Abstract
We have found suppressor T cells that inhibit the proliferative response of naive CD4+ T cells in T cell receptor (TCR) Vβ8.1 transgenic mice rendered tolerant in vivo by inoculation of Mls-1a-positive cells. This suppression was mediated by CD4+ T cells but not by CD8+ T cells or double-negative (DN) cells, and splenic CD4+ T cells from tolerant mice displayed a greater suppression than lymph node CD4+ T cells. Cell contact was required for efficient suppression, and known inhibitory cytokines such as IL-4, IL-10, and transforming growth factor β were not involved. Suppressor T cells inhibited IL-2 production by naive CD4+ T cells, and the addition of exogenous IL-2 diminished the suppressed activity while having little activity on tolerant T cells. Suppression was abolished by the elimination of CD25+ T cells in the tolerant CD4+ T cell subset. CD25+CD4+ T cells suppressed the proliferative response of the residual fraction of the nonanergic population, namely, 6C10+CD4+ T cells still present in the tolerant mice. However, 6C10−CD4+ T cells still had reduced reactivity to Mls-1a even after CD25+CD4+ T cells were removed and exogenous IL-2 was added. Suppressor cells appear to affect only residual nonanergic cells in situ, thereby facilitating the maintenance of the unresponsive state in vivo. These data provide a framework for understanding suppressor T cells and explain the difficulties and variables in defining their activity in other systems, because suppressor T cells apparently control only a small population of nonanergic cells in the periphery and may be viewed as a homeostatic mechanism.
Three features of peripheral T cell tolerance, namely, clonal deletion, clonal anergy, and suppression, have been identified. These processes do not work independently but represent components of a larger mechanism that involves combined activities. In several model systems, tolerance of peripheral T cells can be induced by inoculation of antigen in vivo. Injection of minor lymphocyte-stimulating antigen 1a (Mls-1a) spleen cells or Staphylococcal enterotoxin B into mice induces the transient expansion of T cells expressing reactive Vβ T cell receptor (TCR) during the first 3–4 days after injection, followed by a reduction in the number of these cells. This latter process is a form of clonal deletion (1–6). However, not all specific T cells are eliminated, and a majority of the remaining T cells become anergic to TCR stimulation (6–8). Our laboratory has used TCR Vβ8.1 transgenic mice and the Mls-1a antigen to study T cell tolerance in the periphery as well as central tolerance to a self-antigen (7–10). Tolerant T cells obtained from Mls-1a-inoculated TCR Vβ 8.1 transgenic mice display hyporesponsiveness to antigen restimulation in vitro, and proteins from these cells showed altered tyrosine phosphorylation patterns after TCR engagement (7). In addition, hyporesponsiveness induced to Mls-1a also led to delayed allograft rejection in vivo (10). Therefore, Mls-1a-tolerized mice have been informative in terms of the processes of clonal deletion and clonal anergy.
Although a majority of the peripheral CD4+ T cells are rendered anergic in Mls-1a-inoculated mice, a fraction of residual nonanergic cells still retained the ability to proliferate. This T cell fraction was phenotypically distinguished by a T cell differentiation marker, 6C10 (11). 6C10+CD4+ lymph node T cells sorted from tolerant mice displayed a proliferative response against the Mls-1a antigen in vitro, whereas 6C10-negative cells were still unresponsive. These data suggested that the third mechanism alluded to, suppression, also may be involved in peripheral tolerance. We therefore have asked whether suppressor T cells affect the residual nonanergic T cells in the tolerant mice. Recent studies have demonstrated a role of CD25+CD4+ “natural” anergic/suppressor cells in the prevention of organ-specific autoimmune disease (12–18). CD25+CD4+ suppressor T cells may regulate responding CD25−CD4+ T cells by some undefined mechanism.
Here, we examined the role of suppressor T cells in transgenic mice made tolerant to the exogenous Mls-1a antigen. We have uncovered activity of CD25+CD4+ T cells in peripheral T cell regulation. This report also clearly shows that the hyporesponsiveness resulting from suppression is a distinct mechanism from the anergic state induced after antigen sensitization and that these two distinct mechanisms act simultaneously in the maintenance of tolerance in vivo.
Materials and Methods
Mice.
CBA/Ca (H-2k, Mls-1b), CBA/J (H-2k, Mls-1a), and C57BL/6 (H-2b, Mls-1b) mice were obtained from The Jackson Laboratory. TCR Vβ8.1 transgenic mice were bred onto a CBA/Ca background and express the transgene on greater than 98% of peripheral T cells (7, 11).
Antibodies.
