Abstract
Korean vivax malaria had been prevalent for longtime throughout the country with low endemicity. As a result of the Korean war (1950-1953), malaria became epidemic. In 1959-1969 when the National Malaria Eradication Service (NMES) was implemented, malaria rates declined, with low endemicity in the south-west and south plain areas and high endemic foci in north Kyongsangbuk-do (province) and north and east Kyonggi-do. NMES activities greatly contributed in accelerating the control and later eradication of malaria. The Republic of Korea (South Korea) was designated malaria free in 1979. However, malaria re-emerged in 1993 and an outbreak occurred in north Kyonggi-do and north-west Kangwon-do (in and/or near the Demilitarized Zone, DMZ), bordering North Korea. It has been postulated that most of the malaria cases resulted from bites of sporozoite-infected females of An. sinensis dispersed from North Korea across the DMZ. Judging from epidemiological and socio-ecological factors, vivax malaria would not be possible to be endemic in South Korea. Historical data show that vivax malaria in Korea is a typical unstable malaria. Epidemics may occur when environmental, socio-economical, and/or political factors change in favor to malaria transmission, and when such factors change to normal conditions malaria rates become low and may disappear. Passive case detection is a most feasible and recommendable control measure against the unstable vivax malaria in Korea in cost-effect point of view.
Keywords: Plasmodium vivax, Korea, unstable malaria
INTRODUCTION
Malaria, an important communicable disease for many years in Korea, prevailed throughout the country until the 1950s. Since then, cases rapidly declined until it was declared eradicated in 1979 (WHO, 1981). Recently malaria has re-emerged in South Korea and become an important public health threat. Several review papers on malaria in Korea have been published (Paik and Tsai, 1963; Kim, 1982; Paik et al., 1988; Lee et al., 1994; Chai, 1999). Most of these review papers focussed on a specific period. Therefore, a historical review is meaningful for more comprehensive understanding of the epidemiology and potential for malaria control/eradication. In particular, the accomplishments of the National Malaria Eradication Service (NMES) activities, most of which were not officially ducumented are reported rather in detail. Only endemic Plasmodium vivax in Korea was dealt in this review, excluding imported cases of P. malariae, P. falciparum and other imported strains of P. vivax. Though there was a strong evidence that P. malariae was endemic in limited areas of Chungchongnam-do in 1929-1930 (Chiba and Aki, 1930; Oh, 1930), it was no longer considered as a health threat.
MALARIA SITUATION BEFORE THE 1950S
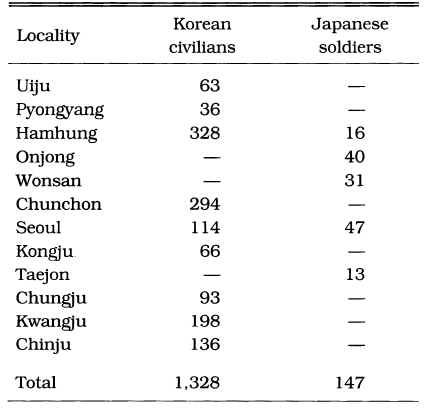
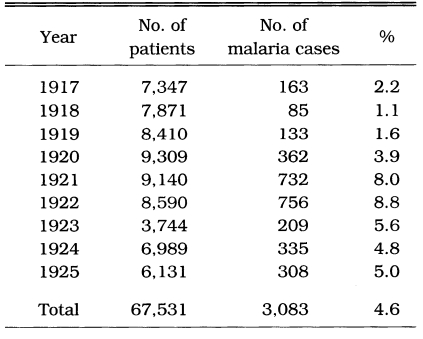
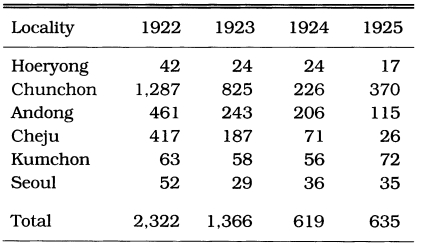
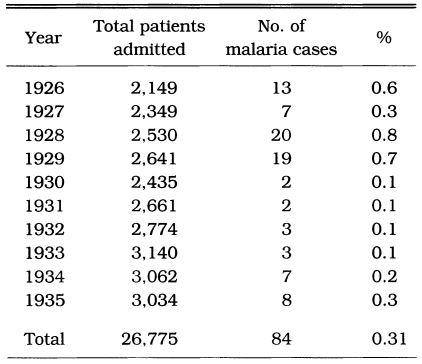
Malaria was prevalent and a major health threat in Korea during previous centuries. The vivax malaria was first reported in 1913 by Hasegawa. He reported 1,328 cases of Korean civilians and 147 cases of Japanese soldiers at 13 locations throughout the country (Table 1). Analysis of 1,201 cases showed that (1) 74.5% was male and 25.5% female, (2) age composition was 44.9% in < 10 years, 20.9% in 11-20 years, 14.5% in 21-30 years, 8.7% in 31-40 years, 5.1% in 41-50 years, and 5.9% in > 51 years, and (3) seasonal occurrence was 1.9% in January, 2.2% in February, 2.4% in March, 5.3% in April, 7.8% in May, 21.4% in June, 15.1% in July, 16.1% in August, 14.8% in September, 9.0% in October, 2.7% in November, and 1.2% in December. Epidemiological significance was that the highest positive rate was shown in the age group under 10 years old (44.8%), and 19.7% of the total cases occurred during the period of non-transmission season (November-May), indicating that a Korean strain would be the delayed type temperate zone strain. Among the 67,531 patients who visited the Kangnung Provincial Hospital in Kangwon-do in 1917-1925, 3,083 cases (4.6%) were diagnosed as vivax malaria (Table 2). Many of the cases were reported during the non-transmission period when daily temperatures were below 15℃, suggesting the protracted incubation of vivax malaria in Korea (Himeno, 1926). Malaria parasite rates of school children in Kangwon-do were 8.1% in Korean students and 3.8% in Japanese students at Cholwon, and 16.1% in Korean students and 7.3% in Japanese students at Chunchon (Tanabe, 1927). Kodama (1928) reported that malaria was prevalent throughout Kangwon-do, particularly in counties bordering the Taibaek mountains, where rice paddies were limited. Kobayashi (1931) reported 2,322 malaria cases in 1922, 1,366 cases in 1923, 619 cases in 1924 and 635 cases in 1925 in 6 different locations (Table 3). He also mentioned that malaria cases occurred throughout the Korean peninsula with an uneven geographical distribution, and malaria was the major endemic disease in some areas, whereas very rare in many other areas, indicating focal malaria transmissions. It is interesting to note that the highest number of malaria cases were reported in Hamhung, Hamkyongnam-do in 1910 (Hasegawa, 1913) and in 1923-1925 (Nagai, 1925). Among a total of 26,775 patients admitted to Severance Hospital, Seoul, during a 10 year period (1926-1935), 84 patients (0.3%) were diagnosed as malaria (Table 4) (Choi, 1936).
Table 1.
Number of malaria patients treated at hospitals in 1910 (Hasegawa, 1913)
Table 2.
Malaria cases treated at the Kangnung (Kangwon-do) provincial hospital (Himeno, 1926)
Table 3.
The malaria cases in different localities in 1922-1925 (Kobayashi, 1931)
Table 4.
Malaria cases admitted in the Severance Hospital, Seoul, 1926-1935 (Choi, 1936)
Available information reported on malaria in Korea prior to 1945 indicates that vivax malaria was endemic and prevalent throughout the Korean peninsula, demonstrating an uneven geographical distribution with a higher incidence rate in mountainous areas rather than large rice growing regions.
There were little data reported for the prevalence of malaria in Korea during the second world war (1941-1945), a period following post-indenpendence (1945-1950) and the Korean war (1950-1953). Among 3,983 students of middle schools in Seoul in 1948, 584 students (14.7%) were diagnosed as malaria (Chun, 1959). In 1952, 1,032 malaria cases were reported from April through September (peak in July) in Yangyang-gun (county), Kangwon-do, of which the highest rate was identified in children under 5 years (Kim and Han, 1953). They also conducted a mass blood survey of 208 soldiers and found 4.8% positive rate. The military medical office reported from a military population of 400,000 that there were 8,855 cases (2.2%) and 5,741 cases (1.4%) of malaria in 1953 and 1954, respectively. US troops dispatched to the Korean war were provided chloroquine chemoprophylaxis (weekly) during the transmission season. Nevertheless 1,513 soldiers were reported with vivax malaria from July 1951 through November 1952 (Hanky et al., 1953). Among 1,350 Canadian soldiers that deployed to Korea during the Korean war in 1952, 152 (11.3%) became ill with vivax malaria after returning home country (Hale and Halpenny, 1953).
