Abstract
We describe a riboprinting scheme for identification of unknown Acanthamoeba isolates at the species level. It involved the use of PCR-RFLP of small subunit ribosomal RNA gene (riboprint) of 24 reference strains by 4 kinds of restriction enzymes. Seven strains in morphological group I and III were identified at species level with their unique sizes of PCR product and riboprint type by Rsa I. Unique RFCP of 17 strains in group II by Dde I, Taq I and Hae III were classified into: (1) four taxa that were identifiable at the species level, (2) a subgroup of 4 taxa and a pair of 2 taxa that were identical with each other, and (3) a species complex of 7 taxa assigned to A. castellanii complex that were closely related. These results were consistent with those obtained by 18s rDNA sequence analysis. This approach provides an alternative to the rDNA sequencing for rapid identification of a new clinical isolate or a large number of environmental isolates of Acanthamoeba.
Keywords: Acanthamoeba, riboprinting scheme, rapid identification
INTRODUCTION
The taxonomy of Acanthamoeba has been revised several times (Pussard and Pons, 1977; De Jonckheere, 1983; Visvesvara, 1991; Stothard et al., 1998; Chung et al., 1998). Pussard and Pons (1977) classified the genus Acanthamoeba into three morphological groups on the basis of cyst size and shape. Group I consists of Acanthamoeba spp. of which cysts are relatively large, with distinctly stellate endocysts and smooth spherical ectocyst. Group II and group III Acanthamoeba spp. have smaller cyst (diameters less than 18 µm): Group II species has polygonal to stellate endocyst with irregular or winkled ectocysts, whereas the cyst of group III species has rounded or slightly angular endocyst with thinner and smooth or slightly wrinkled ectocyst. Classification by Pussard and Pons (1977) has been recognized being practical, but taxonomy of Acanthamoeba at the species level remains unclear. However, De Jonckheere (1983) suggested that some species designated by Pussard and Pons (1977) should be invalid synonyms of the other species on the basis of alloenzyme analysis. Jacobson and Band (1987) reported alloenzyme heterogeneity among strains assigned to A. polyphaga. Therefore, results reported by De Jonckheere (1983) and Jacobson and Band (1987) suggest that a different alloenzyme pattern should not be used as the sole criterion to establish a new species nor a morphological analysis be used alone.
Recently, Stothard et al. (1998) classified 53 strains of Acanthamoeba into 12 sequence types based on 18S rDNA sequence analysis. The results were inconsistent with some species designations. Chung et al. (1998) applied a simpler technique, riboprinting, to subgenus classification of Acanthamoeba and the results coincided well with those of Stothard at al. (1998). Nevertheless, when a number of unidentified Acanthamoeba isolates were collected from clinical samples or environments, identification of the isolates by analysis of 18S rDNA complete sequences would be too labor-intensive, time-consuming, and expensive for most laboratories. Riboprinting can be a substitute of 18S rDNA sequencing as suggested by Chung et al. (1998). In this paper, we provide a new strategy based on the riboprinting for rapid identification of unknown Acanthamoeba isolates.
MATERIALS AND METHODS
Acanthamoeba
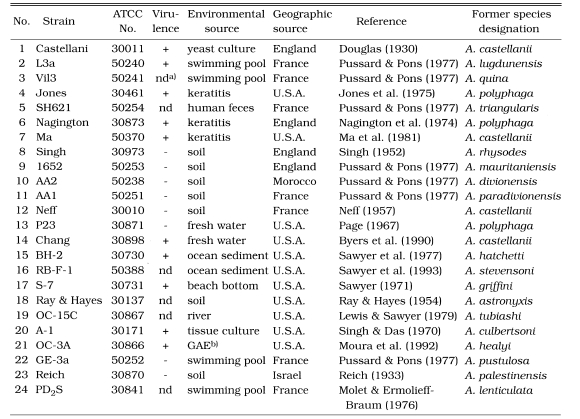
Twenty-four strains including 19 (neo) type strains of the genus Acanthamoeba which were previously assigned to 19 species, were obtained from ATCC (Table 1). According to the morphological grouping of Pussard and Pons (1977), two strains belong to group I, 5 strains to group III and the other 17 strains to group II. They were cultured axenically in Proteose peptone-Yeast extract-Glucose (PYG) medium or Proteose peptone-Yeast extract-Glucose-Cysteine (PYGC) medium at 25℃ or 37℃.
Table 1.