Hybridomas that produce the following mAbs were purchased from the American Type Culture Collection (Manassas, VA): GK1.5 (anti-CD4), 3.155 (anti-CD8), 14-4-4s (anti-I-Ek), J11d.2 (anti-heat-stable antigen), 30-H12 (anti-Thy1.2), 11B11 (anti-mouse IL-4), 7D4 (anti-mouse IL-2Rα, p55), and R4–6A2 (anti-mouse IFN-γ). Culture supernatants of these cells were used for experiments; some were purified further by protein G column chromatography. The hybridoma SM6C10 was a gift of K. Hayakawa (Fox Chase Cancer Center, Philadelphia) (19–21). FITC and biotin-conjugated anti-CD25 (7D4) and anti-CD25 mAb, which react with distinct epitopes of the IL-2Rα (PC61), were obtained from PharMingen. Phycoerythrin anti-CD4 (clone H129.19; GIBCO/BRL) and FITC anti-mouse IgM (Southern Biotechnology Associates) also were used for flow cytometry studies. Neutralizing antibodies of anti-mouse IL-10 (clone 2A5; Endogen, Cambridge, MA), anti-transforming growth factor (TGF)-β1, β2, and β3 (R&D Systems), anti-FasL (clone K10; PharMingen), anti-CD40L (clone MR1; PharMingen), isotype control mouse IgG (anti-KLH; PharMingen), and control rat IgG (Sigma) were used for blocking studies.
Tolerance Induction in Vivo.
Tolerance to Mls-1a was induced as described previously with some modification (7, 11). Briefly, Mls-1a T cell-depleted spleen cells were prepared by treatment of CBA/J spleen cells with anti-Thy 1.2 mAb (30-H12) and rabbit H-2 complement (Pel-Freez Biologicals) followed by centrifugation over a cushion of Lympholyte M (Cedarlane Laboratories). Fifteen million T cell-depleted spleen cells were injected i.v. twice into Vβ8.1 transgenic mice in a 7-day period.
Cell Preparation for Proliferation Assays.
The inguinal, popliteal, brachial, and axillary lymph nodes and the spleens were harvested from control uninoculated and inoculated Vβ8.1 transgenic mice 7 days after the last Mls-1a inoculation. Single-cell suspensions were depleted of RBC. The CD4+ T cells were enriched by depleting non-CD4+ cells with antibodies directed against the heat-stable antigen (J11d.2), I-Ek (14–4-4s), CD8 (3.155), and rabbit complement as described previously (7). Viable cells were recovered by centrifugation over a cushion of Lympholyte M. The purity of the enriched CD4+ T cells from lymph nodes and spleen were >95% and >89%, respectively, in control uninoculated Vβ8.1 transgenic mice and >85% and >75%, respectively, in Mls-1a inoculated Vβ8.1 transgenic mice. The enriched CD4+ T cells were separated further into 6C10− and 6C10+ populations by MACS magnetic cell sorting (type AS column) by using the culture supernatant of SM6C10 and rat anti-mouse IgM MicroBeads according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). The purity of 6C10+ cells in the positive fraction was >92%, and the 6C10− cell purity in the negative fraction was >95%.
Proliferation Assays.
Enriched CD4+ T cells from lymph nodes and spleens (5 × 104) were cultured with irradiated (2,000 rad) T cell-depleted CBA/J spleen cells (5 × 105, unless otherwise indicated) for 96 h in 96-well, flat-bottomed plates in RPMI 1640 medium supplemented with 2 mM l-glutamine/1 mM sodium pyruvate/10 mM Hepes/5 × 10−5 M 2-mercaptoethanol/penicillin/streptomycin/10% heat-inactivated FCS. Cultures were pulsed with 1 μCi/well [3H]thymidine for the last 6–8 h. Transwell (Corning Costar) cultures were performed by using 24-well plates (800 μl) with control naive CD4+ T cells (2 × 105, outer well) and irradiated CBA/J T cell-depleted spleen cells (2 × 106, inner and outer well) in the presence or absence of tolerant CD4+ T cells (2 × 105, inner or outer well).
Flow Cytometry.
Enriched CD4+ T cells (1 × 105 to 5 × 105) were incubated with anti-mouse FcRγII/III mAb (2.4G2, Fc Block; PharMingen) first to prevent nonspecific binding, washed, and then reacted with indicated antibodies. Flow cytometry data were acquired on FACScan (Becton Dickinson) and analyzed by using cellquest (Becton Dickinson).
Cytokine ELISA.
IL-2 in culture supernatants was quantified by ELISA by using two distinct, anti-mouse IL-2 mAbs (JES6–1A12 as the capture antibody and JES6–5H4 as the detection antibody; PharMingen). Recombinant mouse IL-2 (Roche, Gipf-Oberfrick, Switzerland) was used as a standard. The lower limit of the detection was 49 pg/ml.
Results
Suppressive Effect of Tolerant Splenic CD4+ T Cells in Mls-1a Inoculated Vβ8.1 Transgenic Mice.
