Summary
Much effort has been expended on analyzing how microfilament and microtubule cytoskeletons dictate the interaction of cells with matrix at adhesive sites called focal adhesions (FAs). However, vimentin intermediate filaments (IFs) also associate with the cell surface at FAs in endothelial cells. Here, we show that IF recruitment to FAs in endothelial cells requires β3 integrin, plectin and the microtubule cytoskeleton, and is dependent on microtubule motors. In CHO cells, which lack β3 integrin but contain vimentin, IFs appear to be collapsed around the nucleus, whereas in CHO cells expressing β3 integrin (CHOwtβ3), vimentin IFs extend to FAs at the cell periphery. This recruitment is regulated by tyrosine residues in the β3 integrin cytoplasmic tail. Moreover, CHOwtβ3 cells exhibit significantly greater adhesive strength than CHO or CHO cells expressing mutated β3 integrin proteins. These differences require an intact vimentin network. Therefore, vimentin IF recruitment to the cell surface is tightly regulated and modulates the strength of adhesion of cells to their substrate.
Keywords: Intermediate filament, Integrin, Adhesion
Introduction
Angiogenesis is essential for development, tumor survival and tissue reorganization following wounding. This process involves endothelial cell migration from pre-existing blood vessels, formation of adhesive sites between migrating cells and the extracellular matrix (ECM), and assembly of endothelial cells into vessels (Rupp and Little, 2001). Focal adhesion (FA) proteins have a role in each of these processes (Hynes, 2007). For example, each FA is a region of close interaction between cells and the matrix on their substrate, tethers the cytoskeleton to the cell surface and is a hub of signal transduction (Giancotti and Ruoslahti, 1999; Hynes et al., 1999; Wozniak et al., 2004). Many functions of FAs are mediated by the integrin family of αβ heterodimeric transmembrane receptors, which not only bind cytoskeleton linker proteins in the cytoplasm and matrix outside the cell, but also interact with and regulate the activity of various signaling intermediates (Giancotti and Ruoslahti, 1999; Hynes et al., 1999). Typically, integrins mediate the anchorage of actin-containing microfilaments to FAs (Simon and Burridge, 1994; Wozniak et al., 2004). The microtubule cytoskeleton also appears to interact with FAs and is involved in FA disassembly (Ezratty et al., 2005; Small et al., 2002).
In contrast to the extensive literature on actin and microtubules and their relationship to FAs, studies on whether the IF cytoskeleton interacts with FAs and whether IFs have a role in regulating FA structure, function and/or assembly, or vice versa, are few (Bershadsky et al., 1987; Gonzales et al., 2001; Kreis et al., 2005; Tsuruta and Jones, 2003; Windoffer et al., 2006). However, at the edge of a number of different types of endothelial cells, the majority of αvβ3-integrin-rich FAs show precise and complex association with both the microfilament and the vimentin IF cytoskeletons (Gonzales et al., 2001). Moreover, a number of studies have presented indirect evidence that the vimentin IF cytoskeleton is involved in modulating either the structure or function of matrix adhesions in the form of FAs. Indeed, FAs do not distribute geometrically in vimentin-null fibroblasts (Eckes et al., 1998). Furthermore, cells in which vimentin expression has been inhibited by RNA interference assemble smaller than normal FAs (Tsuruta and Jones, 2003). More dramatically, such cells exhibit decreased adhesion to the substratum. These data provide evidence that the vimentin cytoskeleton regulates FA size and might help to stabilize cell-matrix adhesions (Tsuruta and Jones, 2003). This parallels the role of another type of IF - keratin - in determining the structure and function of hemidesmosomes, which link epithelia and the cell matrix (Jones et al., 1998). Since IF-hemidesmosome interaction is mediated through an indirect association between keratin IF and the β4 integrin subunit, we tested the hypothesis that an integrin subunit enriched in the FAs of endothelial cells, namely β3 integrin, is involved in recruiting vimentin IF to the cell surface at FAs. It has already been shown that plectin-β4-integrin tail interactions have an important role in connecting α6β4 with vimentin IFs in endothelial cells (Homan et al., 2002), we therefore also investigated plectin as a possible linker protein that mediates vimentin IF association with FAs. Finally, we analyzed the functional consequences of integrin-regulated IF cell surface association.
Results
β3 integrin mediates IF-FA interaction
Human microvascular and umbilical vein endothelial cells assemble numerous FAs in vitro with over 50% exhibiting interaction with IFs (Gonzales et al., 2001). A comparable number of FAs showed an association with IFs in an endothelial cell type derived from bone marrow (transformed human bone marrow endothelial cell, TrHBMEC) (Gonzales et al., 2001; Tsuruta and Jones, 2003) (Fig. 1A). The ease of maintaining TrHBMECs in culture and their ability to be manipulated at the molecular level makes them an excellent in vitro cell model to study the regulation of IF interaction with FAs.
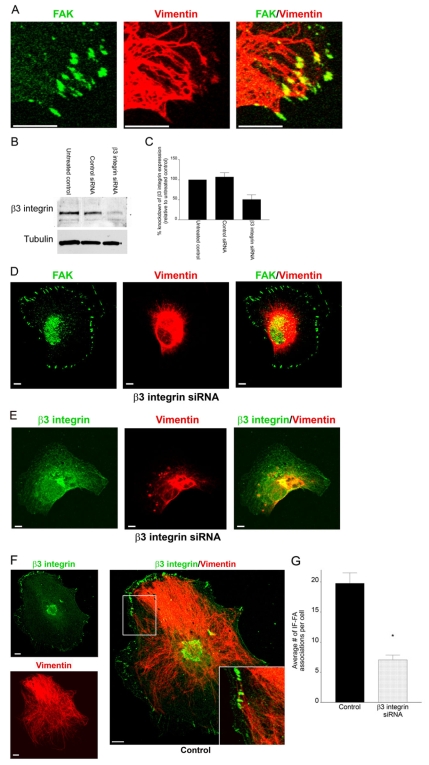
Fig. 1.
Knockdown of β3 integrin in TrHBMECs perturbs interactions between IFs and the cell surface. (A) TrHBMECs were stained for FAK (green) and vimentin (red) as indicated. The panel on the right shows an overlay of the green and red channels. The samples were viewed by confocal microscopy with the focal plane being proximal to the substratum-attached surface of the cells. (B) Extracts of mock-transfected TrHBMECs (untreated control) and TrHBMECs transfected with either control siRNA or β3 integrin siRNA were probed first with a β3 integrin antibody and then reprobed with tubulin. (C) Analysis of densitometric scans of western blots from three different experiments equivalent to those in B. Error bars represent s.e.m. of three experiments. (D) TrHBMECs transfected with β3 integrin siRNA were stained for FAK (green) and vimentin (red) as indicated. (E) TrHBMECs transfected with β3 integrin siRNA were stained for β3 integrin (overexposed to show green) and vimentin (red) as indicated. (F) TrHBMECs transfected with control siRNA were stained for β3 integrin (green) and vimentin (red) as indicated. The inset in the panel on the right is a higher magnification of the boxed area. (G) Quantification of the IF-FA association in TrHBMECs transfected with β3 integrin siRNA compared with the control (*P<0.01). Error bars represent s.e.m. of three experiments, counting a total of 200 cells. Scale bars: 10 μm.
The FAs of TrHBMECs are enriched in β3-subunit-containing integrin heterodimers (Gonzales et al., 2001; Tsuruta and Jones, 2003). To directly assess whether β3 integrin facilitates recruitment of vimentin IF to FAs, TrHBMECs were transfected with a pool of siRNAs designed to silence β3 integrin expression, or nonspecific siRNA as a control. Immunoblotting revealed that cells transfected with the β3 integrin siRNA had ∼49.3% knockdown in β3 integrin expression compared with untreated cells or cells transfected with control siRNA (Fig. 1B,C). Similarly, FACS analysis of TrHBMECs treated with β3 integrin siRNA revealed a 56.6% decrease in cell surface expression levels of αvβ3 integrin compared with levels in untreated cells (supplementary material Fig. S1A). Along with the decrease in αvβ3 integrin cell surface levels, there was a corresponding 29.9% decrease in cell surface expression levels of αv integrin (supplementary material Fig. S1A). There was no obvious change in the expression of β5 integrin at the cell surface in TrHBMECs treated with β3 integrin siRNA compared with controls (supplementary material Fig. S1A). Interestingly, in cells treated with β3 integrin siRNA, IFs were mostly restricted to the perinuclear region (Fig. 1D,E). In sharp contrast, IF showed complex associations with FAs in cells expressing the control siRNA (Fig. 1F). We quantified the association of IFs with FAs in the treated cell populations by counting each instance where vimentin IFs were closely associated with, appeared to be wrapped around or terminated at FAs. Relative to controls, there was a 62% decrease in the association of vimentin IFs with FAs in silenced cells (Fig. 1G).
