Abstract
Excretory/secretory proteins (ESP) from Toxoplasma gondii were analyzed to define the function in the penetration process into host cells. Whole ESP obtained at 37℃ were composed of 15 bands with molecular mass of 110, 97, 86, 80, 70, 60, 54, 42, 40, 36, 30, 28, 26, 22, and 19 kDa. Five ESP of 86, 80, 42, 36, and 28 kDa were reacted with monoclonal antibodies (mAb), named as Tg386 (microneme), Tg485 (surface membrane), Tg786 (rhoptry), Tg378, and Tg556 (both dense granules), respectively. The ESP was released by a temperature-dependent/-independent manner and all at once whenever ready to pour out except Tg786. Each ESP was not exhausted within the parasite but the amount was limited. Tg786 was released continuously with increment, whereas Tg378 and Tg556 were ceased to release after 3 and 4 hr. Dense granular Tg378 and Tg556 were released spontaneously and constitutively before the entry into host cells also. The entry of T. gondii was inhibited by all the mAbs differentially. And the parasite deprived of ESP was inhibited to enter exponentially up to 90.1%. It is suggested that ESP play an essential function to provide appropriate environment for the entry of the parasite into host cells.
Keywords: Toxoplasma gondii, excretory/secretory proteins, temperature-dependent, monoclonal antibody, entry inhibition
INTRODUCTION
Toxoplasma gondii is a world-widely distributed protozoan parasite that infects wide range of warm-blooded animals, including humans. T. gondii can cause severe disease in healthy persons (Choi et al., 1997) in addition to the immunocompromised individuals such as AIDS patients and in newborns during congenital infection. Although most infections are asymptomatic and self-limiting in immunocompetent hosts, these individuals remain chronically infected, which may result in the symptom by reactivation of the tissue-cyst (Kim et al., 2000).
Decoster et al. (1988) first described the recognition of several excretory and secretory antigens (ESA) of T. gondii by sera of toxoplasmosis patients. Since then, many reports have been focused on the ESA as targets of protective cell-mediated immunity (Darcy et al., 1988; Duquesne et al., 1990; Rahmah and Anuar, 1992; Zenner et al., 1999) with little information of each ESA such as the original localities and approximate molecular weights. Recently, cell biological (Cesbron-Delauw and Capron, 1993; Ossorio et al., 1994; Hoppe et al., 2000) and biochemical (Mercier et al., 1998; Nockemann et al., 1998) approaches have classified major proteins of T. gondii into the subcellular components of the parasite. Prigione et al. (2000) demonstrated the T cell clones of protective immunity against components of ESA, especially GRA2 and SAG1.
Now, almost all components of ESA are known as released from highly specialized secretory organelles of T. gondii, micronemes, rhoptries, and dense granules, which function in the penetrating process and during subsequent intracellular interactions within the parasitophorous vacuole (PV). Their secretions are well concerted with a sequential manner (Carruthers and Sibley, 1997; Ngo et al., 2000). First, micronemal proteins are released at the time of apical attachment to the surface of host cells (Fourmaux et al., 1996a). Discharge of rhoptry proteins is followed into the nascent vacuole that localizes at the forming parasitophorous vacuolar membrane (PVM) (Sinai and Joiner, 1997). And then dense granular proteins are secreted into the PV and PVM continuously during the intracellular residence of the parasite (Leriche and Dubremetz, 1990; Cesbron-Delauw, 1994). In this work, we attempted to profile the production, subcellular localization, and exhaustion of excretory/secretory proteins (ESP). And also, we pursued the possible role of the ESP on the penetrating activity of T.gondii into host cells.
MATERIALS AND METHODS
Parasite
The RH strain of T. gondii was maintained by peritoneal passages in Balb/c mice. Tachyzoites were purified by centrifugation over 40% Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) in PBS solution (Sohn and Nam, 1999).
Preparation of ESP
Purified tachyzoites (3×108) were incubated at 37℃ for 1 hr under mild agitation in 1.0 ml Hank's balanced salt solution (Gibco BRL, Rockville, MD). After centrifugation for 5 min at 6,000 rpm, the supernatant was saved as ESP.