List of Acanthamoeba 24 reference strains obtained from ATCC
a)nd; not determined.
b)GAE; granulomatous amebic encephalitis.
Extraction of genomic DNA, PCR amplification of 18S rDNA, and restriction enzyme digestion
Genomic DNA of Acanthamoeba was obtained by the method described by Kong and Chung (1996). The primers and method for PCR amplification of 18S rDNA were the same as described by Chung et al. (1998). In order to check the size of the PCR products, the amplified DNA of 24 strains were electrophoresed on 2.5% agarose gel with DNA size standards (Hind III digested λ phage DNA, Poscochem, Korea; Amplisize, Biorad, U.S.A.). Four kinds of restriction endonucleases (Hae III, Dde I, Rsa I, and Taq I; Poscochem, Korea) which have recognition sequences of four nucleotides were used to generate comparative riboprints. The amplified DNA of each strain was digested with 5-10 units of each restriction enzyme for 2 hr in recommended buffers at 37℃, except for Taq I (67℃). The digested DNA was electrophoresed on 2.5% agarose gel for 1.5 hr with Hae III digested ΦX174 DNA as DNA size marker. The gels were stained with ethidium bromide and photographed under an UV transilluminator.
Development of the riboprinting scheme
The strains were divided into three groups by morphological characteristics of amoeba and cysts. In each group, restriction phenotypes of strains were determined for each restriction enzyme used. Therefore, differences of PCR product sizes and restriction phenotypes were used to build a scheme for distinguishing and identifying unknown Acanthamoeba isolates.
RESULTS
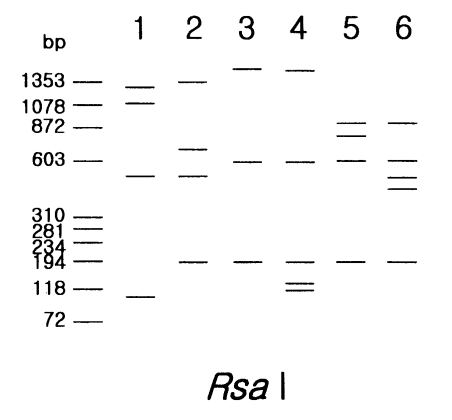
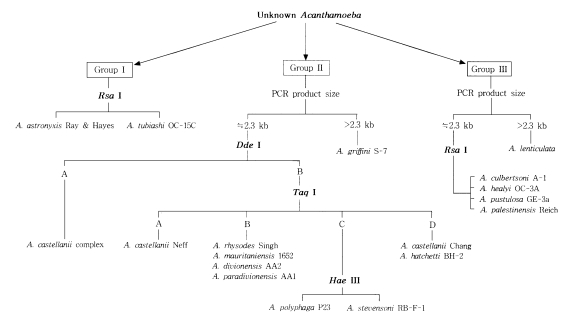
Two strains in morphological group I, Acanthamoeba astronyxis and A. tubiashi, had bigger PCR product size of 18s rDNA than that of other strains in group II or group III. The size of their PCR products was approximately 2.7 kb for A. astronyxis and 2.6 kb for A. tubiashi. In addition to the difference of PCR product size, the riboprint of each strain by restriction enzyme Rsa I was unique (Fig. 1).
Fig. 1.
Schematic representation of riboprinting patterns of Acanthamoeba strains, belonging to morphoolgical group I and III by Rsa I restriction enzyme. Lane 1, A. astronyxis Ray & Hayes; 2, A. tubiashi OC-15C; 3, A. culbertsoni A-1; 4, A. healyi OC-3A; 5, A. pustulosa GE-3a; 6, A. palestinensis Reich.
