 36.69 | F1,6=0.55 | F1,47=7.69 | F4,47=0.26 | F1,47=3.42 | F1,47=0.10 | F1,47=1.45 | |
| P=0.4876 | P=0.0079 | P=0.9018 | P=0.0708 | P=0.7476 | P=0.2349 [+] | |||||||
| 4 | None | 45 | 15.93 | 10.08 | 40.98 | F1,6=2.05 | F1,30=42.99 | F4,30=0.64 | F1,30=2.16 | F1,30=0.02 | ||
| P=0.2025 | P<0.0001 | P=0.6356 | P=0.1524 | P=0.8973 | ||||||||
| Glycogen soleus | 1 | ^0.2 | 65 | 21.95 | 25.83 | 32.52 | F1,6=0.52 | F1,49=1.61 | F4,49=1.05 | F1,49=0.02 | F1,49=2.00 | F1,49=0.17 |
| P=0.4960 | P=0.2101 | P=0.3923 | P=0.8790 | P=0.1631 | P=0.6828 [+] | |||||||
| 2 | ^0.8 | 67 | 18.06 | 20.72 | 42.02 | F1,6=0.30 | F1,50=24.00 | F4,50=3.46 | F1,50=0.00 | F1,50=9.14 | F1,50=2.47 | |
| P=0.6061 | P<0.0001 | P=0.0142 | P=0.9607 | P=0.0039 | P=0.1226 [–] | |||||||
| 3 | ^1.3 | 65 | 22.57 | 44.62 | 50.02 | F1,6=10.93 | F1,47=1.23 | F4,47=1.08 | F1,47=1.53 | F1,47=1.18 | F1,47=0.39 | |
| P=0.0163 | P=0.2733 | P=0.3766 | P=0.2224 | P=0.2828 | P=0.5335 [–] | |||||||
| 4 | ^0.2 | 44 | 21.83 | 20.22 | 32.48 | F1,6=0.16 | F1,29=4.04 | F4,29=2.28 | F1,29=1.02 | F1,29=0.02 | ||
| P=0.7013 | P=0.0539 | P=0.0847 | P=0.3216 | P=0.8774 | ||||||||
| Glycogen liver | 1 | ^0.05 | 65 | 76.41 | 67.58 | 55.59 | F1,6=0.09 | F1,49=0.29 | F4,49=3.19 | F1,49=0.01 | F1,49=0.07 | F1,49=0.36 |
| P=0.7791 | P=0.5936 | P=0.0208 | P=0.9389 | P=0.7992 | P=0.5486 [+] | |||||||
| 2 | ^0.3 | 67 | 135.78 | 139.01 | 153.35 | F1,6=0.01 | F1,51=0.30 | F4,51=2.09 | F1,51=2.15 | F1,51=2.35 | F1,51=9.10 | |
| P=0.9235 | P=0.5844 | P=0.0952 | P=0.1486 | P=0.1317 | P=0.0040 [–] | |||||||
| 3 | ^2.3 | 65 | 228.42 | 292.47 | 223.70 | F1,6=3.82 | F1,47=5.46 | F4,47=0.34 | F1,47=0.14 | F1,47=0.04 | F1,47=0.19 | |
| P=0.0983 | P=0.0237 | P=0.8487 | P=0.7125 | P=0.8520 | P=0.6626 [–] | |||||||
| 4 | Log10 | 43 | 26.13 | 29.21 | 56.55 | F1,6=0.25 | F1,28=4.89 | F4,28=3.42 | F1,28=1.19 | F1,28=5.60 | ||
| P=0.6356 | P=0.0353 | P=0.0213 | P=0.2841 | P=0.0251 | ||||||||
| GLUT-4 gastrocnemius | 1 | Log10 | 65 | 15.82 | 37.34 | 39.69 | F1,6=89.61 | F1,49=0.66 | F4,49=0.25 | F1,49=3.28 | F1,49=2.09 | F1,49=0.00 |
| P<0.0001 | P=0.4189 | P=0.9063 | P=0.0761 | P=0.1542 | P=0.9573 [+] | |||||||
| 4 | None | 41 | 8.81 | 11.85 | 8.90 | F1,6=2.31 | F1,26=1.34 | F4,26=2.73 | F1,26=0.26 | F1,26=0.00 | ||
| P=0.1794 | P=0.2584 | P=0.0506 | P=0.6117 | P=0.9765 | ||||||||
| Glycogen synthase total activity | 1 | Log10 | 63 | 1.68 | 1.73 | 2.33 | F1,6=0.06 | F1,48=5.89 | F4,48=0.55 | F1,48=0.42 | F1,48=20.32 | |
| P=0.8118 | P=0.0190 | P=0.7006 | P=0.5179 | P<0.0001a | ||||||||
| 4 | Log10 | 42 | 1.32 | 1.24 | 2.30 | F1,6=0.33 | F1,27=26.43 | F4,27=2.78 | F1,27=1.37 | F1,27=10.27 | ||
| P=0.5850 | P<0.0001 | P=0.0468 | P=0.2519 | P=0.0035a | ||||||||
| Glycogen synthase activity ratio | 1 | Log10 | 63 | 19.44 | 20.65 | 15.90 | F1,6=0.45 | F1,48=5.34 | F4,48=0.55 | F1,48=1.90 | F1,48=2.64 | |
| P=0.5269 | P=0.0252 | P=0.7008 | P=0.1749 | P=0.1110a | ||||||||
| 4 | Log10 | 42 | 19.68 | 21.03 | 9.80 | F1,6=0.37 | F1,27=20.74 | F4,27=0.98 | F1,27=0.64 | F1,27=0.49 | ||
| P=0.5665 | P=0.0001 | P=0.4347 | P=0.4300 | P=0.4919a | ||||||||
| Glycogen synthase fractional velocity | 1 | Log10 | 63 | 85.61 | 85.90 | 78.72 | F1,6=0.01 | F1,48=3.55 | F4,48=0.10 | F1,48=0.60 | F1,48=1.28 | |
| P=0.9263 | P=0.0655 | P=0.9808 | P=0.4433 | P=0.2631a | ||||||||
| 4 | Log10 | 42 | 85.35 | 84.10 | 76.70 | F1,6=0.13 | F1,27=2.10 | F4,27=1.45 | F1,27=0.04 | F1,27=0.72 | ||
| P=0.7328 | P=0.1584 | P=0.2458 | P=0.8422 | P=0.4040a |
Plasma glucose (mmol/l), glycogen (glucosyl units: μmol g–1 wet mass) in gastrocnemius, soleus and liver, and GLUT-4 (% of standard) in gastrocnemius of control (C) and high runner (HR) lines of house mice based on separate analyses of Groups 1–4 (nested ANCOVAs). For the effect of wheel revolutions as a covariate, the sign in brackets indicates the direction of the effect. Data were transformed as indicated to improve normality of residuals. Values are back-transformed least-squares means from SAS Procedure Mixed. Bold font indicates 2-tailed P<0.05, unadjusted for multiple comparisons
Amount of time tissue was stored in freezer used as covariate
Table 3.
