Abstract
African Americans (AAs) tend to have lower total adiponectin levels compared to European Americans (EA); however, it is not known whether race affects adiponectin multimer distribution and their relationships to metabolic traits. We measured total adiponectin, high molecular weight (HMW), low molecular weight (LMW) (i.e., hexamer), and trimer adiponectin in 132 normoglycemic premenopausal women (75 AAs, 57 EAs), together with measures of total and abdominal fat, plasma lipids, insulin sensitivity (Si), and genetic admixture estimates. We found that lower total adiponectin in AAs was explained by reduced LMW, and trimer forms because levels of HMW did not differ between races. In EAs, HMW was highly correlated with multiple metabolic syndrome traits. In contrast, the LMW and trimer forms were most highly correlated with metabolic traits in AAs, including abdominal adiposity, lipids, and Si. At similar levels of visceral adiposity, AAs exhibited significantly lower LMW adiponectin than EAs. Similarly, at comparable levels of HMW and LMW adiponectin, AAs were more insulin resistant than their EA counterparts. In conclusion, (i) serum adiponectin is lower in AAs predominantly as a result of reduced concentrations of LMW and trimers multimeric forms; (ii) LMW and trimer, not HMW, are most broadly correlated with metabolic traits in AAs. Thus, HMW adiponectin may exert less bioactivity in explaining the metabolic syndrome trait cluster in populations of predominant African genetic background.
INTRODUCTION
Adiponectin (Ad) is an abundant circulating adipokine that exerts beneficial metabolic, anti-inflammatory, and antiatherogenic effects (1). Low serum Ad concentrations are associated with multiple phenotypic traits that comprise the metabolic syndrome, including increased abdominal fat distribution, decreased insulin sensitivity (Si), and reduced high-density lipoprotein (HDL) cholesterol levels. As a consequence, low circulating Ad has been proposed as a marker for the metabolic syndrome (1,2). Furthermore, reduced Ad levels have been reported in African-American (AA) children compared to their European-American (EA) counterparts, and has been implicated in the lower Si observed in AA children (3).
Recent attention has been given to the fact that Ad circulates as multimers of various molecular weights with the predominant forms in humans being a high molecular weight (HMW) Ad species consistent with a duodecamer of ~360 kDa, and low molecular weight (LMW) Ad hexamers of ~180 kDa. Different methodologies have been used to measure adiponectin multimers. Several studies have combined size fractionation and immunoblotting (4–11), which are relatively laborious but reliable methods for all multimers, and enzyme-linked immuno-sorbent assay techniques have been more recently developed that are less able to quantify LMW and trimeric forms (12). Evidence from several in vitro studies supports a more robust biological role of HMW compared to smaller molecular weight forms (8,13–15). We have found that the HMW species is most strongly correlated with Si, abdominal adiposity, HDL cholesterol, serum triglycerides, and basal lipid oxidation rates (6). In addition to HMW, although in a lesser extent, LMW forms (i.e., hexamers) have also been associated with favorable metabolic parameters such as Si and very low-density lipoprotein cholesterol (6) and to increase in response to moderate weight reduction (9); whereas trimer Ad has been correlated with waist circumference and monocytic interleukin-6 secretion (16). Thus, in addition to total Ad, the distribution of Ad multimers can independently explain variability in metabolic traits among individuals and populations.
Whether the distribution of Ad multimers varies as a function of race, and affects the relationship with Si and other metabolic traits in AAs, is not known. There is some evidence that race can affect the “phenotype” of the metabolic syndrome by influencing the degree to which the various component traits cluster as a function of the syndrome complex. For example, in AAs, abdominal adiposity and glucose intolerance are associated with insulin resistance, whereas blood pressure and dyslipidemia exist relatively independent of insulin resistance when compared to EAs (17,18). This raises the question as to whether racial differences in Ad multimers could explain differences in associations between metabolic traits and insulin resistance in the context of the metabolic syndrome. Another consideration in addressing this question is the degree to which self-identified race reflects ancestral genetic admixture. This could be important if racial differences in the metabolic syndrome and Ad biology are genetically determined. Studies that categorize subgroups based on self-identified race may not have a direct relationship with ancestral genetics because genetic admixture is extremely variable in self-identified AAs and EAs. Genetic admixture measurements can determine the relative contribution of a parental population (e.g., African) to an individual’s genome using specific ancestry informative DNA markers that differ in frequency among the respective parental populations (19). Thus, ancestral genetic admixture becomes a continuous variable that can be used to differentiate between genetic and environmental components of a phenotype, in a more precise manner that categorical subgroupings based on self-identified race where differences in ancestral admixture are unknown. This technique has been used previously to explore physiological and metabolic population differences (19,20).