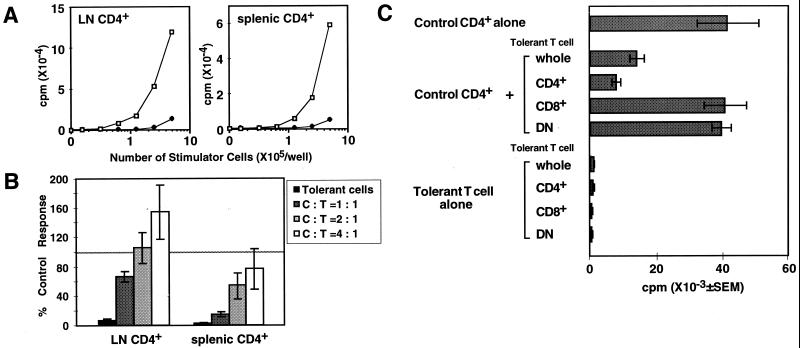
We injected the Mls-1a antigen into TCR Vβ8.1 transgenic mice that have the Mls-1b background to induce peripheral T cell tolerance in vivo. Lymph node and splenic CD4+ T cells in Mls-1a-inoculated TCR Vβ8.1 transgenic mice showed a reduced proliferative response in vitro after restimulation with Mls-1a (Fig. 1A). These tolerant CD4+ T cells, however, could proliferate when stimulated with phorbol 12-myristate 13-acetate and calcium ionophore (data not shown), indicating that the tolerant CD4+ T cells do not lose the ability to proliferate. To examine the regulatory effect of tolerant T cells, peripheral CD4+ T cells from Mls-1a-inoculated mice were cocultured with naive CD4+ T cells in the presence of Mls-1a stimulator cells (Fig. 1B). Proliferation of naive splenic CD4+ T cells is inhibited by the addition of tolerant splenic CD4+ T cells in a dose-dependent manner. On the other hand, lymph node CD4+ T cells from tolerant mice mediated only minimal inhibition of the proliferation of naive lymph node CD4+ T cells. Percentage suppression by splenic CD4+ T cells from tolerant mice that were injected with Mls-1a antigen twice was significantly greater than that of singly injected mice (85.4 ± 10.4%, n = 13, and 68.3 ± 10.3%, n = 7, at 1:1 mixture, respectively; P < 0.05 by t test). To examine whether populations other than CD4+ T cells in the tolerant mice mediate suppression, CD4+, CD8+, and DN cells were enriched and tested for suppressive effect (Fig. 1C). Notably, all fractions of the tolerant cells were unable to proliferate to Mls-1a antigen. However, only CD4+ and the whole population mediated suppression, whereas the CD8+ and DN fraction in which CD4+ T cells were deleted did not have any discernible effect. This result indicates that only CD4+ T cells mediate suppressive activity.
Figure 1.
Tolerant splenic CD4+ T cells suppress proliferation of naive CD4+ T cells. (A) Proliferation of CD4+ T cells in control and Mls-1a-inoculated TCR Vβ 8.1 transgenic mice. A fixed number (5 × 104) of CD4+ T cells from control (□) and Mls-1a-inoculated Vβ8.1 transgenic mice (♦) were stimulated with Mls-1a stimulators for 96 h. Representative data of four experiments are shown. (B) Suppressive effect of tolerant splenic CD4+ T cells in Mls-1a-inoculated TCR Vβ8.1 transgenic mice. Lymph node and splenic CD4+ T cells in uninoculated control mice were cocultured with tolerant CD4+ T cells from lymph nodes and spleen of Mls-1a-inoculated mice, respectively. Proliferation was measured by [3H]thymidine incorporation, and the data are presented as average percentages of the control proliferation ± SEM of five separate experiments. (C) CD4+ T cells but not CD8+ or DN cells in tolerant spleen cells mediate the suppression. CD4+, CD8+, and DN cells were enriched by elimination of other populations in spleen cells from Mls-1a-inoculated Vβ8.1 transgenic mice. The purity of each fraction was as follows: CD4+ fraction (CD4+ > 75%, CD8+ < 0.05%), CD8+ fraction (CD4+ < 0.05%, CD8+ > 65%), and DN fraction (CD4+ < 0.05%, CD8+ < 0.05%). The experiment was repeated three times.
Tolerant CD4+ T Cells Suppress IL-2 Production by Naive CD4+ T Cells in Vitro, and Exogenous IL-2 Abrogates Active Suppression but Does Not Stimulate Anergic T Cells.
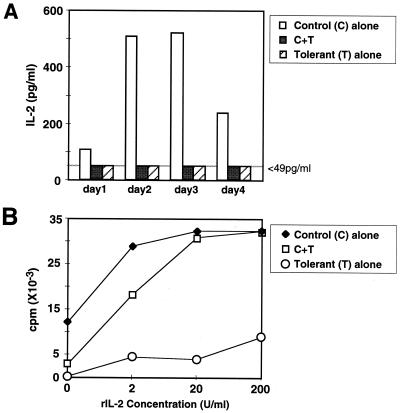
IL-2 production by naive CD4+ T cells cocultured with or without tolerant CD4+ T cells was measured (Fig. 2A). IL-2 in the supernatants of naive CD4+ T cells stimulated with Mls-1a was detected as early as 1 day after antigen stimulation and reached a maximum on day 3, whereas IL-2 production by tolerant CD4+ T cells was lower than the detection limit throughout the culture period. In mixed cultures of naive CD4+ T cells and tolerant CD4+ T cells, IL-2 production by naive CD4+ T cells also was reduced dramatically (Fig. 2A).
Figure 2.
