Abstract
Background
Low serum magnesium levels may cause fatal ventricular arrhythmias. However, their long-term effects on mortality and morbidity in chronic heart failure patients are relatively unknown.
Methods
We studied 1569 chronic systolic and diastolic heart failure patients with normal sinus rhythm who participated in the Digitalis Investigation Group trial and had serum magnesium data available at one month. Of these, 741 patients had normal (>2 mEq/L) and 828 had low (≤2 mEq/L) serum magnesium levels. Propensity scores for having low serum magnesium levels were calculated for each patient using a non-parsimonious multivariable logistic regression model, and were used to match 560 (76%) low-magnesium patients with 560 normal-magnesium patients. Effects of low-magnesium on mortality and hospitalization during a mean follow-up of 36 months were assessed using matched Cox regression analyses.
Results
All-cause mortality occurred in 156 (rate, 915/10,000 person-years) normal- magnesium and 171 (rate, 1034/10,000 person-years) low-magnesium patients, respectively, during 1704 and 1653 years of follow-up (hazard ratio, 1.23; 95% confidence interval, 0.97–1.57; P=0.089). Cardiovascular mortality occurred in 110 (rate, 646/10,000 person-years) normal-magnesium and 133 (rate, 805/10,000 person-years) low-magnesium patients (hazard ratio, 1.38, 95% confidence interval, 1.04–1.83, P=0.024). Hazard ratios and 95% confidence intervals for all-cause and cardiovascular hospitalizations were respectively 1.18 (0.99–1.42; P=0.068) and 1.14 (0.94–1.39; P=0.182).
Conclusions
In a propensity-matched population of ambulatory chronic heart failure patients who were balanced in all measured baseline covariates, serum magnesium level 2 mEq/L or less was associated with increased cardiovascular mortality, but had no association with cardiovascular hospitalization.
Keywords: Heart failure, magnesium, mortality, hospitalization, propensity score
1. Introduction
Magnesium is an important electrolyte that plays an essential role in normal cardiac function, and low serum magnesium levels may be associated with cardiac arrhythmias and sudden death [1–3]. The activation of renin-angiotensin-aldosterone system and the use of diuretics are associated with depletion of serum potassium and magnesium in heart failure patients [4–6]. Studies of the effect of serum magnesium on outcomes in heart failure are limited by traditional regression-based risk adjustment, and inconsistent findings [6–9]. The objective of this study was to determine the long-term effects of low serum magnesium levels on mortality and hospitalization in a propensity-matched chronic heart failure population.
2. Materials and methods
2.1. Study patients
In the Digitalis Investigation Group (DIG) trial, 7788 ambulatory chronic heart failure patients (6800 had left ventricular ejection fraction 45% or less) in normal sinus rhythm, enrolled from 302 centers in the United States and Canada between 1991–1993, were randomized to receive digoxin or placebo [10, 11]. Most of these patients were receiving angiotensin-converting enzyme inhibitors and diuretics. Data on beta-blocker use were not collected in the DIG trial. The focus of this study is a random subset of 1569 DIG participants with data on serum magnesium measured in a central laboratory based on blood drawn at one month after randomization [12].
2.2. Baseline serum magnesium
Data on serum magnesium levels were collected from a subset of DIG population at one month after randomization. Mean (±SD) serum magnesium level was 2.04 (±0.21) mEq/L with a range of 1.10 to 2.90 mEq/L. Patients were categorized to have low (≤2 mEq/L) or normal (>2 mEq/L) serum magnesium levels. Of the 1569 patients in the current analysis, 828 (53%) had serum magnesium ≤2 mEq/L.
2.3. Study design: propensity score matching
The propensity score is the conditional probability of receiving an exposure (e.g. having low magnesium) given a set of measured covariates [13–16]. Propensity score matching can be used to assemble a risk-adjusted study cohort in which two groups of patients are well balanced in all measured baseline covariates. As in randomized clinical trials, the investigators remain blinded to study outcomes during the design phase of the study. In a propensity-matched study, the imbalances in baseline covariates before matching and balances achieved after matching can be objectively measured and presented in tabular and graphic formats. Finally, propensity score-matching generally provides relatively conservative estimates of the true association [17].
2.4. Estimation of propensity scores
Propensity scores for low serum magnesium for each of the 1569 patients were calculated using a non-parsimonious multivariable logistic regression model. We used all available baseline characteristics as shown in Table 1 (except for the derived variables of estimated glomerular filtration rate and chronic kidney disease) and clinically plausible interactions (between age and creatinine, age and diuretic use, and creatinine and diuretic use) in our model for the estimation of propensity scores [18–22]. Because our propensity model was used solely to describe the characteristics of patients with and without low magnesium in this study, rather than to predict the likelihood of low magnesium across other samples, the relevant measure of the model’s success was assessed by the quality of the matching using absolute standardized differences, as described below [16].