Nested ANCOVAs comparing Groups 1 (five days wheel access) and 4 (no wheel access)
| Transform | N | Wheel access | Line type | Mini muscle | Wheel access× line type | Wheel access ×mini muscle | Estrous cycle | Age | |
|---|---|---|---|---|---|---|---|---|---|
| Plasma glucose | None | 101 | F1,6=8.31 | F1,6=1.91 | F1,78=0.55 | F1,6=0.10 | F1,78=0.00 | F4,78=1.02 | F1,78=4.11 |
| P=0.0280 | P=0.2163 | P=0.4595 | P=0.7591 | P=0.9901 | P=0.4011 | P=0.0459 | |||
| Glycogen gastrocnemius | ^0.2 | 110 | F1,6=0.48 | F1,6=2.93 | F1,87=82.38 | F1,6=1.06 | F1,87=0.10 | F4,87=1.41 | F1,87=1.73 |
| P=0.5150 | P=0.1376 | P<0.0001 | P=0.3439 | P=0.7552 | P=0.2371 | P=0.1915 | |||
| Glycogen soleus | ^0.3 | 109 | F1,6=0.08 | F1,6=0.59 | F1,86=0.48 | F1,6=0.81 | F1,86=0.28 | F4,86=0.79 | F1,86=0.00 |
| P=0.7863 | P=0.4698 | P=0.4891 | P=0.4037 | P=0.5962 | P=0.5359 | P=0.9974 | |||
| Glycogen liver | ^0.1 | 109 | F1,6=3.39 | F1,6=0.00 | F1,86=0.71 | F1,6=0.01 | F1,86=0.59 | F4,86=1.60 | F1,86=0.21 |
| P=0.1150 | P=0.9881 | P=0.4007 | P=0.9197 | P=0.4456 | P=0.1823 | P=0.6485 | |||
| GLUT-4 gastrocnemius | ^0.5 | 106 | F1,6=33.79 | F1,6=72.17 | F1,83=0.22 | F1,6=34.48 | F1,83=3.61 | F4,83=0.70 | F1,83=4.59 |
| P=0.0011 | P<0.0001 | P=0.6390 | P=0.0011 | P=0.0610 | P=0.5921 | P=0.0351 | |||
| Glycogen synthase activity ratio | Log10 | 105 | F1,6=4.01 | F1,6=0.29 | F1,82=25.51 | F1,6=0.02 | F1,82=3.44 | F4,82=0.33 | F1,86=0.57 |
| P=0.0921 | P=0.6085 | P<0.0001 | P=0.8934 | P=0.0672 | P=0.8572 | P=0.4518 | |||
| Glycogen synthase fractional velocity | Log10 | 105 | F1,6=0.08 | F1,6=0.05 | F1,82=4.49 | F1,6=0.17 | F1,82=0.16 | F4,82=0.68 | F1,86=0.32 |
| P=0.7809 | P=0.8365 | P=0.0370 | P=0.6919 | P=0.6923 | P=0.6055 | P=0.5715 |
Data were transformed as indicated to improve normality of residuals. Bold font indicates 2-tailed P<0.05, unadjusted for multiple comparisons
Fig. 1.
GLUT-4 abundance in gastrocnemius muscle was much higher in the selectively bred `high runner' (HR) lines of mice than in non-selected control (C) lines after five days of wheel access (Group 1, as outlined in Table 1). Clearly, this differential was not a simple linear function of the amount of wheel running performed by individual mice on the previous night (Table 2, P=0.9573 for effect of wheel revolutions). As discussed in the text, this seems to represent a case of increased `self-induced adaptive plasticity' in the HR lines of mice (Swallow et al., 2005; Garland and Kelly, 2006). A representative western blot for GLUT-4 from six individuals is also shown.
Gastrocnemius glycogen synthase activity
Glycogen synthase total activity was significantly increased, whereas the activity ratio was significantly reduced in mini-muscle gastrocnemius, in comparison with both HR normal and C mice, but was not generally affected by line type or wheel access (Tables 2, 3). Because freezer storage time significantly affected total activity within both groups (Table 2), and time was confounded with group membership, groups 1 and 4 were not compared statistically (Table 3). Inspection of the least-squares means shows that, uniquely, HR mini exhibited a 60% increase in activity ratio following five days of wheel access (from 9.80 to 15.90; Tables 2, 3). Glycogen synthase fractional velocity was also significantly reduced in HR mini (Table 3).
Glycogen concentration
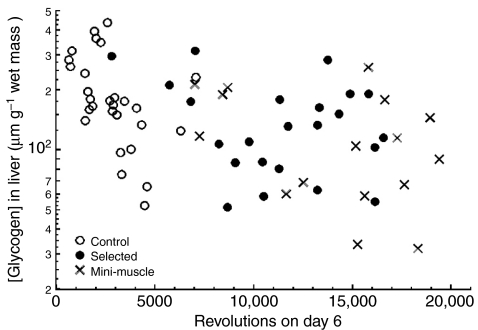
As shown in Table 2, the mini-muscle phenotype was associated with higher glycogen concentration in gastrocnemius, soleus and liver in mice without wheel access (Group 4). When mice had access to wheels for 5–6 days, HR mini also showed significantly elevated gastrocnemius glycogen at all three time points (Tables 2, 3, 4). Although HR mini also showed elevated soleus glycogen at all time points, the effect was statistically significantly only at 02:00 h (Group 2), when values for HR mini were approximately twice those of other mice. HR also had significantly higher soleus glycogen concentration at 07:00 h (Group 3; Table 2). In contrast to results for muscles, liver glycogen concentration was significantly lower in HR mini than HR normal at 07:00 h (Group 3; Table 2). Finally, the number of wheel revolutions was negatively related to glycogen concentration in liver at 02:00 h (Group 2; see Fig. 2), and this was the only significant relationship between [glycogen] and the amount of running (Table 2).
Table 4.
Nested ANCOVAs comparing Groups 1, 2 and 3 sacrificed at different times on the sixth day of wheel access
| Transform | N | Group | Line type | Mini muscle | Group×line type | Group×mini muscle | Estrous cycle | Age | |
|---|---|---|---|---|---|---|---|---|---|
| Plasma glucose | None | 186 | F2,12=7.10 | F1,6=3.27 | F1,154=0.01 | F2,12=1.1 | F2,154=2.37 | F4,154=085 | F1,154=6.12 |
| P=0.0092 | P=0.1203 | P=0.9196 | P=0.3625 | P=0.0969 | P=0.4972 | P=0.0145 | |||
| Glycogen gastrocnemius | ^0.4 | 197 | F2,12=0.13 | F1,6=1.24 | F1,165=92.39 | F2,12=0.98 | F2,165=3.04 | F4,165=0.88 | F1,165=0.01 |
| P=0.8761 | P=0.3084 | P<0.0001 | P=0.4045 | P=0.0503 | P=0.4754 | P=0.9140 | |||
| Glycogen soleus | ^0.6 | 196 | F2,12=1.27 | F1,6=6.53 | F1,164=4.06 | F2,12=4.63 | F2,164=3.58 | F4,164=3.20 | F1,164=0.03 |
| P=0.3170 | P=0.0431 | P=0.0456 | P=0.0324 | P=0.0302 | P=0.0146 | P=0.8708 | |||
| Glycogen liver | ^0.5 | 197 | F2,12=11.06 | F1,6=0.68 | F1,165=1.25 | F2,12=3.28 | F2,165=0.12 | F4,165=4.00 | F1,165=3.88 |
| P=0.0019 | P=0.4422 | P=0.2659 | P=0.0729 | P=0.8854 | P=0.0040 | P=0.0505 |
Data were transformed as indicated to improve normality of residuals. Bold font indicates 2-tailed P<0.05, unadjusted for multiple comparisons
Fig. 2.
Liver glycogen concentration showed a significant (P=0.0040) negative relation with amount of wheel revolutions in the 02:00 h group (Group 2, as outlined in Table 1; see Table 2 for statistical analysis), but no differences among control (C), `high runner' (HR) normal (`selected') or HR mini-muscle mice.
Analyses comparing Groups 1 and 4 failed to show an effect of wheel access on [glycogen] in any of the organs measured (Table 3), despite the fact that liver glycogen in normal mice (i.e. not mini) with wheel access was on average higher than in mice without wheels, regardless of selection history (2.92 and 2.31 times higher for C and HR normal, respectively; Table 2).
