Abstract
Pneumocystis carinii causes serious pulmonary infection in immunosuppressed patients. This study was undertaken to observe the cytoskeletal proteins of P. carinii by immuno-electron microscopy. P. carinii infection was experimentally induced by immunosuppression of Sprague-Dawley rats for seven weeks, and their lungs were used for the observations of this study. The gold particles localized actin, tropomyosin, and tubulin. The actin was irregularly scattered in the cytoplasm of the trophic forms but was much more concentrated in the inner space of the cell wall of the cystic forms called the inner electron-lucent layer. No significant amount of tropomyosin was observed in either trophic forms or cystic forms. The tubulin was distributed along the peripheral cytoplasm and filopodia of both the trophic and cystic forms rather than in the inner side of the cytoplasm. Particularly, in the cystic forms, the amount of tubulin was increased and located mainly in the inner electron-lucent layer of the cell wall where the actin was concentrated as well. The results of this study showed that the cell wall of P. carinii cystic forms is a structure whose inner side is rich in actin and tubulin. The location of the actin and tubulin in P. carinii suggests that the main role of these proteins is an involvement in the protection of cystic forms from the outside environment by maintaining rigidity of the cystic forms.
Keywords: Pneumocystis carinii, cytoskeletal proteins, actin, tubulin, tropomyosin, immuno-electron microscopy
INTRODUCTION
Pneumocystis carinii being one of the major opportunistic protists often causes life-threatening pneumonia in immunocompromised human hosts especially after the advent of the AIDS era. The life cycle of P. carinii usually proceeds in the alveoli, but extrapulmonary spreading is also known in other organs as well.
The cytoskeletal proteins are known to be essential in the motility and reproduction of eukaryotes. The eukaryotic cytoskeletal proteins consist of three major components, microfilament, microtubule, and intermediate filament. Some papers recorded that microtubules and microfilaments were absent in P. carinii (ul Haque et al., 1987; Bedrossian, 1989; Ruffolo et al., 1989; Cushion et al., 1991). However, some investigators have noted a movement of intracystic bodies within the cyst (Yoshida et al., 1981; Shiota, 1984; Cushion et al., 1985; Newsome et al., 1991). Vossen et al. (1976) observed the microtubule in the nucleus, mitochondria, ribosomes, and intracystic bodies by transmission electron microscopy. The cytoskeletal elements in P. carinii are still not fully understood.
The reproduction of P. carinii has not been defined exactly either. Previous reports have observed a release of intracystic bodies after the disruption of the cell wall of cystic forms, meiosis in precystic stages, endodyogeny and binary fission (Campbell et al., 1972; Matsumoto et al., 1984; Vavra and Kucera, 1970; Vossen et al., 1977). In the process of reproduction and transmission, cytoskeletal proteins are no doubt essential in P. carinii as like in other organisms.
This study was performed to determine the variation in cytoskeletal proteins and their location according to their life cycle using immuno-electron microscopy.
MATERIALS AND METHODS
Preparation of Pneumocystis carinii tissue antigen
Two Sprague-Dawley (SD) rats were injected weekly with 5 mg of methylprednisolone acetate (Depomedrol, Pharmacia & Upjohn Ltd., Hwaseong, Korea) over a seven week preriod. They were bred with a regular commercial diet including 0.1% tetracycline water in the animal quarter of the Seoul National University College of Medicine. Seven weeks after immunosup-pression, their lungs were removed and fixed in a mixture of 2% paraformaldehyde and 0.4% glutaraldehyde (pH 7.4) at 4℃ overnight, washed with 0.1 M PBS. Then, they were dehydrated through ethyl alcohol series from 50 to 95%, embedded in LR gold resin (London Resin Co., UK) followed by polymerization at -20℃ for 72 hr under UV illumination. The polymerized tissue blocks were sectioned at 90 nm thickness by ultramicrotome and mounted onto nickel grids.