Table 1.
Baseline patient characteristics by serum magnesium levels, before and after propensity score matching
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| N (%)/mean (±SD) | Serum magnesium >2 mEq/L (N =741) | Serum magnesium ≤2 mEq/L (N =828) | P value | Serum magnesium >2 mEq/L (N =560) | Serum magnesium ≤2 mEq/L (N =560) | P value |
| Age, years | 65.1 (±10.6) | 62.4 (±10.7) | <0.0001 | 63.3 (±10.5) | 63.5 (±10.6) | 0.835 |
| Women | 177 (24%) | 219 (26%) | 0.243 | 139 (25%) | 140 (25%) | 0.945 |
| Non-whites | 73 (10%) | 128 (16%) | 0.001 | 60 (11%) | 65 (12%) | 0.635 |
| Body mass index, kg/m2 | 26.6 (±4.3) | 28.0 (±4.9) | <0.0001 | 27.1 (±5.2) | 27.2 (±5.1) | 0.706 |
| HF duration, months | 34 (±41) | 27 (±34) | 0.001 | 30 (±34) | 30 (±37) | 0.750 |
| Primary cause of heart failure | ||||||
| Ischemic | 560 (76%) | 559 (68%) | 406 (73%) | 412 (74%) | ||
| Hypertensive | 66 (9%) | 107 (13%) | 0.004 | 56 (10%) | 53 (10%) | 0.979 |
| Idiopathic | 75 (10%) | 113 (14%) | 68 (12%) | 65 (12%) | ||
| Others | 40 (5%) | 49 (6%) | 30 (5%) | 30 (5%) | ||
| Prior myocardial infarction | 507(68%) | 504 (61%) | 0.002 | 367 (66%) | 368 (66%) | 0.950 |
| Current angina pectoris | 214 (29%) | 235 (28%) | 0.827 | 164 (29%) | 166 (29%) | 0.896 |
| Hypertension | 316 (43%) | 429 (52%) | <0.0001 | 250 (45%) | 244 (44%) | 0.718 |
| Diabetes mellitus | 187 (25%) | 284 (34%) | <0.0001 | 155 (28%) | 163 (29%) | 0.596 |
| Chronic kidney disease* | 411 (56%) | 317 (38%) | <0.0001 | 262 (47%) | 261 (45%) | 0.509 |
| Medications | ||||||
| Digoxin (pre-trial use) | 358 (48%) | 343 (41%) | 0.006 | 251 (45%) | 260 (46%) | 0.589 |
| Digoxin (by randomization) | 340 (46%) | 432 (52%) | 0.013 | 279 (50%) | 280 (50%) | 0.952 |
| ACE inhibitors | 690 (93%) | 781 (94%) | 0.324 | 525 (94%) | 525 (94%) | 1.000 |
| Hydralazine and Nitrates | 12 (2%) | 6 (1%) | 0.097 | 5 (1%) | 6 (1%) | 0.762 |
| Non-potassium-sparing diuretics | 598 (81%) | 601 (73%) | <0.0001 | 426 (76%) | 428 (76%) | 0.888 |
| Potassium-sparing diuretics | 63 (9%) | 56 (7%) | 0.194 | 38 (7%) | 40 (7%) | 0.814 |
| Potassium supplement | 227 (31%) | 250 (30%) | 0.850 | 175 (31%) | 174 (31%) | 0.949 |
| Symptoms and signs of HF | ||||||
| Dyspnea at rest | 148 (20%) | 182 (22%) | 0.330 | 117 (21%) | 121 (22%) | 0.770 |
| Dyspnea on exertion | 542 (73%) | 636 (77%) | 0.094 | 422 (75%) | 419 (75%) | 0.836 |
| Jugular venous distension | 101 (14%) | 113 (14%) | 0.992 | 75 (13%) | 70 (13%) | 0.656 |
| Third heart sound | 183 (25%) | 207 (25%) | 0.890 | 134 (24%) | 139 (25%) | 0.728 |
| Pulmonary râles | 126 (17%) | 128 (16%) | 0.407 | 78 (14%) | 83 (15%) | 0.670 |
| Lower extremity edema | 149 (20%) | 191 (23%) | 0.155 | 111 (20%) | 116 (21%) | 0.710 |
| NYHA functional class | ||||||
| I | 115 (16%) | 124 (15%) | 88 (16%) | 82 (15%) | ||
| II | 400 (54%) | 458 (55%) | 0.770 | 306 (55%) | 305 (55%) | 0.851 |
| III | 218 (29%) | 233 (28%) | 159 (28%) | 163 (29%) | ||
| IV | 8 (1%) | 13 (2%) | 7 (1%) | 10 (2%) | ||
| Heart rate, rate per minute | 77 (±12) | 79 (±14) | 0.024 | 78 (±12) | 78 (±13) | 0.468 |
| Systolic blood pressure, mm Hg | 126 (±20) | 129 (±20) | 0.021 | 127 (±20) | 127 (±20) | 0.868 |
| Diastolic blood pressure, mm Hg | 75 (±11) | 76 (±11) | 0.004 | 75 (±11) | 75 (±11) | 0.696 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 93 (13%) | 97 (12%) | 0.612 | 61 (11%) | 63 (11%) | 0.849 |
| CT ratio > 0.5 | 427 (58%) | 499 (60%) | 0.288 | 317 (57%) | 328 (59%) | 0.506 |
| Serum concentrations | ||||||
| Creatinine, mg/dL | 1.4 (±0.4) | 1.2 (±0.3) | <0.0001 | 1.3 (±0.3) | 1.3 (±0.3) | 0.705 |