Despite the dramatic effect that the β3 integrin siRNA had on the vimentin IF network in TrHBMECs, we found that there were no alterations in the microfilament or microtubule networks of these cells (supplementary material Fig. S1B). We also silenced the alternate αv integrin partner, β5 integrin, in TrHBMECs (supplementary material Fig. S1C). β5 integrin in control cells was found in FAs that associate with IF (supplementary material Fig. S1C). In TrHBMECs in which β5 integrin had been silenced, vimentin IFs remained extended to the cell periphery (supplementary material Fig. S1C). Together, these results provide evidence for a specific role for β3 integrin in mediating IF-FA interaction in endothelial cells.
We have discovered a novel method of regulating the clustering of β3 integrin at FAs in TrHBMECs. This method provided a second means of demonstrating a role for β3 integrin in regulating IF-FA association. Specifically, when cells were plated in serum-free medium onto poly-L-lysine (PLL)-coated coverslips and allowed to adhere overnight they established paxillin-rich FA-like structures that associated with the microfilament cytoskeleton (supplementary material Fig. S2). The microtubule network was also apparently normal in similarly treated cells (supplementary material Fig. S2). In sharp contrast, antibodies against β3-integrin or the αvβ3-integrin complex failed to stain any obvious adhesion structures in TrHBMECs under these conditions, and the vimentin IF network of such cells was concentrated in the perinuclear region (Fig. 2A,B). However, in TrHBMECs plated on PLL, left for 16 hours and subsequently treated for 30 minutes with MnCl2, β3 integrin was recruited to FAs (Fig. 2A,D). We assume that MnCl2 activates β3 integrin under this experimental regimen, thereby inducing its localization to FAs (Gailit and Ruoslahti, 1988). Moreover, in cells treated with MnCl2, there was a significant and dramatic increase in IF-FA association (Fig. 2C,D,F). We did not observe the latter increase in TrHBMECs, transfected with β3 integrin siRNA, under the same conditions (Fig. 2E,F).
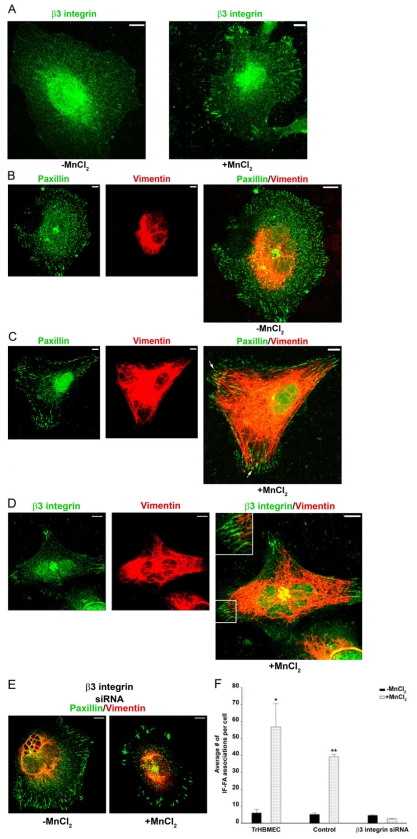
Fig. 2.
β3 integrin recruits IFs to FAs. (A-D) TrHBMECs were plated in serum-free medium on PLL-coated coverslips overnight. Untreated cells (left panel in A;B) or cells incubated for 30 minutes in medium supplemented with 0.5 mM MnCl2 (right panel in A;C,D) were processed for immunofluorescence microscopy using antibodies against β3 integrin (green) (A), a combination of antibodies against paxillin (green) and vimentin (red) (B,C), or a combination of antibodies against β3 integrin (green) and vimentin (red) (D). In B-D individual images and overlays of the green and red channels are presented. The arrows in C indicate the IF-FA association. In D, the boxed area is shown at higher magnification in the inset. (E) TrHBMECs were treated with β3 integrin siRNA 72 hours before being plated in serum-free medium on PLL-coated coverslips overnight. Untreated cells (left panel) or cells incubated for 30 minutes in medium supplemented with 0.5 mM MnCl2 (right panel) were processed for immunofluorescence microscopy using a combination of antibodies against paxillin (green) and vimentin (red). (F) Quantification of the IF-FA interaction, as imaged in B,C,E. Error bars represent s.e.m. of three experiments, counting a total of 200 cells. The number of vimentin IF-FA interactions is significantly higher (*P<0.01) in TrHBMECs plated on PLL-coated substrate and treated with MnCl2 compared with that in untreated cells plated on PLL alone. Control cells (cells treated with transfection reagents alone and no siRNA) treated with MnCl2 had significantly more IF-FA associations per cell than control cells plated on PLL alone or TrHBMECs transfected with β3 integrin siRNA and then plated on PLL-coated substrate and treated with MnCl2 (**P<0.01). Indeed, there is no significant difference in IF-FA associations in TrHBMECs transfected with β3 integrin siRNA and then plated on PLL-coated substrate whether they are treated with MnCl2 or not (P>0.1). Scale bars: 10 μm.
To provide an alternative means of showing that the β3 integrin subunit mediates IF-FA interaction, we also transfected GFP-β3-integrin into CHO cells that lack β3 integrin but express vimentin IF (Fig. 3A,B) (Ylanne et al., 1993). The endogenous αv integrins in CHO cells are known to pair with the expressed β3 integrin subunits, with the resulting αvβ3 integrin heterodimers targeting to FAs at the cell surface (Fig. 3A,B) (Schaffner-Reckinger et al., 1998). In `parental' non-transfected CHO cells, most of the vimentin IF cytoskeleton is concentrated in the perinuclear region (Fig. 3B). By contrast, vimentin IFs extended to FAs at the cell periphery in CHO cells in which we expressed the β3 integrin subunit (CHOwtβ3) (Fig. 3B). Indeed, there was a significant increase in IF-FA association in CHOwtβ3 cells (Fig. 3C). Taken together, these studies reveal that β3 integrin has an important role in regulating vimentin IF-FA interactions, regardless of cell type.
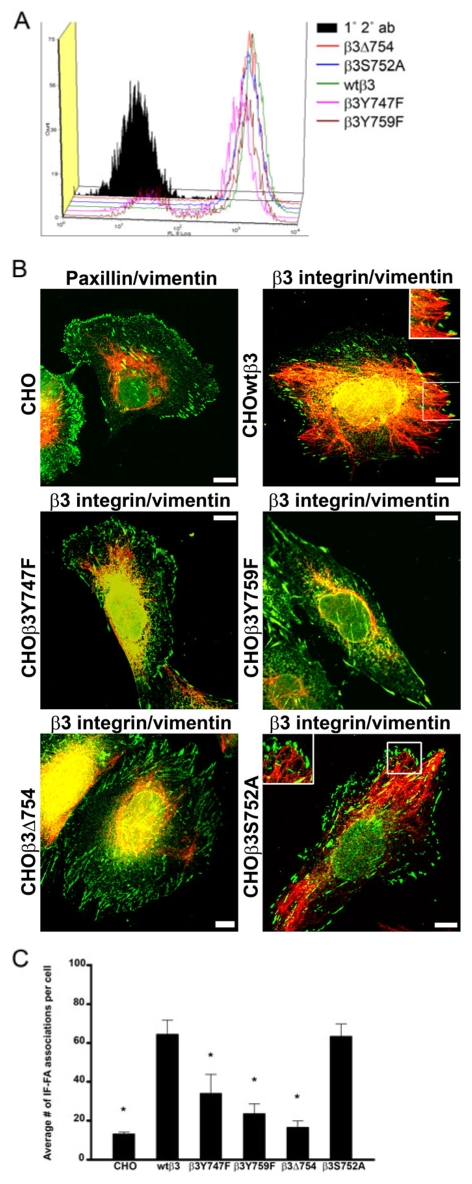
Fig. 3.