The aims of this study were to determine: (i) whether Ad and the distribution of Ad multimers varies as a function of self-identified race in EAs and AAs; (ii) whether Ad and the HMW multimer is similarly related to components of the metabolic syndrome, including insulin resistance and visceral adipose tissue (VAT), in these two racial groups. We hypothesized that AAs, in addition to lower total Ad levels, have distinct distribution of Ad multimers when compared with EAs, and that the relationships between Ad multimers and metabolic traits differ as a function of race.
METHODS AND PROCEDURES
Subject description
Subjects were recruited from studies investigating metabolism and body composition in a biracial group of premenopausal women conducted at the University of Alabama at Birmingham. In these studies, unrelated, premenopausal, self-identified AA and EA women were recruited by newspaper advertisements, radio announcements, and word-of-mouth. The volunteers were not taking any medication known to alter body composition or metabolism (including hormones), were sedentary (defined as exercising less than once per week for the past year), were nonsmokers, were of overall good health, and had regular menstrual cycles. All subjects meeting inclusion criteria were sequentially enrolled and studied. All subjects from the total participant pool were included for analyses (n = 132, 75 AAs, 57 EAs); however, not all metabolic and body composition measures were available on all subjects (Table 1). The study was approved by the University of Alabama at Birmingham Institutional Review Board. All volunteers were screened and briefed about the experimental protocol, and informed consent was obtained before testing. Subjects were admitted to the General Clinic Research Center for 4 days and underwent metabolic and body composition assessment as described below. All testing was performed in the follicular phase of the menstrual cycle (within 10 days of the start of menses).
Table 1.
Body composition and metabolic characteristics of study subjects by race
| Variable | European American | African American |
|---|---|---|
| Age (years) | 35 ± 6 (22–48) | 33 ± 6 (22–44)* |
| BMI (kg/m2) | 27 ± 2 (22–31) | 27 ± 2 (19–31) |
| Fat mass (kg) | 39 ± 9 (19–46) | 37 ± 10 (13–53) |
| Waist (cm) | 86 ± 6 (70–102) | 84 ± 7 (68–97) |
| Total abdominal fat (cm2)a | 412 ± 110 (188–641) | 391 ± 100 (102–534) |
| Subcutaneous abdominal fat (cm2)a | 317 ± 102 (132–553) | 329 ± 91 (69–474) |
| Visceral abdominal fat (cm2)a | 95 ± 29 (25–168) | 62 ± 25 (23–154)** |
| Total cholesterol (mg/dl) | 162 ± 26 (104–212) | 151 ± 35 (78–232) |
| HDL (mg/dl) | 34 ± 8 (13–54) | 40 ± 9 (20–64)** |
| LDL (mg/dl) | 104 ± 24 (52–152) | 98 ± 33 (39–177) |
| Triglycerides (mg/dl) | 121 ± 53 (29–295) | 69 ± 27 (32–144)** |
| Fasting glucose (mg/dl) | 89 ± 5 (75–102) | 88 ± 7 (65–105) |
| Fasting insulin (μIU/ml) | 11 ± 3 (2–20) | 11 ± 4 (3–29) |
| Si (×10−4/min−1/μIU/ml)b | 3.90 ± 2.3 (0.96–13.6) | 2.67 ± 1.7 (0.54–11.9)** |
Results are mean ± s.d. N = 57 EA and 75 AA, unless specified otherwise.
Lipid values (total cholesterol, HDL, LDL, and triglycerides) reflect fasting measurements.
AA, African American; EA, European American; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
N = 51 EA and 69 AA;
N = 51 EA and 54 AA. Race was self-identified. Significantly different from European Americans,
P < 0.05;
P < 0.01.
Body composition and metabolic variables
Total lean and fat masses were determined using dual-energy X-ray absorptiometry (DPX-L; Lunar Radiation, Madison, WI). VAT and subcutaneous abdominal adipose tissue (SCAT) were determined by a single slice measurement (at the level of the umbilicus) using computed tomography. Glucose, insulin, and plasma lipids were measured using commercially available kits as previously reported (3).