Immunogold labeling and electron microscopic observation
Immunogold labeling was performed as described by Yu and Chai (1995). Briefly, the sectioned specimens were washed in PBS-Milk-Tween (3% skim milk and 0.01% Tween 20 in PBS) for 10 min. They were incubated at 4℃ overnight with rabbit anti-actin (chicken back muscle, BioGenex Laboratories, CA), rabbit anti-tropomyosin (chicken gizzard muscle, Sigma) and rabbit anti-tubulin (chicken embryos, Sigma) polyclonal antibodies. The specimens were washed in PBS-BSA-Tween (1% bovine serum albumin and 0.01% Tween 20 in PBS) and then incubated for 2 hr at room temperature with a 5 nm-colloidal gold conjugated goat anti-rabbit IgG (British BioCell International, UK). After washing in PBS-Tween (0.01% Tween 20 in PBS), they were incubated with 2.5% glutaraldehyde to stabilize the gold particles. For silver enhancement, an IntenSE™M Silver enhance-ment kit (Amersham Life Science, UK) was used. After uranyl acetate and lead citrate staining, electronmicroscopic observations were performed with Jeol 1200 EXII.
RESULTS
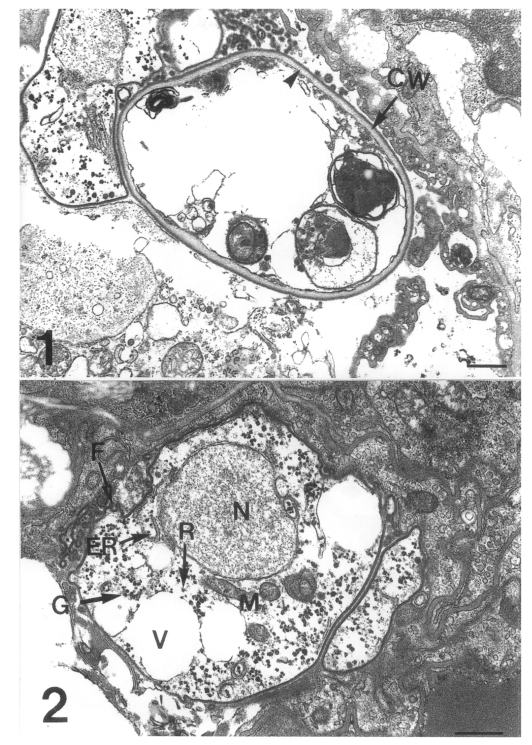
Three developmental stages of Pneumocystis carinii were observed at the surface of the rat lung alveoli: the cystic form with thick wall, the precystic form with intermediate wall, and the trophic form with thin cell wall (Figs. 1 & 2). The cystic form featured a thick cyst wall due to the development of the inner electron-lucent layer beneath the outer electron-dense layer (Fig. 1). The thin cell wall of the trophic form consisted of a well demarcated inner cell membrane and a translucent outer cell wall. The trophic form had many indentations and cilia-like filopodia (Fig. 2). The endoplasmic reticulum and ribosomes were dispersed in the cytoplasm, and the tubular mitochondria could be seen. Inside the cytoplasm, there were some vacuoles and a lot of electron-dense granules called glycogen rosettes (Fig. 2). During the transition from trophic to cystic forms, the cell wall became thicker due to the formation of an inner electron-lucent layer under the outer electron-dense layer. This is a characteristic of the precystic form.
Figs. 1-2.
Fig. 1. A cystic form of Pneumocystis carinii. The cyst wall (CW) is thick because of the formation of an inner electron-lucent layer (arrowhead) under electron-dense layer. Bar = 500 nm. × 10,000. Fig. 2 A trophic form of Pneumocystis carinii. The thin cell wall has many indentations and cilia-like filopodia (F), and the cytoplasm includes endoplasmic reticulum (ER), ribosomes (R), mitochondria (M), some vacuoles (V) and glycogen rosette (G). N, nucleus. Bar = 500 nm. ×12,000.