Monoclonal antibodies and mouse serum
Among our monoclonal antibody (mAb) panel, Tg386, Tg485, Tg786, Tg378, and Tg556 clones reacted with ESP in western blot specifically. And Tg563 clone was used as a control of the major surface membrane protein (SAG1). MAbs were used as ascitic fluid formed after injection of hybridoma clones into Balb/c mouse. For the positive reference serum, mouse was infected with Me49 strain of T. gondii for 8 weeks. Serum was saved from the mouse that had brain cysts of Me49 post-mortem.
Western blot
Western blot was performed by the method of Towbin et al. (1979). ESP was separated in 12% SDS-PAGE gels and transferred onto nitrocellulose sheets (NC, Schlleicher and Shuell, Keene, NH). NC papers blocked by 5% skim milk in PBS/0.05% Tween-20 were incubated with mAbs of 1:1,000 diluted, and then with 1:2,000 diluted HRP-conjugated goat anti-mouse IgG antibody (Cappel, Costa Mesa, CA). They were soaked in enhanced chemiluminescence (ECL) solution (Intron, Daejon, Korea) for 1 min and exposed to an X-ray film (Konica, Tokyo, Japan).
Cell culture and immunofluorescence assay (IFA)
Vero cells (CRL 6318, American Type Culture Collection, Rockville, MD) and A549 cells (CCL 185, ATCC) were maintained in DMEM supplemented with 10% FBS (Gibco BRL). Cells cultured on 18 mm coverslips in 24-well plates were infected with tachyzoites for 24 hr. IFA was done according to the procedure of Sinai et al. (1997). Cells were fixed either with cold absolute methanol for 5 min or with 3% paraformaldehyde for 10 min and then permeabilized by 0.05% Triton X-100 for 5 min, separately. mAbs were diluted in 1:100 of 3% BSA/PBS and FITC-conjugated goat anti-mouse IgG antibody (Sigma Chem. Co., St. Louis, MO) was used in 1:500. Fluorescence was observed under a fluorescence microscopy (Axiophot, Carl Zeiss Co., Oberkochen, Germany).
Penetrating activity of T. gondii into host cells
Host cell entry of T. gondii was assayed by the method of Nam et al. (1990). Host cells on the coverslips were cultured with excess number (about ×107) of T. gondii for 2 hr. The number of parasites was counted at 10 sights under a light microscope after staining with Giemsa solution. Percent inhibition to control was calculated with the fomula: [1 - (No. of T. gondii per host cell treated/No. of T. gondii per host cell untreated)] × 100.
RESULTS
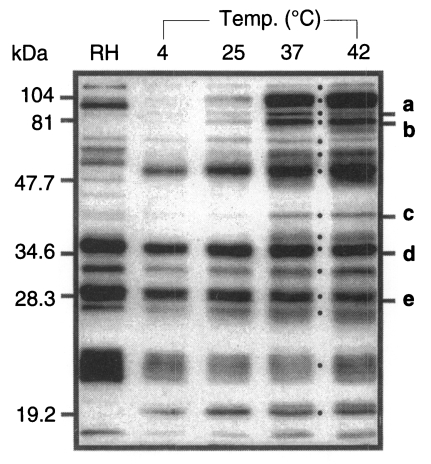
When detected using a reference serum from a mouse infected with Me49 strain, 15 bands were appeared as the whole ESP of T. gondii as the temperature reached 37℃ (Fig. 1). The molecular mass of ESP were estimated as 110, 97, 86, 80, 70, 60, 54, 42, 40, 36, 30, 28, 26, 22, and 19 kDa as dotted besides the lane of 37℃. Among them, 110, 97, 86, 80, 60, 42, and 40 kDa proteins were released temperature-dependently while those of 70, 54, 36, 30, 28, 26, 22, and 19 kDa was released temperature-independently as low as 4℃.
