Abstract
In order to observe the cytotoxicity of Acanthamoeba spp., which were isolated from contact lens containers as ethiological agents for the probable amoebic keratitis in Korea, the crystal violet staining method and LDH release assay were carried out. In the crystal violet staining method, among eight contact lens container isolates, isolate 3 (Acanthamoeba KA/LS5) showed 83.6% and 81.8% of cytotoxicity, and isolate 7 (Acanthamoeba KA/LS37) showed 28.2% and 25.1% of cytotoxicity, in 1 mg/ml and 0.5 mg/ml lysate treatments, respectively. Acanthamoeba culbertsoni and A. healyi showed 84.0% and 82.8% of cytotoxicity. Similar results were observed in A. castellanii and A. hatchetti which showed 83.6% and 75.5% of cytotoxicity. Acanthamoeba royreba and A. polyphaga showed 9.0% and 1.7% of cytotoxicity. In the LDH release assay, isolate 3 (20.4%) showed higher cytotoxicity than other isolates in 1 mg/ml lysate treatment. The results provide that at least isolate 3 has the cytotoxic effect against CHO cells and seems to be the pathogenic strain.
Keywords: Acanthamoeba, acanthamoebic keratitis, cytotoxicity, CHO cell, crystal violet staining, LDH release assay
Acanthamoeba spp. are free-living amoebae found in the natural environments such as the soil, pond, sewage, and the atmosphere. Acanthamoeba culbertsoni is generally known to cause chronic glanulomatous amoebic encephalitis (GAE), whereas A. polyphaga and A. castellanii are causative agents for acanthamoebic keratitis (Visvesvara and Stehr-Green, 1990). In Korea, two GAE cases and one acanthamoebic pneumonia case have been reported (Ringsted et al., 1976; Im and Kim, 1998) as well as several acanthamoebic keratitis cases (Cho et al., 1992; Kim et al., 1995). The source of most amoebae related cases has not been determined, although, for a few patients, the sources of infection have been known to be water, dust, and soil (Ma et al., 1990). However, it seems likely that the contaminated air, water, and contact lens containers are the main sources of infection.
In order to elucidate the pathogenicity of Acanthamoeba spp. isolated from various environments, the observation of experimentally developed GAE in mice was carried out (Im et al., 1999). At the early stage of isolation, however, the GAE observation was not useful since the isolates could not tolerate the temperature at 37℃. Thus, an in vitro cytotoxicity assay against target cells was employed. In general, virulent amoebae that developed GAE in mice had the cytotoxic effect on the target cells (Pidherney et al., 1993; Dove Pettit et al., 1996). The Korean isolate, Acanthamoeba sp. YM-4, isolated from fish gill, had the cytotoxicity against the chinese hamster ovary (CHO) cells shown by the crystal violet staining method and the results were consistent with the in vivo pathogenicity (Shin et al., 1993). Recently, a lactate dehydrogenase (LDH) release assay, which detects LDH released from the cell membrane during the cell lysis of the target cells, was employed to observe the cytotoxicity of microorganisms. In this study, eight strains were isolated from contact lens containers which could be a possible source of infection for amoebic keratitis. In addition, the crystal violet staining method and LDH release assay were carried out to observe the cytotoxicity of Acanthamoeba spp. isolates in Korea.
Eight strains of Acanthamoeba isolated from contact lens containers in Korea (in 1997, by Prof. D.I. Chung and their collaborators) and a few reference amoebae such as A. culbertsoni, A. healyi, A. royreba, A. castellanii, A. hatchetti, and A. polyphaga (Table 1) were axenically cultured in PYG (Proteose Peptone, Yeast extract, Glucose) medium (Visvesvara and Balamuth, 1975) at two different temperatures of 25℃ and 37℃. The lysate preparation of Acanthamoeba was done by the method of Shin et al. (1993). For the cytotoxicity by the crystal violet staining method, CHO cells were cultured as the target cells in Earle's minimal essential medium (EMEM, Sigma) at 37℃ in CO2 incubator. The procedures were also used as the previous paper (Shin et al., 1993). The cytotoxicity by the LDH release assay was observed with CytoTox 96™ Non-Radioactive Cytotoxicity Assay Kit (Promega, WI, USA). About 1×104 CHO cells were prepared in a round-bottomed 96 well plate with 100 µl of EMEM. The amoeba lysates at the concentrations of 0.5 mg/ml, 1 mg/ml and 3 mg/ml were added to each well, and five sets of triplicate wells for the controls were prepared. A casted plate was incubated in a humidified chamber at 37℃ and in a 5% CO2 incubator for 45 min, and centrifuged at 250 g for 4 min. The supernatant (50 µl) from each well was transferred to a fresh 96 well flat-bottom plate. Equal volumes of the reconstituted Substrate Mix (supplied by manufactory) were added to each well. The plate was covered with foil to protect it from light and was incubated at a room temperature for 30 min. Fifty microliters of Stop Solution were added, and after 1 hr, the absorbance was recorded at 490 nm.