Tolerant CD4+ T cells suppress IL-2 production by naive CD4+ T cells, and exogenous IL-2 rescues the suppression. (A) Control naive CD4+ T cells (5 × 104), tolerant CD4+ T cells (5 × 104), or the mixture (1:1) was cultured with Mls-1a stimulators (5 × 105). Culture supernatants were harvested on the indicated day, and IL-2 concentration was measured by ELISA. The lower limit of detection was 49 pg/ml. (B) Control naive CD4+ T cells (5 × 104), tolerant CD4+ T cells (5 × 104), or the mixture (1:1) was cultured with Mls-1a stimulators (5 × 105) in the presence of graded concentrations of rIL-2. Each experiment was repeated three times.
We examined the effect of exogenous IL-2 on the hyporesponsiveness and suppressive effect of tolerant CD4+ T cells. Suppression by tolerant CD4+ T cells could be abrogated completely by the addition of exogenous IL-2 in a dose-dependent manner (Fig. 2B). However, the proliferation of tolerant CD4+ T cells did not recover even in the presence of a high concentration of IL-2.
Suppression Mediated by CD4+ T Cells Requires Cell Contact.
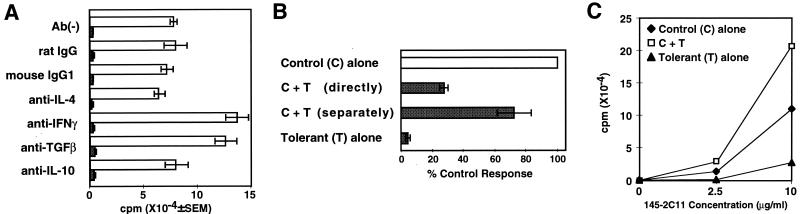
Suppression by tolerant CD4+ T cells was not neutralized with antibodies against various known inhibitory cytokines such as IL-4, IL-10, IFN-γ, and transforming growth factor β (TGF-β) (Fig. 3A). In addition, nitric oxide inhibitors had no effect on the suppression (data not shown). IL-4 secretion by tolerant CD4+ T cells was not detected, and IL-10 production was lower than control naive CD4+ T cells (data not shown). To examine the possibility of a soluble factor other than these inhibitory cytokines, tolerant CD4+ T cells and naive CD4+ T cells were separated in the same culture well by a membrane (Fig. 3B). Suppression by tolerant CD4+ T cells was abrogated by the separation of tolerant cells from naive CD4+ T cells. Therefore, it is unlikely that the suppression in this system is mediated by a soluble factor secreted by tolerant CD4+ T cells.
Figure 3.
Suppression by tolerant CD4+ T cells requires cell contact. (A) Control CD4+ T cells (5 × 104) were cultured with Mls-1a stimulators (5 × 105) and 10 μg/ml of the indicated neutralizing antibodies in the presence (shaded bar) and absence (open bar) of tolerant CD4+ T cells (5 × 104) for 96 h. The experiment was repeated three times. (B) Necessity of cell contact in the suppression. Naive CD4+ T cells were cultured in 24-well Transwell plates with Mls-1a stimulators. Tolerant CD4+ T cells were added directly to the culture or separately to the Transwell. Data are shown as the average of percentage control response of four independent experiments. (C) Tolerant CD4+ T cells could not mediate suppression on proliferative response induced by immobilized anti-CD3 mAb. Control CD4+ T cells (5 × 104) were cultured in 96-well, flat-bottomed plates coated with the indicated concentration of anti-CD3 mAb (145–2C11) in the presence or absence of tolerant CD4+ T cells (5 × 104) for 72 h. The experiment was repeated three times.
Interestingly, tolerant CD4+ T cells did not display suppressive effects after stimulation with immobilized anti-CD3 mAb (Fig. 3C), suggesting the necessity of cell contact with the target responding cells on the antigen-presenting cell (APC). Cell contact-dependent suppression undoubtedly requires a cell surface molecule that can affect naive CD4+ T cells. We have examined the likely candidates, FasL and CD40L. Blocking antibodies against FasL and CD40L, however, failed to affect suppression (data not shown).
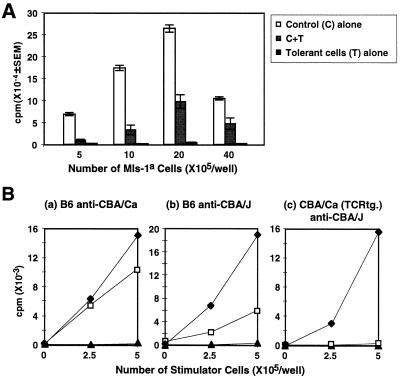
As a next step, we examined whether tolerant CD4+ T cells mediated suppression simply by competition with responding naive CD4+ T cells for the MHC–antigen complex or costimulatory molecules on the same APC. Naive CD4+ T cells and tolerant CD4+ T cells were cocultured with an increasing number of Mls-1a T-depleted spleen cells (Fig. 4A). The extent of the suppression decreased in proportion to increasing the number of stimulators. However, suppression was never abolished completely. Tolerant CD4+ T cells still mediated 55% of the expected suppression even in the presence of an 80-fold excess of APC (40 × 105/well of Mls-1a T-depleted spleen cells). Therefore, it is unlikely that the mechanism of the suppression can be explained by passive interference. In addition, CD80, CD86, and MHC class II expressions on the APC cocultured with tolerant CD4+ T cells were not decreased (data not shown). Therefore, suppression was not caused by down-modulation of known costimulatory molecules on the APC population.