| Potassium, mEq/L | 4.4 (±0.4) | 4.4 (±0.4) | 0.881 | 4.4 (±0.4) | 4.4 (±0.4) | 0.856 |
| Estimated glomerular filtration rate, ml/min per 1.73 m2 | 60 (±22) | 67 (±19) | <0.0001 | 64 (±22) | 64 (±19) | 0.881 |
| Left ventricular ejection fraction, percent | 32 (±112) | 32 (±12) | 0.455 | 32 (±12) | 32 (±12) | 0.636 |
ACE=angiotensin-converting enzyme; CT=cardiothoracic; LV=left ventricular; NYHA=New York Heart Association
Defined as estimated glomerular filtration rate <60 ml/min per 1.73 m2
2.5. Propensity matching and assembly of study cohort
Using a greedy matching protocol, we matched each low-magnesium patient to a normal-magnesium patient who had similar propensity scores to five, four, three, two and one decimal places in five repeated steps [18–22]. Specifically, we first multiplied the raw propensity scores by 100,000, and then rounded it to the nearest value divisible by 0.25. For example, propensity scores of 0.12345678 and 0.12345345 respectively for a normal-magnesium and a low-magnesium patient would be converted to respectively 12345.68 and 12345.45 by multiplying by 10,0000. Both would then be rounded to 12345.50 and matched, and the matched pair would then be removed from the file. When no further five-decimal matches are available, in the second step, the raw propensity scores would be multiplied by 10,000. This process was repeated three more times, each time, multiplying the propensity scores by 1000, 100 and finally 10. In all, we matched 560 (68%) low-magnesium patients with 560 normal-magnesium patients.
2.6. Assessment of covariate balance
We estimated absolute standardized differences to evaluate pre- and post-match balance in the distribution of baseline covariates between patients with normal and low magnesium [18–24]. Absolute standardized differences directly quantify biases in the means (or proportions) of covariates across the groups, and expressed as percentages of the pooled standard deviations. An absolute standardized difference of 0% on any measured covariate indicates no residual bias for that covariate, and any differences below 10% suggest inconsequential residual bias.
2.7. Study outcomes
The primary outcomes were all-cause mortality and all-cause hospitalization. We also studied mortality and hospitalizations due to cardiovascular causes and worsening heart failure. DIG participants were followed for a mean of 36 months and vital status data were complete for 99% of the patients [25].
2.8. Statistical analysis
We used Kaplan-Meier plots and matched Cox regression analysis to estimate associations of low-magnesium with total and cause-specific deaths and hospitalizations in the matched cohort. A matched Cox regression analysis is essentially a stratified analysis that compares survival within each pair of matched patients as a separate stratum and then computes the overall hazard ratio from the individual hazard ratios [26]. We examined the assumption of proportional hazards by a visual examination of the log (minus log) curves [27]. We then repeated our analyses using serum magnesium as a continuous variable. To determine whether the loss of sample size in the matching process affected our results, we estimated the effect of low magnesium on outcomes in the full pre-match cohort of 1569 patients adjusting for raw propensity scores.
2.9. Sensitivity analyses
To determine the effect of a potential unmeasured confounder on our findings, we conducted a formal sensitivity analysis to quantify the degree of a hidden bias that would need to be present to invalidate our main conclusions [19, 22, 28, 29].
