Abstract
Neural progenitor cells (NPs) have shown several promising benefits for the treatment of neurological disorders. To evaluate the therapeutic potential of human neural progenitor cells (hNPs) in amyotrophic lateral sclerosis (ALS), we transplanted hNPs or growth factor (GF)-expressing hNPs into the central nervous system (CNS) of mutant Cu/Zn superoxide dismutase (SOD1G93A) transgenic mice. The hNPs were engineered to express brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), VEGF, neurotrophin-3 (NT-3), or glial cell-derived neurotrophic factor (GDNF), respectively, by adenoviral vector and GDNF by lentiviral vector before transplantation. Donor-derived cells engrafted and migrated into the spinal cord or brain of ALS mice and differentiated into neurons, oligodendrocytes, or glutamate transporter-1 (GLT1)-expressing astrocytes while some cells retained immature markers. Transplantation of GDNF- or IGF-1-expressing hNPs attenuated the loss of motor neurons and induced trophic changes in motor neurons of the spinal cord. However, improvement in motor performance and extension of lifespan were not observed in all hNP transplantation groups compared to vehicle-injected controls. Moreover, the lifespan of GDNF-expressing hNP recipient mice by lentiviral vector was shortened compared to controls, which was largely due to the decreased survival times of female animals. These results imply that although implanted hNPs differentiate into GLT1-expressing astrocytes and secrete GFs, which maintain dying motor neurons, inadequate trophic support could be harmful and there is sexual dimorphism in response to GDNF delivery in ALS mice. Therefore, additional therapeutic approaches may be required for full functional recovery.
Keywords: amyotrophic lateral sclerosis, cell differentiation, glial cell line-derived neurotrophic factor, nerve growth factors, stem cell transplantation, stem cells
Introduction
Amyotrophic lateral sclerosis (ALS) is a late-onset neurodegenerative disease characterized by progressive and lethal motor neuronal death. The disease's hallmark is a selective degeneration and loss of motor neurons in the brain and spinal cord. Most cases (90%) are sporadic and about 10% are inherited (familial ALS). Mutations in the Cu/Zn superoxide dismutase (SOD1) gene are the most common form of inherited ALS, accounting for ~20% of all familial types. This has led to the generation of transgenic mice and rats carrying SOD1G93A that have many characteristics of human ALS pathology, and these are currently the most widely used models of disease (Lepore and Maragakis, 2007). Although several hypotheses have been proposed to explain mutant SOD1-mediated pathogenesis, the exact mechanism responsible for motor neuron degeneration remains unknown (Bruijn et al., 2004; Boillee et al., 2006; Pasinelli and Brown, 2006). However, recent studies in the mutant SOD1 mouse suggest that the disease is non-cell autonomous to motor neurons (Clement et al., 2003), cells neighboring motor neurons such as mutant astrocytes and microglia strongly accelerate disease progression (Beers et al., 2006; Boillee et al., 2006; Yamanaka et al., 2008b), and mutant SOD1 in cell types other than motor neurons and oligodendrocytes contribute to the initiation of motor neuron degeneration (Yamanaka et al., 2008b). Among several possibilities, mutant astrocytes could lead to glutamate-dependent excitotoxicity due to loss of glutamate transporter-1 (GLT1) [excitatory amino acid transporter-2 (EAAT2) in humans] (Howland et al., 2002), enhance inflammatory response from microglia (Yamanaka et al., 2008b), be unable to regulate glutamate receptor subunit 2 expression in motor neurons (Van Damme et al., 2007), or release 1 or more unknown toxic factors that accelerate the death of embryonic stem cell-derived motor neurons (Di Giorgio et al., 2007; Nagai et al., 2007).
Many clinical trials and studies have been undertaken to find effective therapies for ALS, but proven methods still do not exist. Recently, some trials of effective growth factor (GF) delivery have been reported in ALS model based on the assumption that the provision of GF, which could be trophic for motor neurons, would be of benefit in motor neuron disorders. Injection of adeno-associated virus (AAV) encoding insulin-like growth factor-1 (IGF-1) gene into muscle slowed disease progression in ALS mice (Kaspar et al., 2003), and delivery of a lentivirus encoding VEGF extended survival of ALS mice (Azzouz et al., 2004). However, current viral vectors cannot alter dosage or discontinue therapy and have significant limitations for reaching widespread areas of the CNS. In addition, retrograde transport may be affected early in the disease (Williamson et al., 1999), and larger animals such as monkeys may be less efficient at taking back the protein to the spinal cord, thus limiting this approach in patients. Another approach for GF delivery is the direct infusion of trophic molecules into the spinal cord or brain, which yields widespread distribution throughout the CNS. Continuous intracerebroventricular (ICV) infusion of VEGF or IGF-1 extended survival time in ALS mice, and a phase I clinical trial using the same strategy showed a modest benefit in patients without adverse effects (Nagano et al., 2005a, b; Storkebaum et al., 2005). However, direct delivery of GF in vivo has been hindered by limited bioavailability, inability to penetrate gray matter, and a relatively short half-life.
Neural progenitor cells (NPs) possess a range of therapeutic properties for neural repair: functional neuronal or glial replacement, delivery of therapeutic gene products synthesized by stem cells, mitigation of toxic or inflammatory components of the neural environment, and replacement of multiple neural elements that define a given CNS region (Snyder et al., 1995; Flax et al., 1998; Imitola et al., 2004; Park et al., 2002, 2006a, b; Lee et al., 2007). The human neural progenitor cells (hNPs) can be expanded in long-term cultures, and differentiate into neural cell types and integrate into host neural circuitry after transplantation into the developing and adult rodent CNS (Svendsen et al., 1997; Flax et al., 1998; Imitola et al., 2004; Kim et al., 2006; Lee et al., 2007). The hNPs can also be engineered to release glial cell-derived neurotrophic factor (GDNF) and survive transplantation into the lumbar spinal cord of the SOD1G93A rat. These cells continue to release GDNF within the degenerative environment of the spinal cord and show a protective effect on motor neuron cell death (Klein et al., 2005; Suzuki et al., 2007a). However, this was not through the differentiation of donor-derived cells into GLT1-expressing astrocytes around the surviving motor neurons, and GDNF-expressing hNP grafts did not extend survival times in ALS rats. Therefore, in the present study, simultaneous combined strategies of hNP transplantation that could not only replace non-neuronal cells but also effectively deliver GF to the CNS were performed in ALS mice. Both replacement of GLT1-expressing astrocytes and delivery of GF such as IGF-1 or GDNF maintain dying motor neurons. However, improvement in motor performance and extension of lifespan were not observed. These results indicate possible hNP treatment strategies to maintain dying motor neurons for ALS, however, other therapeutic approaches are required for full functional recovery.