IF recruitment to FAs is regulated by β3 integrin in CHO cells and specifically by tyrosine residues within the tail of the β3 integrin. (A) Constructs encoding wild-type and mutant GFP-tagged β3 integrin were transfected into CHO cells and surface expression of αvβ3 integrin was evaluated by FACS using LM609 antibody (1° 2° ab, nontransfected CHO cells labeled with primary and secondary antibodies). (B) CHO cells (top left) were prepared for immunofluorescence using a combination of paxillin (green) and vimentin (red) antibodies. In addition, CHO cells expressing the indicated GFP-tagged β3 integrin proteins were stained for vimentin (red). The insets (top right, bottom right) are higher magnifications of the boxed areas. (C) Quantification of IF-FA association for all of the CHO cell lines. Over 200 cells were analyzed per cell line in at least three separate experiments. Error bars represent s.e.m. of three experiments. *P<0.03; statistically significant decrease in IF-FA association compared with CHO cells expressing wild-type β3 integrin.
Tyrosine residues within the β3 integrin cytoplasmic tail regulate the IF-FA association
We next determined whether specific residues or domains of the β3 integrin cytoplasmic tail are involved in mediating IF association with FAs. Tyrosine residues at positions 747 and 759 and Ser752 within the cytoplasmic tail are important regulatory sites within the cytoplasmic domain of the β3 integrin subunit (Blystone et al., 1996; Blystone et al., 1997; Chen et al., 1992; Faccio et al., 2003; Hughes et al., 1995). Thus, we assayed IF-FA interaction in CHO cell lines expressing various mutants of β3 integrin, including β3Y747F, β3Y759F, β3S752A and β3Δ754 (a deletion mutant). All of the mutant β3 integrin proteins targeted to the surface of CHO cells, associated with αv integrin subunits and localized to FAs, as determined by FACS analyses using an antibody against the αvβ3 complex, and by fluorescence microscopy (Fig. 3A,B). In CHOβ3Y747F, CHOβ3Y759F and CHOβ3Δ754 cells, IFs appeared to be primarily concentrated in the perinuclear region of the cells, with few IF-FA interactions detected (Fig. 3B,C). By contrast, IFs were found to be associated with FAs in CHOβ3S752A cells, at levels comparable with those observed in CHOwtβ3 cells (Fig. 3B,C). These data indicate that Tyr747 and Tyr759 in the β3 integrin cytoplasmic tail are key regulators of IF-FA interaction.
In addition to confocal microscopy, we utilized total internal reflection fluorescence microscopy (TIRFM) to analyze the IF-FA interaction in CHOwtβ3 and CHOβ3Y747F cells, because this technique has been previously used to demonstrate targeting of microtubules to FAs (Krylyshkina et al., 2003). In TIRFM, the excitation light typically penetrates <150 nm above the reflecting surface and is ideal for studying cellular processes that take place close to the growth substrate, such as cell adhesion (Axelrod, 1989; Steyer and Almers, 2001; Toomre and Manstein, 2001). CHOwtβ3-GFP and CHOβ3Y747F-GFP cells were transfected with vimentin-CFP and visualized live using TIRFM. At the basal surface of the cell, vimentin IFs colocalized with wild-type β3 integrin, whereas there was no interaction between IFs and the mutated β3 integrin (Fig. 4). Indeed, the vimentin in CHOβ3Y747F cells was located above the TIRFM plane. Thus, our data demonstrate that vimentin IFs are closely associated with β3 integrin at sites of adhesion at the basal surface of the cell and that the tyrosine residues in the β3 integrin cytoplasmic tail have an important role in recruitment of IFs to FAs.
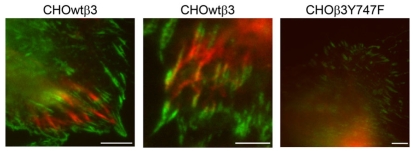
Fig. 4.
TIRFM reveals close interaction between vimentin and β3 integrin. CHOwtβ3-GFP cells (left and center panels) and a CHOβ3Y747F-GFP cell (right panel) transfected with vimentin-CFP and imaged by TIRFM. The left and center panels show the close interaction of vimentin IF with FAs at the basal surface of the cell (note regions of yellow in the center panel indicating close association between IFs and β3 integrin). In contrast, vimentin IFs in the CHOβ3Y747F cell are above the level of the plane visualized by TIRFM. Scale bars: 10 μm.
The microtubule cytoskeleton is involved in recruitment of IFs to FAs
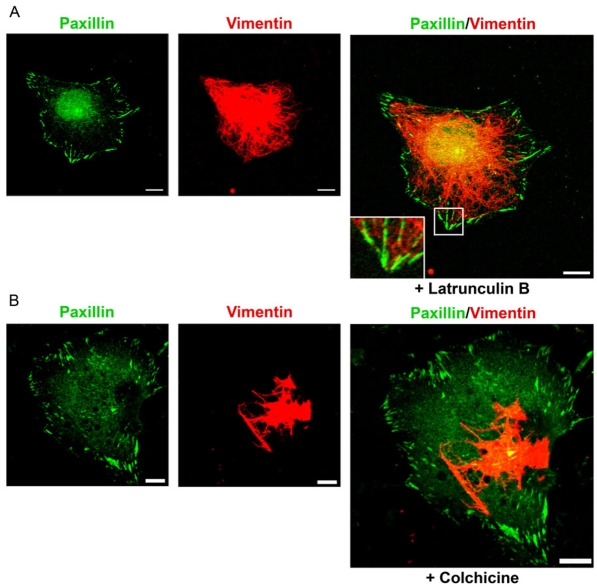
IFs show complex interactions with both the microfilament and microtubule cytoskeleton, and the latter is known to be involved in the distribution of vimentin to diverse parts of the cell (Helfand et al., 2004). Thus, we were interested in assessing whether either or both of these cytoskeletal systems might function to deliver IFs to FAs. To assess this possibility, we used the PLL-MnCl2 technique mentioned previously to regulate IF-FA association. TrHBMECs were plated in serum-free medium onto PLL-coated coverslips, the cells were allowed to adhere overnight and were then treated with either latrunculin B or colchicine at concentrations known to perturb the actin or microtubule networks of TrHBMECs (Tsuruta et al., 2002). The disruption of actin and microtubule networks was verified by fluorescence microscopy (data not shown). The drug-treated cells were subsequently incubated in MnCl2 for 30 minutes to induce β3 integrin recruitment to FAs, as before. Following latrunculin B and MnCl2 treatment, the vimentin cytoskeletal network was well dispersed throughout each cell and IFs extended to and interacted with peripheral FAs, which were stained with antibodies against paxillin (Fig. 5A). However, following colchicine and MnCl2 treatment, the IF cytoskeleton appeared to be collapsed around the nucleus, with few IF fibers extending to the cell periphery and interacting with FAs (Fig. 5B). Nonetheless, β3 integrin was found in the latter (data not shown).
Fig. 5.
IF recruitment to FAs is regulated by the microtubule cytoskeleton. (A,B) TrHBMECs were plated in serum-free medium on PLL-coated coverslips overnight. The cells were treated with 0.1 μM latrunculin B (A) or 0.1 μM colchicine (B) for 15 or 30 minutes, respectively, and then incubated in the same drug for an additional 30 minutes with addition of 0.5 mM MnCl2. The treated cells were subsequently processed for double labeling using a combination of antibodies against paxillin (green) and vimentin (red). The inset in A is a higher magnification of the boxed area. Scale bars: 10 μm.
