Abstract
There have been some reports about the prevalence of anti-Toxoplasma gondii antibody among Koreans, and most of all data were taken from patients visiting hospitals. However, the epidemiological data of the community-based study in Korea are rare. This study was performed to evaluate the seroprevalence of toxoplasmosis among the inhabitants of the rural area Okcheon-gun, Korea. A total of 1,109 serum samples (499 males, 610 females) were examined for the IgG antibodies by ELISA. To set up the cut-off point for ELISA, we used a commercial latex agglutination (LA) kit. The sensitivity and specificity of ELISA against LA test were 89.5%, and 98.6% respectively. Among 1,109 sera, 6.9% showed seropositivity by ELISA. The positive rates of males and females were 6.0% and 7.2%, respectively. However, there were no significant differences between sexes. Comparing the age groups, the highest seropositive rate showed in the seventies or higher, and their rates had a tendency to increase with age (0.05 < p < 0.3). These results revealed that the seroprevalence of toxoplasmosis in rural inhabitants is similar to previous reports in Korea; however we need further investigation to clarify the prevalence of toxoplasmosis in the general population.
Keywords: Toxoplasma gondii, toxoplasmosis, seroepidemiology, latex agglutination, ELISA, Korea
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan parasite occurring with a global distribution among humans and animals. Transmission to humans occurs either through ingestion of T. gondii oocysts shed into the environment by cats, or by eating meat of infected animals. In Korea, foodborne outbreaks of human toxoplasmosis linked to ingesting undercooked pork were reported by Choi et al. (1997). Under normal immune conditions, T. gondii infection is largely asymptomatic, but in those individuals who are immunocompromised, such as patients with AIDS, the parasite can become widely disseminated, causing severe toxoplasmosis and/or encephalitis (Kasper 1997).
It has been known that 15-85% of humans are infected with T. gondii, but the rate of infection varies widely by location, age and other factors (Walzer and Genta, 1989). In Korea, Soh et al. (1960) reported for the first time the seropositive rate of 5.6% in 373 Koreans by skin test. Thereafter, the prevalence of Toxoplasma antibodies has been surveyed in humans by many authors (Choi et al., 1982, 1985, 1989, and 1992; Shim et al., 1991; Ryu et al., 1996; Kook et al., 1999), but mostly based on convenience samples from hospitalized patients. We have found only one report of comminity-based study of anti-Toxoplasma antibody levels in high school students, not in general population, in Cheju island, Korea (Yang et al., 2000). Rural areas have generally worse sanitary conditions than urban areas, and rural inhabitants are easily in contact with reservoir hosts of T. gondii. Therefore, this study was designed to reveal the prevalence rate of anti-Toxoplasma antibody among inhabitants in rural areas, in particular Okcheon-gun, and compare it to the previously reported data in Korea.
MATERIALS AND METHODS
Surveyed areas and population
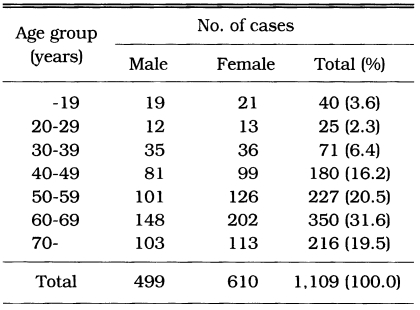
This epidemiologic study was undertaken from February to March 2000. A total of 1,109 inhabitants (499 males, 610 females) in 5 villages of Okcheon-gun, Chungcheongbuk-do were examined. The subjected villages were as follows; Subuk-ri and Okgag-ri Okcheon-eup, Ryegog-ri Cheongsan-myeon, Eunhaeng-ri Gunseo-myeon, Usan-ri Dongi-myeon. Age range of subjected population was 7-88 years old, and their average was 56.9 ± 15.2 (Table 1).
Table 1.
Age and sex distribution of surveyed population in Okcheon-gun, Korea
Latex agglutination (LA) test
The LA test for toxoplasmosis was performed by the procedures carried out by the manufacturer's instruction of TOXOTEST-MT kit (Eiken Chemical Co., Japan). Briefly, serum samples and positive serum were diluted 2-fold serially in a U-shaped 96 microtiter plates using dilution buffer (0.2M amino-2-methyl-1-propanol), and reacted with Toxoplasma antigen-adsorbed polyethylene latex suspension overnight at room temperature. Antibody titers were determined by the last dilution number of sera which precipitated latex in the middle class dispersion. Based on the manufacturer's recommendation, agglutination at dilution of 1:32 or higher was regarded as positive.