Results
In vitro characterization of viruses and virus-infected hNPs
The titer of each adenovirus was about 2-6 × 1012 pfu/ml. To demonstrate and optimize adenoviral infection on hNPs, we infected hNPs with AdGF at different MOI ranges between 0 and 250 pfu per cell. When cells were infected with AdGF at 80 MOI, nearly 100% of hNPs appeared to be GFP-positive and healthy. The amount of secreted brain-derived neurotrophic factor (BDNF), GDNF, and IGF-1 from 1 × 106 cells for 48 h was 75.67, 82.13, and 84.68 ng, respectively. In contrast, AdVEGF was used at 8 MOI because transplantation of hNPs expressing high amounts of VEGF induced death in some experimental mice. The amount of secreted VEGF was 9.04 ng from 1 × 106 cells for 48 h. The titer of lentivirus was 1.08 × 105 TU/µl, and the cells were infected at 3 MOI. According to ELISA, hNPs-LtGDNF secreted 31.89 ng of GDNF from 1 × 106 cells during 48 h under proliferative conditions.
We performed immunocytochemistry to characterize hNPs-AdGF and hNPs-LtGDNF under differentiation conditions. Transduction of the viral vector was confirmed by GFP and GF expression (Figure 1). The majority of viral vector-infected hNPs (~90%) expressed immature cell marker nestin, and many of them expressed more than 1 of the cell type-specific proteins studied, which suggests that cells are double-labeled with glial and neuronal markers are multipotent progenitors (Kim et al., 2006) (data not shown). For example, according to types of infected viruses and secreting GF, about 30-90% of cells expressed GFAP, a protein that is expressed by astrocytes and neural stem cell/progenitor cells, and about 25-45% of cells expressed TUJ1, a neuronal marker. However, only a low percentage (~1%) of cells expressed O4, an immature oligodendrocyte marker (data not shown). These results indicate that a major portion of virus-infected GF-secreting hNPs still remain immature state under differentiation conditions in vitro.
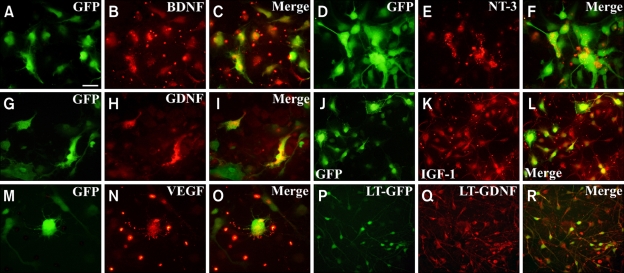
Figure 1.
In vitro immunocytochemistry of viral vector-infected hNPs. Growth factors are secreted by GFP-expressing hNPs. GFP-positive cells (green) are identified by various GF markers that are visualized by Texas red. Double labeling, seen as yellow/orange fluorescence, is observed by dual bypass filter microscopy. (A-C) hNPs-AdBDNF. (D-F) hNPs-AdNT-3. (G-I) hNPs-AdGDNF. (J-L) hNPs-AdIGF-1. (M-O) hNPs-AdVEGF. (P-R) hNPs-LtGDNF. Scale bar: 10 µm.
Engraftment and distribution of hNPs in vivo following transplantation
The animals were sacrificed at 2 or 4 weeks following transplantation and the end point of the disease. In all experimental groups, hNPs and GF-expressing hNPs showed migration and engraftment throughout the spinal cord or brain when implanted via the cisterna magna or cerebral lateral ventricles, respectively (Figure 2-5). In vivo distribution of the donor-derived cells was identified by the expression of GFP, human cell-specific markers (hHSP27, hNuc and hGFAP), or BrdU. GFP-expressing donor-derived cells in vivo indicate that they also express GF simultaneously. Donor-derived cells migrated from the injection site to the adjacent brain stem and whole spinal cord when injected into the cisterna magna. GFP-expressing donor derived cells appeared to be migrating toward the adjacent brain stem from the fourth ventricle and engrafted within the medulla when mice were analyzed 2 weeks after transplantation (Figure 2A and B). Some donor-derived cells might migrate through the subarachnoid space and become engrafted into the white matter of the cervical or thoracic spines (Figure 2C-E). In a few cases, some engrafted cells could be found in the area of the lumbar spine of ALS mice. Moreover, human-specific GFAP-positive cells showed migration from white matter to gray matter of the cervical or thoracic spine of mice 4 weeks following transplantation (Figure 2F-H).
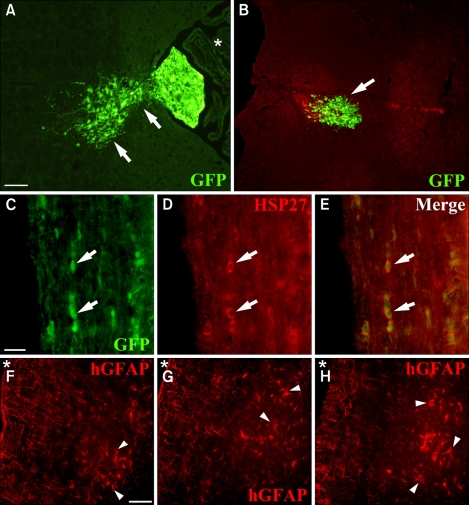
Figure 2.
Engraftment and distribution of hNPs in vivo following transplantation. (A) GFP-expressing donor-derived cells (green) show migration from the fourth ventricle (asterisk) to the adjacent brain stem when the mice were analyzed 2 weeks after transplantation. Arrows indicate migrating cells into the parenchyma of the brain stem. (B) GFP-positive cells engraft within the medulla (an arrow). (C-E) Some donor-derived cells show engraftment in the white matter of the thoracic spine of mice (arrows). (D) GFP-positive cells were identified by human cell-specific marker hHSP27 visualized by Texas red. (E) Double labeling, seen as yellow/orange fluorescence, is observed by dual bypass filter microscopy. (F-H) Human specific GFAP-positive cells (arrow heads, red) appear to have migrated from the white matter to the gray matter of the thoracic spine of mice when analyzed at 4 weeks following transplantation. Asterisk markers indicate the subarachnoid space of the spinal cord. Scale bar: A, 40 µm; C, 10 µm; F, 20 µm.
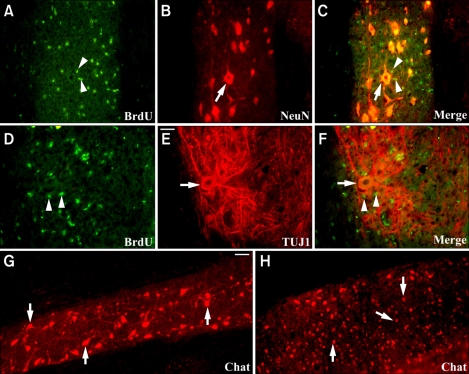
Figure 5.