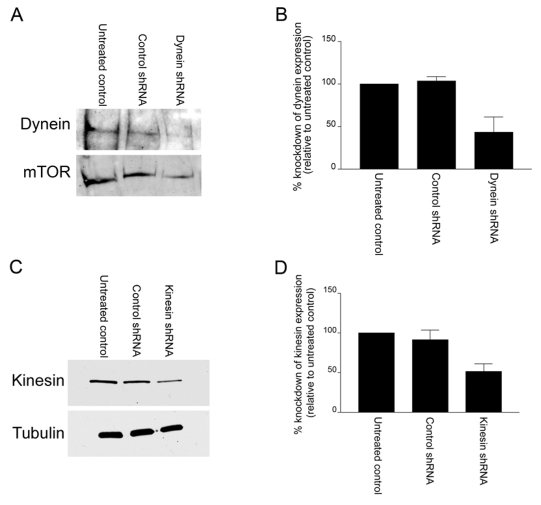
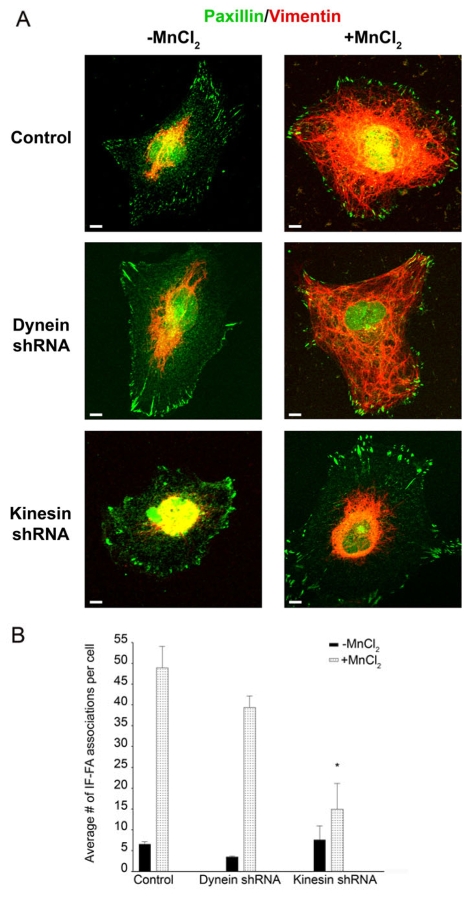
Microtubule motors have been reported to interact with IFs (Helfand et al., 2004). Thus, we determined whether knockdown of specific microtubule motor proteins would inhibit the interaction of IFs with FAs in TrHBMECs. To test this possibility, TrHBMECs were treated with shRNA specific for either the heavy chain dynein or the ubiquitous heavy chain kinesin. Western blot analyses demonstrated a 56.6% and 48.6% knockdown in protein expression, respectively, compared with controls (Fig. 6A,B,C,D). Next, shRNA-treated cells were plated in serum-free medium onto PLL-coated coverslips, followed by incubation in MnCl2. Under these conditions, heavy chain kinesin shRNA inhibited the MnCl2-induced IF-FA interaction in TrHBMECs, whereas treatment with heavy chain dynein shRNA had no effect on the association of IFs with FAs (Fig. 7A,B). By contrast, when dynein or kinesin shRNA-treated cells were plated in serum-free medium onto PLL-coated coverslips, the microtubule networks remained intact in the absence or presence of MnCl2 (supplementary material Fig. S3). We therefore conclude that an intact microtubule network is required for recruitment of IFs to FAs in endothelial cells and, more specifically, plus-end-directed microtubule motor proteins facilitate this process.
Fig. 6.
The use of shRNAs to effectively knockdown dynein and kinesin heavy chain protein expression in TrHBMECs. (A) Extracts of mock electroporated TrHBMECs (untreated control) and cells electroporated with either control (heavy chain kinesin) shRNA or heavy chain dynein shRNA were processed for western immunoblotting, probed first with a dynein heavy chain antibody and then reprobed with an antibody against mTOR. (B) Analysis of densitometric scans of western blots from three different experiments as shown in A. Error bars represent s.e.m. of three experiments. (C) Extracts of mock electroporated TrHBMECs (untreated control) and cells electroporated with either control (heavy chain dynein) shRNA or heavy chain kinesin shRNA were processed for western immunoblotting, probed first with a heavy chain kinesin antibody and then reprobed with an antibody against tubulin. (D) Analysis of densitometric scans of western blots from three different experiments equivalent to that shown in C. Error bars represent s.e.m. of three experiments.
Fig. 7.
Kinesin, but not dynein, has a role in IF recruitment to FAs. (A) TrHBMECs electroporated with the control vector, heavy chain dynein shRNA or heavy chain kinesin shRNA were plated in serum-free medium on PLL-coated coverslips overnight. Untreated cells (left panels) or cells incubated for 30 minutes in medium supplemented with 0.5 mM MnCl2 (right panels) were processed for immunofluorescence microscopy using a combination of antibodies against paxillin (green) and vimentin (red). (B) Quantification of the IF-FA association in TrHBMECs electroporated with heavy chain dynein or heavy chain kinesin shRNA compared with cells electroporated with the control vector. Error bars represent s.e.m. of three experiments, counting a total of 200 cells. Upon addition of MnCl2, cells electroporated with heavy chain kinesin shRNA exhibit significantly fewer IF-FA associations per cell than control cells and cells electroporated with heavy chain dynein shRNA (*P<0.01). Scale bars: 10 μm.
Plectin is required for recruitment of IFs to FAs
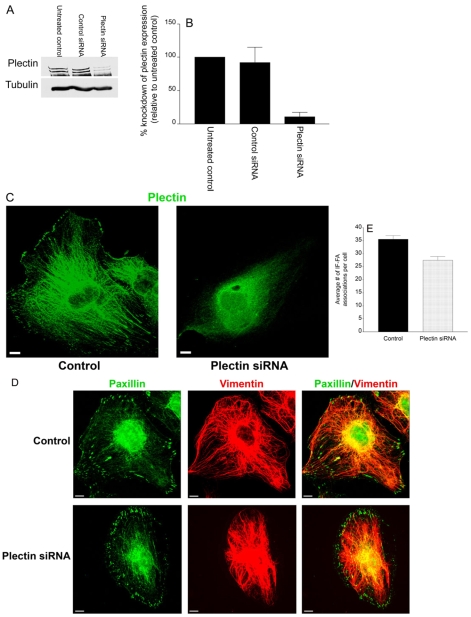
It has already been shown that plectin is associated with vimentin in a variety of cell types (Homan et al., 2002; Spurny et al., 2007; Winter et al., 2008). Moreover, plectin is enriched in the FAs of various cell types, and has been suggested to mediate IF-FA association (Gonzales et al., 2001; Seifert et al., 1992; Wiche et al., 1982). Therefore, we sought to determine whether plectin provides an essential linkage between β3 integrin and vimentin IFs at FAs in endothelial cells. First, TrHBMECs were treated with plectin siRNA and an 89.7% knockdown in protein expression was achieved, as determined by western blotting (Fig. 8A,B). Knockdown of plectin protein expression in TrHBMECs was also demonstrated by immunofluorescence microscopy (Fig. 8C). When IF-FA association was analyzed in cells allowed to spread on uncoated glass coverslips, there was no significant difference between control or plectin siRNA-treated TrHBMECs (Fig. 8D,E). This implies that plectin is not essential for maintaining IF-FA association.
Fig. 8.
Plectin is not required to maintain IF-FA association. (A) Extracts of mock-transfected TrHBMECs (untreated control) and cells transfected with either control siRNA or plectin siRNA were processed for western blotting, probed first with a plectin antibody and then reprobed with a tubulin antibody. (B) Analysis of densitometric scans of western blots from three different experiments equivalent to that shown in A. Error bars represent s.e.m. of three experiments. (C) Mock-transfected (control) TrHBMECs or TrHBMECs transfected with plectin siRNA were stained for plectin (green). (D) Mock-transfected (control) TrHBMECs or TrHBMECs transfected with plectin siRNA were allowed to spread on glass coverslips before being processed for immunofluorescence microscopy using a combination of antibodies against paxillin (green) and vimentin (red). (E) Quantification of IF-FA association in TrHBMECs transfected with plectin siRNA compared with mock-transfected (control) cells. There is no significant difference in IF-FA association between plectin siRNA-treated cells and control cells (P>0.1). Error bars represent s.e.m. of three experiments. Scale bars: 10 μm.
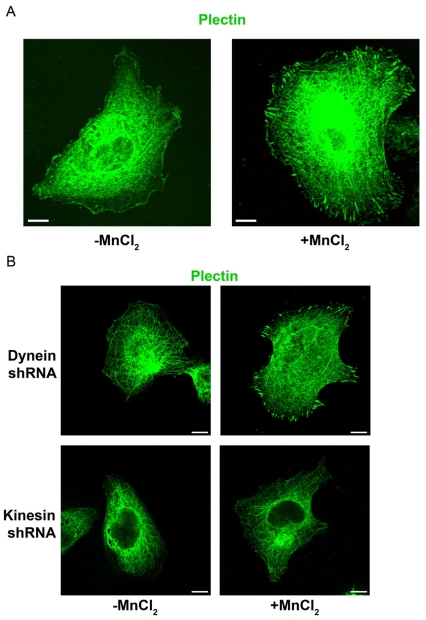
In cells maintained on PLL, plectin failed to localize to FA-like structures (Fig. 9A). However, within 30 minutes of MnCl2 addition, plectin exhibited a robust localization to FAs (Fig. 9A). The recruitment of plectin to FAs is dependent on kinesin, but not dynein. Specifically, when cells treated with heavy chain kinesin shRNA were plated in serum-free medium onto PLL-coated coverslips and treated with MnCl2, plectin did not localize to FA-like structures (Fig. 9B). By contrast, antibodies against plectin strongly stained the FAs of TrHBMECs treated with heavy chain dynein shRNA under the same experimental regimen (Fig. 9B). Although the heavy chain kinesin shRNA prevents plectin from localizing to FA-like structures, the plectin does not seemingly collapse with vimentin to the perinuclear region of the cell and displayed a filamentous pattern throughout the cell interior.
