BRONCHIOLITIS
Given the burden bronchiolitis places on both families and the health care system, it is both appropriate and laudable that Canadian pediatricians in the past 15 years have published a substantial proportion of the world literature on bronchiolitis. In particular, members of PERC and Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC) have made major contributions regarding acute therapy, practice variation as well as predicting severe outcomes and quantifying economic burden. The four articles summarized below follow on the ‘heels’ of previous Canadian studies and represent only a few of the many important articles published by Canadian paediatricians.
Hartling L, Wiebe N, Russell K, Patel H, Klassen TP. A meta-analysis of randomized controlled trials evaluating the efficacy of epinephrine for the treatment of acute viral bronchiolitis. Arch Pediatr Adolesc Med 2003;157:957–64.
This meta-analysis by Hartling et al sought to clarify the controversy surrounding the use of epinephrine for bronchiolitis by systematically reviewing the available evidence from randomized controlled trials (RCTs) comparing epinephrine to placebo or other bronchodilators. Data sources were MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, primary authors and reference lists of selected articles. RCTs were selected if they evaluated the efficacy of epinephrine against placebo or other bronchodilators; involved infants and children two years of age or younger with bronchiolitis; and presented quantitative outcomes. Fourteen studies were included, of which seven were inpatient studies, six were outpatient studies, and one was unknown. Epinephrine and placebo were compared in five inpatient and three outpatient studies. Only one of 10 inpatient outcomes (change in clinical score at 60 min) showed a significant difference between treatment groups (standardised mean difference −0.81, 95% CI −1.56 to −0.07) and it favoured epinephrine. Among outpatients, five of 10 outcomes were significant. Four outcomes (change in clinical score at 60 min, change in oxygen saturation at 30 min, respiratory rate at 30 min after treatment, and improvement) favoured epinephrine, while heart rate at 60 min after treatment favoured placebo. Epinephrine and albuterol were compared in four inpatient and four outpatient studies. Among outpatients, results favoured epinephrine for oxygen saturation at 60 min (weighted mean difference [WMD] 1.91, 95% CI 0.38 to 3.44), heart rate at 90 min (WMD −14.00; 95% CI −22.95 to −5.05), respiratory rate at 60 min (WMD −7.76, 95% CI −11.35 to −4.17) and improvement (odds ratio 4.51, 95% CI 1.93 to 10.53). Among inpatients, results favoured epinephrine over albuterol for respiratory rate at 30 min (WMD −5.12, 95% CI −6.83 to −3.41). Some evidence suggests that epinephrine may be favourable compared with albuterol and placebo among outpatients. There is insufficient evidence to support the use of epinephrine among inpatients. The review concludes that large, multi-centred trials are needed to examine the effectiveness of epinephrine compared with placebo and albuterol before routine use among outpatients can be strongly recommended.
Comment: The first two randomized trials that suggested nebulized epinephrine might be more effective than nebulized beta-agonists in bronchiolitis were conducted in Winnipeg and Ottawa (3,4). Since then, another 10 studies have been published, including an additional trial conducted in Montreal (5). In contrast to the initial promising results from the first two small single centre studies, subsequent studies, as summarized by Hartling’s meta-analysis, have suggested little or no benefit from nebulized epinephrine. Our experience with trying to answer this clinical question highlights one of the major problems resulting from conducting relatively small randomized trials in a single centre: reported results are more likely (by chance alone) to yield inaccurate information. For important clinical questions like this, there is a strong case for conducting larger multi-centre trials that are much more likely to yield a clear, definitive answer. In line with this, a paediatric emergency physician, Amy Plint, has recently been funded by the Canadian Institutes of Health Research to conduct a randomized trial comparing nebulized epinephrine and oral dexamethasone with placebo in 800 children enrolled from eight PERC hospitals over two bronchiolitis seasons.
Patel H, Gouin S, Platt RW. Randomized, double-blind, placebo-controlled trial of oral albuterol in infants with mild-to-moderate acute viral bronchiolitis. J Pediatr 2003;142:509–14.
Infants with bronchiolitis are commonly prescribed oral albuterol for five to six days; however, there is little evidence to support this practice. Patel et al conducted in Montreal an RCT to determine whether oral albuterol is effective in reducing the time to symptom resolution of acute viral bronchiolitis in infants with mild to moderate illness. One hundred twenty-nine infants younger than one year were studied; 64 were allocated to receive albuterol and 65 to receive placebo syrup three times per day for seven days. The mean time (± SD) to resolution of illness (primary outcome) was similar in the two groups; 8.9±4.0 days for albuterol and 8.4±3.7 days for placebo (Student’s t-test P=0.5). There were no significant differences for the secondary outcomes (duration of poor feeding, cough, coryza and noisy breathing). Moreover, there were no significant differences in the median time in days to normal infant sleeping or normal parental sleeping. Due to the lack of significant difference in both primary and secondary outcomes between placebo and treatment groups, the authors do not recommend the widespread use of oral albuterol in infants.