Transplantation of IGF-1-expressing hNPs attenuates motor neuron loss and induces trophic effects in motor neurons of ALS mice. (A-F) BrdU-positive donor-derived cells (arrow heads in A, C, D, and F, green) are shown to be engrafted juxtapositionally to NeuN- or TUJ1-postive host motor neurons (arrows in B, C, E, and F, red) in the gray matter of the thoracic spine of ALS mice. Host motor neurons appear to have larger cell bodies, longer neuronal processes, and enhanced neurites branching and elongation. (G and H) While Chat-positive host motor neurons show larger cell bodies and elongated and branching neuronal processes in hNPs-AdIGF-1-transplanted mice (arrows in G, red), the number of large Chat-positive host motor neurons is decreased and the morphology of cell bodies appears to have shrunken (arrows in H, red) in vehicle-injected controls. Scale bar: E, 40 µm; G, 20 µm.
Differentiation patterns of hNPs in vivo following transplantation
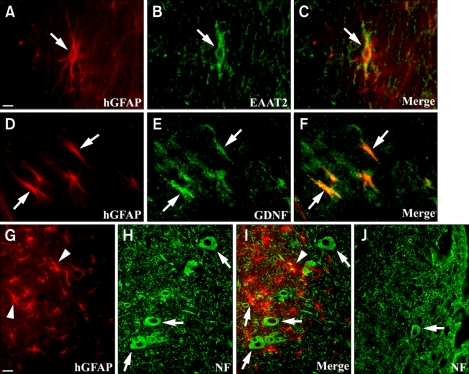
To investigate the patterns of differentiation of donor-derived cells, the brain stem and whole spinal cord of ALS mice were analyzed by immunohistochemistry at 4 weeks following transplantation. About 20% of implanted hNPs could be found to differentiate into TUJ1-positive neurons in the cervical or thoracic spine of mice (n = 10) (20.4 ± 1.3%, mean ± SEM) (Figure 3A-C), however, a major portion of the engrafted cells remained hNestin-expressing immature cells in the spinal cord (n = 10) (50.6 ± 1.5%) (Figure 3G-I). Some donor-derived cells also differentiated into GFAP-expressing astrocytes (n = 10) (28.5 ± 1.7%) but there was hardly any CNPase-positive oligodendrocytes (less than 1%) (data not shown). When hNPs-LtGDNF was injected into the mice, only ~1% of donor-derived cells differentiated into TUJ1-positive neurons (Figure 3D-F), however, more than 50% of implanted cells expressed hGFAP in the brain stem and spinal cord of the mice (n = 8) (53.5 ± 2.1%) (Figure 3J-L). In addition, ~30% and ~5% of GFAP-expressing donor-derived astrocytes expressed EAAT2 and EAAT1, respectively, in the cervical or thoracic spine of the mice (n = 5) (30.8 ± 1.1% and 4.7 ± 0.5%) (Figure 3M-O). These results suggest that hNPs-LtGDNF-derived astrocytes could protect host motor neurons by reducing glutamate mediated excitotoxicity.
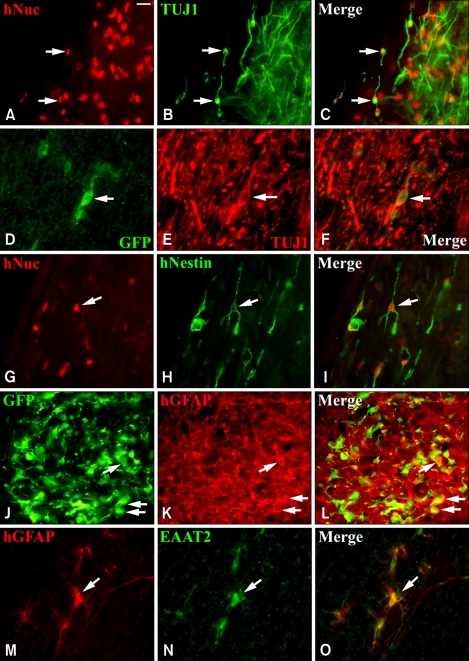
Figure 3.
Differentiation patterns of hNPs in vivo following transplantation. (A-C) The hNuc-positive donor-derived cells visualized by Texas red (arrows in A and C) are co-labeled with TUJ1, a neuronal marker that is identified by fluorescein (arrows in B and C, green) in the cervical and thoracic spine of mice. (G-I) A major portion of hNuc-positive cells express hNestin (arrows in G and I, red), an immature cell marker in the thoracic spine. (D-F) When hNPs-LtGDNF are injected into mice, a small number of GFP-positive donor-derived cells (arrows in D and F) express TUJ1 (arrows in E and F, red) in the spinal cord, (J-L), however, a major portion of GFP-positive cells express hGFAP (arrows in K and L, red) in the brain stem and spinal cord of mice. (M-O) Some donor-derived hGFAP-positive cells (arrows in M and O, red) express EAAT2 (arrows in N and O, green) in the cervical and thoracic spine of mice. Scale bar: A, 10 µm.
Survival and trophic effects of GF-expressing hNP transplantation on motor neurons of the spinal cord
Among all transplantation groups, GDNF- and IGF-1-expressing hNP implantation showed survival and trophic effects on motor neurons of the spinal cord of mice. When mice were analyzed 4 weeks following hNPs-LtGDNF transplantation, some donor-derived astrocytes could be found to express EAAT2 and GDNF in the cervical or thoracic spine (Figure 4A-F). To investigate whether GDNF- and/or EAAT2-expressing donor-derived astrocytes could attenuate motor neuron loss and induce trophic effects on motor neurons, we counted the number of choline acetyltransferase (Chat), NF, or NeuN-positive cells and observed the size of their cell bodies in the thoracic spine of both the hNPs-LtGDNF and vehicle-injected groups. By 4 weeks post grafting (~103-day-old animals), there was a significant (~23%) reduction in the number of motor neurons in the vehicle-injected group (Figure 4J) compared to the hNPs-LtGDNF-injected group (Figure. 4G-I). The average number of motor neurons per longitudinal section of the thoracic spine in the vehicle and hNPs-LtGDNF injected groups (n = 5) was 423 ± 32 and 550 ± 25, respectively. The hGFAP-positive donor-derived cells were located adjacent to host motor neurons and their cell bodies in the gray matter of thoracic spine, and the size of motor neuron cell bodies appeared to be bigger than that of the control group (Figure 4G-J). In case of hNPs-AdIGF-1 transplantation, BrdU-positive donor-derived cells were shown to be located juxtapositionally to the host motor neurons in the thoracic spine of ALS mice, and the host motor neurons appeared to have larger cell bodies, longer neuronal processes, and enhanced neurites branching and elongation compared to vehicle-injected controls (Figure 5).
