Abstract
A scanning electron microscopic study was performed on the surface ultrastructure of metacercariae and adults of Metagonimus takahashii. Metacercariae were collected from the scale of crucian carp (Carassius auratus), and adult flukes were harvested 1-4 weeks after infection to rats. In excysted metacercariae, the oral sucker had type I (numerous) and type II (seven in total) sensory papillae. Tegumental spines were dense and digitated into 5-7 points on the surface anterior to the ventral sucker, but became sparse and less digitated posteriorly toward the end of the body. In adults, seven type II sensory papillae were characteristically arranged around the lip of the oral sucker, and on the inner side of the lip four small and two large type I sensory papillae were symmetrically seen on each side (12 in total). Tegumental spines on anterior two-thirds of the body, were digitated with 9-12 tips ventrally and 8-13 tips dorsally. Sperms entering into the Laurer's canal were observed. The results show that the surface ultrastructure of M. takahashii is generally similar to those of M. yokogawai and M. miyatai except for the digitation of tegumental spines.
Keywords: Metagonimus takahashii, metacercaria, adult, surface ultrastructure, tegumental spine, sensory papilla
INTRODUCTION
Metagonimus takahashii was originally found from the small intestine of dogs experimentally fed with the flesh of freshwater fish (Takahashi, 1929). A year later, it was described as a new species based on two characteristics; having larger eggs than M. yokogawai (Katsurada, 1912) and taking cyprinoid fish but not sweetfish as the fish intermediate host (Suzuki, 1930). The validity of M. takahashii as a new species had long been debated (Asada, 1934; Ito, 1964). Recently, however, it was recognized as a distinct species based on differences in the morphology of both adults and eggs, and in its life cycle (Saito, 1984; Chai et al., 1993). In the meantime, M. miyatai was described as another distinct species (Saito et al., 1997). All of the three species of Metagonimus are distributed widely in Korea (Chai and Lee, 1990).
In order to support the validity of each species, studies on ultrastructures and on molecular and genetic characteristics are greatly needed. A polymerase chain reaction-based restriction fragment length polymorphism (PCR-RFLP) analysis, for example, suggested that M. takahashii and M. miyatai are genetically different from M. yokogawai (Yu et al., 1997).
Studying surface ultrastructures of trematodes are often helpful to know their taxonomic significance; Cryptocotyle lingua (Køie, 1977), Heterophyes aegualis (Taraschewski, 1984), M. yokogawai (Lee et al., 1984), M. miyatai (Chai et al., 1998), Neodiplostomum seoulense (Lee et al., 1985), Heterophyopsis continua (Hong et al., 1991) and Heterophyes nocens (Chai et al., 1992). It has been revealed that the surface ultrastructure varies according to different species, especially in terms of the size, shape, number and distribution of tegumental spines and sensory papillae, and the differentiation patterns during their development. However, the surface ultrastructure of M. takahashii has not been reported. The present study was performed to observe the surface ultrastructure of M. takahashii metacercariae and adults, and to find differences from those reported previously for M. yokogawai (Lee et al., 1984) and M. miyatai (Chai et al., 1998).
MATERIALS AND METHODS
Metacercariae of M. takahashii were collected from the scale of crucian carp (Carassius auratus) caught in Umsong-gun, Chungchongbuk-do (Province), by artificial digestion technique. Other procedures were essentially the same as described in our previous paper (Chai et al., 1998). Briefly, metacercariae for ultrastructural observations were excysted in trypsin solution (Tris buffer, pH 8.0) at 38℃. Adult flukes were harvested from the small intestine of Sprague-Dawley rats at weeks 1-4 post-infection (PI) with metacercariae. The excysted metacercariae and adult flukes were washed with 0.2 M cacodylate buffer (pH 7.3) and fixed in 2.5% glutaraldehyde. They were dehydrated, dried, and mounted on aluminum stubs, followed by coating with gold. The specimens were observed with a scanning electron microscope (DS-130C, ISI Co., Korea) at an accelerating voltage of 10 kV.