Figure 4.
Suppression is not mediated by simple competition on the same APC and required activation through the TCR. (A) A fixed number of naive CD4+ T cells (5 × 104) were cultured with or without tolerant CD4+ T cells (5 × 104) in the presence of the indicated number of Mls-1a stimulators. The experiment was repeated three times. (B) Naive CD4+ splenic T cells from C57BL/6 mice or control Vβ8.1 transgenic mice (5 × 104) (♦), tolerant CD4+ T cells from Mls-1a-inoculated Vβ8.1 transgenic mice (5 × 104) (▴), or mixtures of naive and tolerant CD4+ T cells (□) were stimulated with irradiated, T-depleted spleen cells from CBA/Ca (H-2k, Mls-1b) (a) or CBA/J (H-2k, Mls-1a) (b and c). Representative data are shown from two experiments.
Although suppressor cells appear to require cell contact on the APC, it is unclear whether suppressor cells need to be stimulated through TCR for induction of the suppression. Therefore, in an attempt to show that activation of the suppressor cells requires TCR stimulation, we examined the suppressive effect of tolerant CD4+ T cells in an allogeneic response (Fig. 4B). Tolerant CD4+ T cells from Mls-1a-inoculated TCR Vβ8.1 transgenic mice (H-2k, Mls-1b) mediated suppression against the allogeneic response of the B6 anti-CBA/J (H-2k, Mls-1a) combination as well as the anti-CBA/J response of uninoculated TCR Vβ8.1 transgenic mice. However, tolerant CD4+ T cells showed a reduced suppressive effect against an allogeneic response of B6 anti-CBA/Ca (H-2k, Mls-1b). Therefore, stimulation of suppressor cells by Mls-1a antigen on the APC is required for optimal induction of suppression.
Elimination of CD25+ Population in Tolerant Cells Diminishes Suppression.
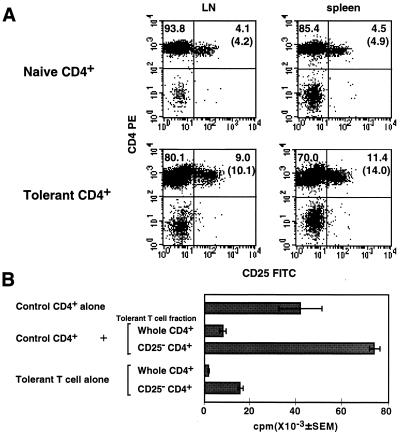
Tolerant CD4+ T cells of Mls-1a-inoculated mice have elevated levels of T cell activation markers such as CD69, CD25, and CTLA-4 (11). A slight increase in the number of CD25+ T cells was observed in Mls-1a-inoculated TCR Vβ8.1 transgenic mice, and this increase was more apparent in spleen cells than in lymph node cells (Fig. 5A). To establish the involvement of CD25+ T cells in the suppression, CD25+ T cells were eliminated with anti-CD25 mAb and rabbit complement from tolerant CD4+ T cells. As shown in Fig. 5B, elimination of the CD25+CD4+ T cells completely abolished suppression and actively augmented the proliferation of the mixed culture of naive CD4+ T cells and tolerant CD4+ T cells. Although the proliferation of tolerant CD4+ T cells partially recovered after removal of CD25+CD4+ T cells, these cells still manifested reduced responses. Hyporesponsiveness of tolerant CD4+ T cells therefore is not mediated by the action of suppressor T cells (Fig. 5B).
Figure 5.
CD25+CD4+ T cells in tolerant Vβ8.1 transgenic mice mediate suppression. (A) CD25 expression of peripheral CD4+ T cells in control naive and Mls-1a-inoculated Vβ8.1 transgenic mice. Tolerant CD4+ T cells were taken from Mls-1a-inoculated Vβ8.1 transgenic mice 7 days after the last inoculation. (B) Elimination of CD25+ cells in tolerant cells abrogates the suppression. Tolerant CD4+ T cells were treated further with anti-CD25 mAb (7D4) and rabbit complement to eliminate CD25+ cells. Control CD4+ T cells (5 × 104) were cultured with the indicated fraction of tolerant CD4+ T cells (5 × 104). Deletion of CD25+ cells was determined by FACS analysis with anti-CD25 mAb (PC61), which reacts with a distinct epitope on IL-2Rα (<0.2% in the CD25−CD4+ fraction). The experiment was repeated three times.