Judging from such limited data, it is certain that the prevalence of malaria in Korea increased in remarkable degree as a result of the Korean war. Because of poor records and population movement, the geographical distribution is not well understood.
ACCOMPLISHMENTS OF THE NATIONAL MALARIA ERADICATION SERVICE
The National Malaria Eradication Service (NMES) was established in April 1959 as a joint project of the Republic of Korea and the UN/WHO Western Pacific Regional Office. The Service was consisted of 28 Korean members and 2-3 WHO consultants (malariologist, parasitologist and/or entomologist), assigned to parasitological laboratory, entomological and epidemiological teams. The results of NMES activities were incompletely reported in published documents (Paik and Tsai, 1963; Whang, 1962, 1963, 1964; Paik et al., 1965; Paik and van der Gugten, 1966; NMES, 1966; Hong, 1967; Chen et al., 1967; Ree and Paik 1967; Hong and Ree, 1968). The followings are the summarized results of NMES activities.
Spleen survey
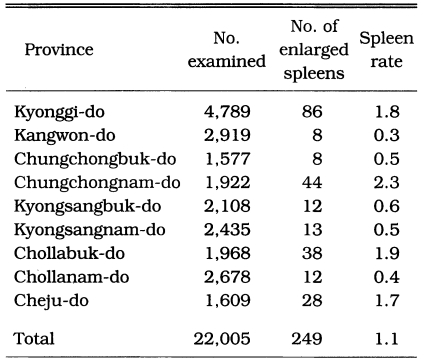
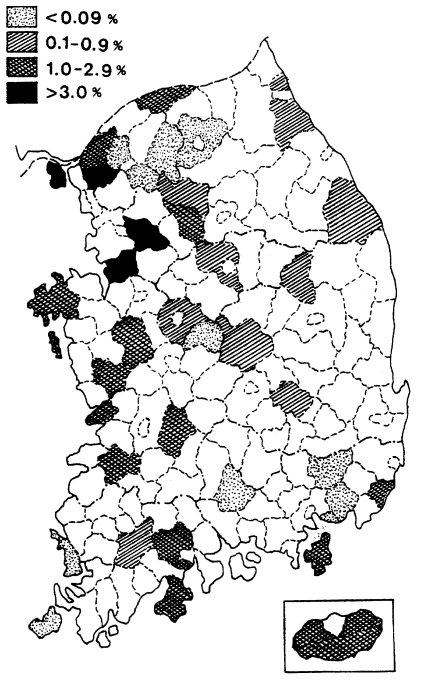
One of the earliest methods used for estimating malaria endemicity in a given lo-cality is to determine the proportion of persons with a palpable enlargement of the spleen. During the period of July-October 1959, a spleen survey was conducted at 37 counties in 9 provinces. A total of 22,005 children under 15 years were examined with an average spleen rate of 1.1% (range 0.0-3.8%). The results of the spleen survey by province are shown in Table 5, and their geographical distribution by county is given in Fig. 1, which revealed that vivax malaria was widely dis-tributed but hypoendemic.
Table 5.
Results of the spleen survey of children under 15 years old in July-October 1959 (NMES, 1966)
Fig. 1.
Map showing the results of spleen survey for malaria endemicity in 1959 (NMES, 1966).
Mass blood survey
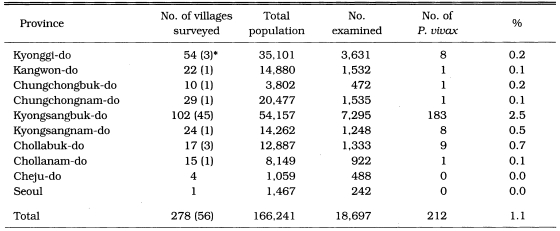
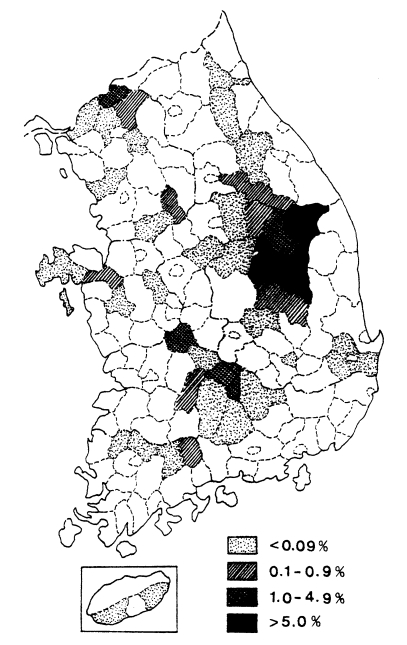
A mass blood survey was carried out at 278 selected villages of 41 cities/counties in 9 provinces during 1960. A total of 18,697 children under 15 years were examined with 212 children (1.1%) positive for P. vivax (Table 6, Fig. 2) (NMES, 1966). The highest slide positive rate was shown in Andong-gun (11.7%), followed by Yechon-gun (10.3%), Bongwha-gun (5.4%), and Yongju-gun (2.8%), all of which are located north Kyongsangbuk-do.
Table 6.
Results of the mass blood survey in 1960 (NMES, 1996)
*Number of positive villages.
Fig. 2.
Distribution of the malaria positive rate by mass blood survey in 1960 (NMES, 1966).
Passive case detection (PCD)
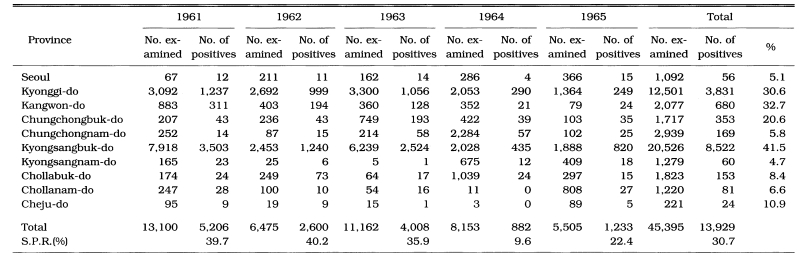
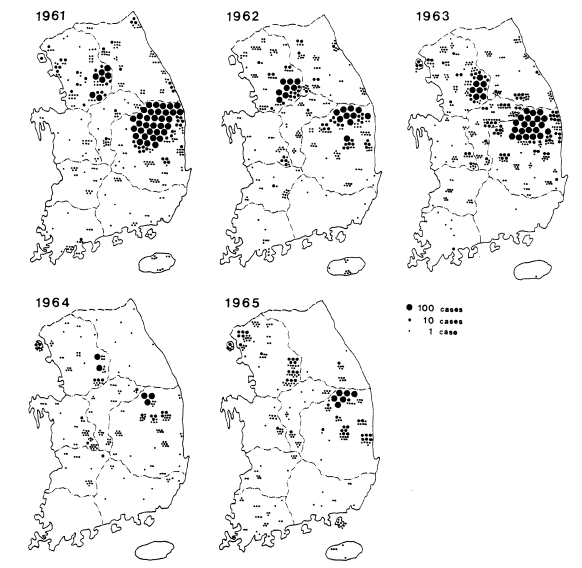
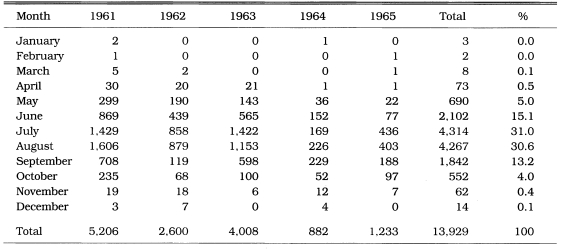
PCD is not only an effective measure of malaria incidence but one of the control measures, particularly in unstable vivax malarial areas. During 1961-1962, PCD units consisted of health centers, hospital/clinic physicians, and school nurses throughout the country. The PCD network was expanded to include villages, training all village chiefs/leaders and voluntary collaborators. Efforts were emphasized especially in high endemic areas, i.e., Kyongsangbuk-do and Kangwon-do. During 1961-1965, a total of 11,259 PCD workers were trained (average 42/100,000 population; range 5-124). Of them, 3,453 workers (30.6%) participated in PCD activities, taking blood smears from all fever cases and sending the slides to NMES laboratory, as well as the administration of chloroquine for suppressive treatment (NMES, 1966). A total 45,395 blood smears from 4,135 PCD units were examined and 13,929 cases were positive for P. vivax. The slide positive rate was 39.7% in 1961, 40.2% in 1962, 35.9% in 1963, 9.6% in 1964 and 22.4% in 1965, with an overall slide positive rate of 30.7% (Table 7). Geographical distribution of the malaria cases detected by PCD is given by year in Fig. 3. The fact that the malaria cases in 1964 and 1965 were significantly lower than those of the previous three years suggests that the early diagnosis and treatment may have played an important role in the decline of malaria occurrence. Monthly occurrence of the malaria cases detected by PCD is shown in Table 8. The peak of the cases was in July and August (31.0% and 30.6%, respectively).