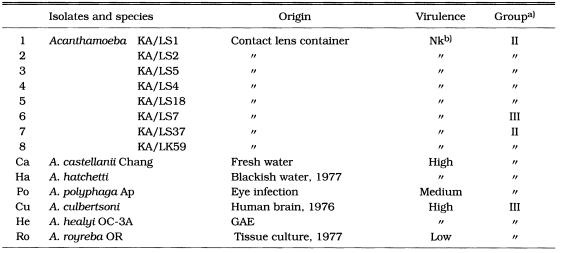
Table 1.
Eight isolates and six reference Acanthamoeba spp.
a)Grouping by Pussard and Ponds (1977). b)not known
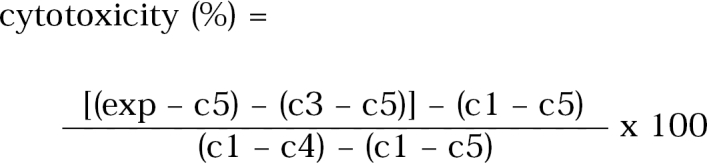
Control 1 (c1) contained only CHO cells in 100 µl of EMEM
Control 2 (c2) contained CHO cells and 10 µl of Lysis Solution
Control 3 (c3) contained only each amoeba lysates
Control 4 (c4) contained 10 µl of Lysis Solution and 100 µl of EMEM
Control 5 (c5) contained only 100 µl of EMEM
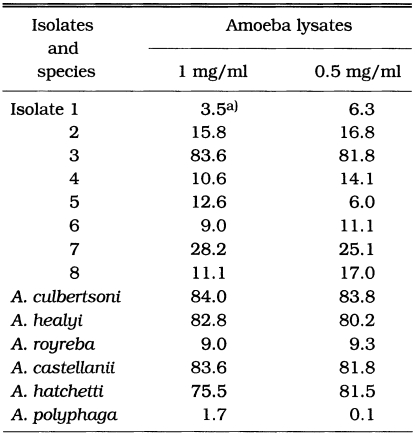
Among eight Korean isolates, isolate 3 showed 83.6% and 81.8% of cytotoxicity by the crystal violet staining method at the concentrations of 1 mg/ml and 0.5 mg/ml lysate treatments, respectively. Isolate 7 showed 28.2% and 25.1% of cytotoxicity at the same lysate concentrations as above (Table 2). In the treatment of 1 mg/ml amoeba lysate, A. culbertsoni and A. healyi which are known as the high virulent species, showed 84.0% and 82.8% of cytotoxicity, respectively (Table 2). Similar results were shown for A. castellanii and A. hatchetti with 83.6% and 75.5% of cytotoxicity. When the concentration of lysates was decreased to 0.5 mg/ml, the same results were observed (Table 2). Acanthamoeba royreba and A. polyphaga, which are known as the low and medium virulent species of Acanthamoeba in experimental GAE developments, showed 9.0% and 1.7% of cytotoxicity in the treatment of 1 mg/ml lysate, respectively (Table 2).
Table 2.
Cytotoxicity of lysates of Acanthamoeba spp. against CHO cells using crystal violet staining method
a)cytotoxicity (%)
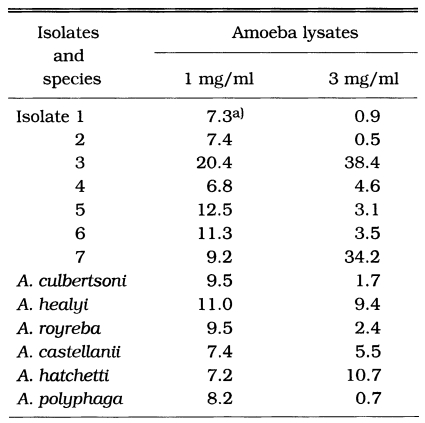
In the LDH release assay, six reference amoebae showed similar degrees of cytotoxicity, which ranged from 7.2% to 11.0%, however it was not a dose-dependent manner (Table 3). Isolate 3 showed relatively higher level of cytotoxicity (20.4%) than the other isolates in the treatment of 1 mg/ml lysates. When the concentration of lysates increased to 3 mg/ml, isolates 3 and 7 showed higher cytotoxicity (38.4% and 34.2%) than the other isolates (Table 3).
Table 3.
Cytotoxicity of lysates of Acanthamoeba spp. against CHO cells using LDH release assay
a)cytotoxicity (%)
In our preliminary study, to elucidate the pathogenicity of Acanthamoeba spp. by the experimental GAE developments, 1×105 of trophozoites of isolate 3 were inoculated intranasally into ten mice, but the death of mouse was not observed for two months (data not shown). It is guessed if isolate 3 is adapted at 37℃ through very long-termed cultivation, amoeba may cause the experimental GAE development.
