Abstract
Cutaneous larva migrans (CLM) is a rare serpiginous cutaneous eruption caused by accidental penetration and migration in the skin with infective larvae of nematode that normally do not have the human as their host. Although CLM has a worldwide distribution, the infection is most frequent in warmer climates. More recently, they have been increasingly imported from the tropics or subtropics by travelers. We experienced two patients who had pruritic serpiginous linear eruption in their skin for a few weeks after traveling to the endemic areas (Brazil and Thailand, respectively). After the treatment with albendazole, the skin lesions resolved with post-inflammatory hyperpigmentation. We report herein two cases of cutaneous larva migrans successfully treated with albendazole.
Keywords: Larva migrans, albendazole
INTRODUCTION
Cutaneous larva migrans (CLM), also known as "creeping eruption," is a distinctive dermatitis caused by infectious larvae of various animal nematodes. The migration of these larvae through the skin causes an eruption of pruritic, serpiginous papulo-vesicular lesions (Thune, 1972; Edelglas et al., 1982; Herbener and Borak, 1988). The organisms that cause CLM are ubiquitous, but are endemic to areas with warm and humid climates, such as the Caribbean, Africa, South America, and Southeast Asia (Davies et al., 1993). More recently they have been increasingly imported from the tropics or subtropics by travelers. We experienced two patients who had eruptions for a few weeks after traveling to the endemic areas. CLM has been treated by physical modalities (freezing the skin with ethyl chloride or solid carbon dioxide), topical drugs, and systemic drugs (Kurgansky and Burnett, 1990). Albendazole has been shown to be effective in the treatment of CLM (Rizzitelli et al.,1997). We report herein the two cases of CLM which were successfully treated with albendazole.
CASE RECORDS
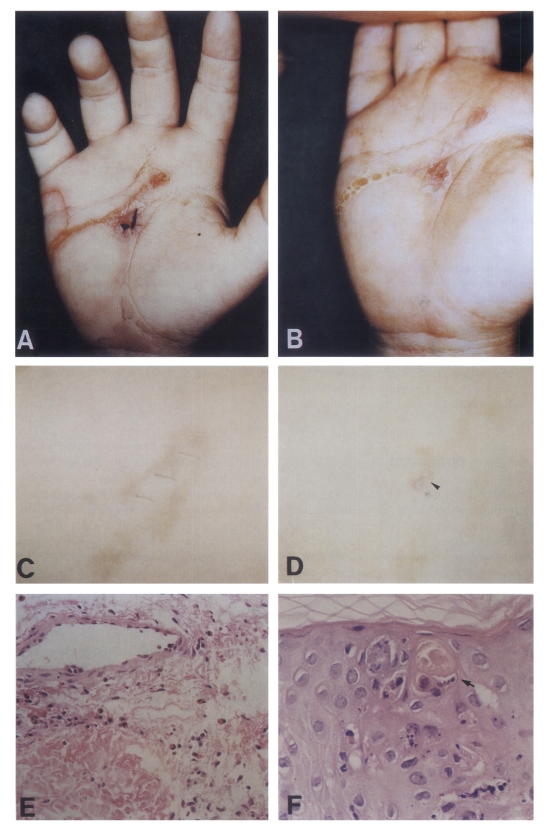
The first patient was a 4-year-old boy who had an eruption on his right palm for two weeks after he had traveled to Brazil for 40 days from April to May, 1999. A physical examination revealed an intensively pruritic, erythematous serpiginous linear skin lesion located on the right palm (Fig. 1A), which had an appearance of a small papule a week prior to his visit. The laboratory findings revealed a leukocyte count of 9,420/mm3 with 4.3% eosinophils and an eosinophil count of 320/mm3. A stool examination showed no ova or parasites. Initial treatment with systemic antihistamine was not helpful. Later, he was treated with albendazole, 10 mg/kg. After three days of albendazole treatment, the lesions improved (Fig. 1B).
Fig. 1.
Linear to serpiginous lesions on the right palm of the first patient (A), and on the right thigh of the second patient before treatment (C). Each of the skin lesions has improved after albendazole treatment (B & D). An arrowhead (D) is the biopsy site. Mild perivascular infiltration of lymphocytes mixed with many eosinophils is observed in the biopsy tissue of the first patient (E, H-E stain, × 200). Cross-sectioned larvae (arrow) are found in the epidermis of the second patient (F, H-E stain, × 400).
The second patient was a 27-year-old female who had an eruption on her right thigh for three weeks after she had traveled to Thailand on November, 1999. On physical examination, there was a pruritic, erythem-atous serpiginous linear skin lesion located on her right thigh (Fig. 1C). The laboratory findings revealed a leukocyte count of 7,500/mm3 with 5.4% eosinophil and an eosinophil count of 391/mm3. A stool examination showed no ova or parasites. She was treated with albendazole, 400 mg/ day for 3 days. After a week of albendazole treatment, the lesions were improved (Fig. 1D).