Comment: Despite the ongoing controversy regarding the effectiveness of nebulized beta-agonists, many clinicians have made the ‘leap’ by assuming that if nebulized beta-agonists provide some benefit, then oral beta-agonists must as well (6). This is despite the lack of any evidence for benefit of beta-agonists when delivered by the oral route (7). Patel et al designed and conducted an elegant randomized trial that went beyond the assessment of a clinical score assessed shortly after treatment and followed children over the course of their illness. Outcomes assessed were time to resolution of illness, normal feeding, normal sleeping, quiet breathing and resolved cough. These issues are more likely to be of practical importance to the parents’ of children with bronchiolitis than a one time assessment of respiratory distress score 30 min after treatment.
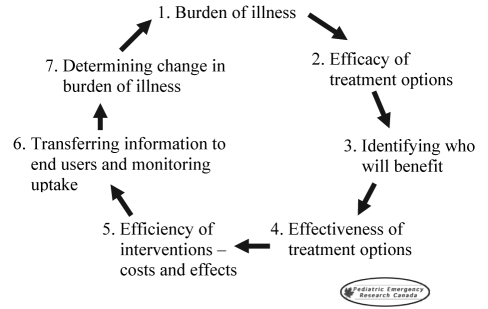
In line with Steps 3 to 5 of the iterative loop of research (Figure 1), we think that outpatient trials assessing these kinds of ‘real world’ outcomes are urgently needed for a host of other outpatient therapies routinely prescribed for bronchiolitis and several other common childhood diseases. Therefore, we urge other paediatric investigators to consider emulating Patel et al’s choice of study outcomes.
Schuh S, Coates AL, Binnie R, et al. Efficacy of oral dexamethasone in outpatients with acute bronchiolitis. J Pediatr 2002;140:27–32.
Schuh et al from Toronto studied the efficacy of oral dexamethasone in acute bronchiolitis in a randomized, double blind, placebo-controlled trial of children younger than 24 months old seen in the ED with moderate to severe respiratory distress (respiratory disease assessment instrument rating of six or greater at baseline). Children were randomly assigned to receive dexamethasone syrup or placebo (patients were given a single dose in hospital and continued to receive either dexamethasone or placebo for five days after discharge). In addition, all children received nebulized albuterol, four times during a 4 h observational period in hospital and four times daily after discharge. Seventy children were analysed, with 36 receiving dexamethasone and 34 receiving placebo. Outcomes were assessed at baseline and at 1 h periods until 4 h. They were also assessed at the patient’s home on day 7. The primary outcome measure was a clinical score, Respiratory Assessment Change Score (RACS), with a decrease indicating improvement. In the ED, RACS from baseline to 4 h showed a significantly greater improvement in the dexamethasone group (dexamethasone RACS −5.0±3.1, placebo RACS −3.2±3.7, P=0.029). The rate of hospitalization from the ED was much lower in the dexamethasone group (19% versus 44% in the placebo group, P=0.039). However, there was no difference in RACS between dexamethasone and placebo groups on day 7 (P=0.75). The authors concluded that treatment of children with moderate to severe bronchiolitis with oral dexamethasone in the first 4 h of therapy is beneficial, resulting in fewer hospitalizations, but that in the long term, its benefit is unclear.
Comment: Numerous clinical trials, including another conducted in Canada (8), have examined whether corticosteroids are effective in the treatment of bronchiolitis. The results of all these previous trials, when examined individually, have suggested no benefit. However, the results of a meta-analysis of these trials suggest that corticosteroids reduce the duration of hospitalization by a modest amount – approximately 10 h (9).
Schuh et al’s findings, though consistent with the results of this meta-analysis, suggest a significantly greater benefit for the use of corticosteroids than any other trial reported to date. Potential explanations for the differences in findings include the use of a very large dose of corticosteroid (dexamethasone 1 mg/kg), treatment of children relatively early in the course of their disease (on average slightly less than 48 h of symptoms), use of a clinical outcome (hospitalization rate) that has not been previously employed, and greater percentage of patients in the dexamethasone group having a family history of atopy (83% versus 53%).
Schuh et al’s study was well-designed and reported, but because it involved only a single centre, it was relatively small. Given our recent experience (discussed above) with the use of epinephrine in bronchiolitis, it is clear that we need the results of a large multicentre RCT with the same study design before we fully accept that steroids are effective in bronchiolitis. Fortunately, two large multicentre trials (Amy Plint’s randomized trial discussed above and another paediatric ED-based trial in the United States) are underway.
Johnson DW, Adair C, Brant R, Holmwood J, Mitchell I. Differences in admission rates of children with bronchiolitis by pediatric and general emergency departments. Pediatrics 2002;110:e49.
Johnson et al in Calgary performed a retrospective review of ED visits involving children with bronchiolitis to quantify the differences in admission practices between paediatric and general EDs in a metropolitan area. In a larger context, this has implications for health care costs, since it would be expected that with a greater base of experience in evaluating children, there would be greater certainty about which children to admit and therefore lower rates of admission in children’s hospitals. The main outcome was population-standardized estimates of admission rates. Three thousand ninety-one charts for children with an International Classification of Diseases, 9th Revision (10) diagnosis of bronchiolitis were reviewed. Two thousand four hundred ninety-six of these children were evaluated at paediatric EDs and 595 at general EDs, with 629 (25%) and 221 (37%) of these children admitted, respectively. When factors such as age, sex, estimated family income, comorbidity rate and clinical severity (estimated by oxygen saturation and respiratory rate) were controlled for with the multiple logistic regression model, population standardized estimates for admission rates at the paediatric and general EDs were 24% (standard error 1%) and 43% (standard error 2%) respectively. Therefore, children diagnosed with bronchiolitis at a paediatric ED, after adjusting for important demographic and clinical information, were approximately half as likely to be admitted to hospital as children diagnosed at general EDs.