Fig. 1.
Profile of ESP of Toxoplasma gondii at various temperatures. Western blot was reacted with a reference serum from a mouse infected with Me49 strain. RH, tachyzoites; 4, 25, 37, and 42, temperature to incubate tachyzoites for 1 hr. Five ESP (a to e) were detected by mAbs (a, Tg386; b, Tg485; c, Tg786; d, Tg378; and e, Tg556) among 15 bands released at 37℃ indicated by dots.
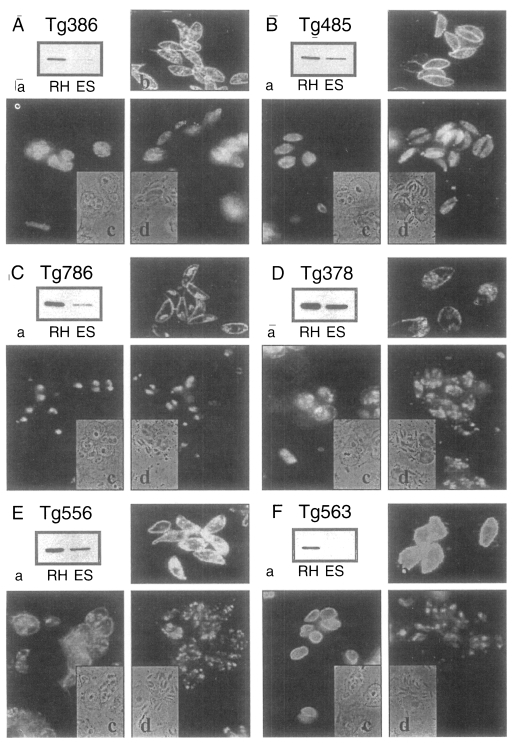
Among the ESP of T. gondii, 5 proteins of 86, 80, 42, 36, and 28 kDa were detected in western blot by mAbs of Tg386, Tg485, Tg786, Tg378, and Tg556 clones (labeled as a to e in Fig. 1) of our mAb panel, respectively. Tg386 clone detected the 86 kDa protein (Fig. 2Aa) and labeled presumably micronemal structure in tachyzoites (Fig. 2Ab). There was no secretion into PVM in methanol fixed intracellular stage (Fig. 2Ac) and apical portion of the parasite was labeled in membrane permeabilzed paraformaldehyde fixed intracellular stage (Fig. 2Ad). Tg485 clone detected the 80 kDa protein and labeled presumably surface protein without involvement into PVM (Fig. 2B). And Tg786 clone detected the 42 kDa protein and labeled rhoptry in the apical portion without secretion into PVM (Fig. 2C). Meanwhile, Tg378 detected the 36 kDa protein (Fig. 2Da) and labeled dense granule (Fig. 2Db) which was secreted into PVM (Fig. 2Dc) and destroyed by permeabilization with Triton X-100 (Fig. 2Dd). And Tg556 detected the 28 kDa protein in dense granule with involvement into PVM (Fig. 2E) as the same pattern of Tg378. For the control of a surface membrane protein, Tg563 clone was used to detect the 30 kDa SAG1 protein (Fig. 2F).
Fig. 2.
Western blots and immunofluorescence images of ESP detected by mAbs. A, ESP detected by Tg386; B, by Tg485; C, by Tg786; D, by Tg378; E, Tg556; and F, SAG1 by Tg563 for control. a, western blot of RH extract (RH) and ESP (ES) by mAbs; b, IFA image of extracellular RH tachyzoites; c, IFA image by the mAb in methanol fixed Vero cells infected with tachyzoites; and d, in paraformaldehyde fixed and Triton X-100 permeabilized Vero cells infected with tachyzoites, respectively.