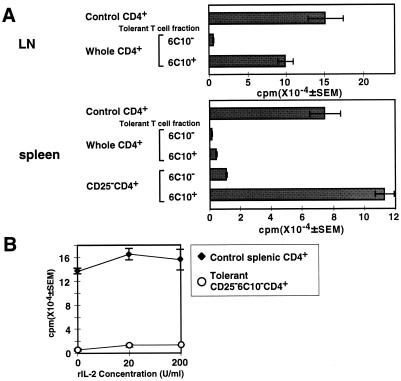
The anergic state of lymph node CD4+ T cells in tolerant mice correlates well with the loss of expression of the T cell differentiation marker, 6C10 (11). Expression of 6C10 on lymph node CD4+ T cells was reduced drastically to less than 25% in tolerant mice, whereas 60–80% of naive lymph node CD4+ T cells express 6C10 (11). A decrease of 6C10+ cells persisted for at least 1 month after inoculation, and functional analysis showed that the remaining 6C10+CD4+ lymph node T cells in tolerant mice retained a proliferative response, whereas 6C10−CD4+ T cells continued to be hyporesponsive (11). To examine whether CD25+ suppressor cells have an effect on these two populations in tolerant mice, proliferation of 6C10+ and 6C10− populations in the presence or absence of CD25+CD4+ T cells was examined. In lymph node cells, 6C10+CD4+ T cells from tolerant mice display reactivity to the Mls-1a antigen, whereas 6C10−CD4+ lymph node T cells had reduced responses (Fig. 6A). The spleen population behavior was more complex. Simple separation of 6C10+ T cells from tolerant splenic CD4+ T cells did not show the comparable differences seen with lymph node cells. However, when CD25+ T cells were eliminated with anti-CD25 mAb and complement, splenic 6C10+CD4+ T cells recovered their proliferative response, whereas CD25−6C10−CD4+ T cells still had reduced responses even after CD25+ suppressor cells were removed. This result is consistent with a dominant-suppressive effect of a subset of splenic CD4+ T cells from Mls-1a-inoculated mice, as shown in Fig. 1B. Collectively, these results show that CD25+CD4+ T cells suppress the 6C10+CD4+ residual nonanergic population but do not affect the hyporesponsiveness of 6C10− anergic cells. CD25−6C10−CD4+ anergic T cells do not recover their proliferative response in the presence of exogenous IL-2 (Fig. 6B). Because the suppression mediated by tolerant CD4+ T cells is abrogated by the addition of exogenous IL-2 (Fig. 2B), reduced reactivity of CD25−6C10−CD4+ T cells after IL-2 exposure also means that hyporesponsiveness of the CD25−6C10−CD4+ T cells is not due to suppression by CD25+ T cells. Taken together, suppression by CD25+ T cells primarily affects nonanergic 6C10+ T cells and facilitates the maintenance of the peripheral tolerant state in vivo.
Figure 6.
CD25+ T cells suppress residual nonanergic 6C10+CD4+ T cells in tolerant mice. (A) Elimination of the CD25+ T cells augmented proliferation of 6C10+CD4+ T cells in the tolerant spleen cells. Splenic CD4+ T cells from Mls-1a-inoculated mice were treated with anti-CD25 mAb (7D4) and rabbit complement. LN CD4+ T cells, CD25-depleted splenic CD4+ T cells, and whole splenic CD4+ T cells were separated further into 6C10− and 6C10+ fractions and stimulated with Mls-1a stimulators. (B) 6C10−CD4+ T cells cannot recover their proliferation even in the presence of exogenous IL-2. Control naive CD4+ T cells and CD25−6C10−CD4+ T cells (5 × 104) from Mls-1a-inoculated mice were cultured with Mls-1a stimulators in the presence of recombinant mouse IL-2. Representative data from three experiments are shown.
Discussion
Evidence from numerous model systems has demonstrated the role of cell-mediated suppression in peripheral T cell tolerance. Our efforts to understand suppressor T cells and their factor(s) began several years ago and have continued to some extent in several systems (22–25). Recent interest in this area once again is obvious. Suppressor populations have been shown to include CD4, CD8, double-negative cells, and APC (26–31), suggesting several distinct mechanisms of suppression. Regulatory cytokines and soluble factor(s) produced by suppressor cells have been reported in various models of autoimmune or inflammatory diseases (28, 32–36). Suppression that requires cell–cell contact at the level of the APC also has been reported (14, 16, 26, 37). Recently, CD25+CD4+ T cells, “natural” anergic/suppressor cells, have been found to be relevant to the prevention of organ-specific autoimmune disease (12–16). The CD25+CD4+ suppressor population regulates self-reacting T cells that have escaped thymic-negative selection and are found in the periphery. Suppressor cells found in our system require cell contact for suppression (Fig. 3B), and exogenous IL-2 completely abrogates suppression (Fig. 2B). These data are consistent with previous reports on the biology of CD25+CD4+ T cells (14, 16). Nevertheless, CD25+CD4+ T cells do not regulate all peripheral CD4+ T cells. In our study, elimination of the CD25+CD4+ T cells clearly abrogated suppression; yet, CD25−6C10−CD4+ T cells still showed a dramatically limited response, even after CD25+CD4+ T cells were removed (Fig. 6A) and exogenous IL-2 was added to the culture (Fig. 6B). Because exogenous IL-2 completely rescues suppression mediated by tolerant CD4+ T cells (Fig. 2B), the hyporesponsiveness of 6C10−CD25−CD4+ T cells is a consequence of distinct biological changes that lead to reduced responses of TCR stimulation. 6C10−CD25−CD4+ T cells thus represent “anergic T cells” induced with antigen sensitization in vivo.