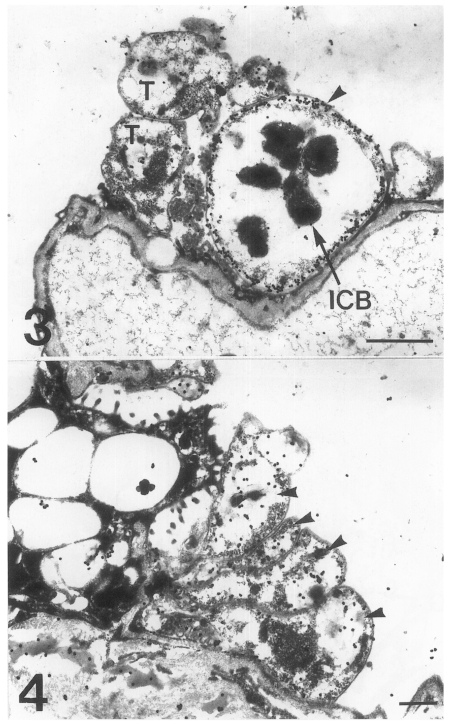
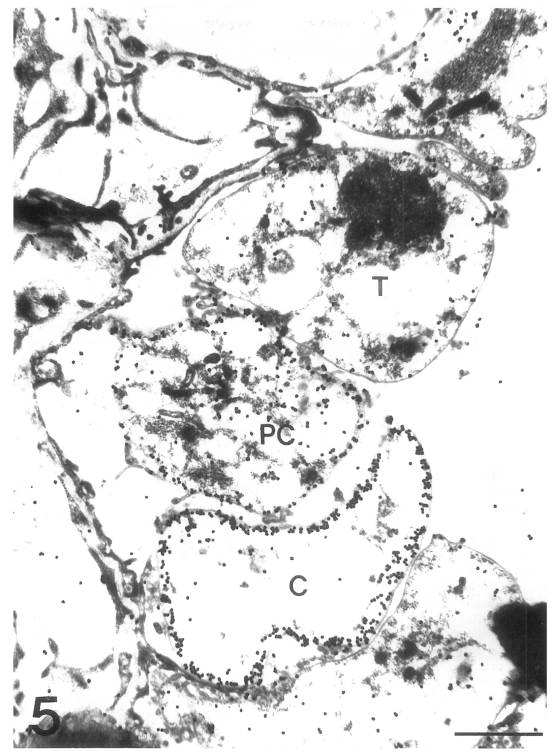
The main distribution of the actin recognized by the antibodies for chicken back muscle actin was the inner electron-lucent layer of the cell wall of the cystic forms (Fig. 3). In comparison, the intracystic bodies did not show gold particles labeled on the actin. In the trophic forms, a small amount of actin was located in the cytoplasm, but not much on the cell wall structure (Fig. 4). In the precystic forms, the gold particles for actin were observed near the cell wall, and the frequency of their presence increased remarkably (Fig. 5). Therefore, the amount of actin was found to increase according to the development of P. carinii from trophic to cystic forms. The antibody for tropomyosin labeled both trophic and cystic forms to an insignificant degree (Fig. 6).
Figs. 3-4.
Fig. 3. Immunogold labelling for actin on a cystic form of Pneumocystis carinii. The gold particles are concentrated on the inner electron-lucent layer (arrowhead) of the cell wall of the cystic forms, and the intracystic bodies show no labelled gold particles. T, trophozoite. Bar = 1 µm. ×8,000. Fig. 4. Immunogold labelling for actin on a trophic form of Pneumocystis carinii. A small amount of actin is located in the cytoplasm, but not much on the cell wall. Arrowheads, trophic forms. Bar = 500 nm. ×10,000.
Fig. 5.
Immunogold labelling for actin in three developmental stages of Pneumocystis carinii. The amount of gold particles labelled on actin is increased according to the development of P. carinii from trophic form (T) to precystic form (PC), and finally to cystic form (C). Bar = 1 µm. ×20,000.