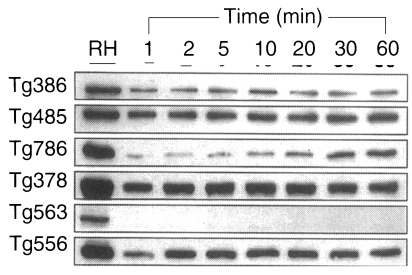
ESP detected by Tg386, Tg485, Tg378, and Tg556 clones were released as early as 1 min without increment in the amount during the experimental time span of 1 hr, while ESP detected by Tg786 clone was secreted after 10 to 20 min with rapid increment in amount (Fig. 3). And ESP of Tg386 and Tg485 clones released as soon as the temperature reached 37℃, whereas those of Tg378 and Tg556 clones released regardless of temperature and time.
Fig. 3.
ESP production at 37℃ detected by mAbs of Fig. 2.
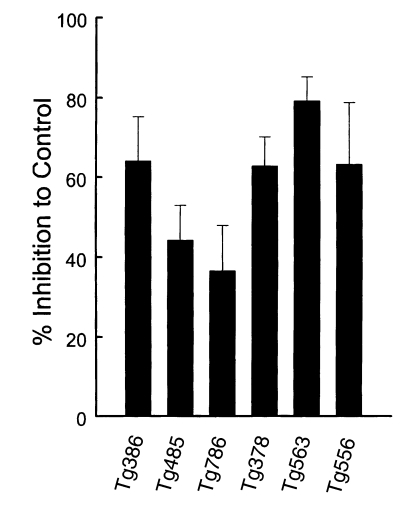
In order to confirm the participation of ESP in the entry mechanism of T. gondii, mAbs were treated in the media before challenge into host cells. The entry of T. gondii was inhibited with Tg386, Tg378, and Tg556 clones by 64.0%, 62.8%, and 63.3%, and with Tg485 and Tg786 clones by 44.3% and 36.6%, respectively (Fig. 4). High inhibition by Tg563 clone might be resulted from the aggregation of T. gondii by binding to the surface membrane proteins each other.
Fig. 4.
Percent inhibition of penetrating activity of Toxoplasma gondii into host cells by mAbs. MAbs were treated in the culture medium before challenge with T. gondii.
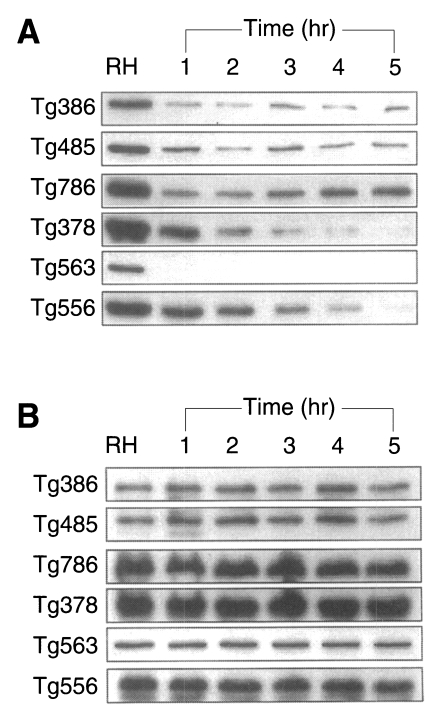
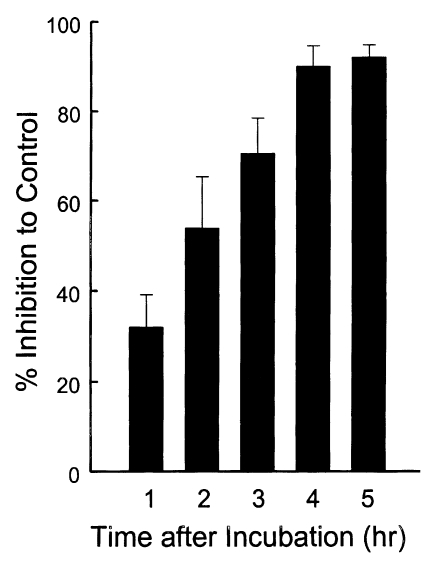
To define whether the ESP was released continuously or not, the incubation medium was changed by new one at every hour for 5 times (Fig. 5). ESP detected by Tg386 and Tg485 clones were released continuously at every change even though a trace level. Meanwhile, ESP by Tg786 clone was released continuously with increment, whereas those by Tg378 and Tg556 clones were ceased to release after 3 and 4 hr changes, respectively, as shown in Fig. 5A. During this time span, there were no changes in residual ESP inside T. gondii (Fig. 5B). And the penetration of T. gondii deprived of ESP was reduced exponentially up to 90.1% at 4 hr (Fig. 6).