Combined analyses of all mice with wheel access for 5–6 days (Groups 1, 2 and 3) showed that [glycogen] was affected in different ways by time of measurement, depending on the tissue (Table 4). Liver [glycogen] was significantly affected by time of measurement (Table 4), as all mice showed a significant increase in its mean value from 16:00 h to 02:00 h and from 02:00 h to 07:00 h (Table 2). In soleus, while HR mini showed an increase in [glycogen] from both 16:00 h to 02:00 h (29.2%) and 02:00 h to 07:00 h (19.0%), C and HR normal showed a decrease from 16:00 h to 02:00 h (17.7% and 19.8% lower values at 02:00 h for C and HR normal, respectively) followed by a rebound from 02:00 h to 07:00 h (25.0% and 115.4% higher values at 07:00 h for C and HR normal, respectively; Table 2). Such differences in pattern and magnitude of variation of soleus [glycogen] with time of measurement were reflected in the significant interaction between time and both line type and mini-muscle factors (Table 4). In comparison with C mice, HR mice in general had higher soleus [glycogen] for Group 3 (Tables 2, 4).
Control, HR normal and HR mini also differed in the way that gastrocnemius [glycogen] varied with measurement time. For example, HR mini showed an increase from 16:00 h to 02:00 h (13.2%), followed by a similar decrease from 02:00 h to 07:00 h (13.6%) (Tables 2, 4). By contrast, C mice showed essentially no change from 16:00 h to 02:00 h (+3.0%), but an increase from 02:00 h to 07:00 h (18.7%), whereas mean values for HR normal increased from both 16:00 h to 02:00 h (34.5%) and 02:00 h to 07:00 h (39.8%; Table 2).
Additionally, [glycogen] was influenced by the estrous cycle in both soleus and liver (Table 4). On average, values in estrous 2 were lowest in liver: values during postestrous, estrous 1, diestrous and proestrous were 13%, 15%, 27% and 46% higher, respectively. In soleus, females during postestrous had the lowest glycogen concentrations, with values for estrous 2, estrous 1, proestrous and diestrous being 2%, 15%, 17% and 37% higher, respectively.
Plasma glucose
Plasma glucose concentration was not affected by line type or mini-muscle phenotype in any group (Table 2). In mice with wheel access for 5 to 6 days, [glucose] was significantly negatively related to the number of revolutions for mice sampled at both 02:00 h and 07:00 h (Groups 2 and 3, respectively).
Comparison of groups 1 and 4 showed a significant increase in plasma [glucose] when mice were housed with access to wheels (Table 3). Mean values for Group 1 were 8.6%, 9.4% and 2.4% higher for C, HR normal and HR mini from Group 4, respectively (Table 2).
Analysis comparing the three groups of mice that had wheel access showed a significant effect of time of measurement on plasma glucose concentration in addition to the positive age influence (Table 4). All mice showed a decrease from 16:00 h to 02:00 h (17.5%, 8.1% and 10.2% for C, HR normal and HR mini, respectively; Table 2). Plasma glucose concentration showed little change from 02:00 h to 07:00 h for C and HR mini, but HR normal showed a mean value 6.8% lower at 07:00 h (Table 2).
DISCUSSION
Wheel running
As expected, HR lines covered much more distance than C lines when given access to wheels for 5–6 days (Tables S1–S5 in supplementary material). As described previously (especially for females), the increased running was mainly caused by a higher average speed and to a lesser degree by an increase in the amount of time spent running (e.g. Swallow et al., 1999; Houle-Leroy et al., 2000; Girard et al., 2001; Koteja and Garland, 2001; Garland et al., 2002; Girard and Garland, 2002; Garland, 2003; Belter et al., 2004; Rezende et al., 2005; Rezende et al., 2006c). The elevated running by HR mice was expressed on the first day of wheel access and persisted across all 6 days [see also fig. 1 in Belter and colleagues (Belter et al., 2004)]. Moreover, HR mini logged more revolutions than HR normal during the entire period of wheel access, mainly through increased running speed. This result differs from previous generations (Garland et al., 2002; Houle-Leroy et al., 2003; Belter et al., 2004; Kelly et al., 2006), when mini-muscle mice did not run significantly more revolutions per day [but see Syme and colleagues (Syme et al., 2005) concerning mice from only the two mini-muscle lines] and might provide an explanation for why the mini-muscle phenotype has been favored by the selection protocol (Garland et al., 2002) (see also Hannon et al., 2008).
The most dramatic differences between HR mini and HR normal occurred in Group 2, which ran on day 6 only until approximately 02:00 h. By this time-point, HR mini had covered a distance 34% greater than HR normal at an average speed 43% higher than HR normal, in a comparatively lower amount of time spent running (90% of time spent by HR normal; Table S5 in supplementary material). Compared with the estimated maximal aerobic speed for these mice (Rezende et al., 2006c), HR mini, HR normal and C were sustaining average voluntary exercise close to 90%, 83% and 71%, respectively, of their V̇O2,max during that period [values calculated from maximum running speeds measured at day 6 (Table S5 in supplementary material) and estimates of V̇O2,max and costs of transport for HR and C lines reported by Rezende and colleagues (Rezende et al., 2006c)]. This difference in wheel running between HR mini, HR normal and C mice from Group 2 had a profound impact on the way these three phenotypes mobilized their liver glycogen depots (e.g. see Fig. 2), as discussed below.
Adaptive plasticity in GLUT-4 abundance
HR and C mice did not differ significantly in gastrocnemius GLUT-4 abundance when housed without wheels; however, after five days of wheel access, HR mice had 2.4-fold more GLUT-4 than C mice (Table 2). As can be seen in Fig. 1, this difference was not a simple linear function of the amount of wheel running on the previous day, which is much higher in HR mice (ANCOVA in Table 2). Thus, GLUT-4 levels in gastrocnemius show a highly significant (Table 3, P=0.0011) genotype–environment interaction: the effect of line type (genetic selection history) depends on the environment in which the animals are housed (without vs with wheel access). In other words, HR lines have evolved a higher degree of (presumably) adaptive plasticity in GLUT-4 expression: five days of wheel access caused an average increase of 271% vs 79% in C mice (Table 2). As discussed elsewhere, plasticity per se is known to be a trait that can evolve, but it is not necessarily the case that plasticity in a given trait will always be in the `good' or `adaptive' direction [i.e. the `beneficial acclimation hypothesis' (Wilson and Franklin, 2002; Swallow et al., 2005; Garland and Kelly, 2006)].
Rapid increases in GLUT-4 following five days of wheel access are consistent with the fact that GLUT-4 has a half-life of only a few hours (Holloszy and Nolte, 2002). In rats, most of the GLUT-4 training-induced increase occurs within 18 h after a single bout of exercise, and essentially the entire response to a continued exercise stimulus can be induced by two days of exercise (Ren et al., 1994; Host et al., 1998). Rats running voluntarily on wheels showed 39% higher values of GLUT-4 expression in soleus after 1 week and remained stable for an additional three weeks of wheel access, despite a 3–4 fold greater total number of revolutions during this period (Henriksen and Halseth, 1994). The present results considered with those of Henriksen and Halseth (Henriksen and Halseth, 1994) suggest that the increased GLUT-4 abundance generated by voluntary wheel running might resemble more a threshold effect (that differs between the HR and C lines: see Fig. 1) than a quantitative response to subtler individual differences in stimuli, such as the amount of running.
The dramatic increase in GLUT-4 abundance after five days of wheel access might be of fundamental importance under the conditions that HR mice are actually selected for breeding, given that glucose transport is a rate-limiting step for utilization of blood glucose during sustained, aerobically supported exercise in mammals (Weber et al., 1996). An enhanced capacity for transport and utilization of glucose from blood would also spare muscle glycogen during exercise (Houle-Leroy et al., 2000), delaying the onset of exhaustion, and also lead to a higher post-exercise glycogen concentration (Nakatani et al., 1997; Kuo et al., 2004; Ivy et al., 2008). The importance of an increased exogenous glucose uptake and/or increased glycogen content for sustaining high levels of wheel running is supported by the finding that transgenic mice overexpressing GLUT-4 in fast-twitch skeletal muscles exhibit four-fold increased levels of voluntary wheel running when compared with control mice (Tsao et al., 2001).