RESULTS
Excysted metacercariae
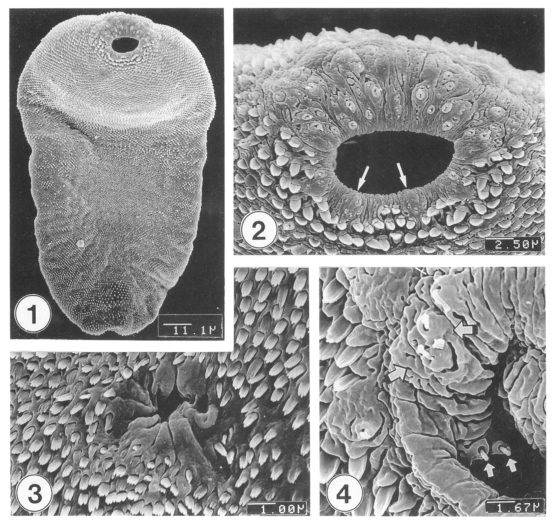
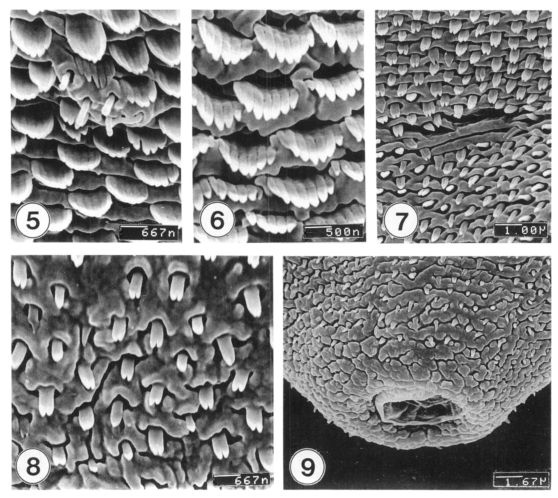
Excysted metacercariae was leaf-like, ventrally concave, ovoid or pyriform in shape (Fig. 1). The oral sucker was big, anteriorly subterminal (Fig. 2). The ventral sucker was positioned in the right side of the median line of the body (Fig. 3). The whole body surface was covered with flat cytoplasmic processes, numerous tegumental spines (Figs. 1-3), and sensory papillae (Figs. 2, 4). The lip of the oral sucker was devoid of spines (Fig. 2) and had many single or grouped type I sensory papillae (ciliated knob-like swellings) and seven type II sensory papillae (simple round swellings of the tegument). Inside the lip of the oral sucker, four small and two large type I papillae were symmetrically seen on each lateral side (Fig. 4), totalling 12 in number. Tegumental spines at the anterior half of the body were dense and digitated into 5-7 points on the ventral surface (Fig. 5) and 7-8 points on the dorsal one (Fig. 6). Around the Laurer's canal, spines were dense but less digitated (Fig. 7). The spines became much sparser and less digitated (twopoints) toward the posterior end of the body (Figs. 8-9). The excretory pore was opened terminally (Fig. 9).
Figs. 1-4.
Scanning electron microscopic (SEM) view of metacercariae of Metagonimus takahashii. Fig. 1. Ventral view of an excysted metacercaria. Fig. 2. Tegument around the oral sucker. Note distribution of seven type II sensory papillae (arrows) on the lip of the oral sucker. Fig. 3. Tegument around the ventral sucker showing dense distribution of tegumental spines. Fig. 4. Inner side of the lip of the oral sucker showing small and large type I sensory papillae (arrows).
Figs. 5-9.
SEM view of metacercariae of M. takahashii. Fig. 5. Tegumental spines and type I sensory papillae at ventral surface between oral and ventral suckers. Fig. 6. Spines at antero-dorsal surface having 7-8 pointed tips. Fig. 7. Dorsal tegument near the Laurer's canal. Fig. 8. Ventral tegument anterior to the excretory pore showing peg-like spines with two-pointed tips. Fig. 9. Tegument around the excretory pore. Spines are sparse, and cytoplasmic processes are cobblestone-like.