Comment: This study by Johnson et al examining differences in admitting practices between paediatric and general EDs relied heavily on findings from studies published in the 1990s by PICNIC investigators (11–15). PICNIC data suggests that, overall, approximately 1% of Canadian children with bronchiolitis are hospitalized and hospitalizations account for more than 60% of health care expenditures for this disease (11). The implication of this finding is that any variation in hospitalization rates would have a significant impact on total health care expenditures. So in the context of this research, the findings by Johnson et al provide an example of how the experience and expertise provided by a paediatric ED may yield cost savings for the health care system without any decrease in quality.
RESPIRATORY
Asthma remains a major disease burden in children, resulting in many ED visits and hospital visits. This has generated an enormous amount of research interest with industry-sponsored research playing a key role. With such a crowded playing field, it is impressive that Canadian researchers have been able to make a significant mark in advancing knowledge in this area. Croup is a respiratory disease that usually peaks in the fall of each year, showing marked increase every other year; and here, Canadian researchers have led the world in performing some of the key RCTs and transforming the management of this disease.
Neto GM, Kentab O, Klassen TP, Osmond MH. A randomized controlled trial of mist in the acute treatment of moderate croup. Acad Emerg Med 2002;9:873–9.
This RCT by Neto et al took place in the ED of the Children’s Hospital of Eastern Ontario. The purpose was to determine whether the use of mist improves clinical symptoms in children aged three months to six years who presented to the ED with moderate croup. Although the use of mist (humidified air) is routine in many EDs, there have been a limited number of studies investigating its effect. Seventy-one patients were enrolled in the study with 35 receiving mist and 36 receiving no mist. The main outcome measure was croup score. The median baseline croup score was four in both groups and croup scores were measured every 30 min for up to 2 h. Outcomes were measured as change from baseline at each of these time points. Secondary outcomes included heart rate, respiratory rate, oxygen saturation and croup score. The results showed no significant difference in change in croup score between the mist and no mist groups (P=0.39), or in improvement of any other variable. Although there were no adverse affects to using mist, there was no proven benefit of mist in the ED treatment of children with moderate croup.
Comment: Some of the important advances in paediatric emergency medicine have occurred in the management of croup and many have been produced by Canadian researchers. This study by Neto et al is exciting because it addresses the effectiveness of a treatment that has been widely adopted by many centres. The use of mist therapy for children presenting to the ED was thought to be an intervention that was obviously effective. However, mist therapy has never been adequately studied in a clinical trial. In this rigorous study, where Neto et al randomized children to mist or no mist, one of the long held medical myths was debunked. While mist therapy cannot be double blinded, she adopted a sound methodological approach by ensuring outcome assessors (of the croup score) and decision makers (paediatric emergency physicians) were blinded to treatment allocation. In the end, there was no evidence that mist therapy was better than the control in dexamethasone-treated patients with croup.
Before this study, Canadian researchers had done much of the work demonstrating that glucocorticoids (dexamethasone or budesonide) are more effective than placebo in hastening the resolution of croup symptoms, reducing time spent in the ED and significantly decreasing the probability of hospitalization (16). As a ‘real world’ example of how radically the routine use of steroids has had on the management of the disease, in western Australia, the rate of intubation in patients with severe disease was reduced by approximately fivefold (17). Where steroids have been widely adopted, the management of croup has been transformed into largely an outpatient disease, with very few children requiring hospital admission. Furthermore, a recently completed multicentre PERC trial shows that dexamethasone is clearly effective, even in children with the mildest symptoms (18).
However, the existence of high-quality evidence does not guarantee that the evidence will be used. David Johnson, funded by Canadian Institutes of Health Research, is examining three methods of changing provider behaviour in croup management. In a cluster RCT, the evidence-based guidelines produced by the Alberta Medical Association (19) have been disseminated using three approaches, ranging from sending out a pamphlet outlining the guidelines (most passive) to a complex, multi-facetted intervention, using opinion leaders along with a series of educational workshops. It has already been demonstrated that the admission rate for croup and the use of effective interventions such as dexamethasone vary significantly across Alberta (20).
Ducharme FM, Chabot G, Polychronakos C, Glorieux F, Mazer B. Safety profile of frequent short courses of oral glucocorticoids in acute pediatric asthma: Impact on bone metabolism, bone density, and adrenal function. Pediatrics 2003;111:376–83.