Frequently sampled, intravenous glucose tolerance test
At approximately 7:00 AM, after a 12-h fast, flexible intravenous catheters were placed in the antecubital spaces of both arms. Three blood samples were drawn over a 40-min period, and sera subsequently separated and pooled for analysis of lipids. Three additional blood samples were taken over a 20-min period for determination of basal glucose and insulin (the average of the values was used for basal “fasting” concentrations). At time “0,” glucose (50% dextrose; 11.4 g/m2) was administered intravenously. At minute 20 after glucose administration, subjects received either an intravenous bolus of insulin (0.02 U/kg) or 5-min infusion of insulin (0.02 U/kg). Blood samples were collected at designated intervals over the test period. For the insulin-modified (bolus injection) tests, samples were collected at the following times (min) relative to glucose administration at 0 min: 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, 180. For the insulin-infusion-modified test, samples were collected at 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, 180, 210, and 240 min. Sera were analyzed for glucose and insulin (frequently sampled, intravenous glucose tolerance test), and values were entered into the MINMOD computer program (version 3.0, Richard N. Bergman) for determination of Si. Si results from insulin-modified frequently sampled, intravenous glucose tolerance tests do not differ with mode of insulin administration (bolus vs. infusion) (21). From the total participant pool (n = 132), 21 AA and 6 EA subjects underwent a tolbutamide-modified frequently sampled, intravenous glucose tolerance test. Because Si results from tolbutamide-modified tests are ~16% higher than those from insulin-modified tests, these data were excluded from the analysis (22).
Ad and multimers determination
Separation of serum Ad isoforms was performed using nondenaturing gel electrophoresis and western blotting as previously described (6); samples were randomly analyzed and after adjusting for background activity, density of specific adiponectin oligomers bands were measured. In a given patient all the bands present were measured. Relative distribution of adiponectin oligomers were calculated by dividing band density through total density. Percentage of adiponectin oligomers were multiplied with total adiponectin levels to calculate absolute oligomer values as previously described (6).
Determination of genetic admixture
To decompose the biological and nonbiological aspects of race, we used admixture instead of racial classification as a covariate in statistical models. To quantify African and European ancestral genetic admixture, ancestry informative markers were assessed at Pennsylvania State University by Dr Shriver and colleagues. Ancestry informative markers are single-nucleotide polymorphisms that were genotyped using agarose gel electrophoresis and melting curve analysis as described in detail by Akey et al. (23). The ancestry informative markers used, their chromosomal and centimorgan location, and their ability to discriminate among parental populations (based on the allelic differences between European and African parental populations) have been described elsewhere (24). Further information about these markers is available through dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/) using handle PSA-ANTH. All genetic data were transformed into a single estimate of genetic admixture using the maximum likelihood approach (25) such that the likelihood that a particular multilocus genotype is from each of 100 different combinations of the two parental populations is calculated as the probability of the genotype given the allele frequencies in each parental population. The parental population combination where the specific multilocus genotype for an individual has the highest probability is thus the most likely and represents the admixture estimate for that individual.
Statistical methods
All data are given as means ± s.d. unless otherwise indicated. Differences in outcomes of interest between racial groups were compared using Student’s t-test. Total Ad, HMW, LMW, trimer, and Si were log10 transformed so that each of these variables followed an approximate normal distribution. The correlations between total Ad, HMW, LMW, trimers, vs. body composition variables, Si, and lipid measurements (as continuous variables) were examined using Spearman’s correlation coefficients. In every instance separate analyses by race were conducted. Multiple regression analysis was conducted for dependent variable Si. The independent variables used in these models were race or admixture, total fat mass, fasting insulin, age, VAT and SCAT, total Ad, total and relative amounts of HMW, LMW, trimer, and an Ad form vs. race interaction terms. All statistical tests used a significance level of 5% and were two-tailed. The SAS program version 9.1 (SAS Institute, Cary, NC) was used for analyses.
RESULTS
Body composition and metabolic characteristics
General characteristics of the study subjects are outlined in Table 1. Visceral fat was significantly lower in AAs compared to EAs. AAs had higher HDL cholesterol and lower triglyceride levels and exhibited more insulin resistance (i.e., lower Si values) compared to EAs.