Adult flukes
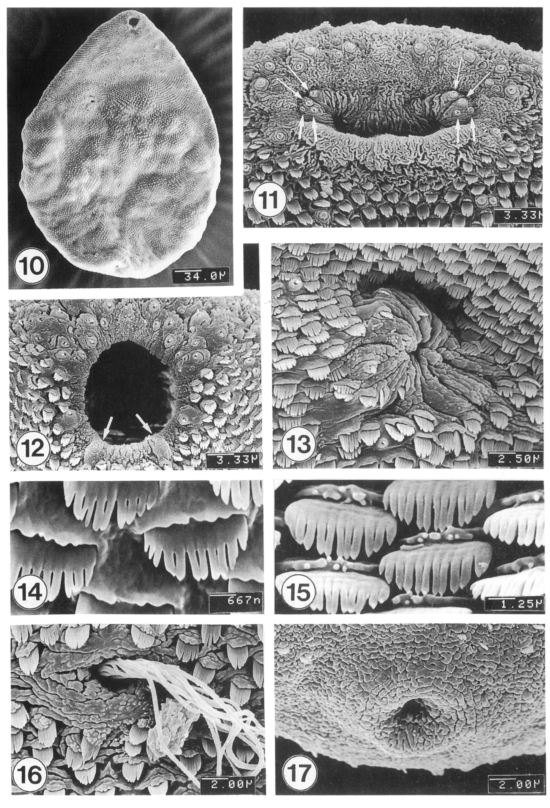
The adult flukes, 1-4 weeks old, became greatly enlarged, and ovoid or leaf-like in shape (Fig. 10). Their surface was covered with knob- or cobblestone-like cytoplasmic processes and tegumental spines. On the lip of the oral sucker, the array of the type I and type II sensory papillae were similar to that of the excysted metacercariae (Figs. 11-12). The ventral sucker was thick, muscular, and located in the right quadrant of the anterior body, having many wrinkles (Fig. 13).
Figs. 10-17.
SEM view of 1-4-week-old adults of M. takahashii. Fig. 10. Ventral view of a whole worm, 2-week-old. Fig. 11. Oral sucker typically showing two pairs each of large (thin arrows) and small type I papillae (thick arrows; other two pairs are hidden at ventrolateral sides) inside the oral sucker. Fig. 12. Tegument around the oral sucker showing type I and type II (seven in number; arrows) sensory papillae on the lip. Fig. 13. Tegument around the ventral sucker showing densely distributed spines. The ventral sucker is muscular and showing many wrinkles. Fig. 14. Ventral tegument between oral and ventral suckers showing broad, brush-like tegumental spines with 9-12 pointed tips. Fig. 15. Mid-dorsal tegument showing brush-like spines with 10-13 tips. Fig. 16. Dorsal tegument around the Laurer's canal. Many sperms are seen entering into the canal. Fig. 17. Tegument around the excretory pore showing very few spines.
The distribution pattern of tegumental spines was also very similar to that of the excysted metacercariae. The ventral surface extending from the anterior to the half way of the body were covered densely with large 9-12 pointed spines (Fig. 14), but near the posterior part, spines were sparse and had only 3-6 points. On the dorsal surface, spines were more easily differentiated into 8-13 points on the anterior one-third of the body and 10-13 points in the middle part (Fig. 15), but became less pointed into 5-7 tips on the posterior onethird of the body. Sperms were observed entering into the Laurer's canal (Fig. 16). The opening of the Laurer's canal was surrounded by several layers of concentric wrinkles forming sphincter-like structures. The excretory pore was anus-shape, devoid of spines, and the surrounding surface had irregular wrinkles (Fig. 17).
DISCUSSION
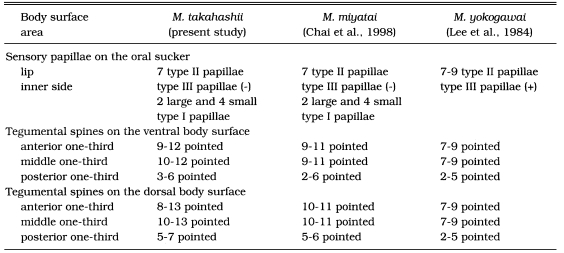
Topography of the surface ultrastructure of M. takahashii was generally similar to those of M. yokogawai (Lee et al., 1984) or M. miyatai (Chai et al., 1998). Especially the presence and characteristic arrangement of type II sensory papillae, seven in total, on the lip of the oral sucker seemed to be a common characteristic of the genus Metagonimus. In M. yokogawai adults, type III sensory papillae (round swelling of cytoplasmic ridges) were observed in the inner side of the lip of the oral sucker (Lee et al., 1984). For M. takahashii and M. miyatai adults (Chai et al., 1998), however, type III papillae were not observed. Instead, total of eight small and four large type I papillae were characteristically arranged at the lateral side of the inner lip of the oral sucker. The presence of these type I sensory papillae is unknown in M. yokogawai and should be verified in the future.