Preparation of Toxoplasma lysate antigen
Infected fibroblasts were scraped, forcibly passed through a 27-gauge needle, and centrifuged at 900 × g for 10 min using Percoll (Sigma Chemical Co., USA) to pellet T. gondii tachyzoites. Then parasites were sonicated on ice and centrifuged at 100,000 ×g for 40 min. The supernatant was pooled, sterile filtered (Gelman Sciences, Ann Arbor, MI, USA) and protein content determined by the Bradford method using bovine serum albumin as the standard. Toxoplasma lysate antigen (TLA) was stored in aliquots at -20℃ until use.
Human sera IgG antibody titers by ELISA
The ELISA was performed by the procedure described by Voller et al. (1976). Each well of a 96-well microtitre plate (Nunc, Denmark) was coated with 100 µl of TLA (10 µg/ml) in 0.05 M carbonate-bicarbonate buffer (pH 9.6), and incubated overnight at 4℃. Each well was blocked with 1% bovine serum albumin in phosphate buffered saline (BSA/PBS). Serum samples were diluted 1:150 with 0.1% BSA/PBS containing 0.05% Tween 20 (BSA/PBS/Tween 20). After 2 hr at room temperature, goat anti-human IgG horseradish peroxidase (1:30,000 dilution; Sigma) was applied and the plates incubated for a further 2 hr. After washing, freshly prepared O-phenylenediamine dihydrochloride (Sigma) was added and the reaction stopped by adding 4N H2SO4. The IgG antibody titers were read the optical density at 490 nm using automatic ELISA reader (SPECTRA, Molecular Devices, USA).
Statistical analysis
We used the SPSS 9.0 software for analyzing data from these experiments. In order to check for statistic difference, chi-square test and Student's t-test were adopted. Differences between two groups were considered significant when p values were < 0.05.
RESULTS
Determination of the cut-off absorbance of ELISA
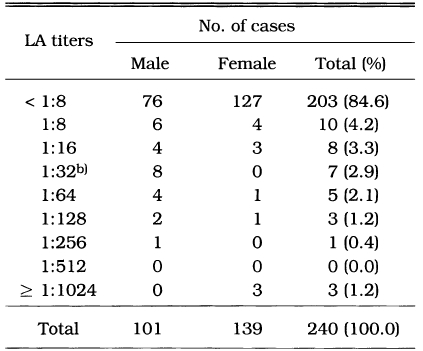
The cut-off value of ELISA was determined by the modification of Choi et al. (1992). Randomly selected 240 sera from surveyed population were subjected to LA test and ELISA. When 240 serum samples were screened by LA test at dilution of 1:8, 37 samples showed agglutination. Selected 37 sera were tested again by LA test at 2-fold dilutions from 1:8 to 1:1,024. Among them, 19 sera showed positive reactions of 1:32 or higher titers (Table 2).
Table 2.
Frequency distribution of anti-Toxoplasma antibody titers in sera of randomly selected 240 cases by LAa) test
a)LA; latex agglutination test.
b)LA titers of 1:32 or higher were regarded as positive.
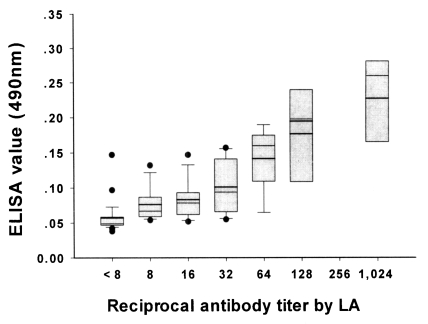
Also, the above mentioned 240 serum samples were tested for the IgG antibodies to T. gondii by ELISA. The correlation between ELISA and LA titers was shown in Fig. 1. In 1:32 LA titer group, the ELISA value of mean ± standard deviation (SD) was 0.101 ± 0.040. The mean ELISA value in 1:32 LA titer group, that is 0.101, did not make statistical difference from 1:16 LA titer group (mean ± SD is 0.083 ± 0.030). Therefore, the cut-off absorbance for positive reactions by ELISA was determined to be 0.141, the mean+one SD of 1:32 LA titer group, which made significant differences between 1:16 and 1:32 LA titer group (p < 0.05). Among 240 serum samples, 20 cases showed positive reaction by ELISA, which was similar in positive rate with the result of LA assay. Most of LA positive cases were positive by ELISA (89.5%). But 1.4% of LA negative sera were positive by ELISA. So, the sensitivity and specificity of ELISA against LA assay were 89.5% and 98.6%, respectively.
Fig. 1.
Relationship between anti-Toxoplasma antibody in sera of randomly selected 240 cases by enzyme-linked immunosorbent assay and latex agglutination test. The data are represented as the mean ± standard deviation.