Fig. 5.
A. ESP production at 37℃ for 1 hr pulsely. Tachyzoites were incubated with new media at every hour for 5 times. B. Residual tachyzoite proteins after ESP production at the same time of (A).
Fig. 6.
Percent inhibition of penetrating activity of Toxoplasma gondii into host cells by the incubation time to deprive of the ESP from T. gondii prior to challenge.
DISCUSSIONS
The ESP from T. gondii was released by temperature-dependent and -independent manner. The molecular mass of 15 ESP at 37℃ were estimated approximately as 110, 97, 86, 80, 70, 60, 54, 42, 40, 36, 30, 28, 26, 22, and 19 kDa, which were compatible with ESA of T. gondii described previously (Darcy et al., 1988; Duquesne et al., 1990) with minor differences. At a glance, although the exact functions of ESP are still unknown, the profile of temperature-dependent ESP production implies T. gondii as a parasite of warm-blooded animals.
During the ESP preparation, surface membrane protein (SAG1) was not detected by Tg563, which minimized the possible contamination of ESP with somatic cell debris (Prigione et al., 2000). Among the 5 proteins of 86, 80, 42, 36, and 28 kDa detected by mAbs of Tg386, Tg485, Tg786, Tg378, and Tg556 clones, only Tg786 were released much longer with rapid increment in amount. It suggests that the ESP are released all at once whenever ready to pour out except that of Tg786 clone. And Tg786 clone was released continuously with increment, whereas those by Tg378 and Tg556 clones were ceased to release after 3 and 4 hr changes, without changes in residual ESP inside T. gondii. This suggests that each ESP is not exhausted within the parasite but the releasing amount of ESP is limited.
ESP detected by Tg378 and Tg556 clones, which localized in dense granules, also were released spontaneously and constitutively. These mAbs inhibited the penetration of T. gondii stronger than the others. In addition, release of these proteins was ceased within 3 or 4 hr, when the penetrating activity of T. gondii reduced rapidly. The term 'excretory/secretory' on the dense granular proteins has been used conventionally the secretion into the PV or PVM formed within the host cells. Although all the dense granular proteins (GRA1-8, Mevelec et al., 1992; Bermudes et al., 1994; Sibley et al., 1995; Fischer et al., 1998; Lecordier et al., 1999; Carey et al., 2000) has not been examined, it is suggested that the dense granular proteins function in the process of penetration into host cells in addition to function during the intracellular stage of this parasite based on our observations.
Many secreted T. gondii proteins apparently associate tightly with membrane after secretion. Microneme (Fourmaux et al., 1996b; Wan et al., 1997) and rhoptry (Hoppe et al., 2000) proteins contain linear hydrophobic sequences with the potential to insert into host cell plasma membrane. Dense granular proteins contain also linear hydrophobic sequences with the potential to insert into PV or PVM (Mercier et al., 1998; Fischer et al., 1998; Lecordier et al., 1999). Dense granular ESP released from extracellular T. gondii may be targeted to the plasma membrane of the host cells also, which provide appropriate environment for the penetration of the parasite in concert with microneme and rhoptry proteins. ESP detected by Tg386 clone (micronemal protein), Tg485, and Tg786 (rhoptry protein) seems to be well regulated by a cascade exocytosis to favor the parasite penetrating into host cells.
The entry of T. gondii was inhibited by treating the parasite with mAbs against ESP before challenge into host cells. And the penetration of T. gondii deprived of ESP was reduced exponentially up to 90.1%. It is suggested that ESP function actually in the entry of T. gondii. Differential inhibition by mAbs implies that each ESP has its specific role in penetration into host cells.