This study by Ducharme et al in Montreal was a cross-sectional study aiming to establish the safety profile of repeated short courses of oral glucocorticoids in children with asthma. Oral glucocorticoids are frequently used to reduce airway inflammation in acute paediatric asthma exacerbations. The factors evaluated were impact on bone mineralization, bone metabolism and adrenal function. Children were evaluated in one to all three of these areas. The study enrolled 83 children between the ages of two and 17. Forty-eight children had been exposed to oral glucocorticoids (median exposure level = four courses, range of three to 11 courses) and 35 had not. For the study, children that had been exposed were also prescribed a new burst of glucocorticoids for the index exacerbation. Impact on bone metabolism was measured through levels of serum osteocalcin, calcium, phosphorus, alkaline phosphatase, urine calcium/creatinine ratio, renal threshold phosphate concentration and urine pyridinoline cross-links. These were measured on day 1, last day of the burst, which was day 4 or 5, and day 30. Impact on bone density was evaluated through determination of bone age and bone mineral density of the lumbar spine, which were obtained one month after the glucocorticoids burst. Adrenal function was evaluated by examining adrenal reserve using basal cortisol and cortisol response to adrenocorticotrophic hormone stimulation one month after the exacerbation. The main finding was that baseline serum osteocalcin level was 30% lower (95% CI 25 to 34) in the exposed children compared with the unexposed. There was a 41% decrease in the baseline serum osteocalcin level between the first and last day of the burst, and then a return to baseline by day 30. There was no such change in the levels of the unexposed group. There was no significant difference in urine pyridinoline levels between the two groups. Bone density scores were similar in the two groups (Z score exposed [± SD] −0.61±1.0; Z score unexposed −0.67±0.9), both of which had scores lower than expected. Measurements of adrenal function were similar between the exposed and unexposed groups. The authors concluded that the practice of giving a short course of oral glucocorticoids for acute asthma was not associated with any lasting effect on bone metabolism, mineralization or adrenal function.
Comment: While glucocorticoids have been shown to have an important role for children with acute asthmatic exacerbations, the safety of this intervention has always been brought into question. This study by Ducharme et al goes a long way to clarifying the question about osteopenia and adrenal suppression. There was no evidence of either of these adverse effects in this study of 83 children (48 of whom had been exposed to short courses of oral glucocorticoids). The implication is that with the evidence supporting the effectiveness of glucocorticoids for patients presenting to the ED, clinicians can now feel somewhat reassured about the safety of this approach.
In 1996, Canadian researchers, Patel et al (21), using a case-control study design determined that in immunocompetent children, recent corticosteroid use did not increase the risk of complicated varicella infection. This study demonstrated that this potential risk was perhaps not as high as previously thought and, hence, judicious and appropriate use of steroids for children with respiratory problems, where the evidence has demonstrated effectiveness, may be used.
Cates CC, Bara A, Crilly JA, Rowe BH. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev 2003;(3):CD000052.
The objective of this Cochrane systematic review by Cates et al in both the United Kingdom and Canada was to assess the effects of holding chambers versus nebulisers for the delivery of beta-2 agonists for acute asthma. The RCTs covered in this review were identified through searches of the Cochrane Airways Group trials register (last searched November 2002) and the Cochrane Central Register of Controlled trials (The Cochrane Library, Issue 3, 2002). The review analyses 1076 children and 44 adults included in 22 trials worldwide. In the latest update, five trials on inpatients with acute asthma (184 children, 28 adults) were added to the review. The primary outcome measures were admission to hospital, or duration of stay in the case of inpatients. Other outcomes were duration in ED, change in respiratory rate, blood gases, pulse rate, tremor, symptom score, lung function, use of steroids and relapse rates. For the primary outcome, method of delivery of medication did not significantly affect hospital admission rates. In adults, relative risk of admission for holding chamber compared with nebuliser was 0.88 (95% CI 0.56 to 1.38); in children, the relative risk was 0.65 (95% CI 0.4 to 1.06). In adults, there was no significant difference between the two methods for length of stay in the ED, whereas in children, length of stay was significantly shorter with use of the holding chamber (WMD −0.47 h, 95% CI −0.58 h to −0.37 h). Also in children, pulse rate was lower for holding chamber (WMD −7.6% baseline, 95% CI −9.9 to −5.3% baseline). Peak flow and forced expiratory volume were similar for holding chamber and nebuliser. In conclusion, the two methods were at least equivalent for most outcomes, but holding chambers may have some advantages over nebulisers for use in children with acute asthma.
Comment: The treatment of acute asthma in ED has been greatly advanced by paediatric emergency researchers. Through their work on demonstrating the effectiveness of frequent, high dose beta-2 agonists, Schuh et al (22) had a major impact in this area, defining the role of ipratropim bromide (23) and examining the role of inhaled steroids in the ED (24). Historically, the switch from injected epinephrine to nebulized salbutamol occurred when Dr Allen Becker (a paediatric allergist) et al (25) demonstrated that injected epinephrine was not superior to nebulized salbutamol with an RTC in the ED.
The Cochrane collaboration is committed to synthesizing all the existing evidence on interventions in health care. Systematic reviews form the basis of this. Canadian researchers have played an important role in the development of Cochrane, with major contributions in the Airways Group and Acute Respiratory Infection review groups. Drs Brian Rowe and Francine Ducharme, co-editors in the Airways groups, have both made important contributions in this area.
This review, which included 1076 children, demonstrated that holding chambers were as effective as standard delivery of beta-2 agonists via nebulization. Indeed, in children, the stay in the ED was shortened by 0.47 h. A cost effectiveness study has shown that holding chambers are less costly than nebulizers for delivery of beta-2 agonists. As with many pieces of evidence, now the key is to ensure that clinicians do use the best evidence for their patients.