Table 7.
Number of malaria cases by province and overall annual slide positive rate (SPR) as reported by the passive case detection (PCD) program in 1961-1965 (NMES, 1996)
Fig. 3.
Distribution of the malaria cases detected by PCD in 1961-1965 (NMES, 1966).
Table 8.
Monthly occurrence of the reported malaria cases by PCD in 1961-1965 (NMES, 1966)
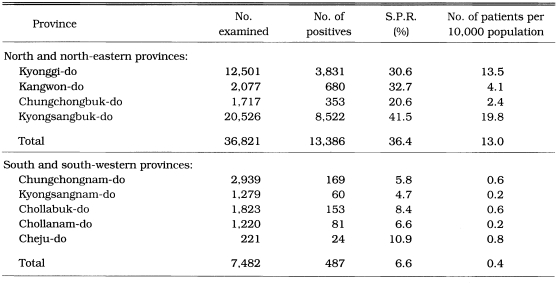
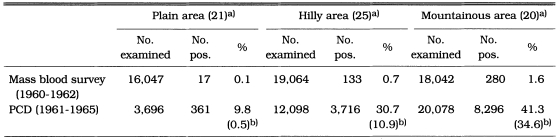
Malaria rate per 10,000 population was significantly higher in the north and north-eastern areas (13.0) than in the south and south-eastern areas (0.4) of South Korea (Table 9). Malaria endemicity was also significantly different among topographically different areas. The slide positive rate of vivax malaria by mass blood survey in 1960-1962 was significantly higher in mountainous areas (1.6%) than in hilly areas (0.7%) or flat areas (0.1%). Similarly, the number of malaria cases per 10,000 population reported by PCD during 1961 through 1965 significantly higher in mountainous areas (34.6%) than in hilly areas (10.9) or flat areas (0.5) as shown in Table 10.
Table 9.
The number of malaria cases by PCD of mid and mid-eastern and south and south-western provinces of South Korea in 1961-1965 (NMES, 1966)
Table 10.
Comparison of the number of malaria cases for topographical regions in 1960-1965 (NMES, 1966)
a)Number of counties investigated.
b)Number of patients per 10,000 population.
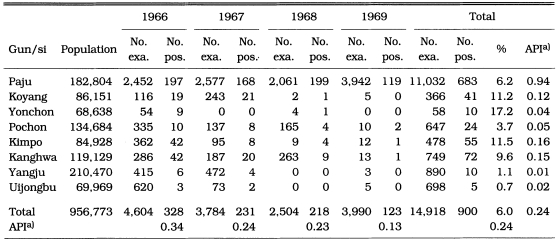
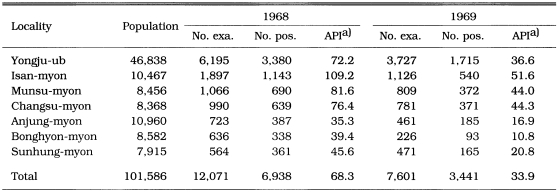
During the period of 1966-1969, PCD activities were carried out in two main endemic areas (north Kyonggi-do and north Kyongsangbuk-do). Of 14,918 blood smears from fever cases, 900 (6.0%) were positive for P. vivax. Annual parasite incidence (API) per 1,000 population was 0.34 in 1966, 0.24 in 1967, 0.23 in 1968 and 0.13 in 1969, which indicates that malaria endemicity was significantly decreasing (Table 11). The API reported by PCD in Yongju-gun, Kyongsangbuk-do in 1968 and 1969 was 68.3 and 33.9, respectively (Table 12)(Lee et al., 1972; Kim, 1982). These API values were so unreasonably high that the source of the data would be not reliable.
Table 11.
Number of reported malaria cases by PCD in north Kyonggi-do in 1966-1969 (Shim and Kim, 1999)
a)Annual parasite incidence per 1,000 population.
Table 12.
Number of malaria cases by PCD in Yongju-gun, Kyongsangbuk-do in 1968 and 1969 (Lee et al., 1972; Kim, 1982).
a)Annual parasite incidence per 1,000 population.
Active case detection (ACD)
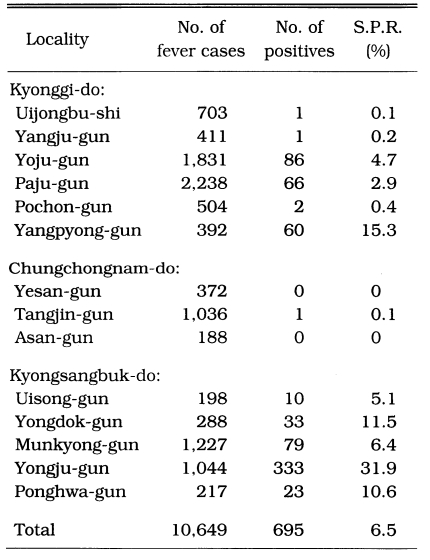
ACD workers periodically visited all households of the assigned villages to identify fever cases, take blood samples and administer antimalaria drugs (chloroquine). ACD was carried out in 3 counties of Chungchongnam-do, 5 counties of Kyongsangbuk-do and 6 counties of Kyonggi-do from 1963 through 1965 (Table 13). The results show that the highest slide positive rate was in Yongju-gun (31.9%). Malaria cases were not reported from in Yesan-gun and Asan-gun, and only one positive case from 1,036 fever cases in Tangjin-gun of Chungchongnam-do.
Table 13.
Number of malaria cases by ACD in 1963-1965 (NMES, 1966)
Control
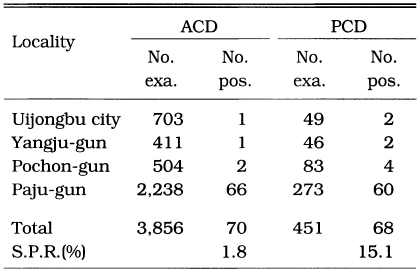
PCD and ACD was carried out at 4 counties in north Kyonggi-do in 1965 in order to evaluate sensitivity and effectiveness in finding malaria cases (Table 14). Of 3,856 fever cases, 70 (1.8%) were positive by ACD while 68 (15.1%) of 451 fever cases were positive by PCD. The result indicated that case detection by PCD and ACD was not different. As ACD is much more expensive and requires much more man-power compared to those of PCD, it was concluded that PCD alone is a recommendable method for malaria control in Korea in cost-effectiveness concept.
Table 14.
Results of ACD and PCD in selected study areas in Kyonggi-do in 1965 (NMES, 1996)
The pilot projects to evaluate the effectiveness of indoor residual spraying for malaria control were conducted twice, first at Ponghyon-myon, Yongju-gun, Kyongsangbuk-do in 1963, and the second at Kaegun-myon, Yangpyong-gun and Taesin-myon, Yoju-gun, Kyonggi-do in 1964. All houses and animal sheds in the study areas were sprayed with DDT at a dosage of 2 g/m2 before the beginning of the mosquito season. Both PCD and mosquito surveys were employed in both the DDT treated and the control (untreated) areas. The results of both pilot projects showed that there was no significant difference in the incidence of malaria and population density of An. sinensis between the DDT sprayed areas and the control areas. However, the life span of vector mosquitoes was shorter in the treated area (47.8% versus 77.8% parous rate of the treated and control area, respectively). Based on these results (ACD, PCD, and DDT residual spray), it was determined that PCD alone would effectively control unstable vivax malaria in Korea.