Fig. 9.
Plectin is recruited to FAs in TrHBMECs in a kinesin-dependent manner. (A) TrHBMECs were plated in serum-free medium on PLL-coated coverslips overnight. Untreated cells (left panel) or cells incubated for 30 minutes in medium supplemented with 0.5 mM MnCl2 (right panel) were processed for immunofluorescence microscopy using an antibody against plectin. (B) TrHBMECs electroporated with heavy chain dynein shRNA or heavy chain kinesin shRNA were plated in serum-free medium on PLL-coated coverslips overnight. Untreated cells (left panels) or cells incubated for 30 minutes in medium supplemented with 0.5 mM MnCl2 (right panels) were processed for immunofluorescence microscopy using an antibody against plectin. Scale bars: 10 μm.
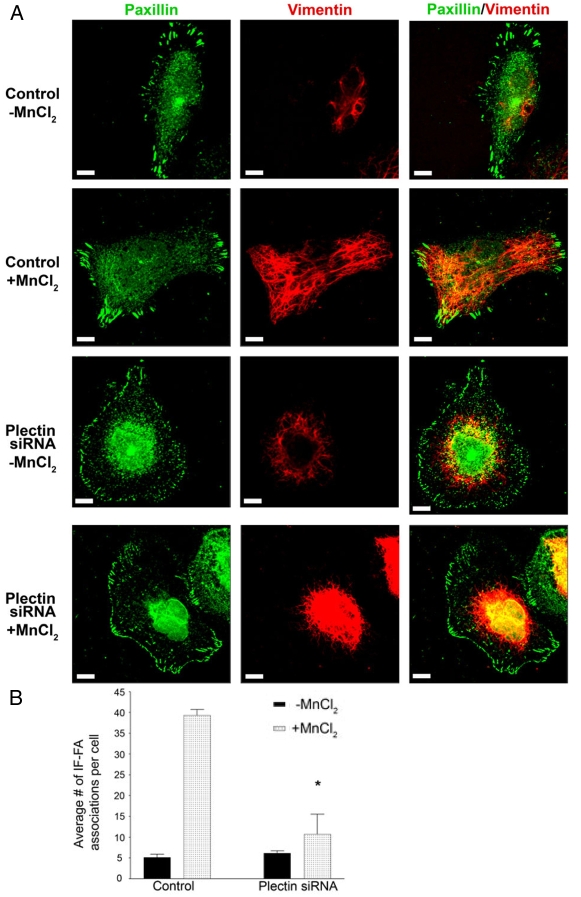
Since plectin relocates from the cell interior to FA-like structures upon the addition of MnCl2, we wanted to assay its role in the recruitment of IFs to FAs. Control or plectin siRNA-treated TrHBMECs were plated in serum-free medium onto PLL-coated coverslips overnight, followed by incubation in MnCl2. Under these conditions, IF-FA association was significantly decreased in plectin siRNA-treated cells compared with controls, when cells were treated with MnCl2 (Fig. 10A,B). Interestingly, plectin siRNA-treated TrHBMECs exhibited no alteration in their microfilament or microtubule networks (supplementary material Fig. S4). These results indicate that plectin is necessary for recruitment of IFs to FAs.
Fig. 10.
Plectin is required for recruitment of IF to FAs. (A) Mock-transfected (control) TrHBMECs or TrHBMECs transfected with plectin siRNA were plated in serum-free medium on PLL-coated coverslips overnight. In addition, cells in the second and fourth rows were incubated for 30 minutes in medium supplemented with 0.5 mM MnCl2. All cells were processed for immunofluorescence microscopy using a combination of antibodies against paxillin (green) and vimentin (red). Overlays are shown in the panels on the right. (B) Quantification of IF-FA association in TrHBMECs transfected with plectin siRNA compared with mock-transfected (control) cells. Upon addition of MnCl2, cells transfected with plectin siRNA had significantly fewer IF-FA associations per cell than control cells (*P<0.01). Error bars represent s.e.m. of three experiments, counting a total of 200 cells. Scale bars: 10 μm.
IFs modulate the functions of FAs
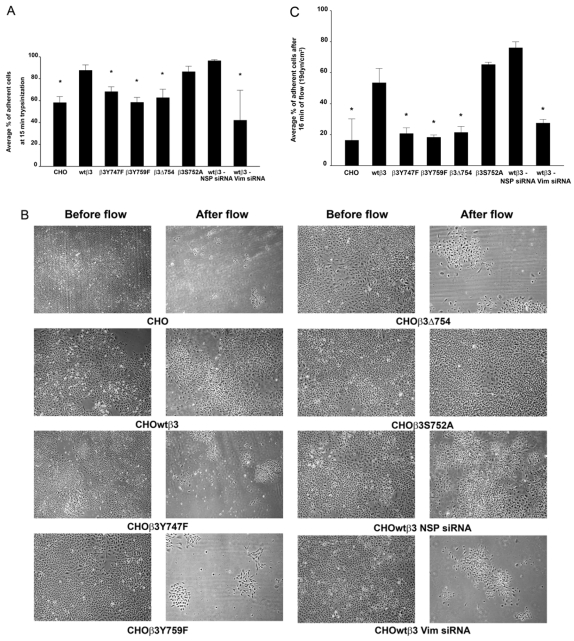
FAs are involved in adhesion to substrate and motility (Wozniak et al., 2004). Therefore, we determined whether IF-FA interaction modulates adhesion strength. This involved testing the strength of adhesion of CHO cells expressing β3 integrin and the various β3 integrin mutants using a trypsinization detachment assay (Ortiz-Urda et al., 2005). CHOwtβ3 and CHOβ3S752A cells exhibited stronger substrate adhesion than parental CHOs, CHOβ3Y747F, CHOβ3Y759F or CHOβ3Δ754 cells (Fig. 11A). To show that these results were a consequence of vimentin IF association with FAs in CHOwtβ3 cells, and not simply β3 integrin expression, CHOwtβ3 cells were transfected with nonspecific control siRNA or vimentin siRNA. We verified the knockdown of vimentin protein expression in these cells by western blot (data not shown). There was no significant difference in adhesion strength of parental CHO, CHOβ3Y747F, CHOβ3Y759F, CHOβ3Δ754 cells and CHOwtβ3 cells transfected with vimentin siRNA (Fig. 11A). However, CHOwtβ3 cells in which vimentin expression has been reduced (CHOwtβ3 Vim siRNA cells) exhibit decreased adhesive strength compared with CHOwtβ3 cells transfected with control siRNA (Fig. 11A).
Fig. 11.
IF-FA interaction enhances cell-substratum adhesion strength of CHO cells. (A) Mock-transfected CHO cells, CHO cells expressing wild-type or mutated β3 integrin, and CHO cells expressing wild-type β3 integrin transfected with control (NSP) or vimentin siRNA were plated into 12-well dishes and allowed to spread for at least 24 hours. They were then treated with trypsin for 15 minutes at room temperature. The percentage of adherent cells was calculated at the end of the treatment protocol. Error bars represent s.e.m. of three experiments. *P<0.03 compared with CHO cells expressing wild-type β3 integrin. (B) The same collection of CHO cells described in A, seeded onto Flexcell glass slides, were subjected to flowing medium (19 dynes/cm2) for 16 minutes. The images show cells before and after flow. (C) The graph represents the percentage of cells, as described in A, remaining adherent to the substrate after they were subjected to flowing medium (19 dynes/cm2) for 16 minutes. Error bars represent s.e.m. of three experiments. *P<0.05 compared with CHO cells expressing wild-type β3 integrin.
As a second measure of adhesion strength, CHO cell lines were subjected to a shear stress of 19 dynes/cm2 for 16 minutes using a flow apparatus. This is within the physiological range of shear stress experienced by endothelial cells in human arteries (DePaola et al., 1999). We chose these parameters because this was the minimal time and force necessary to detach a significant proportion of nontransfected CHO cells (data not shown). At 16 minutes after the initiation of flow, a significantly larger percentage of CHOwtβ3 and CHOβ3S752A cells were retained on the substrate in the flow apparatus than CHO, CHOβ3Y747F, CHOβ3Y759F, CHOβ3Δ754 cells, or CHOwtβ3 Vim siRNA cells under the same flow conditions (Fig. 11B,C). Taken together, the results from these two types of adhesion strength tests demonstrate that IF-FA interactions are important for cell-substrate adhesion strength.