Fig. 6.
Fig. 6. Immunogold labelling for tropomyosin of Pneumocystis carinii. No significant amount of gold particles is found in either trophic (T) or cystic forms (C). Bar = 500 nm. ×8,000.
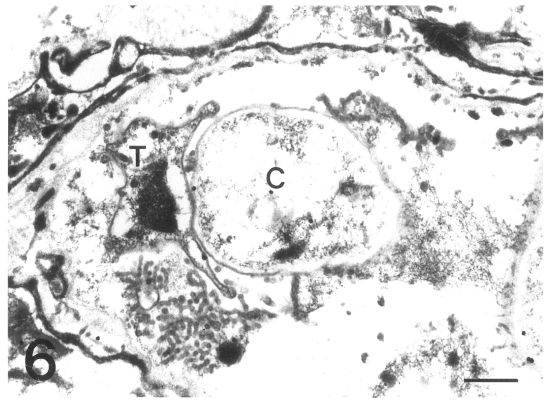
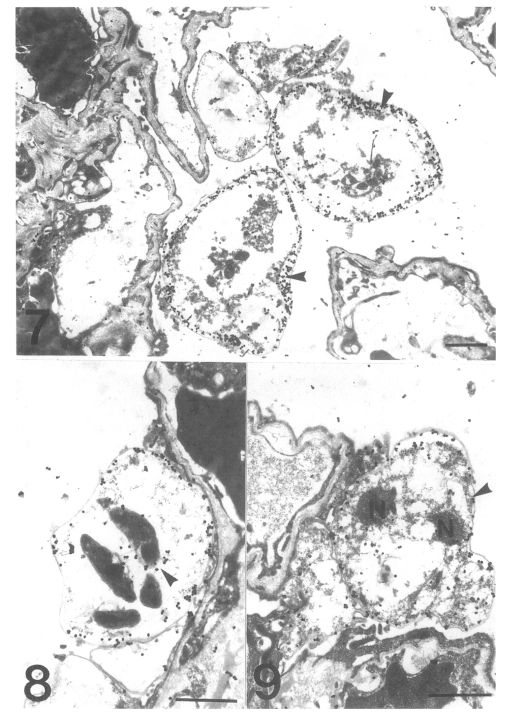
The gold particles for tubulin were dominantly concentrated around the inner electron-lucent layer of the cell wall of the cystic forms, and beneath the pellicle of the intracystic bodies (Figs. 7 & 8). In the trophic forms, the antibody for tubulin labeled most of the filopodia (data not shown) and the thin cell wall (Fig. 9). The amount of tubulin increased remarkably according to the development of P. carinii from trophic to cystic forms in the same manner as actin. The results showed that the actin and tubulin of P. carinii coexisted in the inner electron-lucent layer of the cell wall of the cystic forms.
Figs. 7-9.
Fig. 7. Immunogold labelling for tubulin on a cystic form of Pneumocystis carinii. The number of gold particles is remarkably increased and densely concentrated on the inner electron-lucent layer of the cell wall of the two cystic forms. Bar = 1 µm. ×5,000. Fig. 8 Immunogold labelling for tubulin on a cystic form of Pneumocystis carinii. The gold particles are observed at the pellicle of the intracystic bodies (arrowhead). Bar = 1 µm. ×8,000. Fig. 9 Immunogold labelling for tubulin on a trophic form of Pneumocystis carinii. The gold particles are distributed on the cell wall (arrowhead) and the peripheral cytoplasm. N, nucleus. Bar = 1 µm. ×5,000.