Malaria after NMES
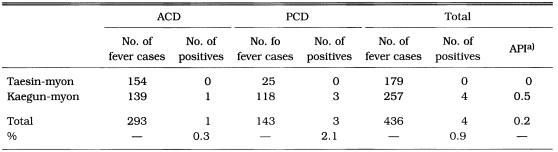
Subsequent to 1969 when NMES was dissolved, there were no specific activities of malaria control/eradication. Both ACD and PCD were carried out with total coverage of the population in Taesin-myon, Yoju-gun and Kaegun-myon, Yangpyong-gun, Kyonggi-do during the period of June-October 1972 to determine malaria endemicity after NMES (Table 15). The API declined to zero (2.5 in 1965) at Taesin-myon, and to 0.5 (9.1 in 1965) at Kaegun-myon (Lee et al., 1972). PCD was carried out at Isan-myon, Yongju-gun, Kyongsangbuk-do in 1976, resulting that 7 malaria cases (1.4%) out of 487 fever cases were detected showing 0.7 of API (Kim, 1982).
Table 15.
Results of ACD and PCD in Taesin-myon, Yoju-gun and Kaegun-myon, Yangpyong-gun, Kyonggi-do in June-October 1972 (Lee et al., 1972)
a)Annual parasite incidence per 1,000 population.
Among 51 malaria cases diagnosed from 29 hospitals located in Seoul in 1970-1982, 38 cases were imported from tropical countries and 13 cases were indigenous. Ten indigenous cases were found in 1970-1974, and one case each in 1976, 1978 and 1981, respectively (Ahn et al., 1982). From the chart of 26 hospitals, 27 indigenous cases were found during the period of 1970-1984, of which 2 cases were recorded in 1984 (Soh et al., 1985). It was not possible to confirm whether these two cases were precisely diagnosed by microscopic confirmation. These data indicate that the incidence of malaria had rapidly declined in 1970s. The Republic of Korea was declared malaria free by the World Health Organization in 1979 (WHO, 1981).
PROLONGED INCUBATION PERIOD OF KOREAN VIVAX STRAIN
A prolonged incubation period has been observed from many strains of P. vivax, mainly in the temperate zone, such as Dutch, Madagascar, USSR and Korea. The first observation of longterm incubation period of Korean P. vivax was made by Hasegawa (1913). He reported that 6 Japanese soldiers returned from Korea to Osaka, Japan in April 1910 and demonstrated the onset of malaria in June 1910. As there was no malaria in Osaka, they were suspected to be infected in Korea during the transmission season of the previous year. He also observed that out of 1,201 malaria cases 251 (20.9%) occurred from December through April during the non-transmission season. In the 1950s, several workers reported both short and long incubation periods of a Korean strain of P. vivax (Eddleman et al., 1951; Hall and Loomis, 1952; Brunetti, 1954; Brunetti et al., 1954; Tiburskaja et al., 1968). Brunetti et al. (1954) reported that an outbreak of vivax malaria in California, USA, resulted from veterans returning from the Korean war. Nine persons became ill after an incubation period of 10-40 days while 26 persons became ill after an incubation period of 217-316 days. Exoerythrocytic stages of the North Korean strain could still be found in the liver 255 days after the inoculation of sporozoites (Garhnam et al., 1975). They mentioned that the North Korean strain resembled the Dutch and St. Elizabeth strains and lied between P. vivax vivax and P. vivax hibernans because it did not always show a long incubation period. Shute et al. (1976) experimentally studied the effect of numbers of sporozoites inoculated on the length of the incubation period using the North Korean strain of P. vivax. All 6 subjects inoculated with 10-100 sporozoites became ill after a long incubation period (262-628 days). Among 5 persons injected with 1,000 sporozoites, 4 became ill after a long incubation (257-386 days) and one 16 days after inoculation. All 5 subjects inoculated with 100,000 sporozoites became ill after a short incubation period (13-16 days). They concluded that there are two strains (populations) of sporozoites, one governing long incubation and the other short incubation, with the strain of short incubation representing a small proportion of the whole population. Tiburskaja and Vrublevskaja (1977) experimentally infected humans with the North Korean strain of P. vivax through bites of the infected mosquitoes. Among 77 patients, 19 (24.7%) became ill after a short incubation period (14-22 days) and 58 (75.3%) after a long incubation period. Among 58 cases of long incubation period, 1 case (1.3%) became ill after 1 month, 11 (14.3%) after 8 months, 14 (18.2%) after 9 months, 14 (18.2%) after 10 months, 13 (16.9%) after 11 months, 3 (3.9%) after 12 months, and 2 (2.6%) after 13 months.
RE-EMERGENCE OF MALARIA SINCE 1993
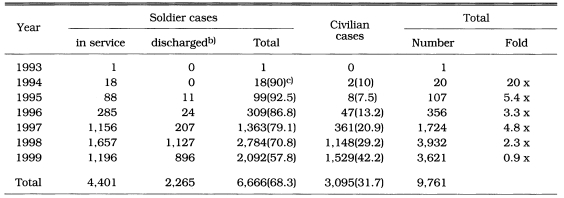
One indigenous case of P. vivax was reported in Paju-gun, Kyonggi-do in July 1993 (Chai et al., 1994). Since then the number of cases has been rapidly increased annually, as shown in Table 16 (National Institute of Health, 1995, 1996, 1997, 1998, 2000; Lee et al., unpublished). The discharged soldiers who were infected while on army service and a paroxism was presented at home after discharge were put in the "soldier" category.
Table 16.
Number of vivax malaria cases in South Korea in 1993-1999a)
a)US Army personnedl (107 cases) infected at/near DMZ are not included.
b)These soldier cases were infected while on service in and/or near the DMZ, and paroxysm was shown at home after discharge.
c)Numbers in parenthesis indicate percentage.
The majority of the cases were reported in military personnel, particularly at beginning of the outbreak, composed of 90% in 1994, 92.5% in 1995, 86.8% in 1996, 79.1% in 1997, 70.8% in 1998, and 57.8% in 1999. The rate of soldiers has been decreasing year after year, and the number of soldier cases decreased from 2,784 in 1998 to 2,092 in 1999. By sociopolitical factors, it was suggested that the rate in military personnel should have been higher than for civilians since soldiers on sentry duty were severely exposed to mosquito bites throughout the night outdoors in and/or near the DMZ where domestic animals were not available for feeding of zoophilic vector mosquitoes. After 1997, many soldiers serving in and/or near the DMZ were placed on chloroquine prophylaxis through the whole mosquito season with primaquine at end of the season (Kim et al., 1997, 1998; Lee, 1998). Chemo-prophylaxis and other measures such as wear of permethrin-treated uniforms resulted in continuous decrease in the number of cases in military personnel in 1999. Lee (1998) reported that chloroquine (300 mg/week) and primaquine (15 mg/day for 14 days) for chemoprophylaxis were administered to soldiers who served in high risk areas (in and/or near the DMZ). None of properly administered soldiers developed malaria, whereas 11% of inadequately administered soldiers and 89% of untreated soldiers developed malaria. The decrease in reported malaria cases in soldiers in 1999 resulted in an overall decrease to 3,621 (0.9x) from 3,932 in 1998, inspite that the malaria cases of civilians increased to 1,529 (1.3x) in 1999 from 1,148 in 1998.
Seasonal occurrence
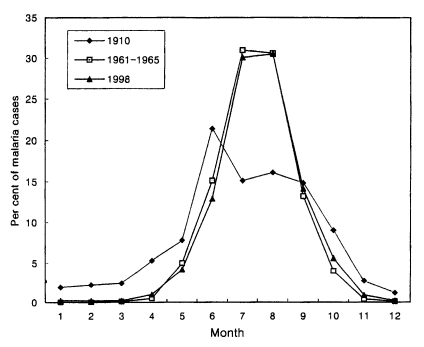
Total 3,932 cases in 1998 were reported throughout the year, totalling 6 cases (0.2%) in January, 8 (0.2%) in February, 9 (0.2%) in March, 40 (1.0%) in April, 164 (4.2%) in May, 509 (12.9%) in June, 1,182 (30.1%) in July, 1,199 (30.5%) in August, 553 (14.1%) in September, 219 (5.6%) in October, 34 (0.9%) in November and 9 (0.2%) in December (Lee et al., unpublished). The pattern of seasonal occurrence of the reemergence of malaria was very similar to that of 1960s (Fig. 4).