3. Results
3.1. Patient characteristics
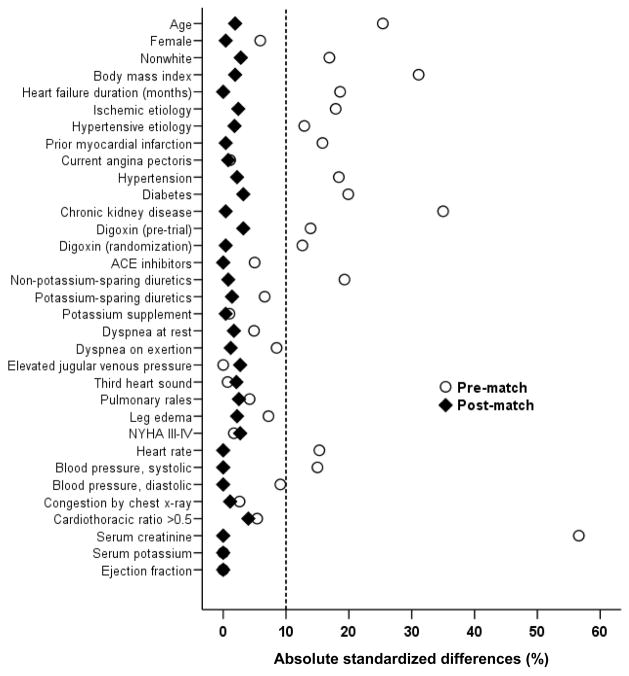
The mean (±SD) age of the 1120 matched patients was 63.5 (±10.6) years, (51% ≥65 years), 25% were women and 11% were non-whites. Before matching, low-magnesium patients were more likely to be younger, non-whites, have hypertension, and less likely to have chronic kidney disease and be receiving non-potassium sparing diuretics (Table 1). After matching, low-magnesium patients were similar in regards to all measured baseline covariates (Table 1 and Figure 1). Post-match absolute standardized differences for all measured covariates were <10% (most were <5%), demonstrating substantial improvement in covariate balance across the treatment groups (Figure 1).
Figure 1.
Absolute standardized differences in covariates between patients with serum magnesium ≤2 mEq/L and >2 mEq/L, before and after propensity score matching
3.2. Magnesium and mortality
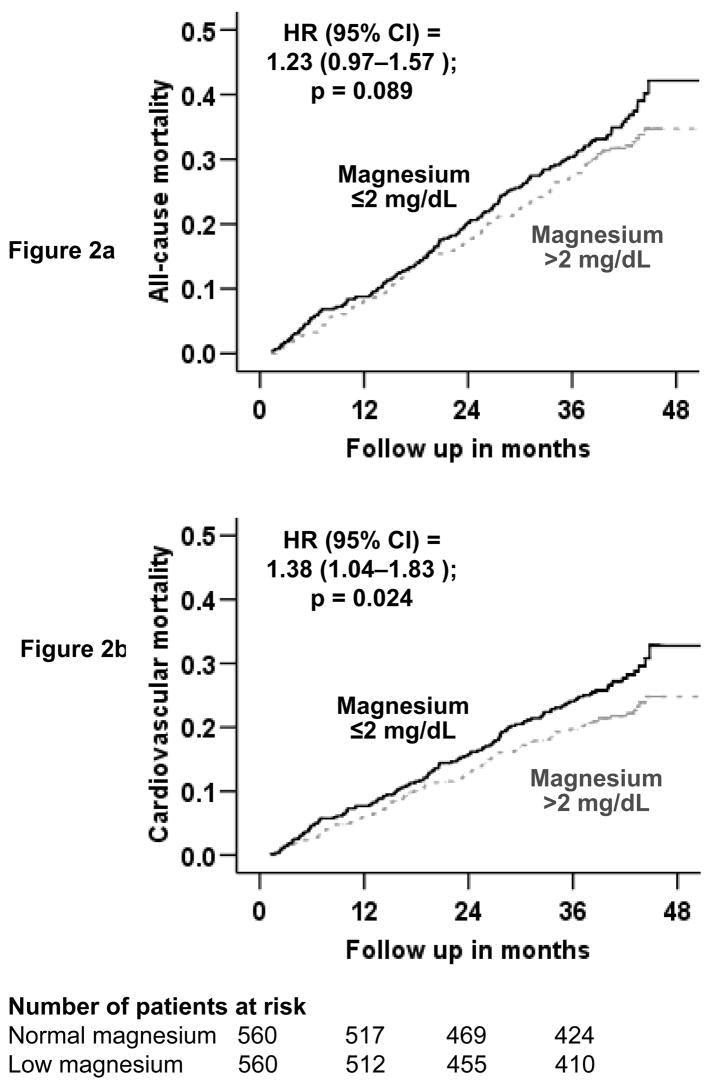
During a mean follow-up of 36 months, 327 (29%) patients in the matched cohort died from all-causes, 243 (22%) due to cardiovascular causes and 93 (8%) due to worsening heart failure. Kaplan-Meier survival curves for all-cause and cardiovascular mortality are displayed in Figure 2. All-cause mortality occurred in 28% normal-magnesium (rate, 915/10,000 person-years) and 31% low-magnesium (rate, 1034/10,000 person-years) patients respectively during 1704 and 1653 years of follow-up (hazard ratio when low-magnesium patients were compared with normal-potassium patients, 1.23; 95% confidence interval, 0.97–1.57; P=0.089; Table 2).