INJURY
Injury is the number one killer of children older than one year and is responsible both for significant morbidity in the paediatric population and a large number of visits to the ED. The sheer magnitude of the problem and the fact that many of these children are first seen in the ED, make injury research a priority for many paediatric emergency researchers. Studies range from injury surveillance studies that recognize new injury patterns (ie, snowboard or all-terrain vehicle injuries), to clinical management trials (such as the derivation of clinical decision rules), to interventional trials in injury prevention (ie, comparing playground surface materials). The three articles summarized below are recent additions to the paediatric emergency medicine literature that have changed clinical practice and improved child health.
Bulloch B, Neto G, Plint A, et al. Validation of the Ottawa Knee Rule in children: A multicenter study. Ann Emerg Med 2003;42:48–55.
This multicentre validation study by Bulloch et al evaluated the sensitivity and specificity of the Ottawa Knee Rule (26) when applied to children and determined whether use of the rule would decrease the number of knee radiographs ordered in the ED. The study included children between the ages of two and 16 years presenting to the ED with an acute injury to the knee occurring within the last seven days and evidence of bony injury to the knee on physical examination. Seven hundred fifty children were enrolled. The mean age (± SD) was 11.8±3.1 years and 59% were male. The children were assessed by physicians or fellows according to the Ottawa Knee Rule, which states that a knee x-ray is required only for patients who have acute knee injury and at least one of the following: tenderness at head of fibula, isolated tenderness of patella, inability to flex to 90 degrees, and inability to bear weight (at least four steps), both immediately and in the ED. Radiographs were ordered at the physicians’ or fellows’ discretion. A positive outcome was defined as any fracture seen on x-ray, regardless of size. A negative outcome was defined as children who had no fracture on radiograph, or were asymptomatic after 14 days if no radiograph was obtained. Seventy children had a positive outcome (fracture). The Ottawa Knee Rule was 100% sensitive (95% CI 94.9% to 100%) and had a specificity of 42.8% (95% CI 39.1% to 46.5%). Radiography was performed for 670 children. If radiographs had been performed according to the Ottawa Knee Rule, 460 children would have required a radiograph, resulting in an absolute reduction of 210 radiographs, or 31%. This study provides evidence that the Ottawa Knee Rule is valid in children and, if used, it could decrease the use of radiography in children with acute knee injuries.
Comment: This study is an example of the growing number of publications in the emergency medicine literature devoted to the developing and testing of clinical decision rules. Clinical decision rules (prediction rules) are designed to help physicians with diagnostic or therapeutic decisions at the bedside. They are derived from original research (as opposed to consensus-based clinical practice guidelines), and incorporate features from history, physical examination or simple tests. These tools help clinicians cope with the uncertainty of medical decision making and improve their efficiency. It has been shown that improved physician efficiency results in better patient care and a more cost-effective medical practice (27).
Dr Ian Stiell, a professor of emergency medicine at the University of Ottawa, is a world-renowned expert in developing clinical decision rules and has contributed in the development of clinical decision rules in adults for the use of radiography for acute ankle and knee injuries, minor head injuries and neck injuries (26,28–32). However, as most pediatricians would not feel comfortable using clinical decision rules derived and validated in adults on the paediatric population, independent validation studies need to be performed in children.
In 1999, Plint et al (33) showed the Ottawa Ankle Rules to be valid in children. In 2001, in an international collaboration involving the ED of The Hospital for Sick Children in Toronto and the Children’s Hospital in Boston, Boutis et al (34) found a “low risk clinical examination” of the ankle to be effective in reducing ankle radiography without missing any “high risk fractures”. The study by Bulloch et al abstracted above has shown that the Ottawa Knee Rule can be confidently used in children older than five years. These findings should improve physician efficiency in the use of ankle and knee radiographs for children with acute injuries. It is interesting to note that to quickly accrue the needed patients, all three studies were multicentre studies, with the Plint et al and Bulloch et al studies using a network of Canadian paediatric emergency medicine researchers known as PERC.
Currently, Dr Martin Osmond is leading a Canadian Institutes of Health Research funded PERC study involving nine paediatric EDs across Canada, working toward deriving a clinical decision rule for the use of CT scan in children with minor head injury. In the future, in an effort to improve physician efficiency and keep down health care costs, we should expect to see the derivation and validation of many more clinical decision rules for use in the acute care of children.
Farion KJ, Osmond MH, Hartling L, et al. Tissue adhesives for traumatic lacerations: A systematic review of randomized controlled trials. Acad Emerg Med 2003;10:110–8.