Fig. 4.
Monthly occurrence of vivax malaria in 1910, 1960s and 1998 (Hasegawa, 1913; NMES, 1966; Lee et al., unpublished).
Geographical distribution
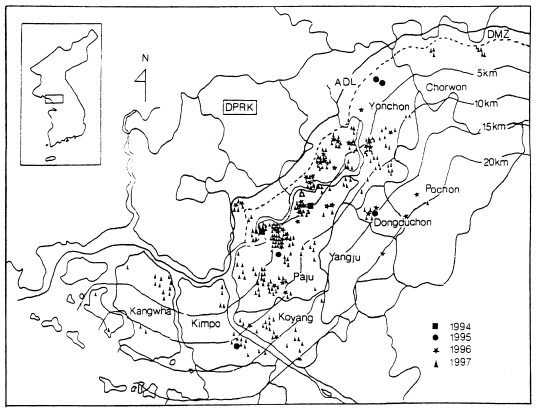
In case of soldiers, data suggest that all cases of soldiers, including discharged soldiers, were infected along the DMZ, the bordering South and North Korea (Yim et al., 1996; Kim et al., 1997; Lee, 1998). Among a total of 389 malaria cases of the soldiers in 1994-1996, 343 (88.2%) soldiers had served in DMZ and the remaining (11.8%) had served at vicinity of the DMZ (Kim et al., 1998). A total of 27 malaria cases in U.S. troops in 1993-1997 stationed along the western corridor near the DMZ (Feighner et al., 1998). Lee et al. (1998) analyzed 650 cases of civilians in 1994-1997, including ex-soldiers discharged from military service where they were potentially infected; 226 cases (34.8%) were ex-soldiers who served in/near DMZ; 317 cases (48.8%) were residents in known malarious areas of north Kyonggi-do and north-west Kangwon-do; 75 cases (11.6%) were residents outside of malaria risk area, but had a history of travel in malarious areas during transmission season; the remaining 32 cases (4.9%) were residents of non-malarial areas. Geographical distri-bution of the civilian cases from 1994 through 1997 was outlined by Kho et al. (1999) as shown in Fig. 5. Among 287 cases examined, 232 (80.8%) were located within 10 km of the southern border of DMZ and the remaining 55 (19.2%) resided between 10-20 km from the DMZ.
Fig. 5.
Distribution of 278 civilian cases of vivax malaria in 1994-1997 (Kho et al., 1999).
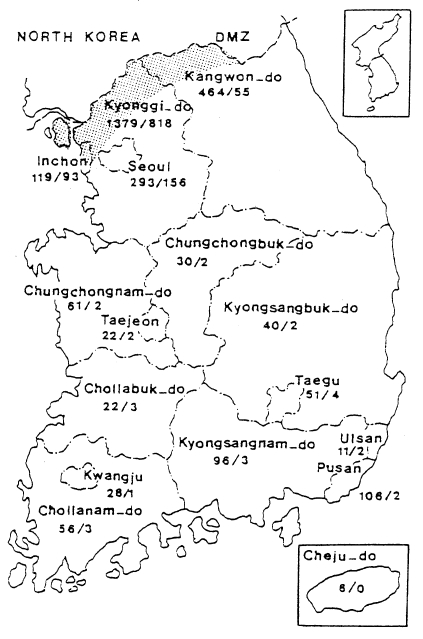
When the malaria cases were plotted on a map by the reported locality, they were widely distributed throughout the country including Cheju-do, as if malaria endemicity were established all over the country. In 1998 a total 3,932 cases were reported, of which 1,196 cases were soldiers serving near the DMZ, and 1,127 cases of ex-soldiers were reported in non-malarious areas (e.g., 293 in Seoul, 106 in Pusan, 96 in Kyongsangnam-do, 6 in Cheju-do, etc.), as shown in Fig. 6. However all the ex-soldiers are believed to be infected during army service in malarious areas near the DMZ. Among the 1,148 civilian cases, 809 (70.5%) patients resided in malarious areas and 339 (29.5%) resided in other areas. Among 339 patients residing in non-malarious areas, 157 responded to a questionaire that they had visited the malarious areas during the transmission season. The site of transmission of the remaining 182 civilians (4.6% of the total malaria cases) was not determined (Lee et al., unpublished).
Fig. 6.
Map showing the number of malaria cases in 1998 by province/special city. No. of sodier cases/No. of civilian cases(Lee et al., unpublished).
Origin of re-emergence and possibility of endemicity
As malaria was eradicated in South Korea in 1979, it was impossible to be re-established of endemicity in 1993 and 1994. No one argues the fact that re-emergence of P. vivax originated from North Korea. As people could not transfer from North to south Korea, the only possible source of infection would be the sporozoite-infected mosquitoes dispersed from North Korea to South across DMZ (Chai, 1997, 1999; Ree, 1998; Kho et al., 1999). This plausible speculation is based on the other postulation that malaria had persisted with low endemicity in North Korea until 1993 and a big epidemic occurred when epidemiological and/or environmental factors changed in favorate of the vector mosquitoes and the parasite (Ree, 1998).
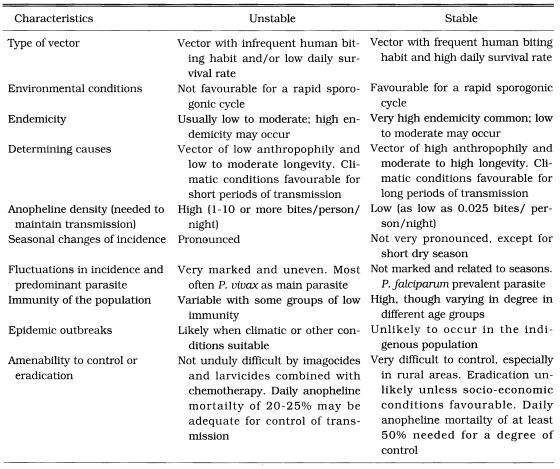
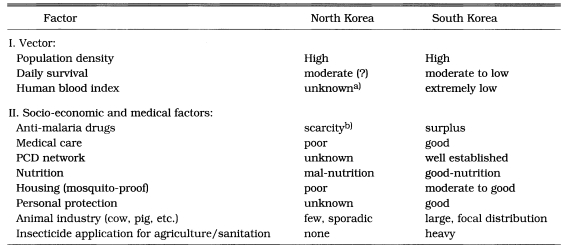
We have a typical unstable malaria in Korea. Some characteristics of the unstable malaria are well summarized by Macdonald (1957) and Gilles and Warrell (1993) (Table 17). The vector species, Anopheles sinensis, is highly zoophilic (Ree et al., 1967), moderate longevity (Hong, 1977) and high population density. Transmission season is short (June-September). Table 18 summarized the differences of entomological, socio-ecological and medical factors between South and North Korea influencing malaria endemicity/epidemicity, which helps to understand malaria epidemiology of South and North Korea. Some recent meteorological data that would cause an epidemic in north Kyonggi-do, Korea were given by Ree (1998).
Table 17.
Some characteristics of unstable and stable malaria (Macdonald, 1957)
Table 18.
Comparison of factors governing malaria endemicity/epidemicity between North and South Korea
a)As there are few heads of cows and pigs in North Korea, human blood index of An. sinensis females might be high.
b)Recently WHO provided chloroquine.