Figure 4.
Motor neurons are protected by EAAT2- and GDNF-expressing donor-derived astrocytes following hNPs-LtGDNF transplantation in the spinal cord of ALS mice. (A-F) The hGFAP-positive donor-derived astrocytes visualized by Texas red (arrows in A, C, D, and F) are co-labeled with EAAT2 and GDNF, which are identified by fluorescein (arrows in B, C, E, and F, green), in the thoracic spine at 4 weeks post-grafting. (G-I) The hGFAP-positive donor-derived astrocytes (arrow heads in G and I, red) located adjacent to NF-positive host motor neurons and their cell bodies (arrows in H and I, green) in the gray matter of the thoracic spine. (H-J) In vehicle-injected mice, the number NF-positive motor neurons is significantly reduced and the size of the motor neuron cell body appears to be smaller (an arrow in J, green) compared to hNPs-LtGDNF-injected mice (arrows in H and I, green). Scale bar: A, 5 µm; G, 10 µm.
Effects of hNPs and GF-expressing hNP transplantation on motor performance
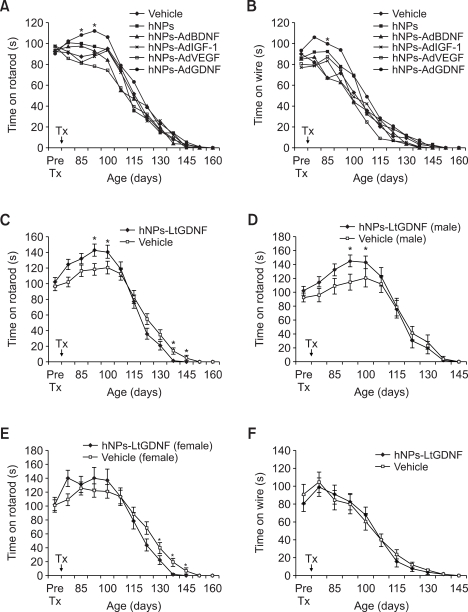
We performed the wire maneuver and rota-rod tests to evaluate the effects of hNPs and GF-expressing hNP transplantation on motor function in ALS mice. We also observed changes in body weight in all transplantation groups and vehicle-injected controls from before transplantation up to death (data not shown). Overall, there was no significant difference in motor performance and body weight transition among the hNPs and hNPs-AdGF transplantation groups and vehicle-injected controls although hNPs-AdGDNF implanted mice showed short-term transient improvement in motor performance during the early period following implantation (Figure 6A and B). The number of mice examined in the vehicle, hNPs, hNPs-AdBDNF, AdIGF-1, hNPs-AdVEGF, and hNPs-AdGDNF transplanted groups was 128, 16, 18, 22, 29, and 46, respectively. In the hNPs-LtGDNF transplanted group (n = 40), motor performance in the rota-rod test was better than in the vehicle-injected controls (n = 37) at 3 and 4 weeks following transplantation, however, after then, performance became worse compared to the control group (Figure 6C). When the results of the rota-rod test were analyzed by gender of the mice, male mice in the hNPs-LtGDNF transplanted group had better performance during the early period following transplantation than male mice in the control group whereas the late deterioration in motor performance in the hNPs-LtGDNF transplanted group was largely due to worsened performance in female mice (Figure 6D and E). However, there was no difference on the wire maneuver test between both groups (Figure 6F).
Figure 6.
Transplantation of hNPs and GF-expressing hNPs do not improve motor performance in ALS mice. (A and B) Although hNPs-AdGDNF-implanted mice show a significant short-term increase in fall down time in the rota-rod and wire maneuver tests at 2 or 3 weeks following transplantation, there is no significant difference overall in fall down time among all hNPs and hNPs-AdGF transplantation groups and vehicle-injected controls. (C) hNPs-LtGDNF-implanted mice also show a significant short-term increase in fall down time in the rota-rod test compared to the control group at 3 and 4 weeks following transplantation, however, after then, fall down time becomes shorter than the control group. (D and E) Following transplantation, the early significant increase in fall down time in the rota-rod test is due to improved performance in male mice of the hNPs-LtGDNF-transplanted group compared to male mice of the control group whereas the late significant decrease in fall down time in the hNPs-LtGDNF-transplanted group is largely due to the worsened performance in female mice compared to female mice of the control group. (F) There is no difference in fall down time in the wire maneuver test between both groups. Value are mean ± SEM, *P < 0.05.
Effects of hNPs and GF-expressing hNP transplantation on the survival of ALS mice
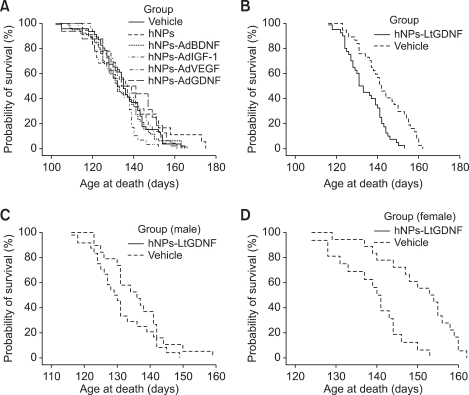
To test the role of transplantation of hNPs and GF-expressing hNPs on disease outcome, we observed the lifespan in all transplantation groups and vehicle-injected controls. There were no statistical differences among hNPs (n = 16) and hNPs-AdGF-transplanted mice (n = 115) and vehicle-injected mice (n = 124) in survival duration (hNPs, 137.1 ± 4.1 d; hNPs-AdBDNF, 139.2 ± 4.1 d, n = 18; hNPs-AdIGF-1, 134.5 ± 2.9 d, n = 22; hNPs-AdVEGF, 133.5 ± 1.6 d, n = 29; hNPs-AdGDNF, 135.8 ± 1.9 d, n = 46; vehicle, 133.8 ± 3.9 d) (Figure 7A). In contrast, hNPs-LtGDNF suspension transplantation significantly decreased overall survival. The average survival times in the vehicle and hNPs-LtGDNF groups were 143.3 ± 2.9 d (n = 21) and 132.8 ± 2.0 d (n = 23) when injected into the cisterna magna and 142.5 ± 2.5 d (n = 16) and 135.5 ± 2.2 d (n = 17) when injected into both the cisterna magna and cerebral lateral ventricle, respectively (Figure 7B). Both data show statistically significant differences between the hNPs-LtGDNF and control groups (P < 0.05).
Figure 7.