Figure 2.
Kaplan-Meier plots for mortality due to (a) all-causes, and (b) cardiovascular causes
Table 2.
Cause-specific mortalities in heart failure patients for serum magnesium ≤2 mEq/L
| Rate/10,000 person-years follow up** (events/follow-up in years) | Rate difference* (/10,000 person-years) | Matched hazard ratio (95% confidence interval) † | P value | ||
|---|---|---|---|---|---|
| Serum magnesium >2 mEq/L (N = 560) | Serum magnesium ≤2 mEq/L (N = 560) | ||||
| All-cause | 915 (156/1704) | 1034 (171/1653) | + 119 | 1.23 (0.97–1.57) | 0.089 |
| Cardiovascular | 646 (110/1704) | 805 (133/1653) | + 159 | 1.38 (1.04–1.83) | 0.024 |
| Worsening heart failure‡ | 252 (43/1704) | 302 (50/1653) | + 50 | 1.42 (0.90–2.25) | 0.135 |
| Other cardio-vascular§ | 393 (67/1704) | 502 (83/1653) | + 109 | 1.36 (0.95–1.94) | 0.090 |
| Non-cardio-vascular¶ | 164 (28/1704) | 175 (29/1653) | + 11 | 1.04 (0.60–1.82) | 0.886 |
| Unknown | 106 (18/1704) | 54 (9/1653) | − 52 | 0.62 (0.26–1.49) | 0.280 |
Absolute rate differences were calculated by subtracting the rates of death in the low-magnesium group from the rates of death in the normal magnesium group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from matched Cox proportional-hazards models.
This category includes patients who died from worsening heart failure, even if the final event was an arrhythmia.
This category includes deaths presumed to result from arrhythmia without evidence of worsening heart failure and deaths due to atherosclerotic coronary disease, bradyarrhythmias, low-output states, and cardiac surgery.
This category includes deaths due to stroke, embolism, peripheral vascular disease, vascular surgery, and carotid endarterectomy.
Total follow up period is same for all cause-specific mortalities as for all-cause mortality
Cardiovascular mortality occurred in 20% normal-magnesium (rate, 646/10,000 person-years) and 24% low-magnesium (rate, 805/10,000 person-years) patients (HR 1.38; 95% CI 1.04–1.83; P=0.024; Table 2). Mortality due to progressive heart failure occurred in 8% normal-magnesium (rate, 252/10,000 person-years) and 9% low-magnesium (rate, 302/10,000 person-years) patients (hazard ratio, 1.42; 95% confidence interval, 0.95–2.25; P=0.135; Table 2). Associations of low magnesium and other cause-specific mortalities are displayed in Table 2.
The results of our sensitivity analysis suggest that the association of low magnesium and cardiovascular mortality is moderately sensitive to a potential unmeasured confounder. An unmeasured binary covariate that would increase the odds of low magnesium by only 4.3% (two-tailed p = 0.024) could potentially explain away our findings of low-magnesium associated cardiovascular mortality. However, for an unmeasured covariate to act as a confounder, it must be strongly correlated with cardiovascular mortality and may not be strongly correlated with any of the covariates displayed in Table 1 [30].
In the full pre-match cohort of 1569 patients, 355 (23%) patients died from cardiovascular causes: 175 (24%) and 180 (22%) respectively in the normal- and low-magnesium groups (propensity-adjusted hazard ratio, 1.12; 95% confidence interval, 0.90–1.40; p =0.303). When serum magnesium was used as a continuous variable, the propensity score adjusted hazard ratio was 0.89 (95% confidence interval, 0.51–1.55; p =0.685).
3.3. Magnesium and hospitalization
All-cause hospitalizations occurred in 746 (67%) patients, hospitalizations due to cardiovascular causes in 585 (52%) and those to worsening heart failure in 315 (28%) patients. All-cause hospitalizations occurred in 65% normal-magnesium (rate, 3596/10,000 person-years) and 68% low-magnesium (rate, 4146/10,000 person-years) patients respectively during 1015 and 919 years of follow-up (hazard ratio, 1.18; 95% confidence interval, 0.99–1.42; P=0.068; Table 3). Associations of low magnesium and other cause-specific hospitalizations are displayed in Table 3.
Table 3.