Footnotes
This work was supported by grant No. 1999-1-20200-002-2 from the Basic Research Program of the Korea Science & Engineering Foundation.
References
- 1.Bermudes D, Dubremetz JF, Achbarou A, Joiner KA. Cloning of a cDNA encoding the dense granule protein GRA3 from Toxoplasma gondii. Mol Biochem Parasitol. 1994;68:247–257. doi: 10.1016/0166-6851(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 2.Carey KL, Donahue CG, Ward GE. Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol Biochem Parasitol. 2000;105:25–37. doi: 10.1016/s0166-6851(99)00160-7. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 4.Cesbron-Delauw MF, Capron A. Excreted/secreted antigens of Toxoplasma gondii - their origin and role in the host-parasite interaction. Res Immunol. 1993;144:41–44. doi: 10.1016/s0923-2494(05)80096-3. [DOI] [PubMed] [Google Scholar]
- 5.Cesbron-Delauw MF. Dense-granule organelles of Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today. 1994;10:293–296. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 6.Choi WY, Nam HW, Kwak NH, et al. Foodborne outbreaks of human toxoplasmosis. J Infect Dis. 1997;175:1280–1282. doi: 10.1086/593702. [DOI] [PubMed] [Google Scholar]
- 7.Darcy F, Deslee D, Santoro F, et al. Induction of a protective antibody-dependent response against toxoplasmosis by in vitro excreted/secreted antigens from tachyzoites of Toxoplasma gondii. Parasite Immunol. 1988;10:553–567. doi: 10.1111/j.1365-3024.1988.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 8.Decoster A, Darcy F, Capron A. Recognition of Toxoplasma gondii excreted and secreted antigens by human sera from acquired and congenital toxoplasmosis: identification of markers of acute and chronic infection. Clin Exp Immunol. 1988;73:376–382. [PMC free article] [PubMed] [Google Scholar]
- 9.Duquesne V, Auriault C, Darcy F, Decaval JP, Capron A. Protection of nude rats against Toxoplasma infection by excreted-secreted antigen-specific helper T cells. Infect Immun. 1990;58:2120–2126. doi: 10.1128/iai.58.7.2120-2126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer HG, Stachelhaus S, Sahm M, Meyer HE, Reichmann G. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol Biochem Parasitol. 1998;91:251–262. doi: 10.1016/s0166-6851(97)00227-2. [DOI] [PubMed] [Google Scholar]
- 11.Fourmaux MN, Achbarou A, Mercereau-Puijalon O, et al. The MIC1 microneme protein of Toxoplasma gondii contains a duplicated receptor-like domain and binds to host cell surface. Mol Biochem Parasitol. 1996a;83:201–210. doi: 10.1016/s0166-6851(96)02773-9. [DOI] [PubMed] [Google Scholar]
- 12.Fourmaux MN, Garcia-Reguet N, Mercereau-Puijalon O, Dubremetz JF. Toxoplasma gondii microneme proteins: gene cloning and possible function. Curr Top Microbiol Immunol. 1996b;219:55–58. doi: 10.1007/978-3-642-51014-4_5. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe HC, Ngo HM, Yang M, Joiner KA. Targeting to rhoptry organelles of Toxoplasma gondii involves evolutionarily conserved mechanisms. Nature Cell Biol. 2000;2:449–456. doi: 10.1038/35017090. [DOI] [PubMed] [Google Scholar]
- 14.Kim MH, Choi YK, Park YK, Nam HW. A toxoplasmic uveitis case of a 60-year-old male in Korea. Korean J Parasitol. 2000;38:29–31. doi: 10.3347/kjp.2000.38.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecordier L, Mercier C, Sibley LD, Cesbron-Delauw MF. Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Mol Biol Cell. 1999;10:1277–1287. doi: 10.1091/mbc.10.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leriche MA, Dubremetz JF. Exocytosis of Toxoplasma gondii dense granules into the parasitophorous vacuole after host cell invasion. Parasitol Res. 1990;76:359–362. doi: 10.1007/BF00932560. [DOI] [PubMed] [Google Scholar]