Our primary concern is that malaria is now endemic in South Korea, particularly in north Kyonggi-do and north-west Kangwon-do (within 20 km from DMZ), and what are the main source of infection of most malaria cases. Malaria outbreak would be resulted from either way, one by direct bites of sporozoite-infected mosquitoes dispersed from North Korea across the DMZ through the entire transmission season (Ree, 1998; Kho et al., 1999), the other by endemicity maintained by local mosquitoes producing secondary, tertiary, and further successive cases (Chai, 1999). For producing a secondary case, the same mosquito should bite humans twice with a 12 day interval. As human blood index of An. sinensis was 0.007 in 1999 (Ree et al., unpublished), proportion of two human bites by the same mosquito would be 0.000049. As proportion of daily survival was 0.871 in 1999 (Ree et al., unpublished), proportion of survival for 12 days of the sporogonic period would be 0.191. Therefore, proportion of two human bites with a 12 day interval would be 0.000009. On the other hand, when we speculate that malaria transmission is resulted from bites of sporozoite-infected mosquitoes dispersed from North Korea, just one bite by chance produces a new case. The proportion of this chance would be 0.007. Although the flight range of mosquitoes differs by species and by environmental factors, it is well known that Anopheles mosquitoes fly quite far distances (20-30 km is not un67common) (Swellengrebel et al., 1938; Simmons et al., 1939; Hocking, 1953). A dispersal range of mosquitoes is decided either by a single flight by wind, or by many times of flight activities. It requires at least 12 times of flights during 12 days of sporogonic period (4 gonotrophic cycles) for seeking hosts, resting places, and breeding places. In 1998, a flight range of An. sinensis was studied in Korea using a mark-release-recapture method, and 20 km of a flight range was observed (unpublished).
A plausible speculation is that many (not all) cases near the DMZ of South Korea may be due to the dispersal of sporozoite-infected mosquitoes from North Korea. If this speculation is true, number of malaria cases in South Korea relies on the degree of epidemicity (infection rate of An. sinensis) in North Korea. The more malaria cases in North, the more in South in a certain degree, because north Kyonggi-do is located at a peripheral edge of North Korea.
SYMPTOMS, TREATMENT AND RELAPSE
Predromal symptoms of 87 patients of vivax malaria was observed (Lim et al., 1996). Thrombocytopenia appeared in 72.4% of the patients with 124,000±71,000/µl, anemia in 92% with 12.4±2.2 g/dl of hemoglobins, and splenomegaly in 72%. High fever (≥ 40℃) was shown in most of the patients, with 48 h periodicity in 72.4%, 24 h periodicity in 10% and no periodicity in 17.6%. Kim and Lim (1997) studied symptoms of 26 patients of ROK Army and observed myalgia (88.5%), vomiting (34.6%), diarrhea (3.8%), and oral herpes simplex (3.8%), besides typical malaria symptoms such as fever, chills, headache, etc. Hematologic findings were anemia (92.3%), thrombocytopenia (61.5%), leukopenia (50%), and leukocytosis (19.2%). Song (1965) reported that parasite density in blood of 171 patients in Korea was 3,853/mm3, of which number of gametocytes were 135/mm3 (3.5% of the total). On the other side, Lim et al. (1996) reported that gametocytes were most frequently found (95.7%), and the next was ring form trophozoites. Kim and Lim (1997) also observed that the majority of parasite stage was gametocytes (92.3%) in their blood smears and the next young trophozoites (26.4%). As these gametocyte rates were unusually high, re-observation of the slides for confirmation is required.
Hankey et al. (1953) reported that 355 veterans infected with P. vivax were treated with 1,500 mg of chloroquine (base) in 3 days (3 doses of 300 mg during the first 24 h, a single dose of 300 mg daily for 2 days), and 137 (38.6%) relapsed at least once. The mean duration from the first attack to the second was 68.6 days in average (range 39-247 days). Tiburskaja and Vrublevskaja (1977) observed that out of 74 patients treated with chloroquine after the first attack, 23 (31.1%) relapsed, of which 11 cases relapsed once, 11 cases twice and 1 case 3 times.
Among 272 patients treated with chloroquine 1,500 mg in 3 days plus primaquine 27 mg/day for 14 days, 2 cases relapsed, whereas no one relapsed among 348 patients treated with chloroquine 1,500 mg in 3 days plus primaquine 15 mg/day for 14 days (Alving et al,. 1953). In the course of these studies, only minimal evidence of drug toxicity was observed. One patient treated with chloroquine alone developed mild generalized pruritus which subsided 4 days after the drug was stopped. A similar mild pruritus developed in a patient treated with chloroquine and primaquine. It disappeared after chloroquine was stopped and before the administration of primaquine was completed. There was no evidence of hemolytic anemia in any patient. Approximately 15% of the patients had a mild to moderately severe anemia (hemoglobin concentration of 6.4-12 gm/100 cc) at the time treatment was instituted.
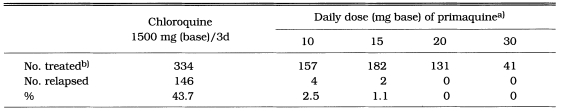
The patients of veterans with vivax malaria acquired in Korea were treated at the time of a late acute attack and followed for more than 4 months thereafter. The effect of a daily dose of 10-30 mg of primaquine for 14 consecutive days on the relapse rate is shown in Table 19. Among a total of 334 patients treated with chloroquine alone, 146 (43.8%) relapsed at least once after treatment. None of the patients treated with 20 or 30 mg of primaquine daily for 14 days relapsed (Jones et al., 1953). A regimen of 20 mg of primaquine daily for 7 days was studied in order to determine whether a shorter period of treatment was equally effective. Thirty-six patients (51.4%) treated with chloroquine alone relapsed, and 2 (2.9%) of 68 patients treated with 20 mg of primaquine daily plus chloroquine 1,500 mg in 3 days relapsed 51 and 68 days after treatment, respectively. A regimen of 15 mg of primaquine daily for 7 days resulted in 1 relapse (3.2%) out of 31 patients 49 days after treatment (Lorenzo et al., 1953). Curative effect of primaquine 15 mg daily for 14 days was tested with 625 veterans of long-term latency, who had been exposed to malaria in Korea in the summer of 1951 and returned to USA before January 1952. None of the 294 men treated with primaqine developed malaria during the 6 months' period following treatment, whereas 58 (17.5%) of 331 men treated with a placebo gave a history of malaria (Coatney et al., 1953).
Table 19.
Fourteen days treatment of Korean vivax malaria with primaquine in 1951-1952 (Jones et al., 1953)
a)Plus chloroquine 1500 mg (base) in 3 days.
b)Only patients who were followed more than 4 months after treatment are included.
VECTOR MOSQUITOES
Anopheles sinensis had been suspected as the vector species of malaria for many years in Korea (Hasegawa, 1913; Kobayashi, 1932; Yokoo, 1944). In 1962, An. sinensis was confirmed for the first time as a malaria vector species; total 5,086 females collected at Kaegun-myon, Yangpyong-gun, Kyonggi-do during the period of 1960-1962 were dissected for salivary gland, and sporozoites were observed from one female, showing a 0.02% sporozoite rate (Ree et al., 1967). In 1967, 4,018 females of An. sinensis were dissected in Chongsong-gun, Kyongsangbuk-do and sporozoites were found in 2 females, showing a 0.05% infection rate (Hong, 1977). He also dissected 89 An. yatsushiroensis and found one positive for oocysts (not sporozoites). In an experimental infection study, sporozoites developed in 2 females out of 9 An. yatsushiroensis (Hong, 1977). These two study results strongly suggest that An. yatsushiroensis is also a vector species of malaria in Korea.
Study results on bionomics and/or behaviour of An. sinensis are available in many references (Yokoo, 1944; Whang, 1962, 1964; Paik et al., 1965; NMES, 1966; Chen et al., 1967; Ree and Paik, 1967; Hong, 1967, 1977; Ree et al., 1981; Lee and Ree, 1991; Ree and Lee, 1993; Shim et al., 1997), summary of which are as follows: (1) broad range of breeding places such as rice paddy, marsh, stream, reservoir, pond, water pool with vegetation, temporary rain pool, etc., (2) highly zoophilic with particular preferences of cows and pigs, (3) feeding activity throughout night with a peak during 1100-0300 hours, (4) rather strong phototaxis, (5) moderate to low daily survival, (6) 2.5-3 days of gonotrophic cycle, (7) mainly outdoor resting habit, (8) seasonal prevalence of May-October, with a large peak in late June-early July and a small peak in late August-early September, (9) population density extremely high, and (10) hibernating mainly outdoors, such as grasses, bushes, dike and others.
CONCLUSION
Korean vivax malaria had been endemic for longtime with low incidence and prevailed throughout the country including Cheju-do and became epidemic during the Korean war (1950-1953). In 1959-1969, when NMES activities was implemented, malaria endemicity was declining, with very low endemicity in south-west and south, plain areas and high endemic foci in north Kyongsangbuk-do and north and east of Kyonggi-do. NMES activities greatly contributed to accelerating disapperance of malaria endemicity particularly in the focal areas, and resulting in the malaria eradication from the Republic of Korea (South Korea) in 1979.