Cause-specific hospitalizations in heart failure patients for serum magnesium ≤2 mEq/L
| Cause for hospitalization * | Rate/10,000 person-years follow up (events/follow-up in years) | Rate difference (per 10,000 person-years) † | Matched hazard ratio (95% confidence interval) ‡ | P value | |
|---|---|---|---|---|---|
| Serum magnesium >2 mEq/L (N =560) | Serum magnesium ≤2 mEq/L (N =560) | ||||
| All-cause | 3596 (365/1015) | 4146 (381/919) | + 550 | 1.18 (0.99–1.42) | 0.068 |
| Cardiovascular | 2361 (284/1203) | 2704 (301/1113) | + 343 | 1.14 (0.94–1.39) | 0.182 |
| Worsening heart failure | 995 (147/1478) | 1203 (168/1396) | + 208 | 1.21 (0.94–1.56) | 0.141 |
| Ventricular arrhythmia, cardiac arrest | 119 (20/1678) | 111 (18/1628) | − 8 | 1.00 (0.52–1.92) | 1.000 |
| SV arrhythmias § | 132 (22/1671) | 149 (24/1613) | + 17 | 1.24 (0.65–2.34) | 0.517 |
| AV block, bradyarrhythmia | 18 (3/1699) | 12 (2/1648) | − 6 | 1.00 (0.14–7.10) | 1.000 |
| Suspected digoxin toxicity | 18 (3/1697) | 30 (5/1645) | + 12 | 1.67 (0.40–6.97) | 0.484 |
| Myocardial infarction | 205 (34/1659) | 216 (35/1619) | + 11 | 0.90 (0.54–1.51) | 0.691 |
| Unstable angina | 534 (82/1536) | 498 (75/1506) | − 36 | 0.97 (0.69–1.37) | 0.862 |
| Stroke | 125 (21/1678) | 179 (29/1617) | + 54 | 1.59 (0.87–2.91) | 0.135 |
| Coronary revascularization¶ | 90 (15/1674) | 86 (14/1624) | − 4 | 1.09 (0.48–2.47) | 0.835 |
| Cardiac transplantation | 6 (1/1701) | 30 (5/1646) | + 24 | 5.00 (0.58–42.80) | 0.142 |
| Other cardiovascular ** | 403 (64/1588) | 446 (68/1523) | + 44 | 1.14 (0.78–1.65) | 0.507 |
| Respiratory infection | 241 (40/1657) | 331 (52/1570) | + 90 | 1.34 (0.85–2.12) | 0.206 |
| Other non-cardiovascular | 1201 (167/1391) | 1520 (198/1302) | + 320 | 1.33 (1.04–1.69) | 0.021 |
| Unspecified | 12 (2/1698) | 24 (4/1645) | + 12 | 2.00 (0.37–10.92) | 0.423 |
| Number of hospitalizations | 9714 (986/1015) | 11893 (1093/919) | + 2179 | ||
Data shown include the first hospitalization of each patient due to each cause.
Absolute differences were calculated by subtracting the percentage of patients hospitalized in the low- magnesium group from the percentage of patients hospitalized in the normal magnesium group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from matched Cox proportional-hazards models that used the first hospitalization of each patient for each reason.
Supraventricular (SV) arrhythmias include Atrioventricular (AV) block and bradyarrhythmias
This category includes coronary-artery bypass grafting and percutaneous transluminal coronary angioplasty
This category includes embolism, venous thrombosis, peripheral vascular disease, hypertension, other vascular surgery, cardiac catheterization, other types of catheterization, pacemaker implantation, installation of automatic implantable cardiac defibrillator, electrophysiologic testing, transplant-related evaluation, nonspecific chest pain, atherosclerotic heart disease, hypotension, orthostatic hypotension, and valve operation
4. Discussion
The results of the current analysis demonstrate that serum magnesium ≤2mEq/L was associated with increased cardiovascular mortality in patients with chronic heart failure. The observed associations are modest, and the significance of these findings is limited by the relatively small sample size. However, to the best of our knowledge, this is the first report of a long-term effect of low magnesium on mortality and hospitalizations in a propensity-matched cohort of chronic heart failure patients.
Our findings that low-magnesium was associated with increased cardiovascular mortality but not with increased cardiovascular hospitalization suggested that most of these deaths were likely sudden in nature. Magnesium deficiency is known to be associated with significant life-threatening ventricular arrhythmias and sudden death, which may be treated with magnesium supplements [1, 31, 32]. In addition, magnesium deficiency may cause myocardial fibrosis and platelet aggregation thus explaining its overall adverse effect on cardiovascular mortality [33, 34]. Finally, low serum magnesium may also be a marker of disease progression in heart failure. Aldosterone, a harmful neurohormone that is activated in heart failure, also increases urinary excretion of magnesium causing low serum magnesium [35].