Serum Ad multimers
Figure 1 is a representative immunoblot from five different patients that illustrates circulating Ad multimeric forms. Two predominant well defined bands were detected, the first with an apparent molecular weight of >360 kDa and designated HMW, and a second band that migrated at a lower molecular weight of ~180 kDa corresponding to the Ad hexamer and designated LMW. We also detected two less well-defined Ad bands with apparent molecular weights of ~140 kDa and 90 kDa, which correspond to Ad trimer, bound and not bound to serum albumin, respectively, as demonstrated by Hada et al. in human plasma using affinity columns and gel filtration (26). The 90 kDa band was not observed in all patients and only 10 out of 57 EAs and 12 out of 75 AAs had a quantifiable band running at this molecular weight. Conversely, the 140 kDa band was observed in almost all patients, 57 EAs and 71 out of 75 AAs. These two bands were collectively quantified as Ad trimer, and as a result the majority of subjects had a quantifiable trimer band. Reference to trimer Ad throughout the article reflects this approach. For those patients with no quantifiable band a value of zero was assigned.
Figure 1.
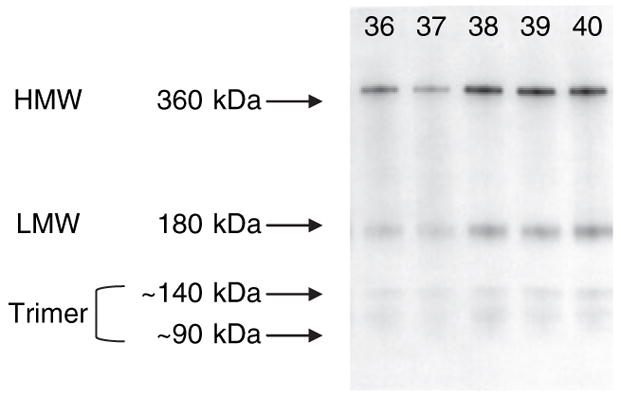
Representative immunoblot illustrating the main adiponectin complexes high molecular weight (HMW), low molecular weight (LMW) (hexamer), and trimer bands, identified from human serum in five different patients.
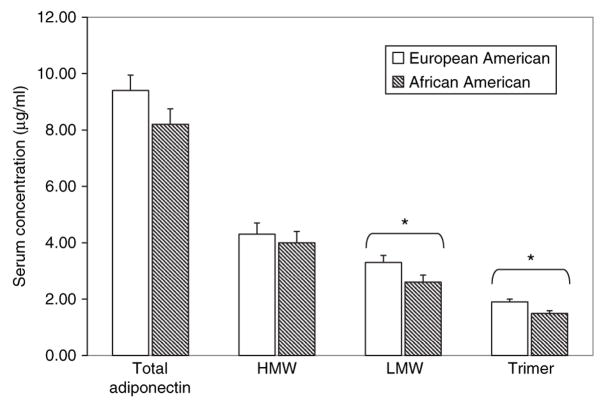
We then analyzed racial differences in total Ad and Ad multimers (Figure 2). Compared to EAs, AAs had lower total Ad (9.4 ± 3.2 μg/ml vs. 8.2 ± 3.4 μg/ml, P < 0.05). The reduced concentration of total Ad could not be explained by any difference in HMW (4.3 ± 1.8 μg/ml vs. 4.0 ± 1.9 μg/ml, P = ns). In contrast, both the LMW (3.3 ± 1.3 μg/ml vs. 2.6 ± 1.5 μg/ml, P < 0.01) and the trimer (1.9 ± 0.9 μg/ml vs. 1.5 ± 0.9 μg/ml, P < 0.01) were reduced in AAs compared with EAs. Lower total Ad levels in AAs due to the reduction in LMW and trimer led in turn to a higher relative proportion of the HMW form as reflected by a higher ratio of HMW to total Ad (0.50 ± 0.13 vs. 0.45 ± 0.07, AAs vs. EAs, respectively, P < 0.01).
Figure 2.
Serum concentrations of total adiponectin, high molecular weight (HMW), low molecular weight (LMW) (hexamer), and trimer (trimer plus trimer bound to albumin) as identified by nondenaturing electrophoresis and immunobloting in European-American (EA) and African-American (AA) women. N = 57 EA and 75 AA. *P < 0.05.