In the morphology and the distribution of tegumental spines, some significant differences were recognized among the three species of Metagonimus (Table 1). In the case of metacercariae, tegumental spines on the antero-ventral surface had 7-8 pointed tips in M. yokogawai (Lee et al., 1984), but in M. miyatai (Chai et al., 1998) and M. takahashii (this study), they were only 5-7 pointed, a less differentiated feature than those of M. yokogawai. In adult flukes, however, the situation became reversed. For example, well differentiated spines had 10-13 pointed tips in M. takahashii, 9-11 tips in M. miyatai (Chai et al., 1998), and only 7-9 tips in M. yokogawai (Lee et al., 1984). Another interesting observation was that the dense distribution of tegumental spines was limited to the anterior half of the body in M. yokogawai (Lee et al., 1984), whereas they extended more posteriorly for M. miyatai (Chai et al., 1998) and M. takahashii (this study).
Table 1.
Comparison of the surface ultrastructures of Metagonimus takahashii, M. miyatai and M. yokogawai adult flukes
Developmental changes in the differentiation of tegumental spines have been well known in trematodes. In the case of Clonorchis sinensis, for example, it was reported that larval flukes which just excysted in the duodenum had double or triple-pointed tegumental spines at the anterior half of the body, but spines gradually disappeared as the worms grew to be adults (Fujino et al., 1979; Lee et al., 1982). On the contrary, for the larval flukes of Fasciola hepatica, tegumental spines having single tip metamorphose into multipointed ones just prior to entry into the bile duct (Bennett and Threadgold, 1975). The conversion of simple shaped spines into serrated ones during the parasite development is also known in Neodiplostomum seoulense (Lee et al., 1985) and Paragonimus iloktsuenensis (Lee et al., 1989).
Considering the fact that the developmental changes in spine differentiation are required to help flukes adapt the microenvironment in the host (Bennett and Threadgold, 1975), characteristics in host-parasite relationships may be different between M. takahashii and M. yokogawai. More active and remarkable differentiation of tegumental spines in M. takahashii during their development may be related to the differences in taking microhabitats in the host intestine, invasiveness into the host mucosa and pathogenicity, and possibly, capability of evasion from the host immunity. It is unfortunate, however, that there have been only a few background data supporting the above suggestion which needs comparative host-parasite studies using the two species of Metagonimus.
In conclusion, the surface ultrastructure of M. takahashii was generally similar to those observed from M. yokogawai (Lee et al., 1984) and M. miyatai (Chai et al., 1998), except for the differences in digitation of spines and differentiation during their development, which may be of taxonomic and bio-ecological significance of M. takahashii.
Footnotes
This study was supported by a Grant from Seoul National University Hospital Research Fund (No. 01-1999-065-0).