Despite a significant increase in cardiovascular mortality, there were no statistically significant differences in individual components of cardiovascular mortality between the groups. This was likely due to small number of cause-specific events. However, the effect of low baseline magnesium on deaths due to non-heart failure-related cardiovascular causes, that included arrhythmias, was of borderline significance (Table 2). This supports the hypothesis that most low-magnesium-related deaths were likely due to life-threatening ventricular arrhythmias. The lack of a statistically significant effect on all-cause mortality, on the other hand, was likely due to lack of an effect of low magnesium on deaths due to non-cardiovascular and unknown causes (Table 2).
Diuretics are commonly used in heart failure and may be associated with poor outcomes, and higher doses of diuretics may be associated with increased risk of death [19, 36]. Despite a common belief that therapy with large doses of loop diuretics may result in low serum magnesium levels, diuretic dose has not been shown to be associated with low serum magnesium levels in heart failure [6]. This may be due to high prevalence of chronic kidney disease in heart failure [21]. While heart failure patients with chronic kidney disease are more likely to use diuretics and may require them in higher doses [21], pre-match data from our study and baseline data from other studies suggest that these patients are also more likely to have normal or high serum magnesium levels [6–8]. A study in patients with hypertension also found that serum magnesium levels were similar regardless of use of diuretics [37].
We used a cut-off point of 2 mEq/L to define low serum magnesium, which might have included many patients with normal serum magnesium, thus underestimating the true effect of low magnesium observed in our analysis. Yet, serum magnesium ≤2mEq/L was associated with increased cardiovascular mortality in chronic heart failure patients who were well balanced in all measured covariates. Given the known association between low serum potassium and low serum magnesium [38, 39], it may be reasonable to check serum magnesium levels in patients with persistent hypokalemia, especially those not responding to therapy. Once detected, hypomagnesemia should be corrected with magnesium supplementation and/or aldosterone antagonists as appropriate [40]. Further prospective studies are needed to understand the true nature of the association between serum magnesium levels and outcomes in chronic heart failure before routine monitoring of serum magnesium can be recommended for these patients.
The prognostic significance of serum magnesium in patients with heart failure has been examined in three previous studies, which reported conflicting findings [6–8]. Larger sample size, longer follow-up, inclusion of both systolic and diastolic heart failure, retrospective randomization based on propensity score matching, and risk adjustment based on a large number of measured covariates distinguish our study from those studies.
Our study has several limitations. Generalizability of this study is limited by patient characteristics (predominantly white, male and relatively young ambulatory patients with mild to moderate heart failure in normal sinus rhythm) and timing of the DIG trial (from pre-beta-blocker era of heart failure therapy). Although our propensity score matching has generated groups with equal number of patients using ACE inhibitors, we were not able to match by their dosages. It is plausible that patients with higher dosage of ACE inhibitor had less severe activation of renin-angiotensin-aldosterone system resulting in less severe hypomagnesemia. However, data on dosages of ACE inhibitors were not collected in the DIG trail. Finally, our study may be sensitive to an unmeasured covariate that is weakly correlated with low serum magnesium. However, for that covariate to be a confounder, it must be also be associated with cardiovascular mortality and not be strongly correlated with any of the covariates in Table 1 [30].
In conclusion, in a propensity-matched population of heart failure patients who were balanced on all measured baseline covariates, the presence of serum magnesium levels ≤2mEq/L was associated with a significant increase in long-term cardiovascular mortality. There is no need for routine screening for serum magnesium in heart failure patients. However, once low serum magnesium is detected in heart failure patients, they should be appropriately treated with magnesium supplements and/or aldosterone antagonists.
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P-50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama..