This systematic review by Farion et al from Ottawa and Edmonton summarizes the evidence for the use of tissue adhesives for the management of traumatic lacerations in children and adults. Studies were selected for review if they were RCTs that enrolled patients of any age with acute (less than 12 h old) linear lacerations managed in an ED or other primary care setting; compared a tissue adhesive (TA) versus standard wound closure (SWC), or two or more TAs; and reported on any measured clinical or physiological outcome. Nine studies, represented by 13 articles, were identified through a comprehensive search of electronic databases, citations and contacting authors. Cosmetic outcome was the principal outcome assessed. Secondary outcomes were pain, procedure time, ease of use and complications. In the eight studies comparing TA with SWC, there was no significant difference in cosmesis at various time points. Subgroup analysis comparing paediatric and adult studies for cosmesis showed no significant difference. Pain scores, as assessed by visual analog scales, significantly favoured TAs. Time to complete the procedure in minutes also favoured TAs with physicians being able to complete the procedure approximately 5 min faster with TAs. In terms of complications, there was a small significant risk of increased dehiscence using TAs (approximately one extra dehiscence for every 25 lacerations treated), and a risk increase for erythema using SWC (one wound with significant erythema for every eight wounds sutured). Only one study compared two TAs (butylcyanoacrylate [Histoacryl Blue, B Braun Melsungen AG, Germany] versus octylcyanoacrylate [Dermabond, Ethicon Inc, USA]) and no significant difference resulted for cosmesis, pain, procedure time or complications. TAs are an acceptable alternative to SWC for repairing simple traumatic lacerations, but further research is needed looking at the characteristics that result in the small increase in dehiscence with TAs and whether this has an effect on cosmetic outcome.
Comment: This systematic review by Farion et al summarizes the evidence for using TAs for simple laceration closure in children and adults. The evidence is clear that the use of TAs for children with acute lacerations would result in a quicker, less painful procedure that results in an equal long-term cosmetic outcome, though none of the original RCTs showed a significant difference in wound dehiscence rates between the two methods of closure, Farion et al have shown that by analyzing the combined data, that there is a slight increased risk of dehiscence with TA closure versus sutures. It is not known whether this results in a poorer cosmetic outcome or whether changes in wound selection, operator technique or type of TA used could minimize the dehiscence rate. Very large RCTs would be needed to evaluate these rare events.
Much of the early research in the area of TA closure was carried out in EDs in Canada. A study by Quinn et al (35) at the University of Ottawa was the first RCT to show the effectiveness of a TA in wound closure in children. Quinn et al (36) were also the first to determine the interrater reliability and validity of the Visual Analog Scale as a tool to assess cosmetic outcome. Another Canadian study by Osmond et al (37) was the first to demonstrate the cost-effectiveness of TAs as an alternative to sutures. As a result of this and other research, TAs are now used daily for laceration closure in most EDs across North America.
The Farion et al’s systematic review was carried out under the aegis of the Cochrane Collaboration, and was also published in The Cochrane Database of Systematic Reviews (38). The Cochrane Library consists of a regularly updated collection of evidence-based medicine databases, including The Cochrane Database of Systematic Reviews, which provided high-quality information to people providing and receiving care and those responsible for research, teaching, funding and administration. As part of the Cochrane Library, this tissue adhesive review will be frequently updated, incorporating new RCTs when they become available. Thus, the review will remain current and will be an excellent source of information for practicing physicians.
Macpherson AK, To TM, Macarthur C, Chipman ML, Wright JG, Parkin PC. Impact of mandatory helmet legislation on bicycle-related head injuries in children: A population-based study. Pediatrics 2002;110:e60.
Macpherson et al from Toronto studied the impact of helmet legislation on bicycle-related head injuries in Canadian children. This was a population-based study including all Canadian children aged five to 19 years who were hospitalized for bicycle-related injuries from 1994 to 1998. Rates of head injury over time were compared among provinces with and without mandatory helmet legislation. The legislation was introduced in four provinces between October 1995 and July 1997. In the four provinces with legislation (Ontario, New Brunswick, British Columbia, and Nova Scotia) there was a 45% reduction in the rate of bicycle-related head injuries from 1994/1995 to 1997/1998. In the remaining provinces without legislation, the rate of head injury also declined but significantly less (27%). Other bicycle-related injury rates also declined in the population but there were no significant differences between provinces with and without legislation.
Comment: Bicycle crashes are a major cause of injury and death among school-aged children and adolescents, with most hospitalizations and deaths attributed to head injuries. It has been well demonstrated that helmets reduce the risk of head injury by up to 88% and reduce the risk of facial injury by 65% among child cyclists. The difficulty lies in how to get children to wear helmets. Studies examining educational initiatives have shown little increase in bicycle helmet ownership and use. Legislation is felt to be the most effective way to implement injury prevention strategies. In their study, Macpherson et al elegantly show the effectiveness of mandatory helmet legislation in the most important outcome – reduction of head injury. By comparing children with head injuries with children without head injuries, they control for potential differences in the children’s bicycling habits and demonstrate that a decrease in head injuries was not likely due to a decrease in cycling.
Other Canadian studies have added to the bicycle helmet literature in the past. Harlos et al (39) observed 2629 cyclists in Winnipeg to identify the demographic characteristics of those not using bicycle helmets. They found a very low usage rate (21.3%) in Winnipeg and reported that adolescents, males, rural cyclists and those in low income areas were less likely to wear helmets. In a similar study carried out in Sudbury, Ontario, Rowe et al (40) observed helmet usage rates of 20% and determined that only 49% of those were worn correctly. They found a high level of support for helmet legislation for children (81%) and for all ages (57%). It is local Canadian studies such as these that identify populations that may benefit from interventions to increase helmet use. In addition, this data is used to make the case to municipal and provincial leaders for the need for legislation. It is encouraging that over the last few decades there has been a decline in the overall childhood injury rate in Canada; however, much work still needs to be done in the future to continue to identify and eliminate potential injury hazards.