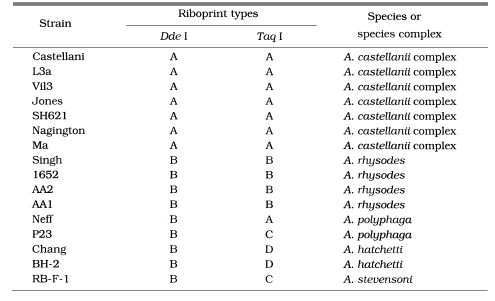
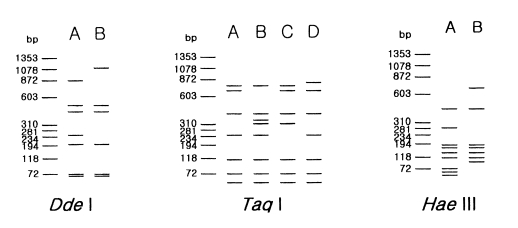
Morphological group II, to which most isolates from clinical and environmental sources belong, contains 17 strains including 12 type or neotype strains. The PCR product size of A. griffini is larger than that of other strains, because of the intron in its 18s rDNA (Gast et al, 1996; Ledee et al., 1996). Sixteen strains whose PCR products were approximately 2.3 kb, were subjected to riboprinting. Table 2 and Fig. 2 show the riboprint types of 16 Acanthamoeba strains by Dde I and Taq I. The ribodemes by Dde I divided 16 strains into two types. Seven strains including Castellani, the type strain of A. castellanii, which showed the same riboprint by Dde I were assigned as A. castellanii complex. Riboprinting by Taq I was applied to differentiate 9 strains with B type by Dde I. By Taq I, nine strains were divided into four subgroups. A. castellanii Neff strain was unique from the other strains. Four strains, including A. rhysodes Singh, A. mauritaniensis 1652, A. divionensis AA2, and A. paradivionensis AA1, were in a subgroup, because of their similar pattern by Taq I. Chang strain fomerly assigned to A. canstellanii and A. hatchetti BH-2 strain was classified to an another subgroup with the same pattern. Riboprint by Hae III or Hha I differenciated A. polyphaga P23 from A. stevensoni RB-F-1, which belonged to the same subgroup by Taq I (Fig. 2).
Table 2.
Riboprint types of Acanthamoeba strains belonging to morphological group II
Fig. 2.
Schematic representation of riboprint types of Acanthamoeba strains, belonging to morphological group II by 3 kinds of restriction enzymes.
Five strains in morphological group III showed differences in PCR product sizes and their riboprints by Rsa I (Fig. 1). Acanthamoeba lenticulata had the largest PCR product. Although the other four strains had similar PCR product size, they were distinguishable from each other by Rsa I riboprints.
Fig. 3 represents the riboprinting scheme of 24 strains analyzed in the present study by their morphological grouping, 18s rDNA PCR product sizes, and its riboprints by restriction enzymes. By this scheme, eleven strains of Acanthamoeba were identified at the species level.
Fig. 3.
Riboprinting scheme with 24 reference strains of Acanthamoeba.
DISCUSSION
This study presents a riboprinting scheme for identification of unknown Acanthamoeba isolates by using PCR/RFLP of the small subunit ribosomal DNA. Considering genetic diversity among strains of genus Acanthamoeba, limited number of strains were applied to build a scheme, however, the present study provides a simple guideline for identification, because 24 strains included 19 type or neotype strains of Acanthamoeba spp. previously assigned.
Pussard and Pons (1977) classified strains in genus Acanthamoeba into three groups, according to their morphological characteristics, and the morphology has recognized to be practical for intrageneric grouping (Stothard et al., 1998; Chung et al., 1998). However, because of variability of cyst morphology by culture conditions (Stratford and Griffiths, 1978), species identification by morphology alone can hardly be possible (Visvesvara, 1991).
This study provides a time-saving alternative to sequence analysis for identification of unknown Acanthamoeba isolates. For mitochondrial (Mt) DNA RFLP and alloenzyme analysis, it takes time to get enough number of trophozoites by culture. Furthermore, the results of MtDNA RFLP and alloenzyme analyses in Acanthamoeba are too heterogeneous to be applied for identifying unknown isolates at the species level (Chung et al., 1996; Kong et al., 1995). Nuclear 18S rDNA sequence analysis is recognized as an objective and reliable method for species identification (Gast et al., 1996; Ledee et al., 1996; Stothard et al., 1998). However, it takes time to sequence approximately 2,300 bp or longer 18S rDNA especially when a large number of isolates are to be tested.
In addition, our study serves a guideline for future studies that can resolve some taxonomic problems in the genus Acanthamoeba. For example, type strains (1652, AA2, and AA1) of A. mauritaniensis, A. divionensis, and A. parasivionensis showed riboprints identical to that of A. rhysodes Singh (Chung et al., 1998). Three former type strains were isolated and recorded as new species by Pussard and Pons (1977). Chung et al. (1998) suggested the A. mauritaniensis, A. divionensis, and A. parasivionensis should be renamed as A. rhysodes under the law of priority. Chang strain previously assigned to A. castellanii was found to be related most closely with BH-2, the type strain of A. hatchetti, by nuclear and Mt riboprinting (Chung et al., 1998; Yu et al., 1999). Furthermore, the Chang and BH-2 had many comigrating DNA fragments on MtDNA RFLP analysis (unpublished data). To confirm these taxonomic revisions, 18S rDNA sequence analysis should be further pursued.