Survival curves. (A) Survival curves of hNPs- and hNPs-AdGF-transplanted mice and vehicle-injected mice. There are no differences in survival duration among all hNPs- and hNPs-AdGF-transplanted groups and vehicle-injected group. (B) Survival curves of hNPs-LtGDNF-transplanted and control mice. The hNPs-LtGDNF transplantation significantly decreases survival times (P < 0.01). (C) Survival curves of hNPs-LtGDNF-transplanted male and control male mice. There is no difference between both groups. (D) Survival curves of hNPs-LtGDNF-transplanted female and control female mice. In female mice, cell transplantation significantly decreases survival times (P < 0.01).
In order to evaluate gender difference on survival duration, we separately analyzed survival times of male and female mice in both the hNPs-LtGDNF and control groups. In male mice, the mean survival times of the vehicle (n = 19) and hNPs-LtGDNF groups (n = 24) were 136.0 ± 2.2 d and 130.8 ± 1.8 d, respectively, when injected into the cisterna magna or both the cisterna magna and cerebral lateral ventricle. There was no statistically difference between both groups (Figure 7C). However, in female mice, the mean survival times of the vehicle (n = 18) and hNPs-LtGDNF groups (n = 16) were 150.3 ± 2.2 d and 138.6 ± 2.2 d, respectively, and there was a significantly difference between both groups (P < 0.05) (Figure 7D). Therefore, the negative effect of hNPs-LtGDNF transplantation into ALS mice on survival time was mainly due to the shortened survival period in female mice.
Discussion
Transplantation of neural stem/progenitor cells has shown a range of therapeutic potential for neurological disorders because engrafted cells could replace lost or dysfunctional neurons and glias, deliver various growth factors or other therapeutic gene products that expressed intrinsically or engineered ex vivo to be overexpressed by the stem/progenitor cells, mitigate toxic or inflammatory components of the neural environment, replace multiple neural elements that define a given CNS region, and integrate seamlessly into host neural circuitry.
Previous studies of motor neuron disease models showed that transplantation of embryonic or neural stem cell-derived neural precursors replaced motor neurons, and established some connection in part with peripheral targets in a functional manner (Wu et al., 2002; Kerr et al., 2003; Harper et al., 2004; Deshpande et al., 2006). However, this strategy still has many limitations associated with appropriate motor neuron differentiation, axonal extension into the peripheral nervous system, formation of functional neuromuscular junction, and establishment of a functional neural network with the host nervous system.
Other cell transplantation approaches that did not replace injured motor neurons, including those with enhanced growth factor expression, also showed some improvement in ALS models (Aebischer et al., 1996; Garbuzova-Davis et al., 2002, 2003; Corti et al., 2004, 2007; Llado et al., 2004; Xu et al., 2006; Yan et al., 2006; Martin and Liu, 2007). Moreover, administration of growth factors was reported to have some beneficial roles on disease outcome in ALS rodent models (Federici and Boulis, 2006). Thus, stem/progenitor cell-based ex vivo gene therapy, employing cells genetically engineered to express growth factors, could provide not only appropriate missing neurons or glias but also simultaneously trophic factors for motor neurons in ALS models after transplantation. However, when GDNF-expressing human neural progenitor cells were directly transplanted into the lumbar spinal cord, there were no benefits in motor performance and animal survival although they provided limited neuroprotection (Klein et al., 2005; Suzuki et al., 2007a).
The diffuse distribution of grafted cells throughout the CNS via direct transplantation of stem/progenitor cells into cerebrospinal fluid (CSF) may be a potential alternative to intraparenchymal injection because ALS is characterized by relatively selective degeneration of motor neurons in the brain and spinal cord. Previous studies have demonstrated that neural stem/progenitor cells can be delivered to the brain and spinal cord via intraventricular and intrathecal injection because of their responsiveness to signals derived from the injured and degenerating CNS (Flax et al., 1998; Park et al., 2002, 2006a, b; Kerr et al., 2003; Lepore and Maragakis, 2007). Therefore, in this study, we initially hypothesized that the intrathecal injection of hNPs and GF-expressing hNPs by adenoviral vector through the cistern magna would show decreased motor neuron death and increase animal survival times. However, these cells overall do not improve motor behavior and animal survival although some donor-derived cells distribute diffusely in the brain stem and spinal cord and differentiate into neurons and astrocytes but largely retain the immature cell marker nestin and show limited protective effects on motor neurons. The only positive result is that GDNF-expressing hNP-implanted mice show short-term improvement in motor performance. As our next experiment, we transplanted GDNF-expressing hNPs by lentiviral vector because the expression of adenovirally delivered GDNF might be transient and insufficient, and hNPs-LtGDNF appear to have gained more glial cells-specific differentiation capacity, especially astrocytes, according to in vitro and in vivo differentiation experiments. However, as a result, motor performance and extension of lifespan were not improved but instead worsened in female mice.
As described earlier, intrathecal and/or ventricular injection of cells have several advantages, such as wide distribution of administered cells, easy application, and noninvasive methods. In this study, some transplanted hNPs-LtGDNF could survive and migrate into host tissues, differentiate into neurons and glias, including astrocytes expressing EAAT2 and GDNF, and exert neuroprotective influences on neighboring host motor neurons. However, many cells remained in the ventricular region and CSF tract of the spinal cord until 4 weeks after transplantation, and their engraftments and integrations seemed to be very limited. Because of the low nutrient level of CSF, the survival rate of transplanted cells might be low (Habisch et al., 2007). Furthermore, disruption of the blood-brain barrier and blood-spinal cord barrier in SOD1G93A transgenic mice was reported (Garbuzova-Davis et al., 2007). Thus, use of high doses of immune suppressants or combined immune suppressants would be required to increase the viability of donor-derived cells affected by immune rejection and chances for functional recovery.
In ALS mice models, axonopathy is known to induce loss of neural connection to the muscle at the neuromuscular junction long before motor neuron degeneration and initiation of symptoms (Fischer, et al., 2004; Pun et al., 2006). Transplantation of GDNF-expressing human neural precursor cells in ALS rat models induced survival of motor neurons in the spinal cord, but did not reduce the number of denervated muscle end plates (Suzuki et al., 2007). In addition, a recent study showed that when motor neurons were transplanted, axonal outgrowth and attachment to the muscle could be achieved by using GDNF-secreting cells implanted into the sciatic nerve (Deshpande et al., 2006). These findings suggest that combinatorial approaches of the protection of motor neuron cell bodies and nerve terminals in the muscle may lead to potential improvements in limb function and survival of ALS mice.