Seroprevalence of anti-Toxoplasma antibody by ELISA
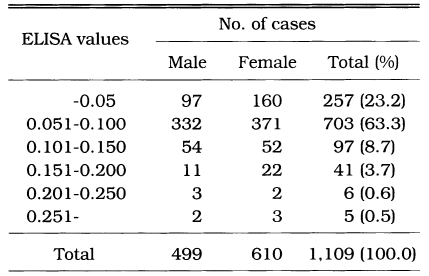
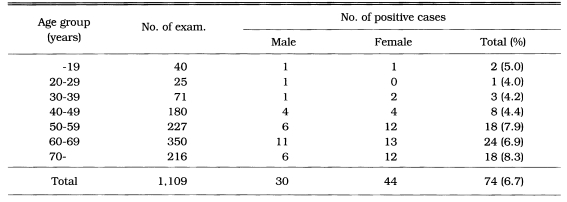
The distribution of IgG antibody titers by ELISA was shown in Table 3. Fig 2. showed the mean ± SD of absorbance in the subjected population according to sex and seroreactivity. The mean absorbance of 1,109 cases was 0.078 ± 0.032. There were significant difference between 74 seropositive cases (0.180 ± 0.034) and 1,109 surveyed population (0.078 ± 0.032) (p < 0.01). Among 1,109 serum specimens, 74 cases revealed seropositive antibody titers which was absorbance of 0.141 or higher. The age and sex distribution of positive reactions of anti-Toxoplasma antibodies shown in Table 4. The positve rates of males and females were 6.0%, and 7.2%, respectively. However, these were not significantly different. There was an overall rise of positive rate with increasing age from 4.0% of subjected population in the forties to 8.3% of population in the seventies or higher.
Table 3.
Frequency distribution of anti-Toxoplasma antibody titers in sera of 1,109 cases by ELISA
Fig. 2.
The mean ± standard deviation of ELISA values in the surveyed 1,109 cases and seropositive 74 cases according to sex and seroreactivity. The cut-off absorbance for positive reaction by ELISA was 0.141. TM, total males (499 cases); TF, total females (610 cases); TB, total both sexes (1,109 cases); PM, seropositive males (30 cases); PF, seropositive females (44 cases); PB, seropositive both sexes (74 cases).
Table 4.
Age and sex distribution of seropositive cases of anti-Toxoplasma antibody by ELISA in surveyed population
DISCUSSION
The prevalence of toxoplasmosis in the rural area Okcheon-gun was 6.7% by ELISA, and this rate was similar to that of Yang et al. (2000), who reported 5.6-8.8% positive rate in the rural area, Cheju island. However, it is difficult to make an exact comparison with previous reports on the survey in Korea because of the difference in assay system or age distribution of sample population. The prevalence rate of this survey was roughly higher than those of pregnant women (0.5-4.3%) and outpatients of general hospital (6.5%), which groups were similar to the general population (Choi et al., 1985; Shim et al., 1991; Ryu et al., 1996). Also this rate was higher or similar with the prevalences of urban areas in Cheju island (4.6-6.9%) (Yang et al., 2000). The positive rates of anti-Toxoplasma antibody in Korean people vary from 0.5% (Choi et al., 1985) to 12.9% (Yang et al., 2000). Soh et al. (1960) first reported 5.6% positive human case out of 373 subjects by skin test. After that, Choi et al. (1982) obtained 7.2% positive cases out of 412 patients, and Choi et al. (1985) screened 377 pregnant women and 43 pelvic tumor patients which resulted in 0.5% in the former and 7.0% in the latter. Ryu et al. (1996) reported 4.3% positive rate among 899 pregnant women by ELISA. Recently, Kook et al. (1999) showed 7.7% positive cases in 542 children patients, and Yang et al. (2000) reported seropositive rate of 5.5% (high school students) and 12.9% (adult patients). Comparing the seropositivity of toxoplasmosis, positive rate of Korean population is lower than those of European or American countries (Walzer and Genta, 1989; Kasper et al., 1997).
In the present study, we used LA test to set up the cut-off absorbance for ELISA. The seropositive rates by LA and ELISA were 8.0%, and 8.3%, respectively. This difference of positive rates may have resulted partly from different antigenic epitope recognized by LA and ELISA and partly from difference of limits of sensitivity of both tests (Walzer and Genta, 1989; Choi et al., 1992; Kasper et al., 1997).
The positive rate of females were higher than that of males, whereas the absorbance of male population was not statistically significant to absorbance of female in this study (p < 0.348). Similar result was obtained in other survey performed in Cheju island (Yang et al., 2000). In contrast to this result, male population showed a higher seropositive rate than female population did in all age groups although mean antibody levels in positive groups were almost the same for male and female (Takahashi et al., 1985). Seroprevalence of toxoplasmosis is known to increase with age (Takahashi et al., 1985, Shim et al, 1991; Taylor et al., 1997). In this study, the lowest positive rate was seen in the age of 20-29 years, and the positive rates were slowly increased with age, and the peak level revealed in the seventies or higher. The rate in older people over 70 years was 8.3% which were 2 times higher than the younger people. The reason for the rise in quantitative titers with age is not clear. A hypothesis would be that the increase is a reflection of increasing exposure years as the humans get older. Multiple minor infections might at first produce low antibody levels and later higher levels (Taylor et al., 1997).