Mini-muscle phenotype
The mini-muscle phenotype exhibited approximately 2–3-fold more glycogen than the normal phenotype per gram wet mass of muscle, even when mice had no access to wheels (Table 2). The magnitude of this difference is such that, despite their 50% smaller gastrocnemius mass (Garland et al., 2002), HR mini mice still have more glycogen than mice of the normal phenotype on an absolute (whole-muscle) basis. In spite of their elevated glycogen levels, HR mini did not differ significantly from HR normal or C mice in gastrocnemius GLUT-4 abundance when housed without wheels (Tables 2, 3, 4). HR mini also do not differ from normal in mass-specific glycogen phosphorylase (GP) activity, measured as either the activated (GPa) or total forms (GPtot=GPa+GPinactive) (Houle-Leroy et al., 2000; Guderley et al., 2008). However, HR mini exhibit almost two-fold higher specific activity of hexokinase in mixed hindlimb muscle that excluded gastrocnemius, even without wheel access (Houle-Leroy et al., 2003) (see also Guderley et al., 2006), and here we found that glycogen synthase activity differs in HR mini (Tables 2, 3).
Hexokinase activity (primarily HK II in skeletal muscle) is essential to glucose uptake, trapping glucose inside the cells and maintaining the downhill gradient from capillaries to the intracellular compartment (Halseth et al., 1999). The importance of this step in determining the rates of muscle glucose uptake has been demonstrated in mice overexpressing HK II under hyperglycemic-hyperinsulinemic clamp conditions (Chang et al., 1996) and during moderate-intensity exercise (Halseth et al., 1999). In this way, the higher capacity for muscle glucose phosphorylation already demonstrated for HR mini through a higher activity of HK (Houle-Leroy et al., 2003) might explain their higher glycogen content despite the similar levels of GLUT-4. Determination of muscle glucose transport using the glucose analog 3-O-methylglucose would be necessary to test this prediction. A decrease in glycogen synthase activity ratio in skeletal muscle coincident with elevated glycogen concentration has been described in previous studies (Danforth, 1965; Nakatani et al., 1997). Such feedback control probably occurs as a result of inhibition of glycogen synthase phosphatase by elevated glycogen levels (Villar-Palasi, 1995; Nielsen et al., 2001). Thus, the lower GS I activity of HR mini when compared with mice of the normal phenotype might be interpreted as a result of feedback control by their higher glycogen levels. Irrespective of the processes actually limiting glycogen storage in HR mini and HR normal, the present study shows that these two muscle phenotypes must have a different regulatory set-point for glycogen concentration in different organs.
Wheel access for five days resulted in disproportionately higher levels of GLUT-4 and 60% increased activity ratio of glycogen synthase in gastrocnemius from HR mini (Table 3) – two previously described effects of aerobic training (Kuo et al., 2004; Christ-Roberts et al., 2004). The increase in GS I activity along with GLUT-4 expression in HR mini is probably related to their higher dependence on glycogen depots from gastrocnemius, in comparison with both C and HR normal: HR mini was the only phenotype showing a decrease in glycogen concentration in this muscle from 02:00 h to 07:00 h. Moreover, HR mini ran significantly more revolutions than HR normal, and at higher speeds, which might explain the fact that this was the only phenotype that exhibited a reduction in gastrocnemius [glycogen] from 02:00 h to 07:00 h.
Presumably, the increased glycogen concentration represents an advantage for the HR mini, being one component of a set of subordinate traits (see also Wong et al., in revision) that enable them to sustain aerobic exercise of even higher intensity than HR normal (Hultman and Harris, 1988) (but see Pederson et al., 2005a; Pederson et al., 2005b). By contrast, a recent study of endurance during forced treadmill exercise, in which mice ran on average for ∼30 min, found that HR had higher endurance than C lines for both sexes, but HR mini did not have a significantly higher endurance capacity than HR normal (Meek et al., 2007). From a different perspective, a structural role for high [glycogen] in HR mini cannot be discounted (Hargreaves and Spriet, 2006).
Glycogen concentration and circadian patterns
Comparing lines of rats bred for high (HCR) vs low (LCR) treadmill endurance-running capacity, Howlett and colleagues reported no difference in gastrocnemius [glycogen] measured after exhaustive treadmill exercise [apparently during the day (Howlett et al., 2003)]. Similarly, Noland and colleagues found no significant difference in either basal or insulin-stimulated glycogen synthesis between chow-fed HCR and LCR rats (Noland et al., 2007). In the present study, glycogen concentration varied in complex ways in relation to time of measurement. This complexity is attributable to the combined effects of voluntary exercise that lasted for different amounts of time and an inherent circadian cycle of glycogen concentration. Additionally, the way these effects are expressed in C, HR normal and HR mini is also affected by their different intensities of voluntary exercise (Table S5 in supplementary material).
Previous studies have shown variation of up to 100% in [glycogen] in a 24 h period, both in muscles and liver from rats and mice. In mouse diaphragm, for example, it peaks around 05:00 h and shows lowest values around 17:00 h [values clock-shifted to match our light cycle (Pessacq and Gagliardino, 1975)]. In rat liver, the highest values are also observed early in the morning, and lowest values late in the afternoon (Palmer et al., 1979; Escobar et al., 1998). Therefore, the general pattern of circadian variation in [glycogen] in muscle and liver found in the present study (Table 2) agrees with that reported in the literature. Moreover, the observed increase in [glycogen] from 16:00 h to 07:00 h (particularly evident for liver of all mice, for gastrocnemius of HR normal and for soleus of HR mini; Table 2) implies that a net glycogen deposition occurs concomitantly with the period of active wheel running in these mice. Although the classic theory of glycogen metabolism is that complete activation of glycogen phosphorylase through phosphorylation is coupled with the complete inactivation of glycogen synthase (Stryer, 1995), several studies of glycogen kinetics in vivo have shown that simultaneous glycogen synthesis and degradation occurs both in liver and muscle during exercise in rats (Constable et al., 1984; David et al., 1990; Azevedo et al., 1998) and humans (Magnusson et al., 1994; Price et al., 1994).
The temporal pattern of variation in [glycogen] in gastrocnemius, soleus and liver differed among C, HR normal and HR mini mice, probably due to differences in glycogen turnover associated with their disparate muscle characteristics and patterns of voluntary running. The normal phenotype seemed to rely mostly on glycogen derived from soleus to sustain peak running (C and HR normal presented a net decrease in soleus glycogen concentration from 16:00 h to 02:00 h; Table 2), whereas HR mini seemed to depend more on gastrocnemius glycogen stores (HR mini showed a net decrease in gastrocnemius glycogen concentration from 02:00 h to 07:00 h; Table 2). As soleus comprises mainly fibers presenting a low recruitment threshold, we actually would expect this muscle to be recruited even during low-intensity exercise (Saltin and Gollnick, 1983; Ivy et al., 1989). Consequently, the intriguing and remaining question to be answered is why HR mini did not show a net decrease in soleus [glycogen] in association with voluntary running activity. The relative demands placed on soleus and gastrocnemius to sustain locomotion change with speed in rats running on a treadmill: the demand placed on soleus can be unaffected by speed at 0% incline and can even decrease with speed at higher inclines (Roy et al., 1991). As mice do not run constantly at 0% incline when in a wheel (see movie at: http://www.biology.ucr.edu/people/faculty/Garland/Girard01.mov), and HR mini run voluntarily at higher speeds [this study (Syme et al., 2005; Kelly et al., 2006)], the demand placed on soleus by HR mini (particularly females) might be less than for HR normal. HR mini also have larger soleus muscles than mice of the normal phenotype (Gomes et al., 2004; Syme et al., 2005; Guderley et al., 2006), with a higher glycogen concentration (Table 2), which could help preserving their glycogen levels during activity. Additionally, HR mini might rely proportionally more on gastrocnemius glycogen depots to sustain moderate exercise intensities than the normal phenotype for the following reasons. When compared with the normal phenotype, gastrocnemius fibers from HR mini present a substitution of almost half of their type IIb isoforms by slower myosin heavy chain (MHC) isoforms, including type IIa (Guderley et al., 2006) (see also Guderley et al., 2008). Gastrocnemius from HR mini also presents reduced force per cross-sectional area when compared with the normal phenotype (Syme et al., 2005) and a higher mass-specific aerobic capacity (Houle-Leroy et al., 2003; Rezende et al., 2006b; Guderley et al., 2008) and capillarity [in medial gastrocnemius (Wong et al., in revision)]. These features of the gastrocnemius from HR mini bring this muscle to a physiological condition closer to that presented by the normal soleus of mice, as the typical percentage of type IIa in soleus is much higher in mice [∼42.3% (Tsao et al., 2001)] than in rats [∼4% (Rice et al., 1988)]. Thus, given that IIa fibers have a lower recruitment threshold than type IIb fibers (Saltin and Gollnick, 1983; Ivy et al., 1989) and that HR mini gastrocnemius has a higher proportion of type IIa isoforms and a higher glycogen concentration, HR mini might rely more on their gastrocnemius glycogen depots to sustain moderate exercise in comparison with the normal phenotype. Studies of muscle use and substrate turnover during voluntary running would be necessary to test these hypotheses.