A histological examination of the first case showed only intraepidermal bullae with mild perivascular lymphocytic infiltrations without any larva (Fig. 1E). In case of the second patient, burrows in the epidermis which had larva (Fig. 1F) was observed.
DISCUSSION
Cutaneous larva migrans are caused by the invasion into skin of the larvae of certain nematodes parasitic in animals. The most common causative nematode is Ancylostoma braziliensis, a cat and dog hookworm (Miller, 1979; Allen, 1990). Other nematodes, such as Ancylostoma caninum (dog hookworm), A. tubaeformis (cat hookworm), Uncinaria stenocephala (European dog hookworm), Bunostomum phlebotomum (cattle hookworm), Gnathostomum spinigerum and Strongyloides stercoralis can also cause CLM (Edelglas et al., 1982; Herbener and Borak, 1988).
Six cases have hitherto been recorded in Korea since the first report in 1995 (Jang et al., 1995; Lim et al., 1996; Lee et al., 1998; Kim et al., 1999; Chang et al., 1999). Thus, the two patients in this report are the seventh and eighth human cases of CLM in Korea. Six cases including these two cases occurred after traveling to Thailand, Indonesia, and Brazil.
The infection occurs when humans come in contact with infected soil. The larvae penetrate the epidermis, producing a pruritic, erythematous, and urticarial papule or papulovesicle at the site of the entry (Edelglas et al., 1982). A 2- to 3-mm-wide serpiginous or linear erythematous tracts develop as the larvae migrate through the skin. With the larvae about 1 cm in length from the advancing border, the tract becomes lengthened by 2 to 3 cm daily (Edelglas et al., 1982; Davies et al., 1993). Because of the elaboration of toxic antigens, pruritus gets severe during this migratory phase. Most patients with CLM have a peripheral eosinophilia. Diagnosis is usually made on the bases of history and clinical evaluation, since laboratory tests are often not helpful.
While the clinical features of CLM are distinctive and characteristic, the histopathologic changes are not. Biopsy specimens of a erythematous tract reveal tunnels confined to the epidermis. The larvae are usually found in tunnels at the dermoepidermal junction. Usually, identification of the species of the parasite is not possible in tissue section. Lateral double alae of Ancylostoma sp. can be rarely observed. (Meyers and Neafie, 1976; Orihel and Ash, 1995). In this report, it was fortunate that we found a cross-sectioned larva in the second case, albeit we could not observe the double alae. Hematoxylin and Eosin stain reveals a mild and perivascular dermal infiltrate. It is primarily comprised of polymorphonuclear leukocytes and eosinophils, with occasional lymphocytes and plasma cells (Elder et al., 1997). These two cases would be cutaneous larva migrans by clinical featutes and histopathologic examination although larva could not find in the first case.
Cutaneous larva migrans usually shows self-limited course, since human is an accidental host. If untreated, larvae usually die within 2 to 8 weeks, but occasionally can persist for up to a year (Edelglas et al., 1982). For the symptomatic disease, local measures such as Burow's soaks and topical antibiotics, in addition to systemic antihistamine therapy are often helpful (Jacksonville Dermatology Society, 1965). Liquid nitrogen has been traditionally the mainstay of therapy for CLM among dermatologists. The larval track represents an allergic reaction to the larva and does not correlate with the exact location of the parasite. For this reason, the use of liquid nitrogen on the leading edge of the track is a very imprecise way to localize and kill the migrating larva (Kurgansky and Burnett, 1990). Treatment with topical and systemic thiabendazole appears to be highly successful (Edelglas et al., 1982; Jacksonville Dermatology Society, 1965; Stone and Mullins, 1965). However, side effects seen in oral thiabendazole administration include dizziness, anorexia, nausea, vomiting, and diarrhea, which resolve once the therapy is discontinued (Stone and Mullins, 1965). Ivermectin, a new derivative of avermectin B, looks very promising as a new therapeutic modality, but more experience is required before recommending it for routine use in treating CLM (Davies et al., 1993). Albendazole, a new benzimidazole derivative, has also been shown to be very effective in the treatment of CLM when given as a single 400 mg dose or 200 mg twice a day for 3 days. Side effects are infrequent and mild, and appear only after high-dosage and long-term administration (Williams and Monk, 1989; Jones et al., 1990; Rizzitelli et al., 1997). In this report, albendazole was given to a 4-year-old child at a single dosage of 10 mg/kg and to 27-year-old patient at a dosage of 400 mg/day for 3 days, respectively. The albendazole supposedly cured CLM of the two patients without adverse effects.