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Enselberg CD, Simmons HG, Mintz AA. The effects of magnesium upon cardiac arrhythmias. Am Heart J. 1950;39:703–12. doi: 10.1016/0002-8703(50)90130-x. [DOI] [PubMed] [Google Scholar]
- 2.Burch GE, Giles TD. The importance of magnesium deficiency in cardiovascular disease. Am Heart J. 1977;94:649–57. doi: 10.1016/s0002-8703(77)80137-3. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb SS. Importance of magnesium in congestive heart failure. Am J Cardiol. 1989;63:39G–42G. doi: 10.1016/0002-9149(89)90218-x. [DOI] [PubMed] [Google Scholar]
- 4.Sueta CA, Patterson JH, Adams KF., Jr Antiarrhythmic action of pharmacological administration of magnesium in heart failure: a critical review of new data. Magnes Res. 1995;8:389–401. [PubMed] [Google Scholar]
- 5.Zehender M, Meinertz T, Faber T, et al. Antiarrhythmic effects of increasing the daily intake of magnesium and potassium in patients with frequent ventricular arrhythmias. Magnesium in Cardiac Arrhythmias (MAGICA) Investigators. J Am Coll Cardiol. 1997;29:1028–34. doi: 10.1016/s0735-1097(97)00053-3. [DOI] [PubMed] [Google Scholar]
- 6.Cohen N, Almoznino-Sarafian D, Zaidenstein R, et al. Serum magnesium aberrations in furosemide (frusemide) treated patients with congestive heart failure: pathophysiological correlates and prognostic evaluation. Heart. 2003;89:411–6. doi: 10.1136/heart.89.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichhorn EJ, Tandon PK, DiBianco R, et al. Clinical and prognostic significance of serum magnesium concentration in patients with severe chronic congestive heart failure: the PROMISE Study. J Am Coll Cardiol. 1993;21:634–40. doi: 10.1016/0735-1097(93)90095-i. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb SS, Baruch L, Kukin ML, Bernstein JL, Fisher ML, Packer M. Prognostic importance of the serum magnesium concentration in patients with congestive heart failure. J Am Coll Cardiol. 1990;16:827–31. doi: 10.1016/s0735-1097(10)80329-8. [DOI] [PubMed] [Google Scholar]
- 9.Ralston MA, Murnane MR, Unverferth DV, Leier CV. Serum and tissue magnesium concentrations in patients with heart failure and serious ventricular arrhythmias. Ann Intern Med. 1990;113:841–6. doi: 10.7326/0003-4819-113-11-841. [DOI] [PubMed] [Google Scholar]
- 10.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 11.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 12.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–8. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 14.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Asso. 1984;79:516–524. [Google Scholar]
- 15.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 16.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–7. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–53. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–53. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–9. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: A propensity score analysis. Am Heart J. 2006;152:956–66. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A, Zannad F, Love TE, et al. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–43. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 25.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–30. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Gorfine M, Hsu L, Prentice RL. Nonparametric correction for covariate measurement error in a stratified Cox model. Biostatistics. 2004;5:75–87. doi: 10.1093/biostatistics/5.1.75. [DOI] [PubMed] [Google Scholar]
- 27.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–23. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum PR. Sensitivity analysis for matched case-control studies. Biometrics. 1991;47:87–100. [PubMed] [Google Scholar]
- 29.Rosenbaum PR. Sensitivity to Hidden Bias. In: Rosenbaum PR, editor. Observational Studies. 2. New York: Springer-Verlag; 2002. pp. 110–124. [Google Scholar]
- 30.Rothman KJ, Greenland S. Precision and validity in epidemiological studies. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. 2. Philadelphia: Lippincott - Raven; 1998. pp. 115–134. [Google Scholar]
- 31.Wester PO. Electrolyte balance in heart failure and the role for magnesium ions. Am J Cardiol. 1992;70:44C–49C. doi: 10.1016/0002-9149(92)91357-a. [DOI] [PubMed] [Google Scholar]
- 32.Ceremuzynski L, Gebalska J, Wolk R, Makowska E. Hypomagnesemia in heart failure with ventricular arrhythmias. Beneficial effects of magnesium supplementation. J Intern Med. 2000;247:78–86. doi: 10.1046/j.1365-2796.2000.00585.x. [DOI] [PubMed] [Google Scholar]
- 33.Valiathan SM, Kartha CC. Endomyocardial fibrosis--the possible connexion with myocardial levels of magnesium and cerium. Int J Cardiol. 1990;28:1–5. doi: 10.1016/0167-5273(90)90002-m. [DOI] [PubMed] [Google Scholar]
- 34.Shechter M, Merz CN, Paul-Labrador M, et al. Oral magnesium supplementation inhibits platelet-dependent thrombosis in patients with coronary artery disease. Am J Cardiol. 1999;84:152–6. doi: 10.1016/s0002-9149(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 35.Horton R, Biglieri EG. Effect of aldosterone on the metabolism of magnesium. J Clin Endocrinol Metab. 1962;22:1187–92. doi: 10.1210/jcem-22-12-1187. [DOI] [PubMed] [Google Scholar]
- 36.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–64. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke K, Wood DR, Hanley JF. The value of serum magnesium determination in hypertensive patients receiving diuretics. Arch Intern Med. 1987;147:1553–6. [PubMed] [Google Scholar]
- 38.Whang R. Magnesium and potassium interrelationships in cardiac arrhythmias. Magnesium. 1986;5:127–33. [PubMed] [Google Scholar]
- 39.Whang R, Oei TO, Aikawa JK, et al. Predictors of clinical hypomagnesemia. Hypokalemia, hypophosphatemia, hyponatremia, and hypocalcemia. Arch Intern Med. 1984;144:1794–6. [PubMed] [Google Scholar]
- 40.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]