In this study, the effects of transplantation of hNPs-LtGDNF in motor performance and survival of ALS mice were negative. The main reason was worsened motor performance and survival in female mice. Late disease onset and slow progression of female SOD1 mice has been reported (Suzuki et al., 2007b), and the protective role of estrogen in female mice appear to influence disease progression (Choi et al., 2008). Interestingly, estrogen stimulates the expression of GDNF in the developing hypothalamus (Ivanova et al., 2002). Therefore, excessive expression of GDNF in the CNS due to hNPs-LtGDNF transplantation could be one of the reasons for the negative effects on motor performance and survival of female SOD1 mice. We also observed some irritable behaviors, such as jumping, squeaking, and turning, in female mice after cells were injected through both the cisterna magna and intracerebral ventricle. The delivery of growth factors for a long period in the CNS could be harmful. In patients with Parkinson's disease, side effects of centrally delivered GDNF have been reported (Kordower et al., 1999; Nutt et al., 2003). As we observed in mice transplanted with NPs-AdVEGF, the delivery of low-dose VEGF would be better than a high dose in Parkinson's models (Yasuhara et al., 2005). Therefore, modulation of expression level of growth factors in vivo seems to be very important. However, the precise mechanisms of the negative effects of hNPs-LtGDNF transplantation of female SOD1 mice in this study need to be clarified.
In chimeric mutant SOD1 mice, the aberrant function of non-neuronal cells (microglia and astrocytes) appears to contribute to disease progression progression (Clement et al., 2003; Boillee et al., 2006; Yamanaka, et al., 2008a). Chimeric mutant SOD1 animals suggest that increasing the proportion of healthy wild-type non-neuronal cells is related to the prolongation of animal survival times. Selective reduction of mutant SOD1 from astrocytes results in prolongation of disease duration (Yamanaka, et al., 2008a). Astrocytes in ALS patients and animal models have been revealed to have functional abnormalities in glutamate transport, specifically GLT1 (EAAT2) (Rothstein et al., 1995; Howland et al., 2002). Loss of this astroglial protein is known to cause excitotoxic motor neuron degeneration (Rothstein et al., 1996). Furthermore, ALS astrocytes alter the expression of dendritic glutamate (AMPA) receptors in motor neurons, also making them more susceptible to excitotoxicity (Lepore et al., 2008). More recent studies also suggest that human mutant SOD1 carrying astrocytes release several toxic factors, including the activation of NOX2 to produce oxygen radicals, which induce embryonic stem cell-derived motor neuron degeneration (Di Giorgio et al., 2007, 2008; Nagai et al., 2007; Marchetto, et al., 2008). Thus, these results indicate that any approach to replace altered astroglial function may be of therapeutic benefit. A recent study showed that focal transplantation of glial-restricted precursors into the cervical spine of ALS models efficiently differentiate into GLT1-expressing normal astrocytes, attenuate motor neuron loss, slow declines in motor functions, and extend survival and disease duration of animals (Lepore et al., 2008). In the present study, some transplanted hNPs-LtGDNF also differentiated into EAAT2- and/or EAAT1-expressing astrocytes in the brain stem and spinal cord of mice, and appeared to play a role in attenuating motor neuron death. However, compared to the study mentioned above, intrathecal transplantation of GDNF-expressing human neural progenitors in this study showed decreased engraftment and survival of donor-derived cells in the spinal cord, inaccurate targeting to key motor neuron pools that ultimately affect survival in ALS models, and inefficient differentiation into functional mature astrocytes that reduce excitotoxic motor neuron death.
Methods
Animals
We used SOD1 transgenic mice (Jackson Laboratories, Bar Harbor, ME) expressing the human SOD1G93A gene (G93A; Gly93 → Ala mutation) for the ALS model. The mutation has a dominant gain-of-function effect. Male transgenic mice were bred with background-matched B6C3/F1 wild-type females. The progeny were genotyped by PCR at 4-5 weeks after birth as previously described (Gurney, 1994), and maintained as SOD1G93A heterozygotes for subsequent studies. All transgenic ALS mice received the same care and housing and were evenly distributed to control and transplantation groups.
Cell culture
Human fetal tissue from cadavers at 13 weeks of gestation was obtained with full parental consent and approval of the research ethics committee of Yonsei University College of Medicine, Seoul, Korea. The methods of acquisition conformed to NIH and Korean government guidelines. The telencephalic brain tissue was dissected, dissociated in trypsin, and seeded into tissue culture-treated 100-mm plates (Corning) with serum-free growth medium. Mitogenic stimulation was achieved by adding fibroblast growth factor-2 (FGF2; R&D, Minneapolis, MI) and leukemia inhibitory factor (LIF; Sigma, St. Louis, MO). The concrete manners of the hNP culture as neurospheres are described in a previously published paper (Kim et al., 2006).
Viral vector construction
We constructed recombinant adenoviral vectors bearing human BDNF, IGF-1, VEGF, NT-3, or GDNF under the control of the constitutive cytomegalovirus (CMV) promoter, and placed these in tandem with a sequence of humanized version of the recombinant green fluorescent protein (hrGFP; Stratagene, La Jolla, CA) gene under the control of an internal ribosomal entry site (IRES) (Supplemental Data Figure S1A). Adenoviral vectors constructed in this fashion were designated as AdBDNF, AdIGF-1, AdVEGF, AdNT-3, AdGDNF, or AdGF as a whole, and produced in conformity with the AdEasy Adenoviral Vector System manual (Stratagene). Infectious recombinant viruses were purified by CsCl gradient centrifugation and titrated on 293 cells by Tissue Culture Infecting Dose 50 (TCID50; QBiogene).
We constructed a lentiviral vector carrying the GDNF gene, which was designated as LtGDNF. LtGDNF was produced by calcium-precipitate-transfection (transient transfection) method using vectors that had been obtained from Trono lab (http://tronolab.epfl.ch/) in accordance with the method offered by the manufacturer. The backbone of transfer vector is pWPI (Supplemental Data Figure S1B). The packaging and envelope plasmids are psPAX2 and pMD2G, respectively. Enhanced green fluorescent protein (eGFP) is a reporter gene. The transfection was conducted to the 293FT cell line and produced viral vectors were concentrated by ultracentrifugation under the sucrose cushion. The viral titer was checked as transducing units (TU) by FACS analysis method offered by Trono lab.
Viral vector infection
For adenoviral vector transduction, hNPs were seeded onto cell culture dishes with serum-free growth medium containing FGF2, LIF, and heparin. One hour later, recombinant adenoviruses encoding BDNF, IGF-1, VEGF, NT-3, or GDNF were added to each well with a multiplicity of infection (MOI) of 80. The medium was replaced with fresh one the next day, and transplantation of cells into mice was conducted 3 days following viral infection. Conditioned media were collected from adenoviral infected-hNP cultures to determine the levels of secreted adenovirus-derived BDNF, IGF-1, VEGF, NT-3, or GDNF via ELISA as previously described (Woodhall et al., 2001).