Hepatic gluconeogenesis (John-Alder et al., 1986) and especially increased rates of glycogenolysis (Bagby et al., 1978) are essential to sustain prolonged submaximal locomotor activity in rats. Even when faced with an exogenous glucose infusion [final concentration 25% (w/w)], both trained and untrained rats experience significant hepatic glycogenolysis during exercise (Azevedo et al., 1998). In our study, although all three phenotypes showed a net glycogenesis in liver from 16:00 h to 07:00 h (Table 2), there was a negative relationship between the number of revolutions at 02:00 h and glycogen concentration in liver (Fig. 2). Such a negative relationship is clear evidence of the importance of liver glycogen depots in sustaining voluntary wheel running. Hence, the patterns of variation observed for liver glycogen concentration could be interpreted as reflecting differences in the rate of glycogenolysis for the different phenotypes (in proportion to their voluntary exercise intensity), circumventing part of the normal net circadian glycogenesis occurring during this period.
Unlike glycogen, basal blood glucose peaks in the late afternoon and early night – just before the onset of the activity period – with the lowest values found in the late morning in both rats and mice (Fleur et al., 1999; Challet et al., 2004; Mendoza et al., 2005). Consistent with this general pattern, we found the highest values of plasma glucose at 16:00 h (Group 1) and lower values at 02:00 h and 07:00 h (Groups 2 and 3; Table 2). We do not know how much of the decrease in plasma [glucose] during the night is simply due to the circadian rhythm of basal glucose as opposed to being caused by muscle uptake to sustain running. However, the negative relation between plasma [glucose] and revolutions at both 02:00 h and 07:00 h (Table 2) is strong evidence for the importance of plasma glucose for sustaining voluntary running in our mice. Rats treated with 3-mercaptopicolinic acid (3-MPA; a drug that inhibits gluconeogenesis) showed severe hypoglycemia during an endurance test (values 37% lower than during resting), associated with virtually depleted liver glycogen at exhaustion, as well as 25–30% lower endurance time than sham-treated rats (John-Alder et al., 1986). Although we also observed a negative relationship between liver [glycogen] and revolutions just after the peak of wheel running (Group 2), the fact that the liver [glycogen] actually increased from 16:00 h to 02:00 h – and then increased even more to 07:00 h – for all three phenotypes indicates that our mice were not glucose restricted. Mice maintained in cages with wheels and ad libitum food exhibited 75% of their spontaneous food intake during the night, when almost all of the running occurred (Mendoza et al., 2005). Thus, it is probable that part of the plasma glucose available to sustain voluntary running is derived from food ingested during the night, thus helping to spare liver glycogen depots (Holloszy and Kohrt, 1996).
Conclusions
Glycogen concentration and GLUT-4 showed distinct and interesting relationships with genetic selection history and short-term wheel access. Voluntary wheel running affected glycogen concentration in muscles and liver, altering the normal circadian pattern of variation and generating a complex and different trajectory for each organ and phenotype. Five days of wheel access caused a 2.4-fold difference in gastrocnemius GLUT-4 expression between HR and C lines of mice, and we interpret this as an example of enhanced adaptive plasticity that has evolved in response to the selective breeding protocol [see also Swallow and colleagues (Swallow et al., 2005) on `self-induced adaptive plasticity' and Garland and Kelly (Garland and Kelly, 2006)]. Although wheel access generated a large line-type effect on the capacity for glucose transport in muscles, it did not affect [glycogen] in any of the organs studied. Although all mice showed increased capacity for glucose transport after wheel access and some evidence of glycogen use to sustain exercise, their amount of voluntary wheel running does not seem to be limited by a lack of carbohydrates locally available to sustain muscle contraction. Even in the liver, where a clear negative relationship between glycogen concentration and number of revolutions was observed after the peak of activity (Fig. 2), a concomitant net glycogen deposition occurred from 16:00 h to 02:00 h and from then to 07:00 h. Therefore, contrary to our hypothesis, glycogen depletion does not seem to be responsible for the apparent selection limit observed since reaching approximately generation 16 in the HR lines (see Garland, 2003).
Supplementary Material
We thank Michelle Park for help with dissections, and Richard Cardullo and Kimberly Hammond for kindly providing equipment. The study was supported by U.S. National Science Foundation Grant IOB-0543429 to T.G. and National Institutes of Health Grants GM-48680 and GM-08395 to B.B.Y. E.L.R. is currently a Ramón y Cajal Fellow supported by the Spanish Ministry of Science and Innovation. All animal procedures are in compliance with the UCR Institutional Animal Care and Use Committee and US laws. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/212/2/238/DC1
References
- Asdell, S. A., Dorrnenbal, H. and Sperling, G. A. (1962). Sex steroid hormones and voluntary exercise in rats. J. Reprod. Fertil. 3, 26-36. [DOI] [PubMed] [Google Scholar]
- Azevedo, J. L., Jr, Linderman, J. K., Lehman, S. L. and Brooks, G. A. (1998). Training decreases muscle glycogen turnover during exercise. Eur. J. Appl. Physiol. 78, 479-486. [DOI] [PubMed] [Google Scholar]
- Bagby, G. J., Green, H. J., Katsuta, S. and Gollnick, P. D. (1978). Glycogen depletion in exercising rats infused with glucose, lactate, or pyruvate. J. Appl. Physiol. 45, 425-429. [DOI] [PubMed] [Google Scholar]