Since 1993, malaria has re-emerged mainly in north Kyonggi-do and north-west Kangwon-do, located bordering the DMZ. It is postulated that malaria in North Korea had persisted with low endemicity until an epidemic started in 1994, as meteorological and socio-economical factors changed in favor of vectors and parasites, resulting in an outbreak in north Kyonggi-do. This outbreak may have resulted from sporozoite-infected mosquitoes dispersing from North Korea across the DMZ to South Korea.
All historic records tell that vivax malaria in Korea results in unstable malaria, with epidemics occurring when environmental factors such as climatic, socio-ecological, political (wars), entomological and parasitological factors change in favor of vectors and/or parasites. PCD is a feasible and recommendable control measure in cost-effect point of view against the unstable vivax malaria in Korea.
ACKNOWLEDGEMENT
The author is grateful to all staffs of the Department of Parasitology, Yonsei University College of Medicine for their assistance in preparing this review. The author deeply appreciates to the Editorial Committee, the Korean Society for Parasitology for being invited to write the present review paper.
References
- 1.Ahn MH, Shim HJ, Im KI, Soh CT. Imported malaria cases in Korea. Yonsei Rep Trop Med. 1982;13:23–29. [Google Scholar]
- 2.Alving AS, Hankey DD, Coatney GR, Jones R, Coker WG, Garrison PL, Donovan WN. Korean vivax malaria II. Curative treatment with pamaquine and primaquine. Am J Trop Med Hyg. 1953;2:970–976. [PubMed] [Google Scholar]
- 3.Brunetti R. Outbreak malaria with prolonged incubation period in California, a nonendemic area. Science. 1954;119:74–75. doi: 10.1126/science.119.3080.74. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti R, Fritz RF, Hollister AC. An outbreak of malaria in California, 1952-1953. Am J Trop Med Hyg. 1954;3:779–788. doi: 10.4269/ajtmh.1954.3.779. [DOI] [PubMed] [Google Scholar]
- 5.Chai IH, Lim GI, Yoon SN, Oh WI, Kim SJ, Chai JY. Occurrence of tertian malaria in a male patient who has never been abroad. Korean J Parasitol. 1994;32:195–200. doi: 10.3347/kjp.1994.32.3.195. (in Korean) [DOI] [PubMed] [Google Scholar]
- 6.Chai JY. Re-emerging malaria. J Korean Med Assoc. 1997;40:728–733. (in Korean) [Google Scholar]
- 7.Chai JY. Re-emerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol. 1999;37:129–143. doi: 10.3347/kjp.1999.37.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YK, Ree HI, Hong HK, Chow CY. WHO/VBC/67·41. 1967. Bionomics and vector efficiency of Anopheles sinensis in Korea. [Google Scholar]
- 9.Chiba E, Akai K. On the existence of quartan malaria in Korea. Mansen no Ikai. 1930;112:2–19. (in Japanese) [Google Scholar]
- 10.Choi T. A case of tropical malaria in Korea. J Chosun Med Soc. 1936;26:491–498. (in Japanese) [Google Scholar]
- 11.Chun CH. Malaria in Korea. Korean Med. 1959;2:63–66. (in Korean) [Google Scholar]
- 12.Coatney GR, Alving AS, Jones R, Hankey DD, Robinson DH, Garrison PL, et al. Korean vivax malaria V. Cure of the infection by primaquine administered during long-term latency. Am J Trop Med Hyg. 1953;2:985–988. [PubMed] [Google Scholar]
- 13.Eddleman EE, Hale WH, Snowden WM. Vivax malaria with long incubation period. Report of seven cases. US Armed Forces Med J. 1951;2:1693–1698. [PubMed] [Google Scholar]
- 14.Feighner BH, Pak SI, Novakoski WL, Kelsey LL, Strickman D. Reemergence of Plasmodium vivax malaria in the Republic of Korea. Emerg Infect Dis. 1998;4:295–297. doi: 10.3201/eid0402.980219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnham PCC, Bray RS, Bruce-Chwatt LJ, Draper CC, Killick-kendrick R, Sergiev PG, et al. A strain of Plasmodium vivax characterized by prolonged incubation: morphological and biological characteristics. Bull World Health Organ. 1975;52:21–32. [PMC free article] [PubMed] [Google Scholar]
- 16.Gilles HM, Warrell DA. Bruce-Chwatt's essential malariology. 3rd ed. London: Edward Arnold; 1993. p. 340. [Google Scholar]
- 17.Hale TR, Halpenny GW. Malaria in Korean veterans. Canad Med Ass J. 1953;68:444–448. [PMC free article] [PubMed] [Google Scholar]
- 18.Hall WH, Loomis GW. Vivax malaria in veterans of the Korean war. A preliminary report of 25 cases. New Engl J Med. 1952;246:90–93. doi: 10.1056/NEJM195201172460304. [DOI] [PubMed] [Google Scholar]
- 19.Hankey DD, Jones R, Coatney GR, Alving AS, Coker WG, Garrison PL, Donovan WN. Korean vivax malaria I. Natural history and response to chloroquie. Am J Trop Med Hyg. 1953;2:958–988. [PubMed] [Google Scholar]
- 20.Hasegawa Y. Malaria in Korea. J Chosun Med Soc. 1913;4:53–69. (in Japanese) [Google Scholar]
- 21.Himeno K. Malaria occurring at Kangnung area, Kangwon-do. Mansen no Ikai. 1926;62:59–66. (in Japanese) [Google Scholar]
- 22.Hocking B. The intrinsic range and speed of flights of insects. Trans R Ent Soc Lond. 1953;104:223–345. [Google Scholar]
- 23.Hong HK. Bionomics of Anopheles sinensis Wiedmann in western plain area in Korea. Korean J Zool. 1967;10:18–22. (in Korean) [Google Scholar]
- 24.Hong HK. Ecological studies on Korean mosquitoes mainly found in the human habitation. Tongkook University; 1977. p. 57. Ph thesis. (in Korean) [Google Scholar]
- 25.Hong HK, Ree HI. Two unreported Anopheles mosquitoes in Korea. Korean J Zool. 1968;11:16–18. [Google Scholar]
- 26.Jones R, Jackson LS, Lorenzo AD, Marx RL, Levy BL, Kenny EC, et al. Korean vivax malaria III. Curative effect and toxicity of primaquine in doses from 10 to 30 mg daily. Am J Trop Med Hyg. 1953;2:977–982. [PubMed] [Google Scholar]
- 27.Kho WG, Jang JY, Hong ST, Lee HW, Lee WJ, Lee JS. Border malaria characters of remerging vivax malaria in the Republic of Korea. Korean J Parasitol. 1999;37:71–76. doi: 10.3347/kjp.1999.37.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim CJ, Han HM. A note on malaria in Korea. Korean Med Pharmacol J. 1953;2:1248–1250. (in Korean) [Google Scholar]
- 29.Kim DC. Status of malaria infection in the Republic of Korea. Yonsei Rep Trop Med. 1982;13:59–62. [Google Scholar]
- 30.Kim DI, Kim DS, Ahn YS. Epidemiological study of malaria outbreak in the DMZ of ROK. J Korean Mil Med Assoc. 1997;28:110–119. (in Korean) [Google Scholar]
- 31.Kim DI, Lee JC, Koo SH. Epidemiological study of malaria outbreak in the DMZ of ROK (II) J Korean Mil Med Assoc. 1998;29:26–40. (in Korean) [Google Scholar]
- 32.Kim KH, Lim CS. Clinical observation in 26 cases of indigenous malaria in 1995. Korean J Med. 1997;52:577–583. (in Korean) [Google Scholar]
- 33.Kobayashi H. Characteristics of malaria in Korea. Tokyo Iji Shinshi. 1931;2750:2491–2493. (in Japanese) [Google Scholar]
- 34.Kobayashi H. Review on malaria and Anopheles in Korea. J Chosun Med Soc. 1932;22:107–111. (in Japanese) [Google Scholar]
- 35.Kodama R. On malaria disease in Korea. Mansen no Ikai. 1928;86:17–30. (in Japanese) [Google Scholar]