The transduction method of lentiviral vector encoding GDNF into hNPs was the same as for adenovirus. The titer of lentivirus was 3 MOI, and the level of GDNF secreted from hNPs was checked by PCR and ELISA. To confirm the safety of lentiviral infection, we had observed the survival, cellular morphology, and GFP and GDNF expression patterns of lentivirus-infected hNPs for at least 10 weeks prior to transplantation. In addition, neural cell types and morphologies of lentivirus-infected hNPs under proliferation and differentiation conditions were observed and identified by immunocytochemistry.
Transplantation
Adenovirus encoding GF or lentivirus encoding GDNF infected hNPs (designated as hNPs-AdGF or hNPs-LtGDNF) were prelabeled by ex vivo exposure to bromodeoxyuridine (BrdU, 3 µM/L; Sigma) for 5 days before transplantation. After anesthesia with an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg), the mice were fixed on a Benchmark digital stereotaxic instrument (myNeuroLab.com, St. Louis, MO). Ten µl of hNPs, hNPs-AdGF, or hNPs-AdGDNF suspended in H-H buffer (1 × Hanks' balanced salt solution, 10 mM HEPES, pH 7.4; GIBCO) were slowly injected into the cisterna magna of ALS mice using a 26-gauge needle about 75 days after birth. In some ALS mice, hNPs-LtGDNF suspension was injected not only into the cisterna magna but also the bilateral cerebral ventricles (4 µl into each cerebral ventricle). The ventricular injection sites were 0.9 mm lateral and 0.1 mm anterior to the bregma and 2 mm deep from the dura. H-H buffer was injected into the littermate heterozygote mice in the control group. Cyclosporine was injected (10 mg/kg) intraperitoneally daily from the day before transplantation to the day of death or sacrifice in all hNP transplantation groups and vehicle-injected controls.
Behavior tests
To evaluate impairment in motor function in ALS mice, we measured body weight and motor performance using a wire-maneuver and rota-rod (Columbus Instruments, Columbus, OH) test once a week from the week before hNP transplantation. The maximum time allowed to an animal was 180 s in the wire-maneuver test and 300 s in the rota-rod test. Survival analysis was also conducted along with the behavioral tests. Mouse mortality was determined by the day of death or inability to right itself within 30 s when the mouse was placed on its side. During the behavior test, we also evaluated whether hNP transplantation could have an influence on the normal behavior of mice.
Immunochemistry
Using various cell type-specific markers to characterize the virus-infected hNPs in vitro, we performed immunocytochemistry with the following antibodies: anti-hNestin (human-specific Nestin, 1:200; Chemicon, Temecula, CA), anti-GFAP (glial fibrillary acidic protein, 1:1500; Dako, Carpinteria, CA), anti-hGFAP (human-specific GFAP, 1:500; Sternberger, Lutherville, MD), anti-Tuj1 (neuronal class β-Tubulin III, 1:500; Covance, Berkeley, CA), anti-NeuN (neuronal nuclear protein, 1:40; Chemicon), anti-CNPase (2'3'-cyclic nucleotide 3'-phosphodiesterase, 1:100; Chemicon), anti-O4 (oligodendrocyte marker O4, 1:200; Chemicon), anti-GFP (1:300; Invitrogen), anti-Chat (choline acetyltransferase, 1:200; Chemicon), anti-PDGFR (platelet-derived growth factor receptor, 1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), FITC-conjugated anti-BrdU (1:20; Roche, Mannheim, Germany), biotin-conjugated anti-hHSP27 (human-specific heat shock protein 27, 1:40; Stressgen, Ann Arbor, MI), anti-EAAT1 (1:100; Santa Cruz Biotechnology), anti-EAAT2 (1:100; Santa Cruz Biotechnology), and anti-GDNF (1:50; Santa Cruz Biotechnology).
After transplantation, immunohistochemical analysis of brain and spinal cord tissue of ALS mice model was performed. We first perfused the mice with chilled 1× PBS and 4% paraformaldehyde (PFA; Sigma) in 0.1M PIPES buffer, and then the brain and spinal cord were removed and post-fixed. These tissues were cut in 16-µm sections on cryostat and stained with the antibodies described above. The stained sections were mounted and examined by immunofluorescence microscopy (B×51; OLYMPUS, Tokyo, Japan).
Statistics
Because transgenic mice have different characters according to litter cage, data were analyzed primarily by unpaired t-test. Survival analysis was conducted by Kaplan-Meier method. All data are shown as mean ± standard error of the mean (SEM).
Supplemental Data
Supplemental Data include a figure and can be found with this article online athttp://e-emm.or.kr/article/article_files/SP-41-7-05.pdf.
Acknowledgements
This research was supported by a grant from Stem Cell Research Center (SC-4130) and the Korean Science and Engineering Foundation through the National Core Research Center for Nanomedical Technology.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- BDNF
brain-derived neurotrophic factor
- EAAT1
excitatory amino acid transporter-1
- EAAT2
excitatory amino acid transporter-2
- GDNF
glial cell-derived neurotrophic factor
- GF
growth factor
- GLT1
glutamate transporter-1
- hNPs
human neural progenitor cells
- ICV
intracerebroventricular
- IGF-1
insulin-like growth factor-1
- NPs
neural progenitor cells
- NT-3
neurotrophin-3
- SOD1
Cu/Zn superoxide dismutase
Supplementary Material
Supplemental Data
References
- 1.Aebischer P, Schluep M, Déglon N, Joseph JM, Hirt L, Heyd B, Goddard M, Hammang JP, Zurn AD, Kato AC, Regli F, Baetge EE. Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med. 1996;2:696–699. doi: 10.1038/nm0696-696. [DOI] [PubMed] [Google Scholar]
- 2.Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 3.Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 6.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 7.Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci. 2008;268:40–47. doi: 10.1016/j.jns.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Jr, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 9.Corti S, Locatelli F, Donadoni C, Guglieri M, Papadimitriou D, Strazzer S, Del Bo R, Comi GP. Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain. 2004;127:2518–2532. doi: 10.1093/brain/awh273. [DOI] [PubMed] [Google Scholar]
- 10.Corti S, Locatelli F, Papadimitriou D, Del Bo R, Nizzardo M, Nardini M, Donadoni C, Salani S, Fortunato F, Strazzer S, Bresolin N, Comi GP. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain. 2007;130:1289–1305. doi: 10.1093/brain/awm043. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande DM, Kim YS, Martinez T, Carmen J, Dike S, Shats I, Rubin LL, Drummond J, Krishnan C, Hoke A, Maragakis N, Shefner J, Rothstein JD, Kerr DA. Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol. 2006;60:32–44. doi: 10.1002/ana.20901. [DOI] [PubMed] [Google Scholar]
- 12.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effects of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Federici T, Boulis NM. Gene-based treatment of motor neuron diseases. Muscle Nerve. 2006;33:302–323. doi: 10.1002/mus.20439. [DOI] [PubMed] [Google Scholar]
- 15.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 17.Garbuzova-Davis S, Willing AE, Milliken M, Saporta S, Zigova T, Cahill DW, Sanberg PR. Positive effect of transplantation of hNT neurons (NTera 2/D1cell line) in a model of familial amyotrophic lateral sclerosis. Exp Neurol. 2002;174:169–180. doi: 10.1006/exnr.2002.7860. [DOI] [PubMed] [Google Scholar]