- Belter, J. G., Carey, H. V. and Garland, T., Jr (2004). Effects of voluntary exercise and genetic selection for high activity levels on HSP72 expression in house mice. J. Appl. Physiol. 96, 1270-1276. [DOI] [PubMed] [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254. [DOI] [PubMed] [Google Scholar]
- Brooks, G. A. (1998). Mammalian fuel utilization during sustained exercise. Comp. Biochem. Physiol. 120, 89-107. [DOI] [PubMed] [Google Scholar]
- Brooks, G. A. and Mercier, J. (1994). The balance of carbohydrate and lipid utilization during exercise: the `crossover' concept. J. Appl. Physiol. 76, 2253-2261. [DOI] [PubMed] [Google Scholar]
- Challet, E., Malan, A., Turek, F. W. and Reeth, O. V. (2004). Daily variations of blood glucose, acid-base state and PCO2 in rats: effect of light exposure. Neurosci. Letters 355, 131-135. [DOI] [PubMed] [Google Scholar]
- Chang, P. Y., Jensen, J., Printz, R. L., Granner, D. K., Ivy, J. L. and Moller, D. E. (1996). Overexpression of hexokinase II in transgenic mice. Evidence that increased phosphorylation augments muscle glucose uptake. J. Biol. Chem. 271, 14834-14839. [PubMed] [Google Scholar]
- Christ-Roberts, C. Y., Pratipanawatr, T., Pratipanawatr, W., Berria, R., Belfort, R., Kashyap, S. and Mandarino, L. J. (2004). Exercise training increases glycogen synthase activity and GLUT-4 expression but not insulin receptor signaling in obese and type 2 diabetic subjects. Metabolism 53, 1233-1242. [DOI] [PubMed] [Google Scholar]
- Constable, S. H., Young, J. C., Higuchi, M. and Holloszy, J. O. (1984). Glycogen resynthesis in leg muscles of rats during exercise. Am. J. Physiol. 247, R880-R883. [DOI] [PubMed] [Google Scholar]
- Danforth, W. H. (1965). Glycogen synthetase activity in skeletal muscle: interconversion of two forms and control of glycogen synthesis. J. Biol. Chem. 240, 588-593. [PubMed] [Google Scholar]
- David, M., Petit, W. A., Laughlin, M. R., Shulman, R. G., King, J. E. and Barret, E. J. (1990). Simultaneous synthesis and degradation of rat liver glycogen. An in vivo nuclear magnetic resonance spectroscopy study. J. Clin. Invest. 86, 612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumke, C. L., Rhodes, J. S., Garland, T., Jr, Maslowski, E., Swallow, J. G., Wetter, A. C. and Cartee, G. D. (2001). Genetic selection of mice for voluntary wheel running: effect on skeletal muscle glucose uptake. J. Appl. Physiol. 91, 1289-1297. [DOI] [PubMed] [Google Scholar]
- Escobar, C., Díaz-Munoz, M., Encinas, F. and Aguilar-Roblero, R. (1998). Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am. J. Physiol. 274, R1309-R1316. [DOI] [PubMed] [Google Scholar]
- Fleur, S. E., la, Kalsbeek, A. and Buijs, R. M. (1999). A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J. Neuroendocrinol. 11, 643-652. [DOI] [PubMed] [Google Scholar]
- Garetto, L. P. and Armstrong, R. B. (1983). Influence of circadian rhythms on rat muscle glycogen metabolism during and after exercise. J. Exp. Biol. 102, 211-222. [DOI] [PubMed] [Google Scholar]
- Garland, T., Jr (2003). Selection experiments: an under-utilized tool in biomechanics and organismal biology. In Vertebrate Biomechanics and Evolution (ed. V. L. Bels, J.-P. Gasc and A. Casinos), pp. 23-56. Oxford, UK: BIOS Scientific Publishers.
- Garland, T., Jr and Kelly, S. A. (2006). Phenotypic plasticity and experimental evolution. J. Exp. Biol. 209, 2234-2261. [DOI] [PubMed] [Google Scholar]
- Garland, T., Jr and Rose, M. R. (ed.) (2009). Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments. Berkeley, California: University of California Press (in press).
- Garland, T., Jr, Morgan, M. T., Swallow, J. G., Rhodes, J. S., Girard, I., Belter, J. G. and Carter, P. A. (2002). Evolution of a small muscle polymorphism in lines of house mice selected for high activity levels. Evolution 56, 1267-1275. [DOI] [PubMed] [Google Scholar]
- Girard, I. and Garland, T., Jr (2002). Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. J. Appl. Physiol. 92, 1553-1561. [DOI] [PubMed] [Google Scholar]
- Girard, I., McAleer, M. W., Rhodes, J. S. and Garland, T., Jr (2001). Selection for high voluntary wheel-running increases speed and intermittency in house mice (Mus domesticus). J. Exp. Biol. 204, 4311-4320. [DOI] [PubMed] [Google Scholar]
- Gomes, F. R., Rezende, E. L., Bunkers, J. L. and Garland, T., Jr (2004). Organ masses and carbohydrate metabolism of mice artificially selected for high voluntary wheel running. Integr. Comp. Biol. 43, 912 [abstract]. [Google Scholar]
- Guderley, H., Houle-Leroy, P., Diffee, G. M., Camp, D. M. and Garland, T., Jr (2006). Morphometry, ultrastructure, myosin isoforms, and metabolic capacities of the “mini muscles” favoured by selection for high activity in house mice. Comp. Biochem. Physiol. B 144, 271-282. [DOI] [PubMed] [Google Scholar]
- Guderley, H., Joanisse, D. R., Mokas, S., Bilodeau, G. M. and Garland, T., Jr (2008). Altered fiber types in gastrocnemius muscle of high wheel-running selected mice with mini muscle phenotypes. Comp. Biochem. Physiol. B 149, 490-500. [DOI] [PubMed] [Google Scholar]
- Halseth, A. E., Bracy, D. P. and Wasserman, D. H. (1999). Overexpression of hexokinase II increases insulin and exercise-stimulated muscle glucose uptake in vivo. Am. J. Physiol. Endocrinol. Metab. 276, E70-E77. [DOI] [PubMed] [Google Scholar]
- Hannon, R. M., Kelly, S. A., Middleton, K. M., Kolb, E. M., Pomp, D. and Garland, T., Jr (2008). Phenotypic effects of the “mini-muscle” allele in a large HR x C57BL/6J mouse backcross. J. Heredity 99, 349-354. [DOI] [PubMed] [Google Scholar]
- Hargreaves, M. and Spriet, L. L. (ed.) (2006). Exercise Metabolism, 2nd edn. Champaign, Illinois: Human Kinetics.