Acanthamoeba castellanii complex corresponds to T4 sequence type of Byers group. Stothard et al. (1998) suggested that various species in T4 might be reclassified as A. castellanii because the sequence type includes the type strain for that species. However, the molecular characteristics of strains in T4 were quite heterogeneous and many species which belonged to this clad have already been assigned. We would like to assign this subgroup (T4 sequence type) as species complex as Costas and Griffith suggested (1986).
Although the scheme proposed here is useful, some caution should be taken in when applying the scheme to identify environmental or clinical isolates of Acanthamoeba, when the isolate contains intron in 18S rDNA. Among 17 strains in group II examined in this study, only A. griffini had intron in its 18S rDNA. However, we found two clinical isolates containing intron in 18S rDNA and both isolates showed the highest sequence homology of 18S rDNA with the type strain of A. castellanii (unpublished data). In such cases, riboprinting of 16S-like Mt rDNA which has no intron can be helpful, for confirmation of assignment by sequence analysis of nuclear 18S rDNA. Additionally, investigators should determine the morphological grouping of the isolates before applying the scheme for identification.
In conclusion, the riboprinting scheme supplemented with morphological grouping could provide rapid identification of unknown Acanthamoeba isolates. This scheme would provide an alternative to rDNA sequence analysis especially when many isolates are to be identified.
References
- 1.Byers TJ, Hugo ER, Stewart VJ. Genes of Acanthamoeba: DNA, RNA and protein sequences (A Review) J Protozool. 1990;37:s17–s25. doi: 10.1111/j.1550-7408.1990.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 2.Chung DI, Kong HH, Yu HS, Oh YM, Yee ST, Lim YJ. Biochemical and molecular characterization of a strain KA/S2 of Acanthamoeba castellanii isolated from Korean soil. Korean J Parasitol. 1996;34:79–85. doi: 10.3347/kjp.1996.34.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Chung DI, Yu HS, Hwang MY, et al. Subgenus classification of Acanthamoeba by riboprinting. Korean J Parasitol. 1998;36:69–80. doi: 10.3347/kjp.1998.36.2.69. [DOI] [PubMed] [Google Scholar]
- 4.Costas M, Griffiths AJ. Physiological characterization of Acanthameoba strains. J Protozool. 1986;33:304–309. [Google Scholar]
- 5.De Jonckheere Isoenzyme and total protein analysis by agarose isoelectric focusing, and toxonomy of the genus Acanthamoeba. J Protozool. 1983;30:701–706. [Google Scholar]
- 6.Douglas M. Notes on the classification of the amoeba found by Castellani in cultures of a yeast-like fungus. J Trop Med London. 1930;33:258–259. [Google Scholar]
- 7.Gast RJ, LeDee D, Fuerst PA, Byers TJ. Subgenus systematics of Acanthamoeba: Four nuclear 18S rDNA sequence types. J Euk Microbiol. 1996;43:498–504. doi: 10.1111/j.1550-7408.1996.tb04510.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson LM, Band RN. Genetic heterogeneity in a natural population of Acanthamoeba polyphaga from soil, an isoenzyme analysis. J Protozool. 1987;34:83–86. [Google Scholar]
- 9.Jones DB, Visvesvara GS, Robinson NM. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc UK. 1975;95:221–232. [PubMed] [Google Scholar]
- 10.Kong HH, Chung DI. PCR and RFLP variation of conserved region of small subunit ribosomal DNA among Acanthamoeba isolates assigned to either A. castellanii or A. polyphaga. Korean J Parasitol. 1996;34:127–134. doi: 10.3347/kjp.1996.34.2.127. [DOI] [PubMed] [Google Scholar]
- 11.Kong HH, Park JH, Chung DI. Interstrain polymorphisms of isoenzyme profiles and mitochondrial DNA fingerprints among seven strains assigned to Acanthamoeba polyphaga. Korean J Parasitol. 1995;33:331–340. doi: 10.3347/kjp.1995.33.4.331. [DOI] [PubMed] [Google Scholar]