- 18.Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, Hudson JE, Chen N, Davis CD, Sanberg PR. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J Hematother Stem Cell Res. 2003;12:255–270. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- 19.Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, Sanberg PR. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE. 2007;2:e1205. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurney ME. Transgenic-mouse model of amyotrophic lateral sclerosis. N Engl J Med. 1994;331:1721–1722. doi: 10.1056/NEJM199412223312516. [DOI] [PubMed] [Google Scholar]
- 21.Habisch HJ, Janowski M, Binder D, Kuzma-Kozakiewicz M, Widmann A, Habich A, Schwalenstöcker B, Hermann A, Brenner R, Lukomska B, Domanska-Janik K, Ludolph AC, Storch A. Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: limited intraparenchymal migration and survival narrows therapeutic effects. J Neural Transm. 2007;114:1395–1406. doi: 10.1007/s00702-007-0748-y. [DOI] [PubMed] [Google Scholar]
- 22.Harper JM, Krishnan C, Darman JS, Deshpande DM, Peck S, Shats I, Backovic S, Rothstein JD, Kerr DA. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc Natl Acad Sci USA. 2004;101:7123–7128. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanova T, Karolczak M, Beyer C. Estradiol stimulates GDNF expression in developing hypothalamic neurons. Endocrinology. 2002;143:3175–3178. doi: 10.1210/endo.143.8.8794. [DOI] [PubMed] [Google Scholar]
- 26.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2008;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 27.Kerr DA, Llado J, Shamblott MJ, Maragakis NJ, Irani DN, Crawford TO, Krishnan C, Dike S, Gearhart JD, Rothstein JD. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci. 2003;23:5131–5140. doi: 10.1523/JNEUROSCI.23-12-05131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HT, Kim IS, Lee IS, Lee JP, Snyder EY, Park KI. Human neurospheres derived from the fetal central nervous system are regionally and temporally specified but are not committed. Exp Neurol. 2006;199:222–235. doi: 10.1016/j.expneurol.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 30.Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, Cochran EJ, Mufson EJ, Penn R, Goetz CG, Comella CD. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease. Ann Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 31.Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, Clark D, Rose H, Fu G, Clarke J, McKercher S, Meerloo J, Muller FJ, Park KI, Butters TD, Dwek RA, Schwartz P, Tong G, Wenger D, Lipton SA, Seyfried TN, Platt FM, Snyder EY. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 32.Lepore AC, Maragakis NJ. Targeted stem cell transplantaiton strategies in ALS. Neurochem Int. 2007;50:966–975. doi: 10.1016/j.neuint.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llado J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004;27:322–331. doi: 10.1016/j.mcn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Marchetto MCN, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Martin LJ, Liu Z. Adult olfactory bulb neural precursor cell grafts provide temporary protection from motor neuron degeneration, improve motor function and extend survival in amyotrophic lateral sclerosis mice. J Neuropathol Exp Neurol. 2007;66:1002–1018. doi: 10.1097/nen.0b013e318158822b. [DOI] [PubMed] [Google Scholar]
- 37.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagano I, Ilieva H, Shiote M, Murakami T, Yokoyama M, Shoji M, Abe K. Therapeutic benefit of intrathecal injection of insulin-like growth factor-1 in a mouse model of amyotrophic lateral sclerosis. J Neurol Sci. 2005a;235:61–68. doi: 10.1016/j.jns.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Nagano I, Shiote M, Murakami T, Kamada H, Hamakawa Y, Matsubara E, Yokoyama M, Moritaz K, Shoji M, Abe K. Beneficial effects of intrathecal IGF-1 administration in patients with amyotrophic lateral sclerosis. Neurol Res. 2005b;27:768–772. doi: 10.1179/016164105X39860. [DOI] [PubMed] [Google Scholar]
- 40.Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF ICV GDNF Study Group. Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 41.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 42.Park KI, Hack MA, Ourednik J, Yandava B, Flax JD, Stieg PE, Gullans S, Jensen FE, Sidman RL, Ourednik V, Snyder EY. Acute injury directs the migration, proliferation, & differentiation of solid organ stem cells: Evidence from the effect of hypoxia-ischemia in the CNS on clonal "reporter" neural stem cells. Exp Neurol. 2006a;199:156–178. doi: 10.1016/j.expneurol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Park KI, Himes BT, Stieg PE, Tessler A, Fischer I, Snyder EY. Neural stem cells may be uniquely suited for combined gene therapy & cell replacement: Evidence from engraftment of Neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp Neurol. 2006b;199:179–190. doi: 10.1016/j.expneurol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 45.Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 46.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 47.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 48.Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 49.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 50.Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007a;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki M, Tork C, Shelley B, McHugh J, Wallace K, Klein SM, Lindstrom MJ, Svendsen CN. Sexual dimorphism in disease onset and progression of a rat model of ALS. Amyotroph Lateral Scler. 2007b;8:20–25. doi: 10.1080/17482960600982447. [DOI] [PubMed] [Google Scholar]
- 53.Van Damme P, Bogaert E, Dewil M, Hersmus N, Kiraly D, Scheveneels W, Bockx I, Braeken D, Verpoorten N, Verhoeven K, Timmerman V, Herijgers P, Callewaert G, Carmeliet P, Van DenBosch L, Robberecht W. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc Natl Acad Sci USA. 2007;104:14825–14830. doi: 10.1073/pnas.0705046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 55.Woodhall E, West AK, Chuah MI. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Brain Res Mol Brain Res. 2001;88:203–213. doi: 10.1016/s0169-328x(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 56.Wu P, Tarasenko YI, Gu Y, Huang LY, Coggeshall RE, Yu Y. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rats. Nat Neurosci. 2002;5:1271–1278. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]
- 57.Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, Hatfield G, Koliatsos VE. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82:865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 58.Yasuhara T, Shingo T, Muraoka K, wen Ji Y, Kameda M, Takeuchi A, Yano A, Nishio S, Matsui T, Miyoshi Y, Hamada H, Date I. The differences between high and low-dose administration of VEGF to dopaminergic neurons of in vitro and in vivo Parkinson's disease model. Brain Res. 2005;1038:1–10. doi: 10.1016/j.brainres.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 59.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008a;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, Cleveland DW, Goldstein LS. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc Natl Acad Sci USA. 2008b;105:7594–7599. doi: 10.1073/pnas.0802556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, Koliatsos VE. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells. 2006;24:1976–1985. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data