- Hartmann, J., Garland, T., Jr, Hannon, R. M., Kelly, S. A., Muñoz, G. and Pomp, D. (2008). Fine mapping of `Mini-Muscle,' a recessive mutation causing reduced hind-limb muscle mass in mice. J. Heredity 99, 679-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen, E. J. and Halseth, A. E. (1994). Early alterations in soleus GLUT-4, glucose transport, and glycogen in voluntary running rats. J. Appl. Physiol. 76, 1862-1867. [DOI] [PubMed] [Google Scholar]
- Holloszy, J. O. and Kohrt, W. M. (1996). Regulation of carbohydrate and fat metabolism during and after exercise. Annu. Rev. Nutr. 16, 121-138. [DOI] [PubMed] [Google Scholar]
- Holloszy, J. O. and Nolte, L. A. (2002). Glucose transport in skeletal muscle. In Muscle Metabolism. Frontiers in Animal Diabetes Research, Vol. 4 (ed. J. R. Zierath and H. Wallberg-Henriksson), pp. 87-112. New York, USA: Taylor and Francis. [Google Scholar]
- Host, H. H., Hansen, P. A., Nolte, L. A., Chen, M. M. and Holloszy, J. O. (1998). Rapid reversal of adaptive increases in muscle GLUT-4 and glucose transport capacity after training cessation. J. Appl. Physiol. 84, 798-802. [DOI] [PubMed] [Google Scholar]
- Houle-Leroy, P., Garland, T., Jr, Swallow, J. G. and Guderley, H. (2000). Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J. Appl. Physiol. 89, 1608-1616. [DOI] [PubMed] [Google Scholar]
- Houle-Leroy, P., Guderley, H., Swallow, J. G. and Garland, T., Jr (2003). Artificial selection for high activity favors mighty mini-muscles in house mice. Am. J. Physiol. 284, R433-R443. [DOI] [PubMed] [Google Scholar]
- Howlett, R. A., Gonzalez, N. C., Wagner, H. E., Fu, Z., Britton, S. L., Koch, L. G. and Wagner, P. D. (2003). Selected Contribution: skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J. Appl. Physiol. 94, 1682-1688. [DOI] [PubMed] [Google Scholar]
- Hultman, E. and Harris, R. C. (1988). Carbohydrate metabolism. In Principles of Exercise Biochemistry. Med. Sport Sci., Vol. 27 (ed. J. R. Portmans), pp. 78-119. Karger, Basel. [Google Scholar]
- Ivy, J. L., Brozinick, J. T., Jr, Torgan, C. E. and Kastello, G. M. (1989). Skeletal glucose transport in obese Zucker rats after exercise training. J. Appl. Physiol. 66, 2635-2641. [DOI] [PubMed] [Google Scholar]
- Ivy, J. L., Ding, Z., Hwang, H., Ciadella-Kam, L. C. and Morrison, P. J. (2008). Post exercise carbohydrate-protein supplementation: phosphorylation of muscle proteins involved in glycogen synthesis and protein translation. Amino Acids 35, 89-97. [DOI] [PubMed] [Google Scholar]
- John-Alder, H. B., McAllister, R. M. and Terjung, R. L. (1986). Reduced running endurance in gluconeogenesis-inhibited rats. Am J. Physiol. 251, R137-R142. [DOI] [PubMed] [Google Scholar]
- Kelly, S. A., Czech, P. P., Wight, J. T., Blank, K. M. and Garland, T., Jr (2006). Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J. Morphol. 267, 360-374. [DOI] [PubMed] [Google Scholar]
- Kochan, R. G., Lamb, D. R., Lutz, S. A., Perrill, C. V., Reimann, E. M. and Schlender, K. K. (1979). Glycogen synthase activation in human skeletal muscle: effects of diet and exercise. Am. J. Physiol. 236, E660-E666. [DOI] [PubMed] [Google Scholar]
- Koteja, P. and Garland, T., Jr (2001). Response to R. Eikelboom. Anim. Behav. 61, F25-F26. [Google Scholar]
- Kuo, C.-H., Hwang, H., Lee, M.-C., Castle, A. L. and Ivy, J. L. (2004). Role of insulin on exercise-induced GLUT-4 protein expression and glycogen supercompensation in rat skeletal muscle. J. Appl. Physiol. 96, 621-627. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Magnusson, I., Rothman, D. L., Jucker, B., Cline, G. W., Shulman, R. G. and Shulman, G. I. (1994). Liver glycogen turnover in fed and fasted humans. Am. J. Physiol. 266, E796-E803. [DOI] [PubMed] [Google Scholar]
- Malisch, J. L., Breuner, C. W., Gomes, F. R., Chappell, M. A. and Garland, T., Jr (2008). Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen. Comp. Endocrinol. 156, 210-217. [DOI] [PubMed] [Google Scholar]
- Meek, T. H., Hannon, R. M., Lonquich, B., Marsik, R. L., Wijeratne, R. S. and Garland, T., Jr (2007). Endurance capacity of mice selectively bred for high voluntary wheel running. Integr. Comp. Biol. 47, e81 [abstract]. [DOI] [PubMed] [Google Scholar]
- Mendoza, J., Graff, C., Dardente, H., Pevet, P. and Challet, E. (2005). Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J. Neurosci. 25, 1514-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, K. M., Shubin, C. E., Moore, D. C., Carter, P. A., Garland, T., Jr and Swartz, S. M. (2008). The relative importance of genetics and phenotypic plasticity in dictating bone morphology and mechanics in aged mice: evidence from an artificial selection experiment. Zoology 111, 135-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge, C. and León-Velarde, F. (1991). Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol. Rev. 71, 1135-1172. [DOI] [PubMed] [Google Scholar]
- Morgan, M. A. and Pffaf, D. W. (2002). Estrogen's effects on activity, anxiety, and fear in two mouse strains. Behav. Brain Res. 132, 85-93. [DOI] [PubMed] [Google Scholar]
- Nakatani, A., Han, D.-H., Hansen, P. A., Nolte, L. A., Host, H. H., Hickner, R. C. and Holloszy, J. O. (1997). Effect of endurance exercise training on muscle glycogen supercompensation in rats. J. Appl. Physiol. 82, 711-715. [DOI] [PubMed] [Google Scholar]
- Nehrenberg, D. L., Hua, K., Estrada-Smith, D., Garland, T., Jr and Pomp, D. (In review). Voluntary exercise and its effects on body composition depend on genetic selection history. Obesity [DOI] [PubMed]
- Nesher, R., Karl, I. E. and Kipnis, D. M. (1985). Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am. J. Physiol. Cell Physiol. 249, C226-C232. [DOI] [PubMed] [Google Scholar]
- Newsholme, E. A. (1988). Application of knowledge of metabolic integration to the problem of metabolic limitations in sprints, middle distance and marathon running. In Principles of Exercise Biochemistry. Med. Sport Sci. Vol. 27 (ed. J. R. Portmans), pp. 194-211. Karger, Basel. [Google Scholar]
- Nielsen, J. N., Derave, W., Kristiansen, S., Ralston, E., Ploug, T. and Richter, E. A. (2001). Glycogen synthase localization and activity in rat skeletal muscle is strongly dependent on glycogen content. J. Physiol. 531, 757-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland, R. C., Thyfault, J. P., Henes, S. T., Whitfield, B. R., Woodlief, T. L., Evans, J. R., Lust, J. A., Britton, S. L., Koch, L. G., Dudek, R. W. et al. (2007). Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 293, E31-E41. [DOI] [PubMed] [Google Scholar]
- Ogawa, S., Chan, J., Gustafsson, J.-A., Korach, K. S. and Pffaf, D. W. (2003). Estrogen increases locomotor activity in ice through estrogen receptor α: specificity for the type of activity. Endocrinology 144, 230-239. [DOI] [PubMed] [Google Scholar]
- Palmer, W. K., Goldfarb, A. H. and Ivy, J. L. (1979). Circadian and ovarian influences on tissue glycogen in female rats. Endocrinology 105, 1254-1261. [DOI] [PubMed] [Google Scholar]
- Passoneau, J. V. and Lauderdale, V. R. (1974). A comparison of three methods of glycogen measurement in tissues. Anal. Biochem. 60, 405-412. [DOI] [PubMed] [Google Scholar]
- Pederson, B. A., Cope, C. R., Irimia, J. M., Schroeder, J. M., Thurberg, B. L., DePaoli-Roach, A. A. and Roach, P. J. (2005a). Mice with elevated muscle glycogen stores do not have improved exercise performance. Biochem. Biophys. Res. Commun. 331, 491-496. [DOI] [PubMed] [Google Scholar]
- Pederson, B. A., Cope, C. R., Irimia, J. M., Schroeder, J. M., Smith, M. W., Thurberg, B. L., DePaoli-Roach, A. A. and Roach, P. J. (2005b). Exercise of mice genetically lacking muscle glycogen synthase – in mice, muscle glycogen is not essential for exercise. J. Biol. Chem. 280, 17260-17265. [DOI] [PubMed] [Google Scholar]