Survey of Toxoplasma antibody performed in this study showed 6.7% of prevalence of rural inhabitants in Korea. This prevalence rate ranged within the values of the previously reported data. The positive rate in the fifties or higher were two times higher than the younger people, and their rates had tendency to increase with age. However there were not seen sex difference. This survey is meaningful as a community and general population based study to reveal the seroprevalence of toxoplasmosis in Koreans, but further survey from various areas will be necessary to clarify the seroepidemiological status of toxoplasmosis in the Korean population.
References
- 1.Choi WY, Yoo JE, Kim WK. Toxoplasma antibodies by indirect latex agglutination tests in St. Mary's Hospital patients. Korean J Parasitol. 1982;23:300–304. doi: 10.3347/kjp.1982.20.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Choi WY, Choi HR, Rha JG. Significance of Toxoplasma antibody titers by indirect latex agglutination tests in pregnant women and pelvic tumor patients. Korean J Parasitol. 1985;23:300–304. doi: 10.3347/kjp.1985.23.2.300. [DOI] [PubMed] [Google Scholar]
- 3.Choi WY, Nam HW, Youn JH, Kim WS, Kim WK. Toxoplasma antibody titers by indirect latex agglutination test in patients of Kangnam St. Mary's Hospital and Cheju Medical Center. Korean J Parasitol. 1989;27:171–175. doi: 10.3347/kjp.1989.27.3.171. [DOI] [PubMed] [Google Scholar]
- 4.Choi WY, Nam HW, Youn JH, et al. Dectection of antibodies in serum and cerebrospinal fluid to Toxoplasma gondii by indirect latex aggluination test and enzyme-linked immunosorbent assay. Korean J Parasitol. 1992;30:83–90. doi: 10.3347/kjp.1992.30.2.83. [DOI] [PubMed] [Google Scholar]
- 5.Choi WY, Nam HW, Kwak NH, et al. Foodborne outbreaks of human toxoplasmosis. J Infect Dis. 1997;175:1280–1282. doi: 10.1086/593702. [DOI] [PubMed] [Google Scholar]
- 6.Kasper LH. Harrison's principles of internal medicine. 14th ed. New York: McGraw-Hill; 1997. Toxoplasma infection; pp. 1197–1202. [Google Scholar]
- 7.Kook J, Lee HJ, Kim BI, et al. Toxoplasma gondii antibody titers in sera of children admitted to the Seoul National University Children's Hospital. Korean J Parasitol. 1999;37:27–32. doi: 10.3347/kjp.1999.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu JS, Min DK, Ahn MH, et al. Toxoplasma antibody titers by ELISA and indirect latex agglutination test in pregnant women. Korean J Parasitol. 1996;34:233–238. doi: 10.3347/kjp.1996.34.4.233. [DOI] [PubMed] [Google Scholar]
- 9.Shim HS, Na YE, Lee YH, Shin DW. Toxoplasma antibody titers of general outpatients and pig sera by indirect latexagglutination test. Chungnam Med J. 1991;18:77–85. [Google Scholar]
- 10.Soh CT, Lee SJ, Ahn YK. Latent infection by Toxoplasma gondii in Korea. Yonsei Med J. 1960;1:52–54. [Google Scholar]
- 11.Takahashi J, Konishi E, Matsumura T. A survey of antibody to Toxoplasma gondii among patients of a hospital in Hyogo prefecture, Japan, by enzyme-linked immunosorbent assay. Jpn J Parasitol. 1985;34:87–92. [Google Scholar]
- 12.Taylor MR, Lennon B, Holland CV, Cafferkey M. Community study of Toxoplasma antibodies in urban and rural schoolchildrenaged 4 to 18 years. Arch Dis Child. 1997;77:406–409. doi: 10.1136/adc.77.5.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voller A, Bidwell DE, Bartlett A, Fleck DG, Perkins M, Oladenkin B. A microplate enzyme immunoassaay for Toxoplasma antibody. J Clin Pathol. 1976;29:150–153. doi: 10.1136/jcp.29.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walzer PD, Genta RM. parasitic infection in the compromised host. New York: Marcel Dekker, Inc; 1989. Toxoplasma gondii. [Google Scholar]
- 15.Yang HJ, Jin KN, Park YK, et al. Seroprevalence of toxoplasmosis in the residents of Cheju island, Korea. Korean J Parasitol. 2000;38:91–93. doi: 10.3347/kjp.2000.38.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]