It has been shown that in vivo induced anergic T cells differ from T cells rendered anergic in vitro with respect to their response to exogenous IL-2. Anergic T cells induced in vitro in the absence of a costimulatory signal recover their proliferative responses when cultured with exogenous IL-2 (38, 39), whereas tolerant CD4+ T cells induced in vivo are less able to use exogenous IL-2 (1, 7). CD25+CD4+ “natural” anergic/suppressor T cells also recover their reactivity in the presence of IL-2 (14, 16, 40). Anergic T cells induced after antigen sensitization in vivo thereby can be distinguished from CD25+CD4+ “natural” anergic/suppressor T cells.
Although reduced expression of the 6C10 antigen correlates with the anergic state induced in antigen-inoculated mice (11, 41), the molecular mechanisms underlying this expression pattern is unknown. The 6C10 antigen has been thought to be a carbohydrate epitope on Thy-1 glycoprotein and is expressed on approximately 90% of thymocytes and 60–80% of peripheral T lymphocytes (11, 19–21). Because the Thy-1 glycoprotein is a glycosylphosphatidylinositol-anchored protein bound to lipid rafts on the cytoplasmic membrane (19–21), it is conceivable that the 6C10 antigen could normally facilitate the accumulation of signaling molecules at the site of TCR stimulation. Involvement of Thy-1 antigens in T cell activation, in fact, has been demonstrated in several systems (42–44). However, in the naive state, 6C10−CD4+ T cells can proliferate comparably to 6C10+CD4+ T cells when stimulated with Mls-1a in Vβ8.1 transgenic mice (data not shown). Therefore, reduced responses of 6C10−CD4+ T cells after anergy induction cannot be completely explained simply by the loss of 6C10 antigen. Loss of the 6C10 antigen may relate to the biological alteration or disorganization of the much larger T cell signaling complex in anergic T cells.
Suppressor cells in our system require cell contact to mediate inhibition. Failure of suppression after stimulation with immobilized anti-CD3 mAb also suggests the requirement of cell contact at the APC. Although it remains to be clarified whether suppressor cells affect naive CD4+ T cells directly or by modulating the function of the APC, it is apparent that regulation by CD25+CD4+ T cells is executed at the site of antigen presentation in the periphery. We have suggested previously that some APCs that survive UV radiation in mice induce suppressor T cells (31). This type of APC might be involved in peripheral T cell regulation by inducing or activating suppressor T cells. Therefore, it is also possible that some APCs that interact with suppressor T cells might have a regulatory role in this system.
An active role of suppression has been demonstrated clearly in adoptive transfer models in vivo (25, 27). In contrast, it has been difficult to show whether unresponsiveness in anergic cells is functionally independent from suppression in tolerant animals. In this report, we have demonstrated clearly that the anergic state after antigen exposure in vivo is not brought about solely by suppressor T cells. In addition, we have shown that suppressor T cells apparently contribute to the down-regulation of nonanergic CD4+ T cells in situ in tolerant mice. Suppressor T cells, therefore, may inhibit the nonanergic cells remaining in the periphery as well as newly emerging T cells and, synergistically with anergic cells, maintain the unresponsive state induced after excessive antigen sensitization. Our present study suggests a physiological role of suppressor T cells in lymphocyte homeostasis in vivo.
Acknowledgments
We greatly appreciate Drs. A. Bhandoola and C. Maier for their thoughtful suggestions and Dr. M. Wysocka for help in cytokine measurement. We thank Drs. G. Massey, J. Davis, M. Katsumata, T. Horie, T. Kumagai, C. Kirk, and A. Hasegawa for their help and advice. This work was supported by Grant 1 PO1 AI43620 from the National Institutes of Health to M.I.G.