The number of travelers who take a trip to subtropic or tropic areas is increasing each year in Korea. Therefore CLM should be included in the differential diagnosis of patients who develop typical pruritic serpiginous lesion and have a recent travel history to a tropical or subtropical areas.
In conclusion, we described two cases of CLM in Korea, which were successfully treated with albendazole.
References
- 1.Allen C. Cutaneous larva migrans: a traveler's disease. NZ Med J. 1990;103:345. [PubMed] [Google Scholar]
- 2.Chang SE, Seo CW, Choi JH, Sung KJ, Moon KC, Koh JK. A case of cutaneous larva migrans showing a larva on biopsy. Korean J Dermatol. 1999;37:547–549. (in Korean) [Google Scholar]
- 3.Davies HD, Sakulus P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129:588–591. doi: 10.1001/archderm.129.5.588. [DOI] [PubMed] [Google Scholar]
- 4.Edelglas JW, Douglas MC, Stiefler R, Tessler M. Cutaneous larva migrans in a nothern climate. A souvenir of your dream vacation. J Am Acad Dermatol. 1982;7:353–358. doi: 10.1016/s0190-9622(82)70122-7. [DOI] [PubMed] [Google Scholar]
- 5.Elder D, Elenitsas R, Johnson B, Jr, Jaworsky C. Histopathology of the Skin. Philadelphia, USA: Lippincott-Raven; 1997. Parasitic infestations of the skin; p. 560. [Google Scholar]
- 6.Herbener D, Borak J. Cutaneous larva migrans in nothern climates. Am J Emerg Med. 1988;6:462–464. doi: 10.1016/0735-6757(88)90247-1. [DOI] [PubMed] [Google Scholar]
- 7.Jacksonville Dermatology Society. Creeping eruption treated with thiabendazole. Arch Dermatol. 1965;91:425–426. [PubMed] [Google Scholar]
- 8.Jang IK, Lee DW, Cho BK. A case of cutaneous larva migrans. Korean J Dermatol. 1995;33:396–400. (in Korean) [Google Scholar]
- 9.Jones S, Reynolds N, Oliwiecki S, Harman R. Oral albendazole for the treatment of cutaneous larva migrans. Br J Dermatol. 1990;122:99–101. doi: 10.1111/j.1365-2133.1990.tb08245.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim JW, Kim DJ, Kim IH, Song HJ, Oh CH. A case of cutaneous larva migrans. Korean J Dermatol. 1999;37:423–426. (in Korean) [Google Scholar]
- 11.Kurgansky D, Burnett JW. Creeping eruption. Cutis. 1990;45:399–400. [PubMed] [Google Scholar]
- 12.Lee SJ, Moon TK, Hann SK. Two cases of cutaneous larva migrans. Ann Dermatol. 1998;10:61–63. [Google Scholar]
- 13.Lim MH, Lee SC, Won YH, Jun IK. A case of creeping eruption. Korean J Dermatol. 1996;34:485–488. (in Korean) [Google Scholar]
- 14.Meyers WM, Neafie RC. Creeping eruption. In: Binford CH, Connor DH, editors. Pathology of Tropical and Extraordinary Diseases. Vol 2. Washington D.C., USA: Armed Forces Institute of Pathology; 1976. pp. 437–439. [Google Scholar]
- 15.Miller TA. Hookworm infection in man. Adv Parasitol. 1979;17:315–384. doi: 10.1016/s0065-308x(08)60552-7. [DOI] [PubMed] [Google Scholar]
- 16.Orihel TC, Ash LR. Parasites in Human Tissues. Chicago, USA: American Society of Clinical Pathologists; 1995. pp. 116–119. [Google Scholar]
- 17.Rizzitelli G, Scarabelli G, Veraldi S. Albendazole: a new therapeutic regimen in cutaneous larva migrans. Int J Dermatol. 1997;36:700–703. doi: 10.1046/j.1365-4362.1997.00263.x. [DOI] [PubMed] [Google Scholar]
- 18.Stone OJ, Mullins JF. Thiabendazole effectiveness in creeping eruption. Arch Dermatol. 1965;91:427–429. doi: 10.1001/archderm.1965.01600110013004. [DOI] [PubMed] [Google Scholar]
- 19.Thune PO. Creeping eruption of larva migrans. Int J Dermatol. 1972;11:231–232. doi: 10.1111/j.1365-4362.1972.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams H, Monk B. Creeping eruption stopped in its tracks by albendazole. Clin Exp Dermatol. 1989;14:355–356. doi: 10.1111/j.1365-2230.1989.tb02583.x. [DOI] [PubMed] [Google Scholar]