REFERENCES
-
1.Tugwell P, Bennett KJ, Sackett DL, Haynes RB. The measurement iterative loop: A framework for the critical appraisal of need, benefits and costs of health interventions. J Chronic Dis. 1985;38:339–51. doi: 10.1016/0021-9681(85)90080-3. [DOI] [PubMed] [Google Scholar]
-
2.Bennett KJ, Tugwell P. Iterative loop gives framework for assessing technology. Dimens Health Serv. 1986;63:68, 71. [PubMed] [Google Scholar]
-
3.Menon K, Sutcliffe T, Klassen TP. A randomized trial comparing the efficacy of epinephrine with salbutamol in the treatment of acute bronchiolitis. J Pediatr. 1995;160:1004–7. doi: 10.1016/s0022-3476(95)70234-2. [DOI] [PubMed] [Google Scholar]
-
4.Sanchez I, De Koster J, Powell RE, Wolstein R, Chernick V. Effect of racemic epinephrine and salbutamol on clinical score and pulmonary mechanics in infants with bronchiolitis. J Pediatr. 1993;122:145–51. doi: 10.1016/s0022-3476(05)83508-5. [DOI] [PubMed] [Google Scholar]
-
5.Patel H, Platt RW, Pekeles GS, Ducharme FM. A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–24. doi: 10.1067/mpd.2002.129844. [DOI] [PubMed] [Google Scholar]
-
6.Nahata MC, Schad PA. Pattern of drug usage in bronchiolitis. J Clin Pharm Ther. 1994;19:117–8. doi: 10.1111/j.1365-2710.1994.tb01122.x. [DOI] [PubMed] [Google Scholar]
-
7.Gadomski AM, Lichenstein R, Horton L, King J, Keane V, Permutt T. Efficacy of albuterol in the management of bronchiolitis. Pediatrics. 1994;93:907–12. [PubMed] [Google Scholar]
-
8.Klassen TP, Sutcliffe T, Watters LK, Wells GA, Allen UD, Li MM. Dexamethasone in salbutamol-treated inpatients with acute bronchiolitis: A randomized, controlled trial. J Pediatr. 1997;130:191–6. doi: 10.1016/s0022-3476(97)70342-1. [DOI] [PubMed] [Google Scholar]
-
9.Garrison MM, Christakis DA, Harvey E, Cummings P, Davis RL. Systematic corticosteroids in infant bronchiolitis: A meta-analysis. Pediatrics. 2000;105:E44. doi: 10.1542/peds.105.4.e44. [DOI] [PubMed] [Google Scholar]
-
10.International Classification of Diseases, 9th Revision. Geneva: World Health Organization; 1979. [Google Scholar]
-
11.Langley JM, Wang EE, Law BJ, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: A Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. J Pediatr. 1997;131:113–7. doi: 10.1016/s0022-3476(97)70133-1. [DOI] [PubMed] [Google Scholar]
-
12.Opavsky MA, Stephens D, Wang EEL. Testing models predicting severity of respiratory syncytial virus infection on the PICNIC RSV database. Arch Pediatr Adolesc Med. 1995;149:1217–20. doi: 10.1001/archpedi.1995.02170240035005. [DOI] [PubMed] [Google Scholar]
-
13.Wang EE, Law BJ, Boucher FD, et al. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study of admission and management variation in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1996;129:390–5. doi: 10.1016/s0022-3476(96)70071-9. [DOI] [PubMed] [Google Scholar]
-
14.Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1995;126:212–9. doi: 10.1016/s0022-3476(95)70547-3. [DOI] [PubMed] [Google Scholar]
-
15.Wang EE, Law BJ, Stephens D, et al. Study of interobserver reliability in clinical assessment of RSV lower respiratory illness: A Pediatric Investigators Collaborative Network for Infections in Canada (PICNIC) Study. Pediatr Pulmonol. 1996;22:23–7. doi: 10.1002/(SICI)1099-0496(199607)22:1<23::AID-PPUL4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
-
16.Russell K, Wiebe N, Saenz A, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2004;(1):CD001955. doi: 10.1002/14651858.CD001955.pub2. [DOI] [PubMed] [Google Scholar]
-
17.Geelhoed GC. Sixteen years of croup in a Western Australian teaching hospital: Effects of routine steroid treatment. Ann Emerg Med. 1996;28:621–6. doi: 10.1016/s0196-0644(96)70084-7. [DOI] [PubMed] [Google Scholar]
-
18.Bjornson C, Klassen TP, Williamson J, et al. Treatment of mild croup with a single dose of oral dexamethasone: A multi-center, randomized, placebo-controlled trial N Engl J Med(In press) [DOI] [PubMed] [Google Scholar]
-
19.Alberta Medical Association Guideline for the Diagnosis and Management of Croup <wwwalbertadoctorsorg/bcm/ama/ama-website.nsf/0/3EA50DCC10AAD9F187256E1A0067025A?OpenDocument> (Version current at June 30, 2004). [Google Scholar]
-
20.Johnson DW, Williamson J, Craig W, et al. Management of croup: Practice variation among 21 Alberta hospitals. Pediatric Academic Societies’ Annual Meeting; San Francisco. May 1 to 4; 2004. (Poster) [Google Scholar]
-