- 36.Lee JS, Kho WG, Lee HW, Seo M, Lee WJ. Current status of vivax malaria among civilians in Korea. Korean J Parasitol. 1998;36:241–248. doi: 10.3347/kjp.1998.36.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MS. Recent (1993-1997) epidemiology and control strategy of malaria in Korean soldiers. Keimyung Med J. 1998;17:173–185. (in Korean) [Google Scholar]
- 38.Lee SK, Ree HI. Studies on mosquito population dynamics in Chollabug-do, Korea (1985-1990) I. Seasonal and annual fluctuations in population size. Korean J Entomol. 1991;21:141–155. (in Korean) [Google Scholar]
- 39.Lee SW, Shim JC, Kang YB, Ree HI. Investigation of malaria prevalence in Daesin-myon, Yoju-gun, and Kaegun-myon, Yangpyong-gun, Kyonggi-do province. Rep NIH, Korea. 1972;9:135–138. [Google Scholar]
- 40.Lee TU, Cho YJ, Paik YH. An epidemio-historical study on malaria in Korea. Kyung Hee Univ Med J. 1994;19:205–220. (in Korean) [Google Scholar]
- 41.Lim CS, Kim YK, Lee KN, Kim KH, Kim HS, Lim HW. Hematologic findings of reappeared Plasmodium vivax in Korea. Korean J Clin Pathol. 1996;16:836–843. (in Korean) [Google Scholar]
- 42.Lorenzo AD, Marx RL, Alving AS, Jones R. Korean vivax malaria IV. Curative effect of 15 milligrams of primaquine daily for 7 days. Am J Trop Med Hyg. 1953;2:983–984. [PubMed] [Google Scholar]
- 43.Macdonald G. The epidemiology and control of malaria. Oxford Univ. Press; 1957. [Google Scholar]
- 44.Nagai H. Malaria cases of Japanese soldiers stationed in Korea. J Chosun Med Soc. 1925;17:854–861. [Google Scholar]
- 45.National Institute of Health. Communicable disease monthly report. 1995;6(3):33. (in Korean) [Google Scholar]
- 46.National Institute of Health. Communicable disease monthly report. 1996;7(3):32. (in Korean) [Google Scholar]
- 47.National Institute of Health. Communicable disease monthly report. 1997;8(2):22. (in Korean) [Google Scholar]
- 48.National Institute of Health. Communicable disease monthly report. 1998;9(2):23. (in Korean) [Google Scholar]
- 49.National Institute of Health. Communicable disease monthly report. 2000;11(2):24. (in Korean) [Google Scholar]
- 50.NMES. Malaria pre-eradication programme in Korea. Progress report, 1961-1965. Ministry of Health and Social Affairs; 1966. p. 75. (in Korean) [Google Scholar]
- 51.Oh SN. On quartan malaria in Korea. Mansen no Ikai. 1930;112:18–19. (in Japanese) [Google Scholar]
- 52.Paik YH, Ree HI, Shim JC. Malaria in Korea. Jpn J Exp Med. 1988;58:55–66. [PubMed] [Google Scholar]
- 53.Paik YH, Song JH, Ree HI, Hong HK. Epidemiological studies on malaria situation in Korea. Part 1. On the bionomics of Anopheles sinensis and its relation to malaria in Korea. New Med. 1965;8:1043–1049. (in Korean) [Google Scholar]
- 54.Paik YH, Tsai FC. A note on the epidemiology of Korean vivax malaria. New Med. 1963;6:189–196. (in Korean) [Google Scholar]
- 55.Paik YH, van der Gugten AC. Evaluation report on the results of the passive casedetection conducted in the Korea Malaria Pre-Eradication Programme during the period 1960-1965. Korean J Parasitol. 1966;4:1–9. doi: 10.3347/kjp.1966.4.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Ree HI. Can malaria be endemic in South Korea? Korean J Inf Dis. 1998;30:397–400. [Google Scholar]
- 57.Ree HI, Hong HK, Paik YH. Study on natural infection of Plasmodium vivax in Anopheles sinensis in Korea. Korean J Parasitol. 1967;5:3–4. doi: 10.3347/kjp.1967.5.1.3. (in Korean) [DOI] [PubMed] [Google Scholar]
- 58.Ree HI, Hong HK, Shim JC, Lee JS, Cho HW, Kim CL. A study on seasonal prevalence of the populations of the mosquito larvae and other aquatic invertebrates in rice fields in Korea. Korean J Zool. 1981;24:151–161. [Google Scholar]
- 59.Ree HI, Lee SK. Studies on mosquito population dynamics in Chollabug-do, Korea (1985-1990) II. Factors influencing population sizes of Culex tritaeniorhynchus and Anopheles sinensis. Korean J Entomol. 1993;23:185–194. [Google Scholar]
- 60.Ree HI, Paik YH. Insecticide susceptibility tests on adults on Anopheles sinensis in Korea. Korean J Parasitol. 1967;5:65–68. doi: 10.3347/kjp.1967.5.1.65. [DOI] [PubMed] [Google Scholar]
- 61.Shim JC, Kim DS. Resurgence of the vivax malaria cases in Korea. Inf Dis. 1999;31:25–34. [Google Scholar]
- 62.Shim JC, Shin EH, Yang DS, Lee WK. Seasonal prevalence and feeding time of mosquitoes at outbreak regions of domestic malaria (P. vivax) in Korea. Korean J Entomol. 1997;27:265–277. (in Korean) [Google Scholar]
- 63.Shute PG, Lupascu G, Branzei P, Maryon M, Constantinescu P, Bruce-Chwatt LJ, et al. A strain of Plasmodium vivax characterized by prolonged incubation: the effect of numbers of sporozoites on the length of the prepatent period. Trans R Soc Trop Med Hyg. 1976;70:474–481. doi: 10.1016/0035-9203(76)90132-2. [DOI] [PubMed] [Google Scholar]
- 64.Simmons JS, Callender GR, Curry DP, Schwartz SC. Malaria in Panama. Baltimore: Johns Hopkins Press; 1939. p. 326. [Google Scholar]
- 65.Soh CT, Lee KT, Im KI, Min DY, Ahn MH, Kim JJ, Yong TS. Current status of malaria in Korea. Yonsei Rep Trop Med. 1985;16:11–18. [Google Scholar]
- 66.Song JH. Studies of malaria parasite densities found in Korea in relation with endemicity. J Pub Hlth. 1965;2:1–9. (in Korean) [Google Scholar]
- 67.Swellengrebel NH, Buck A. Malaria in the Nederlands. Amsterdam: Scheltema and Holkema; 1938. p. 259. [Google Scholar]
- 68.Tanabe Y. Distribution of malaria in Korea: on malaria transmission in Chunchon and Cholwon, Kangwon-do. J Chosun Med Soc. 1927;19:882–885. (in Japanese) [Google Scholar]
- 69.Tiburskaja NA, Sergiev PG, Vrublevskaja OS. Dates of onset of relapses and the duration of infection in induced tertian malaria with short and long incubation periods. Bull World Health Organ. 1968;38:447–457. [PMC free article] [PubMed] [Google Scholar]
- 70.Tiburskaja NA, Vrublevskaja OS. The course of infection caused by the North Korean strain of Plasmodium vivax. WHO/MAL/77. 1977;895:1–19. [Google Scholar]
- 71.Whang CH. Biological observation on Anopheline mosquitoes in Korea, with special reference to Anopheles (Anopheles) sinensis Wiedmann. Yonsei Med J. 1962;3:39–50. [Google Scholar]
- 72.Whang CH. Induced malaria in Korea. Yonsei Med J. 1963;4:51–57. doi: 10.3349/ymj.1963.4.1.51. [DOI] [PubMed] [Google Scholar]
- 73.Whang CH. Studies on bionomics of Anopheline mosquitoes in Korea. Korean Med J. 1964;9:49–74. (in Korean) [Google Scholar]
- 74.WHO. Synopsis of the world malaria situation, 1979. Wkly Epid Rec. 1981;56:145–149. [Google Scholar]
- 75.Yim HW, Suh GY, Ahn YS, Oh SY, Kim DL, Lim CS. Epidemiologic and clinical analysis of 87 indigenous malaria in Korean soldiers in 1995. Korean J Infect Dis. 1996;28:219–224. (in Korean) [Google Scholar]
- 76.Yokoo T. An investigation of the distribution and biology of mosquitoes, especially Anopheles hycanus sinensis. J Jpn Soc Appl Zool. 1944;15:43–85. (in Japanese) [Google Scholar]