DISCUSSION
Eukaryote cytoskeleton provides mechanical support to cells and is also often engaged in motile activities (Alberts et al., 1994). In this study, the actin was concentrated on the inner electron-lucent layer of the cell wall of the cystic forms, which suggests that the actin protein is the major component of the cell wall. As a matter of fact, since the cystic form is known to be responsible for the air transmission (Hong et al., 1999), the cyst wall should be rigid and chemically stable for survival of P. carinii in adverse environmental conditions. Mostly, β-glucan cellulose is thought to fulfill this function, but the observed increase of actin gold particles on the cell wall according to maturation of cystic forms indicates that the actin is also involved in the protection of P. carinii from outside conditions by strengthening the cell membrane rigidity.
In addition to maintaining cell shape, actin is known to be involved in cell invasion by Plasmodium falciparum (Webb et al., 1996). In Cryptosporidium parvum and C. muris infections, host cell actin is involved in the process of invasion (Yu and Choi, 2000). The role of actin in the attachment of P. carinii on host pneumocytes has neither been confirmed nor ascertained yet and further study is necessary.
As one of the actin binding proteins, tropomyosin has been thought to be present in most eukaryotic cells. Given such a presence however, the result of this study that P. carinii did not show tropomyosin labeled to a significant degree is a somehow unexpected one. However, there are some points worth considering about this negative tropomyosin result. One is the possibility that the primary antibody used in this study did not cross react with P. carinii tropomyosin. Another is that the tropomyosin of P. carinii was not properly preserved during the tissue processing. Finally, P. carinii does not have tropomyosin. In fact it has been reported that there are several kinds of isotypes of non-muscle type tropomysin (Lin et al., 1997), so the possibility of failed cross reactivity between chicken gizzard muscle tropomyosin and the P. carinii one can not be ruled out.
In this study, the tubulin was uniformly distributed in the inner electron-lucent layer of the cell wall of the cystic forms to an amount greater than in the trophic forms. The location was exactly the same as that of the actin. Therefore, maintaining the cell wall structure of cystic forms may be the most important function of microtubules also. However, the function of the microtubule of P. carinii may not be limited to maintaining the cell shape. Barlett et al. (1992) reported that benzimidazoles repress in vitro growth of P. carinii by inhibiting the formation of the microtubule. The location of tubulin in the trophic forms was along the cell wall and filopodia, which suggests that the tubulin seems to be related with the process of attachment of the parasite to the host cell membrane.
Many theories about the reproduction of P. carinii have been proposed, but the exact mechanism has not been established. The cyst wall of cystic forms needs to be broken for the commencement of another phase of the life cycle of the intracystic bodies. The manner in which the intracystic bodies excyst is also not clear. We speculate that the actin and the tubulin in the inner electron-lucent layer of the cell wall of the cystic forms may be involved in the process of opening the cyst wall by making the cell wall mobile and that the distribution of tubulin gold particles on pellicles of intracystic bodies is related with the propagation process of intracystic bodies.
In conclusion, the present study revealed that the cyst wall of P. carinii cystic forms is a structure whose inner electron-lucent layer is rich in actin and tubulin. The location of actin and tubulin in P. carinii suggests that the main role of these proteins is related with the protection of cystic forms from the outside environment by maintaining the rigidity of the cystic forms.
Footnotes
This study was supported by a research grant from the Seoul National University College of Medicine (#97110192).