21.Patel H, Macarthur C, Johnson D. Recent corticosteroid use and the risk of complicated varicella in otherwise immunocompetent children. Arch Pediatr Adolesc Med. 1996;150:409–14. doi: 10.1001/archpedi.1996.02170290075012. [DOI] [PubMed] [Google Scholar]
-
22.Schuh S, Parkin P, Rajan A, et al. High- versus low-dose frequently administered, nebulized albuterol in children with severe, acute asthma. Pediatrics. 1989;83:513–8. [PubMed] [Google Scholar]
-
23.Schuh S, Johnson DW, Callahan S, et al. Efficacy of frequent nebulized ipratropium bromide added to frequent high-dose albuterol therapy in severe childhood asthma. J Pediatr. 1995;127:639–45. doi: 10.1016/s0022-3476(95)70368-3. [DOI] [PubMed] [Google Scholar]
-
24.Schuh S, Reisman J, Alshehri M, et al. A comparison of inhaled fluticasone and oral prednisone in children with severe acute asthma. N Engl J Med. 2000;343:689–94. doi: 10.1056/NEJM200009073431003. [DOI] [PubMed] [Google Scholar]
-
25.Becker AB, Nelson NA, Simons FE. Inhaled sabutamol (albuterol) vs injected epinephrine in the treatment of acute asthma in children. J Pediatr. 1983;102:465–9. doi: 10.1016/s0022-3476(83)80679-9. [DOI] [PubMed] [Google Scholar]
-
26.Stiell IG, Greenberg GH, Wells GA, et al. Derivation of a decision rule for the use of radiography in acute knee injuries. Ann Emerg Med. 1995;26:405–13. doi: 10.1016/s0196-0644(95)70106-0. [DOI] [PubMed] [Google Scholar]
-
27.Stiell IG, Wells GA. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med. 1999;33:437–47. doi: 10.1016/s0196-0644(99)70309-4. [DOI] [PubMed] [Google Scholar]
-
28.Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127–32. doi: 10.1001/jama.269.9.1127. [DOI] [PubMed] [Google Scholar]
-
29.Stiell IG, Lesiuk H, Wells GA, et al. Canadian CT Head and C-Spine Study Group Canadian CT head rule study for patients with minor head injury: Methodology for phase II (validation and economic analysis) Ann Emerg Med. 2001;38:317–22. doi: 10.1067/mem.2001.116795. [DOI] [PubMed] [Google Scholar]
-
30.Stiell IG, Lesiuk H, Wells GA, et al. Canadian CT Head and C-Spine Study Group The Canadian CT Head Rule Study for patients with minor head injury: Rationale, objectives, and methodology for phase I (derivation) Ann Emerg Med. 2001;38:160–9. doi: 10.1067/mem.2001.116796. [DOI] [PubMed] [Google Scholar]
-
31.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357:1391–6. doi: 10.1016/s0140-6736(00)04561-x. [DOI] [PubMed] [Google Scholar]
-
32.Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001;286:1841–8. doi: 10.1001/jama.286.15.1841. [DOI] [PubMed] [Google Scholar]
-
33.Plint AC, Bulloch B, Osmond MH, et al. Validation of the Ottawa Ankle Rules in children with ankle injuries. Acad Emerg Med. 1999;6:1005–9. doi: 10.1111/j.1553-2712.1999.tb01183.x. [DOI] [PubMed] [Google Scholar]
-
34.Boutis K, Komar L, Jaramillo D, et al. Sensitivity of a clinical examination to predict need for radiography in children with ankle injuries: A prospective study. Lancet. 2001;358:2118–21. doi: 10.1016/S0140-6736(01)07218-X. [DOI] [PubMed] [Google Scholar]
-
35.Quinn JV, Drzewiecki A, Li MM, et al. A randomized controlled trial comparing a tissue adhesive with suturing in the repair of pediatric facial lacerations. Ann Emerg Med. 1993;22:1130–5. doi: 10.1016/s0196-0644(05)80977-1. [DOI] [PubMed] [Google Scholar]
-
36.Quinn JV, Drzewiecki AE, Stiell IG, Elmslie TJ. Appearance scales to measure cosmetic outcomes of healed lacerations. Am J Emerg Med. 1995;13:229–31. doi: 10.1016/0735-6757(95)90100-0. [DOI] [PubMed] [Google Scholar]
-
37.Osmond MH, Klassen TP, Quinn JV. Economic comparison of a tissue adhesive and suturing in the repair of pediatric facial lacerations. J Pediatr. 1995;126:892–5. doi: 10.1016/s0022-3476(95)70203-2. [DOI] [PubMed] [Google Scholar]
-
38.Farion K, Osmond MH, Hartling L, et al. The Cochrane Library, Issue 2, 2004. Chichester, United Kingdom: John Wiley & Sons, Ltd; Tissue adhesives for traumatic lacerations in children and adults (Cochrane Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
-
39.Harlos S, Warda L, Buchan N, Klassen TP, Koop VL, Moffatt ME. Urban and rural patterns of bicycle helmet use: Factors predicting usage. Inj Prev. 1999;5:183–8. doi: 10.1136/ip.5.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
40.Rowe BH, Thorsteinson K, Bota GW. Bicycle helmet use and compliance: A northeastern Ontario roadside survey. Can J Public Health. 1995;86:57–61. [PubMed] [Google Scholar]