- 12.Ledee DR, Hay J, Byers TJ, et al. Acanthamoeba griffini Molecular characterization of a new corneal pathogen. Invest Ophthalmol Vis Sci. 1996;37:544–550. [PubMed] [Google Scholar]
- 13.Lewis EJ, Sawyer TK. Acanthamoeba tubiashi n. sp. a new species of fresh-water Amoebida (Acanthamoebidae) Trans Am Microsc Soc. 1979;98:543–549. [Google Scholar]
- 14.Ma PE, Willaert KB, Stevens AR. A case of keratitis due to Acanthamoeba in New York, and features of 10 cases. J Inf Dis. 1981;143:662–667. doi: 10.1093/infdis/143.5.662. [DOI] [PubMed] [Google Scholar]
- 15.Molet B, Ermolieff-Braum Description d'une amibe d'deau douce: Acanthamoeba lenticulata n. sp. (Amoebida) Protistologica. 1976;12:571–576. [Google Scholar]
- 16.Moura H, Wallace S, Visvesvara GS. Acanthamoeba healyi n. sp. and the isoenzyme and immunoblot profiles of Acanthamoeba spp., Groups 1 and 3. J Protozool. 1992;39:573–583. doi: 10.1111/j.1550-7408.1992.tb04853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagington J, Watson PG, Playfair TJ, McGill J, Jones BR. Amebic infection of the eye. Lancet. 1974;ii:1537–1540. doi: 10.1016/s0140-6736(74)90285-2. [DOI] [PubMed] [Google Scholar]
- 18.Neff R. Purification, axenic cultivation and description of a soil amoeba, Acanthamoeba sp. J Protozool. 1957;4:176–182. [Google Scholar]
- 19.Page FC. Re-definition of the genus Acanthamoeba with descriptions of three species. J Protozool. 1967;14:709–724. doi: 10.1111/j.1550-7408.1967.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 20.Pussard M, Pons R. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida) Protistologica. 1977;13:557–598. [Google Scholar]
- 21.Ray DL, Hayes RE. Hartmannella astronyxis: a new species of free-living amoeba. Cytology and life cycle. J Morphol. 1954;95:159–188. [Google Scholar]
- 22.Reich K. Studien er die Bodenprotozoen palstinos. Arch Protistenkd. 1933;79:76–98. [Google Scholar]
- 23.Sawyer TK. Acanthamoeba griffini, a new species of marine amoeba. J Protozool. 1971;18:650–654. [Google Scholar]
- 24.Sawyer TK, Nerad TA, Lewis EJ, McLaughlin SM. Acanthamoeba stevensoni n. sp. (Protozoa: Amoebida) from sewage-contaminated shellfish beds in Raritan bay, New York. J Protozool. 1993;40:742–746. [Google Scholar]
- 25.Sawyer TK, Visvesvara GS, Harke BA. Pathogenic amoebas from brackish and ocean sediments, with a description of Acanthamoeba hatchetti, n. sp. Science. 1977;196:1324–1325. doi: 10.1126/science.867031. [DOI] [PubMed] [Google Scholar]
- 26.Singh BN. Nuclear division in nine species of small free-living amoebae and its bearing on the classification of the order Amoebida. Phil Trans Roy Soc London Ser Biol Sci. 1952;236:405–461. doi: 10.1098/rstb.1970.0063. [DOI] [PubMed] [Google Scholar]
- 27.Singh BN, Das SR. Studies on pathogenic and nonpathogenic small free-living amoebae and the bearing of nuclear division on the classification of the order Amoebida. Phil Trans Roy Soc London Ser Biol Sci. 1970;259:435–476. doi: 10.1098/rstb.1970.0063. [DOI] [PubMed] [Google Scholar]
- 28.Stothard DR, Schroeder-Diedrich JM, Awwad MH, et al. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J Euk Microbiol. 1998;45:45–54. doi: 10.1111/j.1550-7408.1998.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stratford MP, Griffiths AJ. Variations in the properties and morphology of cysts of Acanthamoeba castellanii. J Gen Microbiol. 1978;108:33–37. [Google Scholar]
- 30.Visvesvara GS. Classification of Acanthamoeba. Rev Infect Dis. 1991;13(suppl. 5):s369–s372. doi: 10.1093/clind/13.supplement_5.s369. [DOI] [PubMed] [Google Scholar]
- 31.Yu HS, Hwang MY, Kim TO, et al. Phylogenetic relationships among Acanthamoeba spp. based on PCR-RFLP analyses of mitochondrial small subunit rRNA gene. Korean J Parasitol. 1999;37:181–188. doi: 10.3347/kjp.1999.37.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]