Abbreviations
- TCR
T cell receptor
- Mls
minor lymphocyte-stimulating antigen
- APC
antigen-presenting cell
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230449097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230449097
References
- 1.Rammensee H G, Kroschewski R, Frangoulis B. Nature (London) 1989;339:541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- 2.Webb S, Morris C, Sprent J. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 3.Dannecker G, Mecheri S, Staiano-Coico L, Hoffmann M K. J Immunol. 1991;146:2083–2087. [PubMed] [Google Scholar]
- 4.Kawabe Y, Ochi A. J Exp Med. 1990;172:1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rellahan B L, Jones L A, Kruisbeek A M, Fry A M, Matis L A. J Exp Med. 1990;172:1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald H R, Baschieri S, Lees R K. Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 7.Bhandoola A, Cho E A, Yui K, Saragovi H U, Greene M I, Quill H. J Immunol. 1993;151:2355–2367. [PubMed] [Google Scholar]
- 8.Yui K, Ishida Y, Katsumata M, Komori S, Chused T M, Abe R. J Immunol. 1993;151:6062–6075. [PubMed] [Google Scholar]
- 9.Yui K, Komori S, Katsumata M, Siegel R M, Greene M I. Proc Natl Acad Sci USA. 1990;87:7135–7139. doi: 10.1073/pnas.87.18.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhandoola A, Bassiri H, Markmann J F, Yui K, Hashimoto Y, Greene M I. Eur J Immunol. 1994;24:1710–1713. doi: 10.1002/eji.1830240739. [DOI] [PubMed] [Google Scholar]
- 11.Maier C C, Bhandoola A, Borden W, Yui K, Hayakawa K, Greene M I. Proc Natl Acad Sci USA. 1998;95:4499–4503. doi: 10.1073/pnas.95.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 13.Asano M, Toda M, Sakaguchi N, Sakaguchi S. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 15.Suri-Payer E, Amar A Z, Thornton A M, Shevach E M. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 16.Thornton A M, Shevach E M. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 18.Thornton A M, Shevach E M. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa K, Hardy R R. J Exp Med. 1988;168:1825–1838. doi: 10.1084/jem.168.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayakawa K, Hardy R R. Immunol Rev. 1991;123:145–168. doi: 10.1111/j.1600-065x.1991.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 21.Gui M, Wiest D L, Li J, Kappes D, Hardy R R, Hayakawa K. J Immunol. 1999;163:4796–4804. [PubMed] [Google Scholar]
- 22.Monroe J G, Lowy A, Granstein R D, Greene M I. Immunol Rev. 1984;80:103–131. doi: 10.1111/j.1600-065x.1984.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 23.Weiner D B, Liu J, Hanna N, Bluestone J A, Coligan J E, Williams W V, Greene M I. Proc Natl Acad Sci USA. 1988;85:6077–6081. doi: 10.1073/pnas.85.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner D B, Williams W V, Siegel R M, Jerrold-Jones S, Greene M I. Transplant Proc. 1988;20:1151–1153. [PubMed] [Google Scholar]
- 25.Saouaf S J, Zerva L, Greene M I. Transgenics. 1994;1:171–183. [Google Scholar]
- 26.Qin S, Cobbold S P, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 27.Maeda H, Takata M, Takahashi S, Ogoshi S, Fujimoto S. Int Immunol. 1994;6:855–862. doi: 10.1093/intimm/6.6.855. [DOI] [PubMed] [Google Scholar]
- 28.Miller C, Ragheb J A, Schwartz R H. J Exp Med. 1999;190:53–64. doi: 10.1084/jem.190.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue T, Asano Y, Matsuoka S, Furutani-Seiki M, Aizawa S, Nishimura H, Shirai T, Tada T. J Immunol. 1993;150:2121–2128. [PubMed] [Google Scholar]
- 30.Cauley L S, Cauley K A, Shub F, Huston G, Swain S L. J Exp Med. 1997;186:71–81. doi: 10.1084/jem.186.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene M I, Sy M S, Kripke M, Benacerraf B. Proc Natl Acad Sci USA. 1979;76:6591–6595. doi: 10.1073/pnas.76.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano T, Ishii Y, Ishizaka K. J Immunol. 1996;156:1728–1734. [PubMed] [Google Scholar]
- 33.Ishii Y, Nakano T, Ishizaka K. J Immunol. 1996;156:1735–1742. [PubMed] [Google Scholar]
- 34.Chen Y, Takata M, Maiti P K, Mohapatra S, Mohapatra S S, Sehon A H. J Immunol. 1994;152:3–11. [PubMed] [Google Scholar]
- 35.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J E, Roncarolo M G. Nature (London) 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 36.Bridoux F, Badou A, Saoudi A, Bernard I, Druet E, Pasquier R, Druet P, Pelletier L. J Exp Med. 1997;185:1769–1775. doi: 10.1084/jem.185.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldmann H, Cobbold S. Annu Rev Immunol. 1998;16:619–644. doi: 10.1146/annurev.immunol.16.1.619. [DOI] [PubMed] [Google Scholar]
- 38.Beverly B, Kang S M, Lenardo M J, Schwartz R H. Int Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 39.Lombardi G, Sidhu S, Batchelor R, Lechler R. Science. 1994;264:1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 40.Jordan M S, Riley M P, von Boehmer H, Caton A J. Eur J Immunol. 2000;30:136–144. doi: 10.1002/1521-4141(200001)30:1<136::AID-IMMU136>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Maier C C, Greene M I. Immunol Res. 1998;17:133–140. doi: 10.1007/BF02786438. [DOI] [PubMed] [Google Scholar]
- 42.Thomas P M, Samelson L E. J Biol Chem. 1992;267:12317–12322. [PubMed] [Google Scholar]
- 43.Lehuen A, Beaudoin L, Bernard M, Kearney J F, Bach J F, Monteiro R C. Int Immunol. 1995;7:607–616. doi: 10.1093/intimm/7.4.607. [DOI] [PubMed] [Google Scholar]
- 44.Tentori L, Pardoll D M, Zuniga J C, Hu-Li J, Paul W E, Bluestone J A, Kruisbeek A M. J Immunol. 1988;140:1089–1094. [PubMed] [Google Scholar]