- Pessacq, M. T. and Gagliardino, J. J. (1975). Glycogen metabolism in muscle: its circadian and seasonal variations. Metabolism 24, 737-743. [DOI] [PubMed] [Google Scholar]
- Price, T. B., Taylor, R., Mason, G. F., Rothman, D. L., Shulman, G. L. and Shulman, R. G. (1994). Turnover in human muscle glycogen with low-intensity exercise. Med. Sci. Sports Exerc. 26, 983-991. [PubMed] [Google Scholar]
- Putnam, C. T., Jones, N. L., Lands, L. C., Bragg, T. M., Hollidge-Horvat, M. G. and Heingerhauser, G. J. (1995). Skeletal muscle pyruvate dehydrogenase activity during maximal exercise in humans. Am. J. Physiol. 269, 458-468. [DOI] [PubMed] [Google Scholar]
- Ren, J.-M., Semenkovich, C. F., Gulve, E. A., Gao, J. and Holloszy, J. O. (1994). Exercise-induces rapid increases in GLUT-4 expression, glucose transport capacity and insulin-stimulated glycogen storage in muscle. J. Biol. Chem. 269, 14396-14401. [PubMed] [Google Scholar]
- Rezende, E. L., Chappell, M. A., Gomes, F. R., Malisch, J. L. and Garland, T., Jr (2005). Maximal metabolic rates during voluntary exercise, forced exercise, and cold exposure in house mice selectively bred for high wheel-running. J. Exp. Biol. 208, 2447-2458. [DOI] [PubMed] [Google Scholar]
- Rezende, E. L., Garland, T., Jr, Chappell, M. A., Malisch, J. L. and Gomes, F. R. (2006a). Maximum aerobic performance in lines of Mus selected for high wheel-running activity: effects of selection, oxygen availability, and the mini-muscle phenotype. J. Exp. Biol. 209, 115-127. [DOI] [PubMed] [Google Scholar]
- Rezende, E. L., Gomes, F. R., Malisch, J. L., Chappell, M. A. and Garland, T., Jr (2006b). Maximal oxygen consumption in relation to subordinate traits in lines of house mice selectively bred for high voluntary wheel running. J. Appl. Physiol. 101, 477-485. [DOI] [PubMed] [Google Scholar]
- Rezende, E. L., Kelly, S. A., Gomes, F. R., Chappell, M. A. and Garland, T., Jr (2006c). Effects of size, sex, and voluntary running speeds on costs of locomotion in lines of laboratory mice selectively bred for high wheel-running activity. Physiol. Biochem. Zool. 79, 83-99. [DOI] [PubMed] [Google Scholar]
- Rice, C. L., Pettigrew, F. P., Noble, E. G. and Taylor, A. W. (1988). The fiber composition of skeletal muscle. In Principles of Exercise Biochemistry. Med. Sport Sci., Vol. 27 (ed. J. R. Portmans), pp. 22-39. Karger, Basel. [Google Scholar]
- Roberts, T. J., Weber, J.-M., Hoppeler, H., Weibel, E. R. and Taylor, C. R. (1996). Design of the oxygen and substrate pathways. II. Defining the upper limits of carbohydrate and fat oxidation. J. Exp. Biol. 199, 1651-1658. [DOI] [PubMed] [Google Scholar]
- Roy, R. R., Hutchinson, D. L., Pierotti, D. J., Hodgson, J. A. and Edgerton, V. R. (1991). EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J. Appl. Physiol. 70, 2522-2529. [DOI] [PubMed] [Google Scholar]
- Rugh, R. (1968). The Mouse: Its Reproduction and Development. Minneapolis, MN: Burgess.
- Saltin, B. and Gollnick, P. D. (1983). Skeletal muscle adaptability: significance for metabolism and performance. In Handbook of Physiology. Skeletal Muscle, pp. 555-630. American Physiological Society, Bethesda, MD: Williams and Wilkins, Baltimore.
- Schwarz, E. and Schwarz, H. K. (1943). The wild and commercial stocks of the house mouse, Mus musculus Linnaeus. J. Mammal. 24, 59-72. [Google Scholar]
- Singh, M. K., Krisan, A. D., Crain, A. M., Collins, D. E. and Yaspelkis, B. B., III (2003). High-fat diet and leptin treatment alter skeletal muscle insulin-stimulated phosphatidylinositol 3-kinase activity and glucose transport. Metabolism 52, 1196-1205. [DOI] [PubMed] [Google Scholar]
- Stryer, L. (1995). Biochemistry. New York: W. H Freeman and Company.
- Swallow, J. G. and Garland, T., Jr (2005). Selection experiments as a tool in evolutionary and comparative physiology: insights into complex traits – an introduction to the symposium. Integr. Comp. Biol. 45, 387-390. [DOI] [PubMed] [Google Scholar]
- Swallow, J. G., Carter, P. A. and Garland, T., Jr (1998). Artificial selection for increased wheel-running behavior in house mice. Behav. Genet. 28, 227-236. [DOI] [PubMed] [Google Scholar]
- Swallow, J. G., Koteja, P., Carter, P. A. and Garland, T., Jr (1999). Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. J. Exp. Biol. 202, 2513-2520. [DOI] [PubMed] [Google Scholar]
- Swallow, J. G., Koteja, P., Carter, P. A. and Garland, T., Jr (2001). Food consumption and body composition in mice selected for high wheel-running activity. J. Comp. Physiol. B 171, 651-659. [DOI] [PubMed] [Google Scholar]
- Swallow, J. G., Rhodes, J. S. and Garland, T., Jr (2005). Phenotypic and evolutionary plasticity of organ masses in response to voluntary exercise in house mice. Integr. Comp. Biol. 45, 426-437. [DOI] [PubMed] [Google Scholar]
- Swallow, J. G., Hayes, J. P., Koteja, P. and Garland, T., Jr (2009). Selection experiments and experimental evolution of performance and physiology. In Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments. (ed. T. Garland, Jr. and M. R. Rose). Berkeley, California: University of California Press. In press.
- Syme, D. A., Evashuk, K., Grintuch, B., Rezende, E. L. and Garland, T., Jr (2005). Contractile abilities of normal and `mini' triceps surae muscles from mice (Mus domesticus) selectively bred for high voluntary wheel running. J. Appl. Physiol. 99, 1308-1316. [DOI] [PubMed] [Google Scholar]
- Thomas, J. A., Schlender, K. K. and Larner, J. (1968). A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal. Biochem. 25, 486-499. [DOI] [PubMed] [Google Scholar]
- Thorburn, A. W., Gumbiner, B., Bulacan, F., Wallace, P. and Henry, R. R. (1990). Intracellular glucose oxidation and glycogen synthase activity are reduced in non-insulin-dependent (Type II) diabetes independent of impaired glucose uptake. J. Clin. Invest. 85, 522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao, T.-S., Li, J., Chang, K. S., Stenbit, A. E., Galuska, D., Anderson, J. E., Zierath, J. R., McCarter, R. J. and Charron, M. J. (2001). Metabolic adaptations in skeletal muscle overexpressing GLUT-4: effects on muscle and physical activity. FASEB J. 15, 958-969. [DOI] [PubMed] [Google Scholar]
- Villar-Palasi, C. (1995). Effect of glucose phosphorylation on the activation by insulin of skeletal muscle glycogen synthase. Biochim. Biophys. Acta 1244, 203-208. [DOI] [PubMed] [Google Scholar]
- Vock, R., Hoppeler, H., Claassen, H., Wu, D. X. Y., Billeter, R., Weber, J.-M., Taylor, C. R. and Weibel, E. R. (1996). Design of the oxygen and substrate pathways. VI. Structural basis of intracellular substrate supply to mitochondria in muscle cells. J. Exp. Biol. 199, 1689-1697. [DOI] [PubMed] [Google Scholar]
- Wasserman, D. H. and Cherrington, A. D. (1991). Hepatic fuel metabolism during muscular work, role and regulation. Am. J. Physiol. 260, E811-E824. [DOI] [PubMed] [Google Scholar]
- Weber, J. M., Roberts, T. J., Vock, R., Weibel, E. R. and Taylor, C. R. (1996). Design of the oxygen and substrate pathways. III. Partitioning energy provision by carbohydrates. J. Exp. Biol. 199, 1659-1666. [DOI] [PubMed] [Google Scholar]
- Wilson, R. S. and Franklin, C. E. (2002). Testing the beneficial acclimation hypothesis. Trend. Ecol. Evol. 17, 66-70. [Google Scholar]
- Wong, L. E., Garland, T., Jr and Hepple, R. T. (In revision). Anatomic capillarization is elevated in the medial gastrocnemius muscle of mighty mini mice. J. Appl. Physiol. [DOI] [PubMed]
- Yaspelkis, B. B., III, Singh, M. K., Krisan, A. D., Collins, D. E., Kwong, C. C., Bernard, J. R. and Crain, A. M. (2004). Chronic leptin treatment enhances insulin-stimulated glucose disposal in skeletal muscle of high-fat fed rodents. Life Sci. 74, 1801-1816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.