Discussion
The possibility that FAs are involved in IF organization has been suggested by previous work (Tsuruta and Jones, 2003; Windoffer et al., 2006). The results of the current study build on these studies and demonstrate that IF-FA associations at the cell surface are determined by an integrin. Specifically, we have shown that β3 integrin regulates interaction of IFs with the cell surface at the site of FAs in several different cell types. Moreover, our data also show that tyrosine phosphorylation of the β3 integrin cytoplasmic tail is an important regulator of IF association with FAs. These same residues are known to have important roles in ligand binding, migration, regulation of actin cytoskeleton assembly and the ability of αvβ3 integrin to act as a scaffolding molecule for signaling intermediates (Blystone, 2004; Blystone et al., 1997; Boettiger et al., 2001; Jenkins et al., 1998; Schaffner-Reckinger et al., 1998).
In addition to β3 integrin, our data demonstrate that the plakin molecule plectin is required to initiate the interaction of vimentin with FAs. These results are consistent with previous work demonstrating that plectin is enriched at FAs (Gonzales et al., 2001; Seifert et al., 1992; Wiche et al., 1982). However, one surprising aspect of our study is that once the vimentin-FA association has been established, loss of plectin from FAs has no apparent impact on IF-FA interaction. This implies that other molecules associated with both the vimentin network and FAs might stabilize and maintain interactions of IFs with the cell surface. The nature of these molecules is unclear at this time.
Whether plectin binds directly or indirectly to β3 integrin at FAs remains to be determined. Interestingly in this regard, Src, a member of the Src family of protein tyrosine kinases (SFKs), binds constitutively and selectively to β3 integrins through an interaction involving its SH3 domain and the C-terminal region of the β3 cytoplasmic tail (Arias-Salgado et al., 2003). Since plectin also possesses a putative SH3 domain, we speculate that plectin might interact directly with β3 integrin in a similar fashion (Jefferson et al., 2007). However, we cannot rule out the idea that plectin interacts indirectly with β3 integrin via another FA protein.
Our results also indicate that an intact microtubule network and the microtubule motor protein kinesin are necessary for the recruitment of plectin and IFs to FAs, with such recruitment functioning to stabilize FAs. This appears at odds with reports that the microtubule network directs FA disassembly by delivering a `relaxing' factor to substrate adhesive sites (Ezratty et al., 2005; Small et al., 2002). However, our data are consistent with several studies showing that the microtubule cytoskeleton, via microtubule motors, regulates the delivery of IFs to different regions of the cell (Helfand et al., 2004; Prahlad et al., 1998; Yoon et al., 1998). Based on this precedent we suggest that microtubules `transport' IFs to FAs in a kinesin-dependent fashion. Whether kinesin is actively moving IFs along the microtubule network or whether kinesin is merely acting as a linker molecule between IF and microtubules, allowing IF to piggy-back along the microtubule network to FAs, remains to be determined. In this regard, recent data have shed light on the molecules involved in microtubule interaction with FAs. Specifically, the targeting of microtubules to FAs is inhibited in cells lacking the spectroplakin molecule ACF7, which is able to bind both microtubules and actin filaments (Wu et al., 2008). ACF7 might therefore conceivably indirectly mediate the recruitment of IFs to FAs.
A previous report from this laboratory demonstrated that IF association with FAs leads to the assembly of larger FAs than those observed in cells lacking vimentin IF (Tsuruta and Jones, 2003). Indeed, these data, taken together with the results we have presented here, lead us to propose that, under certain circumstances, the microtubule cytoskeleton has a role in stabilizing the structure of FAs by its ability to transport IFs to sites of cell-substratum adhesion. Furthermore, we suggest that the ability of microtubules to either induce disassembly of FAs or stabilize them by mediating their recruitment of IFs is not only tightly regulated but also depends on cellular context and the nature of the forces to which cells are subject (Tsuruta and Jones, 2003).
Our functional assays measuring strength of adhesion demonstrate that integrin-mediated IF association with FAs enhances the CHO strength of adhesion to the substrate. This result mirrors what is known about the function of IF linkage to integrins at the site of hemidesmosomes in certain epithelial cells. This linkage stabilizes cell adhesion to matrix and also mediates cell-matrix signaling (Jones et al., 1998). By analogy, we suggest that the interaction of IFs with β3 integrin at FAs, in cells such as angiogenic endothelial cells, not only enhances their adhesive strength, but also probably contributes to cell growth, survival and differentiation.
In summary, we have presented evidence that β3 integrin and plectin are important regulators of the organization and distribution of the vimentin IF cytoskeleton in several cell types. Moreover, we demonstrated that β3-integrin-mediated recruitment of IFs to the cell surface regulates adhesion strength. In the case of endothelial cells, this has important implications for angiogenesis, where precise control of adhesion is central to blood vessel development and blood vessel maturation.
Materials and Methods
Cell culture and drug treatment
Immortalized human bone marrow endothelial cells (TrHBMECs) were provided by Babette Weksler (Weill Medical College of Cornell University, Ithaca, NY) and Denise Paulin (University of Paris VII and Institute Pasteur, Paris, France) (Schweitzer et al., 1997). TrHBMECs were maintained in DMEM, containing 10% fetal bovine serum, 1% penicillin-streptomycin, 1% L-glutamine and 1× RPMI 1640 vitamins solution unless otherwise stated (Gonzales et al., 2001; Gonzalez et al., 2002). In some experiments, TrHBMECs were plated onto glass coverslips coated with 0.01% poly-L-lysine solution (Sigma, St Louis, MO) in serum-free medium, as previously described (Zaric and Ruegg, 2005). The cells were allowed to spread overnight at 37°C and, in certain cases, the serum-free medium was supplemented with MnCl2 to a final concentration of 0.5 mM. Cells were also treated with 0.1 μM latrunculin B or 0.1 μM colchicine (final concentrations) (Sigma). CHO cells were maintained in F-12K medium, containing 10% fetal bovine serum, 1% penicillin-streptomycin and 1% L-glutamine. CHO cell lines that were transfected with various β3 integrin constructs were kept under selection with 0.07 mM G418 antibiotic (Sigma).
Constructs
cDNA encoding full-length human GFP-tagged β3 integrin subunit and full-length human CFP-tagged vimentin were prepared as detailed elsewhere (Tsuruta et al., 2002; Tsuruta and Jones, 2003). Mutations at Ser752, Tyr747, Tyr759 and the deletion of the nine C-terminal residues in β3 integrin cDNA were generated using the QuickChange XL Site-Directed Mutagenesis Kit from Stratagene (La Jolla, CA) following the procedure of the manufacturer.
RNA interference and transfections
Cells were transfected with vimentin siRNA, 5′-CTGGCACGTCTTGACCTTGAA-3′ (Qiagen, Valencia, CA), β3 integrin siRNAs (Dharmacon, Boulder, CO), β5 integrin siRNAs, or plectin siRNAs (Dharmacon) using TransMessenger Transfection Reagent (Qiagen), following the company protocol (see supplementary material Table S1 for Dharmacon siRNA target sequences). The Dharmacon siRNAs were purchased as pools composed of four siRNAs targeting one gene. As controls, we used nonspecific 5′-fluorescein-conjugated siRNA (Qiagen), siCONTROL Non-Targeting siRNA pool (Dharmacon), or lamin A/C siRNA (Dharmacon). The shRNA against the ubiquitous human kinesin heavy chain will be described elsewhere (H.E.T. et al., unpublished). The shRNA against the dynein heavy chain 1 was a kind gift from Jacob Sznajder (Department of Medicine, Northwestern University, Chicago, IL). The following target sequence was used: 5′-AGATCAGCCTGCCGATTCA-3′. These shRNAs were transfected into cells by electroporation (Tsuruta et al., 2002). DNA encoding full-length human GFP-tagged β3 integrin subunit, the GFP-tagged β3 integrin mutants, and full-length human CFP-tagged vimentin were transfected into cells by electroporation (Tsuruta et al., 2002). In brief, before transfection, cells were trypsinized and resuspended in HEPES-buffered medium at 2×106 cells/ml. The cells were electroporated with DNA at 960 μFD, 186 ohm and 210 V in a BTX Electro Cell Manipulator 600 (BTX, San Diego, CA). Cells treated with siRNAs or shRNAs were used for experiments 72 hours following transfection or electroporation.