References
- 1.Alberts B, Bray D, Lewis J, et al. Molecular Biology of the Cell. 3rd ed. New York & London: Garland Publishing Inc; 1994. [Google Scholar]
- 2.Barlett MS, Edlind TD, Durkin MM, et al. Antimicrotubule benzimidazoles inhibit in vitro growth of Pneumocystis carinii. Antimicrob Agents Chemother. 1992;36:779–782. doi: 10.1128/aac.36.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedrossian CW. Ultrastructure of Pneumocystis carinii: a review of internal and surface characteristics. Semin Diagn Pathol. 1989;6:212–237. [PubMed] [Google Scholar]
- 4.Campbell WG. Ultrastructure of Pneumocystis in human lung. Life cycle in human pneumocystosis. Arch Pathol. 1972;93:312–324. [PubMed] [Google Scholar]
- 5.Cushion MT, Ruffolo JJ, Linke MJ, Walzer PD. Pneumocystis carinii: growth variables and estimates in the A549 and WI-38 VA13 human cell lines. Exp Parasitol. 1985;60:43–54. doi: 10.1016/s0014-4894(85)80021-7. [DOI] [PubMed] [Google Scholar]
- 6.Cushion MT, Stringer JR, Walzer PD. Cellular and molecular biology of Pneumocystis carinii. Int Rev Cytol. 1991;131:59–107. doi: 10.1016/s0074-7696(08)62017-3. [DOI] [PubMed] [Google Scholar]
- 7.Hong ST, Park YK, Kim J, et al. Is Pneumocystis carinii vertically transmitted to neonatal rats? Korean J Parasitol. 1999;37:149–156. doi: 10.3347/kjp.1999.37.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JJ, Warren KS, Wamboldt DD, Wang T, Lin JL. Tropomyosin isoforms in nonmuscle cells. Int Rev Cytol. 1997;170:1–38. doi: 10.1016/s0074-7696(08)61619-8. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto Y, Yoshida Y. Sporogony in Pneumocystis carinii: synaptonemal complexes and meiotic nuclear divisions observed in precysts. J Protozool. 1984;31:420–428. doi: 10.1111/j.1550-7408.1984.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 10.Newsome AL, Durkin MM, Bartlett MS, Smith JW. Videomicroscopic recording of Pneumocystis carinii motion. J Protozool. 1991;38:207S–208S. [PubMed] [Google Scholar]
- 11.Ruffolo JJ, Cushion MT, Walzer PD. Ultrastructural observations on life cycle stages of Pneumocystis carinii. J Protozool. 1989;36:53S–54S. doi: 10.1111/j.1550-7408.1989.tb02697.x. [DOI] [PubMed] [Google Scholar]
- 12.Shiota T. Morphology and development of Pneumocystis carinii observed by phasecontrast microscopy and semiultrathin section light-microscopy. Jpn J Parasitol. 1984;33:443–455. [Google Scholar]
- 13.ul Haque A, Plattner SB, Cook RT, Hart MN. Pneumocystis carinii. Taxonomy as viewed by electron microscopy. Am J Clin Pathol. 1987;87:504–510. doi: 10.1093/ajcp/87.4.504. [DOI] [PubMed] [Google Scholar]
- 14.Vavra J, Kucera K. Pneumocystis carinii Delanoe its ultrastructure and ultrastructural affinities. J Protozool. 1970;17:463–483. doi: 10.1111/j.1550-7408.1970.tb04715.x. [DOI] [PubMed] [Google Scholar]
- 15.Vossen ME, Beckers PJ, Stadhouders AM, et al. Microtubules in Pneumocystis carinii. Z Parasitenkd. 1976;49:291–292. doi: 10.1007/BF00380598. [DOI] [PubMed] [Google Scholar]
- 16.Vossen ME, Beckers PJ, Stadhouders AM, et al. New aspects of the life cycle of Pneumocystis carinii: an alternative view on the life cycle of parasite. Z Parasitenkd. 1977;51:213–217. doi: 10.1007/BF00500960. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida Y, Shiota T, Yamada M, Matsumoto Y. Further light microscopic studies on morphology and development of Pneumocystis carinii. Zentralbl Bakteriol Mikrobiol Hyg A. 1981;250:213–218. [PubMed] [Google Scholar]
- 18.Yu JR, Chai JY. Localization of actin and myosin in Cryptosporidium parvum using immunogold staining. Korean J Parasitol. 1995;33:155–164. doi: 10.3347/kjp.1995.33.3.155. [DOI] [PubMed] [Google Scholar]
- 19.Webb SE, Fowler RE, O'Shaughnessy C, et al. Contractile protein system in the asexual stages of the malaria parasite Plasmodium falciparum. Parasitology. 1996;112:451–457. doi: 10.1017/s0031182000076915. [DOI] [PubMed] [Google Scholar]