References
- 1.Asada J. On Metagonimus yokogawai and its related species. Clin Med. 1934;22:43–56. (in Japanese) [Google Scholar]
- 2.Bennett CE, Threadgold LT. Fasciola hepatica: development of tegument during migration in mouse. Exp Parasitol. 1975;38:38–55. doi: 10.1016/0014-4894(75)90036-3. [DOI] [PubMed] [Google Scholar]
- 3.Chai JY, Chung HL, Choi MH, et al. Surface ultrastructure of Heterophyes nocens (Trematoda: Heterophyidae) Korean J Parasitol. 1992;30:75–82. doi: 10.3347/kjp.1992.30.2.75. [DOI] [PubMed] [Google Scholar]
- 4.Chai JY, Huh S, Yu JR, et al. An epidemiological study of metagonimiasis along the upper reaches of the Namhan River. Korean J Parasitol. 1993;31:99–108. doi: 10.3347/kjp.1993.31.2.99. [DOI] [PubMed] [Google Scholar]
- 5.Chai JY, Kang YJ, Choi SY, Guk SM, Yu JR, Lee SH. Surface ultrastructure of Metagonimus miyatai metacercariae and adults. Korean J Parasitol. 1998;36:217–225. doi: 10.3347/kjp.1998.36.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai JY, Lee SH. Intestinal trematodes of humans in Korea: Metagonimus, heterophyids and echinostomes. Korean J Parasitol. 1990;28(suppl):103–122. doi: 10.3347/kjp.1990.28.suppl.103. [DOI] [PubMed] [Google Scholar]
- 7.Fujino T, Ishii Y, Choi DW. Surface ultrastructure of the tegument of Clonorchis sinensis newly excysted juveniles and adult worms. J Parasitol. 1979;65:579–590. [PubMed] [Google Scholar]
- 8.Hong SJ, Chai JY, Lee SH. Surface ultrastructure of the developmental stages of Heterophyopsis continua (Trematoda: Heterophyidae) J Parasitol. 1991;77:613–620. [PubMed] [Google Scholar]
- 9.Ito J. Metagonimus and other human heterophyid trematodes. Progress Med Parasitol in Japan. 1964;1:314–393. [Google Scholar]
- 10.Katsurada F. Heterophyes in Japan. II. Creation of a new genus Metagonimus. Okayama Igakkai Zasshi (273) 1912;273:768–778. (in Japanese) [Google Scholar]
- 11.Køie M. Stereoscan studies of cercariae, metacercariae, and adults of Cryptocotyle lingua (Creplin, 1825) Fischoeder, 1903 (Trematoda: Heterophyidae) J Parasitol. 1977;63:835–839. [PubMed] [Google Scholar]
- 12.Lee SH, Hong SJ, Chai JY, Seo BS. Studies on intestinal trematodes in Korea XV. Tegumental ultrastructure of Fibricola seoulensis according to developmental stages. Seoul J Med. 1985;26:52–63. [Google Scholar]
- 13.Lee SH, Hong ST, Seo BS. A study on the fine tegumental structures of the metacercaria and juvenile stages of Clonorchis sinensis. Korean J Parasitol. 1982;20:123–132. doi: 10.3347/kjp.1982.20.2.123. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Kim SJ, Chai JY, Sohn WM. Tegumental ultrastructure of Paragonimus iloktsuenensis according to the developmental stages. Korean J Parasitol. 1989;27:57–66. doi: 10.3347/kjp.1989.27.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Seo BS, Chai JY, Hong SJ. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea. VII. Electron microscopic observation on the tegumental structure. Korean J Parasitol. 1984;22:1–10. doi: 10.3347/kjp.1984.22.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Saito S. On the validity of Metagonimus spp. Proc Jap Parasite Taxon Morphol Conf. 1984;2:1–6. (in Japanese) [Google Scholar]
- 17.Saito S, Chai JY, Kim KH, et al. Metagonimus miyatai sp. nov. (Digenea: Heterophyidae), a new intestinal trematode transmitted by freshwater fishes in Japan and Korea. Korean J Parasitol. 1997;35:223–232. doi: 10.3347/kjp.1997.35.4.223. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S. Yokogawa's Metagonimus. List of publications on special animals in Okayama Prefecture. Okayama Prefectural Report. 1930;1:46–148. (in Japanese) [Google Scholar]
- 19.Takahashi S. On the life history of Metagonimus and Exorchis major. Okayama Igakkai Zasshi. 1929;41:2687–2755. (in Japanese) [Google Scholar]
- 20.Taraschewski H. Die trematoden der gattung Heterophyes taxonomie, biologie, epidemiologie. Germany: Dissertation to Universität Hohenheim; 1984. [Google Scholar]
- 21.Yu JR, Chung JS, Huh S, et al. PCR-RFLP patterns of three kinds of Metagonimus in Korea. Korean J Parasitol. 1997;35:271–276. doi: 10.3347/kjp.1997.35.4.271. [DOI] [PubMed] [Google Scholar]