Antibodies
Antibodies used were mouse anti-αvβ3 integrin (LM609), rabbit anti-β3 integrin, mouse anti-αv integrin (Chemicon, Temecula, CA), mouse anti-β5 integrin (Calbiochem, San Diego, CA), goat anti-β3 integrin (N-20) (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-FAK [pY397] (Invitrogen, Carlsbad, CA), rabbit monoclonal anti-paxillin (Epitomics, Burlingame, CA), rabbit monoclonal anti-plectin (Epitomics), rabbit anti-dynein heavy chain (Santa Cruz Biotechnology), mouse anti-kinesin heavy chain (Chemicon), rabbit monoclonal anti-actin (Epitomics), rat monoclonal anti-tubulin (Novus Biologicals, Littleton, CO), rabbit anti-mTOR (Cell Signaling Technology, Danvers, MA), mouse anti-vimentin (V9) (Sigma), and rhodamine-conjugated phalloidin (Molecular Probes, Eugene, OR). Secondary antibodies conjugated to fluorochromes or horseradish peroxidase were purchased from Jackson ImmunoResearch (West Grove, PA).
Immunofluorescence microscopy analyses
Cells, grown on glass coverslips, were fixed in 3.7% formaldehyde in PBS for 5 minutes and extracted in 0.5% Triton X-100 in PBS for 7 minutes at 4°C to allow antibody penetration. After washing in PBS, the fixed and extracted cells were incubated with primary antibodies (1:50) at 37° C for 1 hour, washed three times in PBS, and then incubated with the secondary antibody (1:50) for 1 hour at 37° C as detailed elsewhere (Tsuruta et al., 2002). All preparations were viewed with a Zeiss laser-scanning microscope (LSM) 510 confocal microscope using a 63×/1.4 NA or a 100×/1.4 NA objective (Zeiss, Thornwood, NY). Images were exported from the Zeiss LSM Image Browser Version 4,0,0,157 as TIFF files, and figures were generated using Adobe Photoshop software. For magnified insets, images were zoomed 2× in the Zeiss LSM Image Browser before being exported. Localization data were analyzed using the LSM Image Browser and Microsoft Excel (Microsoft Corporation, Redmond, WA). Statistical significance was estimated by Student's t-test. A P-value less than 0.05 was considered significant.
Multi-color TIRFM
In brief, live cells were visualized with an HCX PL APO 100×/1.46 NA objective using a Leica AM TIRF MC system (Leica Microsystems, Bannockburn, IL). Cells were maintained in a Bioptechs apparatus on the stage of the microscope. The pH of the system was kept constant by gassing with 5% CO2 95% air and the temperature was maintained at 37°C. The TIRF angle for the GFP channel was 69.43° and the CFP channel was 67.47°. All images were taken within 90 nm of the substrate. The laser angle into the objective was 90°. Red and green images were acquired, and the raw stacks were split into single channels using Leica software. Post-acquisition processing was performed with Leica Advanced Fluorescence Lite Release Candidate software and Adobe Photoshop. All images were taken using a 100×/1.46 NA objective. Using the Leica software, images are shown at 1× or 2× magnification of the original image.
Protein preparations, SDS-PAGE, western immunoblotting and FACS
Cell populations were solubilized in SDS sample buffer consisting of 8 M urea, 1% SDS in 10 mM Tris-HCl, pH 6.8, and 15% β-mercaptoethanol. Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and processed for immunoblotting. All primary and secondary antibodies were used at a concentration of 1:1000. For FACS analyses of the CHO cells, trypsinized cells were resuspended in PBS containing a 50% dilution of normal goat serum, incubated with the monoclonal antibody LM609 (1:50) for 45 minutes at room temperature, washed with PBS, incubated with Cy5-conjugated goat anti-mouse IgG (1:100) for 45 minutes, washed, resuspended in PBS, and analyzed using a Beckman Coulter Elite PCS sorter (Beckman Coulter). For negative controls, primary antibody was omitted from the above procedure, or nontransfected CHO cells were stained with primary and secondary antibody. For FACS analyses of the TrHBMECs, trypsinized cells were resuspended in complete medium and incubated with LM609, mouse anti-αv integrin, or mouse anti-β5 integrin (1:50) for 30 minutes at room temperature, washed with PBS, incubated with FITC-conjugated goat anti-mouse IgG (1:50) for 30 minutes, washed, resuspended in complete medium, and analyzed using the Beckman Coulter Elite PCS sorter. For a negative control, primary antibody was omitted from the above procedure.
Cell detachment assays
Cellular adhesion strength was measured first by a trypsinization assay using the CHO cell lines. In this assay, 50,000 cells were plated per well in 12-well dishes (Becton Dickinson Labware, Franklin Lakes, NJ). Cells were allowed to spread for at least 24 hours. For siRNA-treated cells, CHOwtβ3 cells were transfected with control non-specific (NSP) 5′-fluorescein-conjugated siRNA or vimentin siRNA 2 days before seeding into 12-well dishes. The numbers of detached cells as a percentage of total were determined in three trials after incubation of the cells for 15 minutes in 0.05% trypsin-EDTA (Invitrogen). In a second assay of adhesion, the CHO cell lines were seeded onto 1 mm Flexcell Streamer™ Culture Slips (Flexcell International, Hillsborough, NC) at 160,000 cells per slide. Cells were allowed to spread for 4 days at 37°C before being assembled into the Flexcell Shear Stress Device (Flexcell International). For siRNA-treated cells, CHOwtβ3 cells were transfected with control NSP siRNA or vimentin siRNA 2 days before seeding onto glass slides. The cells were then exposed to fluid shear stress generated by perfusing culture medium over the cells for a period of 16 minutes. The pH of the system was kept constant by gassing with 5% CO2 95% air and the temperature was maintained at 37°C. A shear stress of 19 dynes/cm2 was used in all experiments. Phase-contrast images of cells were taken before and after exposure to flow. Images were collected on a Photometrics Cool SNAP HQ camera (Roper Scientific, Ottobrunn, Germany). All images of the cells were taken using the 10× objective. Cell numbers were counted in images of six different, randomly selected regions for each condition, before and after flow. The percentage of adherent cells remaining after the flow regimen was then calculated.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/9/1390/DC1
We thank S. Hopkinson for assistance and advice. We are grateful to the NIH for grant support (HL067016 and AR054184 to J.C.R.J.). R.B. was supported by an AHA predoctoral training award (0610076Z). Deposited in PMC for release after 12 months.