An in vitro cytotoxicity assay using the crystal violet staining method, Shin et al. (1993) reported that the lysate (1 mg/ml) of Acanthamoeba sp. YM-4 (Korean isolate) had the 99% cytotoxicity against CHO cells in comparison with A. culbertsoni and A. polyphaga, 100% and 31.5%, respectively. In this study, among eight Korean isolates from contact lens containers, isolate 3 showed high degree of cytotoxic effects as much as A. culbertsoni, A. healyi, A. castellanii and A. hatchetti, and isolate 7 showed moderate degree of cytotoxicity.
When using the LDH release assay, six reference amoebae showed no corresponding level of cytotoxicity to the degree of pathogenicity which was shown by the experimental GAE development, although isolates 3 and 7 showed higher levels of cytotoxicity than the other isolates. Unfortunately, the present study suggested that the LDH release assay could not be applied on the observation of cytotoxicity in Acanthamoeba against CHO cells as the target cells. In comparison to the crystal violet staining method that needs the adherent ability of CHO cells, the LDH release assay requires that the cell wall of target cells should be lysed. Thus, in the observation of cytotoxic effects of Acanthamoeba using CHO cells as the target cells, the crystal violet staining method was more useful than the LDH release assay.
Finally, the results revealed that at least isolate 3 has the cytotoxic effect against CHO cells and seemed to be the most pathogenic strain among eight isolates. In the further study, in vivo experimental GAE developments must be observed.
ACKNOWLEDGEMENT
We thank Professor Dong-Il Chung, Department of Parasitology, College of Medicine, Kyungpook National University, for donating reference amoebae including Korean isolates. And we thank Professor Millina Lee and Sun Park, Department of Microbiology, Ajou University School of Medicine, for commenting on this study.
Footnotes
This study was supported by the grant from Ministry of Education for Basic Medical Science, 1997 (#97-L4).
References
- 1.Cho HK, Moon YS, Lee HK, Park AJ, Cho SI. Acanthamoeba keratitis (case report) J Korean Ophthalmol. 1992;33:538–543. (in Korean) [Google Scholar]
- 2.Dove Pettit DA, Williamson J, Cabral GA, Marciano-Cabral F. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: A transmission and scanning electron microscopy study. J Parasitol. 1996;82:769–777. [PubMed] [Google Scholar]
- 3.Im KI, Kim DS. Acanthamoebiasis in Korea: Two new cases with clinical cases review. Yonsei Med J. 1998;39:478–484. doi: 10.3349/ymj.1998.39.5.478. [DOI] [PubMed] [Google Scholar]
- 4.Im KI, Shin HJ, Seo DH, Jeon SH, Kim TE. Pathogenicity of Acanthamoeba spp., Korean isolates; Experimental infection and zymodemes. Korean J Parasitol. 1999;37:85–92. doi: 10.3347/kjp.1999.37.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JJ, Kim MK, Park IW, Lee HB. Acanthamoeba keratitis in contact lens wearer. J Korean Ophthalmol. 1995;36:2042–2047. (in Korean) [Google Scholar]
- 6.Ma P, Visvesvara GS, Martinez AJ, Theodore FH, Daggett PM, Sawyer TK. Naegleria and Acanthamoeba infections: Review. Rev Infect Dis. 1990;12:490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- 7.Martinez AJ. Clinical manifestations of free-living amebic infections. In: Rondanelli EG, editor. Amphizoic amoebae: Human pathology. Padua, Italy: Piccin Nuova Libraria; 1987. pp. 61–177. [Google Scholar]
- 8.Pidherney MS, Alizadeh H, Stewart GL, McCulley JP, Niederkorn JY. In vitro and in vivo tumorcidal properties of a pathogenic/free-living amoeba. Cancer Letters. 1993;72:91–98. doi: 10.1016/0304-3835(93)90016-3. [DOI] [PubMed] [Google Scholar]
- 9.Pussard M, Pons R. Morphologie de la paroi kystique et taxonomie de genre Acanthamoeba (Protozoa, Amoebida) Protistologica. 1977;13:557–598. [Google Scholar]
- 10.Ringsted J, Val Jager B, Suk DS, Visvesvara GS. Probable Acanthamoeba meningoencephalitis in a Korean child. Am J Clin Pathol. 1976;66:723–730. doi: 10.1093/ajcp/66.4.723. [DOI] [PubMed] [Google Scholar]
- 11.Shin HJ, Ra MS, Im KI. Cytotoxicity of Acanthamoeba sp. YM-4 (Korean isolate) Yonsei Rep Trop Med. 1993;24:31–38. [Google Scholar]
- 12.Visvesvara GS, Balamuth W. Comparative studies on related free-living and pathogenic amoebae with special reference to Acanthamoeba. J Protozool. 1975;22:245–256. doi: 10.1111/j.1550-7408.1975.tb05860.x. [DOI] [PubMed] [Google Scholar]
- 13.Visvesvara GS, Stehr-Green JK. Epidemiology of free-living amoeba infections. J Protozool. 1990;37(suppl):25–33. doi: 10.1111/j.1550-7408.1990.tb01142.x. [DOI] [PubMed] [Google Scholar]