- 17.Mercier C, Cesbron-Delauw MF, Sibley LD. The amphipathic alpha helices of the Toxoplasma protein GRA2 mediate post-secretory membrane association. J Cell Sci. 1998;111:2171–2180. doi: 10.1242/jcs.111.15.2171. [DOI] [PubMed] [Google Scholar]
- 18.Mevelec MN, Chardes T, Mercereau-Puijalon O, et al. Molecular cloning of GRA4, a Toxoplasma gondii dense granule protein, recognized by mucosal IgA antibodies. Mol Biochem Parasitol. 1992;56:227–238. doi: 10.1016/0166-6851(92)90172-g. [DOI] [PubMed] [Google Scholar]
- 19.Nam HW, Youn JH, Kim DJ, Choi WY. Tight junctional inhibition of entry of Toxoplasma gondii into MDCK cells. Korean J Parasitol. 1990;28:197–205. doi: 10.3347/kjp.1990.28.4.197. [DOI] [PubMed] [Google Scholar]
- 20.Ngo HM, Hoppe HC, Joiner KA. Differential sorting and post-secretory targeting of proteins in parasitic invasion. Trends Cell Biol. 2000;10:67–72. doi: 10.1016/s0962-8924(99)01698-0. [DOI] [PubMed] [Google Scholar]
- 21.Nockemann S, Dlugonska H, Henrich B, Kitzerow A, Daubener W. Expression, characterization and serological reactivity of a 41 kDa excreted-secreted antigen (ESA) from Toxoplasma gondii. Mol Biochem Parasitol. 1998;97:109–121. doi: 10.1016/s0166-6851(98)00138-8. [DOI] [PubMed] [Google Scholar]
- 22.Ossorio PN, Dubremetz JF, Joiner KA. A soluble secretory protein of the intracellular parasite Toxoplasma gondii associates with the parasitophorous vacuole membrane through hydrophobic interactions. J Biol Chem. 1994;269:15350–15357. [PubMed] [Google Scholar]
- 23.Prigione I, Facchetti P, Lecordier L, et al. T cell clones raised from chronically infected healthy humans by stimulation with Toxoplasma gondii excretory-secretory antigens cross-react with live tachyzoites: characterization of the fine antigenic specificity of the clones and implications for vaccine development. J Immunol. 2000;164:3741–3748. doi: 10.4049/jimmunol.164.7.3741. [DOI] [PubMed] [Google Scholar]
- 24.Rahmah N, Anuar AK. Demonstration of antigenic similarities and variations in excretory/secretory antigens of Toxoplasma gondii. Biochem Biophys Res Commun. 1992;187:294–298. doi: 10.1016/s0006-291x(05)81491-3. [DOI] [PubMed] [Google Scholar]
- 25.Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J Cell Sci. 1995;108:1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- 26.Sinai AP, Joiner KA. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu Rev Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 27.Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110:2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 28.Sohn WM, Nam HW. Western blot analysis of stray cat sera against Toxoplasma gondii and the diagnostic availability of monoclonal antibodies in sandwich-ELISA. Korean J Parasitol. 1999;37:249–256. doi: 10.3347/kjp.1999.37.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan KL, Carruthers VB, Sibley LD, Ajioka JW. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol Biochem Parasitol. 1997;84:203–214. doi: 10.1016/s0166-6851(96)02796-x. [DOI] [PubMed] [Google Scholar]
- 31.Zenner L, Estaquier J, Darcy F, Maes P, Capron A, Cesbron-Delauw MF. Protective immunity in the rat model of congenital toxoplasmosis and the potential of excreted-secreted antigens as vaccine components. Parasite Immunol. 1999;21:261–272. doi: 10.1046/j.1365-3024.1999.00229.x. [DOI] [PubMed] [Google Scholar]