References
- Arias-Salgado, E. G., Lizano, S., Sarkar, S., Brugge, J. S., Ginsberg, M. H. and Shattil, S. J. (2003). Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl. Acad. Sci. USA 100, 13298-13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod, D. (1989). Total internal reflection fluorescence microscopy. Methods Cell Biol. 30, 245-270. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A. D., Tint, I. S. and Svitkina, T. M. (1987). Association of intermediate filaments with vinculin-containing adhesion plaques of fibroblasts. Cell Motil. Cytoskeleton 8, 274-283. [DOI] [PubMed] [Google Scholar]
- Blystone, S. D. (2004). Integrating an integrin: a direct route to actin. Biochim. Biophys. Acta 1692, 47-54. [DOI] [PubMed] [Google Scholar]
- Blystone, S. D., Lindberg, F. P., Williams, M. P., McHugh, K. P. and Brown, E. J. (1996). Inducible tyrosine phosphorylation of the β3 integrin requires the αv integrin cytoplasmic tail. J. Biol. Chem. 271, 31458-31462. [DOI] [PubMed] [Google Scholar]
- Blystone, S. D., Williams, M. P., Slater, S. E. and Brown, E. J. (1997). Requirement of integrin β3 tyrosine 747 for β3 tyrosine phosphorylation and regulation of αvβ3 avidity. J. Biol. Chem. 272, 28757-28761. [DOI] [PubMed] [Google Scholar]
- Boettiger, D., Huber, F., Lynch, L. and Blystone, S. (2001). Activation of αvβ3-vitronectin binding is a multistage process in which increases in bond strength are dependent on Y747 and Y759 in the cytoplasmic domain of β3. Mol. Biol. Cell 12, 1227-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. P., Djaffar, I., Pidard, D., Steiner, B., Cieutat, A. M., Caen, J. P. and Rosa, J. P. (1992). Ser-752->Pro mutation in the cytoplasmic domain of integrin β3 subunit and defective activation of platelet integrin αIIbβ3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc. Natl. Acad. Sci. USA 89, 10169-10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaola, N., Davies, P. F., Pritchard, W. F., Jr, Florez, L., Harbeck, N. and Polacek, D. C. (1999). Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc. Natl. Acad. Sci. USA 96, 3154-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckes, B., Dogic, D., Colucci-Guyon, E., Wang, N., Maniotis, A., Ingber, D., Merckling, A., Langa, F., Aumailley, M., Delouvee, A. et al. (1998). Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 111, 1897-1907. [DOI] [PubMed] [Google Scholar]
- Ezratty, E. J., Partridge, M. A. and Gundersen, G. G. (2005). Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581-590. [DOI] [PubMed] [Google Scholar]
- Faccio, R., Novack, D. V., Zallone, A., Ross, F. P. and Teitelbaum, S. L. (2003). Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by β3 integrin. J. Cell Biol. 162, 499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailit, J. and Ruoslahti, E. (1988). Regulation of the fibronectin receptor affinity by divalent cations. J. Biol. Chem. 263, 12927-12932. [PubMed] [Google Scholar]
- Giancotti, F. G. and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028-1032. [DOI] [PubMed] [Google Scholar]
- Gonzales, M., Weksler, B., Tsuruta, D., Goldman, R. D., Yoon, K. J., Hopkinson, S. B., Flitney, F. W. and Jones, J. C. R. (2001). Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell 12, 85-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, A. M., Gonzales, M., Herron, G. S., Nagavarapu, U., Hopkinson, S. B., Tsuruta, D. and Jones, J. C. R. (2002). Complex interactions between the laminin α4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 99, 16075-16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand, B. T., Chang, L. and Goldman, R. D. (2004). Intermediate filaments are dynamic and motile elements of cellular architecture. J. Cell Sci. 117, 133-141. [DOI] [PubMed] [Google Scholar]
- Homan, S. M., Martinez, R., Benware, A. and LaFlamme, S. E. (2002). Regulation of the association of α6β4 with vimentin intermediate filaments in endothelial cells. Exp. Cell Res. 281, 107-114. [DOI] [PubMed] [Google Scholar]
- Hughes, P. E., O'Toole, T. E., Ylanne, J., Shattil, S. J. and Ginsberg, M. H. (1995). The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J. Biol. Chem. 270, 12411-12417. [DOI] [PubMed] [Google Scholar]
- Hynes, R. O. (2007). Cell-matrix adhesion in vascular development. J. Thromb. Haemost. 5Suppl. 1, 32-40. [DOI] [PubMed] [Google Scholar]
- Hynes, R. O., Bader, B. L. and Hodivala-Dilke, K. (1999). Integrins in vascular development. Braz. J. Med. Biol. Res. 12, 501-510. [DOI] [PubMed] [Google Scholar]
- Jefferson, J. J., Ciatto, C., Shapiro, L. and Liem, R. K. (2007). Structural analysis of the plakin domain of bullous pemphigoid antigen1 (BPAG1) suggests that plakins are members of the spectrin superfamily. J. Mol. Biol. 366, 244-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, A. L., Nannizzi-Alaimo, L., Silver, D., Sellers, J. R., Ginsberg, M. H., Law, D. A. and Phillips, D. R. (1998). Tyrosine phosphorylation of the β3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J. Biol. Chem. 273, 13878-13885. [DOI] [PubMed] [Google Scholar]
- Jones, J. C. R., Hopkinson, S. B. and Goldfinger, L. E. (1998). Structure and assembly of hemidesmosomes. BioEssays 20, 488-494. [DOI] [PubMed] [Google Scholar]
- Kreis, S., Schonfeld, H. J., Melchior, C., Steiner, B. and Kieffer, N. (2005). The intermediate filament protein vimentin binds specifically to a recombinant integrin α2/β1 cytoplasmic tail complex and co-localizes with native α2/β1 in endothelial cell focal adhesions. Exp. Cell Res. 305, 110-121. [DOI] [PubMed] [Google Scholar]
- Krylyshkina, O., Anderson, K. I., Kaverina, I., Upmann, I., Manstein, D. J., Small, J. V. and Toomre, D. K. (2003). Nanometer targeting of microtubules to focal adhesions. J. Cell Biol. 161, 853-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Urda, S., Garcia, J., Green, C. L., Chen, L., Lin, Q., Veitch, D. P., Sakai, L. Y., Lee, H., Marinkovich, M. P. and Khavari, P. A. (2005). Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science 307, 1773-1776. [DOI] [PubMed] [Google Scholar]
- Prahlad, V., Yoon, M., Moir, R. D., Vale, R. D. and Goldman, R. D. (1998). Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143, 159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp, P. A. and Little, C. D. (2001). Integrins in vascular development. Circ. Res. 89, 566-572. [DOI] [PubMed] [Google Scholar]
- Schaffner-Reckinger, E., Gouon, V., Melchior, C., Plancon, S. and Kieffer, N. (1998). Distinct involvement of β3 integrin cytoplasmic domain tyrosine residues 747 and 759 in integrin-mediated cytoskeletal assembly and phosphotyrosine signaling. J. Biol. Chem. 273, 12623-12632. [DOI] [PubMed] [Google Scholar]
- Schweitzer, K. M., Vicart, P., Delouis, C., Paulin, D., Dräger, A. M., Langenhuijsen, M. M. A. C. and Weksler, B. B. (1997). Characterization of a newly established human bone marrow endothelial cell line: Distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab. Invest. 76, 25-36. [PubMed] [Google Scholar]
- Seifert, G. J., Lawson, D. and Wiche, G. (1992). Immunolocalization of the intermediate filament-associated protein plectin at focal contacts and actin stress fibers. Eur. J. Cell Biol. 59, 138-147. [PubMed] [Google Scholar]
- Simon, K. O. and Burridge, K. (1994). Interactions between integrins and the cytoskeleton: structure and regulation. In Integrins: Molecular and Biological Responses to the Extracellular Matrix (ed. D. A. Cheresh and R. P. Mecham), pp. 49-78. San Diego, CA: Academic Press.
- Small, J. V., Geiger, B., Kaverina, I. and Bershadsky, A. (2002). How do microtubules guide migrating cells? Nat. Rev. Mol. Cell. Biol. 3, 957-964. [DOI] [PubMed] [Google Scholar]
- Spurny, R., Abdoulrahman, K., Janda, L., Runzler, D., Kohler, G., Castanon, M. J. and Wiche, G. (2007). Oxidation and nitrosylation of cysteines proximal to the intermediate filament (IF)-binding site of plectin: effects on structure and vimentin binding and involvement in IF collapse. J. Biol. Chem. 282, 8175-8187. [DOI] [PubMed] [Google Scholar]
- Steyer, J. A. and Almers, W. (2001). A real-time view of life within 100 nm of the plasma membrane. Nat. Rev. Mol. Cell. Biol. 2, 268-275. [DOI] [PubMed] [Google Scholar]
- Toomre, D. and Manstein, D. J. (2001). Lighting up the cell surface with evanescent wave microscopy. Trends Cell Biol. 11, 298-303. [DOI] [PubMed] [Google Scholar]
- Tsuruta, D. and Jones, J. C. (2003). The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J. Cell. Sci. 116, 4977-4984. [DOI] [PubMed] [Google Scholar]
- Tsuruta, D., Gonzales, M., Hopkinson, S. B., Otey, C., Khuon, S., Goldman, R. D. and Jones, J. C. (2002). Microfilament-dependent movement of the β3 integrin subunit within focal contacts of endothelial cells. FASEB J. 16, 866-868. [DOI] [PubMed] [Google Scholar]
- Wiche, G., Hermann, H., Leichtfried, F. and Pytela, R. (1982). Plectin: a high-molecular weight cytoskeletal polypeptide component that copurifies with intermediate filaments of the vimentin type. Cold Spring Harb. Symp. Quant. Biol. 46, 475-482. [DOI] [PubMed] [Google Scholar]
- Windoffer, R., Kolsch, A., Woll, S. and Leube, R. E. (2006). Focal adhesions are hotspots for keratin filament precursor formation. J. Cell Biol. 173, 341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, L., Abrahamsberg, C. and Wiche, G. (2008). Plectin isoform 1b mediates mitochondrion-intermediate filament network linkage and controls organelle shape. J. Cell Biol. 181, 903-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak, M. A., Modzelewska, K., Kwong, L. and Keely, P. J. (2004). Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 1692, 103-119. [DOI] [PubMed] [Google Scholar]
- Wu, X., Kodama, A. and Fuchs, E. (2008). ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell 135, 137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylanne, J., Chen, Y., O'Toole, T. E., Loftus, J. C., Takada, Y. and Ginsberg, M. H. (1993). Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J. Cell Biol. 122, 223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, M., Moir, R. D., Prahlad, V. and Goldman, R. D. (1998). Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, 147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaric, J. and Ruegg, C. (2005). Integrin-mediated adhesion and soluble ligand binding stabilize COX-2 protein levels in endothelial cells by inducing expression and preventing degradation. J. Biol. Chem. 280, 1077-1085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.