Association of Ad and multimers with body composition, lipid, and metabolic profiles
We evaluated the relationships between total Ad, HMW, LMW, and trimer with several components of the metabolic syndrome in both racial groups as shown in Table 2. HMW was widely associated with metabolic traits only in EAs. In this group, HMW was highly correlated with BMI, waist circumference, VAT, as well as with HDL cholesterol, fasting insulin, Si, and fat mass (all P ≤ 0.05). Unexpectedly, in AAs, it was the LMW that was more broadly correlated with metabolic syndrome traits including central adiposity, HDL, low-density lipoprotein, fasting insulin, and Si as shown in Table 2. HMW in AAs was correlated with HDL cholesterol, fasting insulin, and Si but not related to BMI, fat mass, waist circumference, VAT, SCAT, or low-density lipoprotein cholesterol. The ratio of HMW to total Ad was not a better correlate of metabolic syndrome traits; in EAs, the ratio was correlated with BMI (r = −0.36, P < 0.01), fat mass, and waist circumference (both r = −0.32, P < 0.05), but not with lipids or Si, whereas in AAs, the ratio was correlated with waist circumference (r = 0.26, P < 0.05), low-density lipoprotein cholesterol (r = 0.29, P < 0.05), and fasting insulin (r= 0.24, P < 0.05).
Table 2.
Correlation coefficients between serum adiponectin, main adiponectin multimers, and body composition, lipids, and metabolic parameters by race
| European American |
African American |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Total | HMW | LMW | Trimer | Total | HMW | LMW | Trimer |
| BMI (kg/m2) | −0.19 | −0.34* | −0.09 | 0.09 | 0.00 | 0.11 | −0.03 | −0.13 |
| Fat mass (kg) | −0.16 | −0.26** | −0.10 | 0.21 | 0.17 | 0.21 | 0.10 | −0.10 |
| Waist (cm)a | −0.44*** | −0.49*** | −0.36* | 0.04 | −0.29* | −0.10 | −0.25* | −0.25* |
| VAT (cm2) | −0.40*** | −0.41*** | −0.34*** | −0.05 | −0.33*** | −0.19 | −0.30*** | −0.25* |
| SCAT (cm2) | −0.10 | −0.18 | 0.10 | −0.05 | −0.10 | 0.04 | −0.16 | −0.20 |
| Triglycerides | −0.21 | −0.17 | −0.11 | −0.07 | −0.18 | −0.14 | −0.09 | −0.20 |
| HDL | 0.45*** | 0.43*** | 0.37*** | 0.19 | 0.37*** | 0.30* | 0.22** | 0.25* |
| LDL | −0.12 | −0.12 | −0.12 | −0.04 | −0.14 | 0.03 | −0.29* | −0.16 |
| Fasting insulin | −0.38*** | −0.29* | −0.30* | −0.20 | −0.43*** | −0.25* | −0.46*** | −0.27* |
| Si | 0.49*** | 0.43*** | 0.28* | 0.37*** | 0.39*** | 0.31* | 0.28* | 0.27* |
Significant values in boldface.
HDL, high-density lipoprotein; HMW, high molecular weight adiponectin; LDL, low-density lipoprotein; LMW, low molecular weight adiponectin; SCAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Adjusted for total fat mass.
P < 0.05;
P = 0.05;
P < 0.01; Serum triglycerides, HDL and LDL levels measured in fasted state.
Effects of race on interrelationships among VAT, Ad levels, and insulin resistance
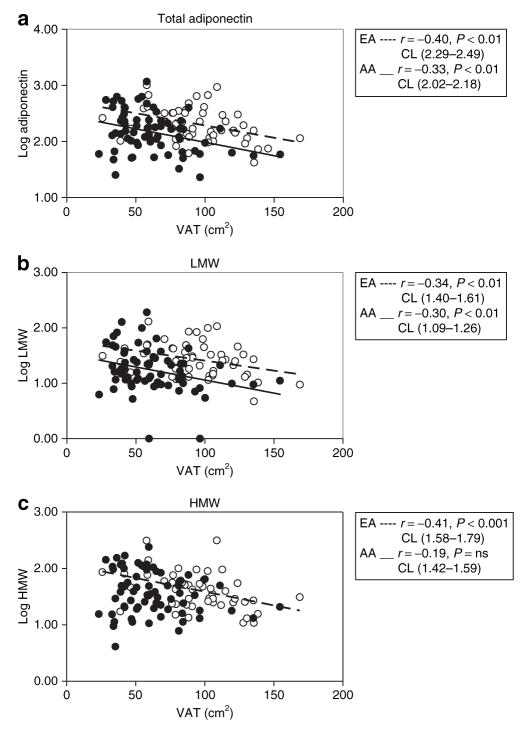
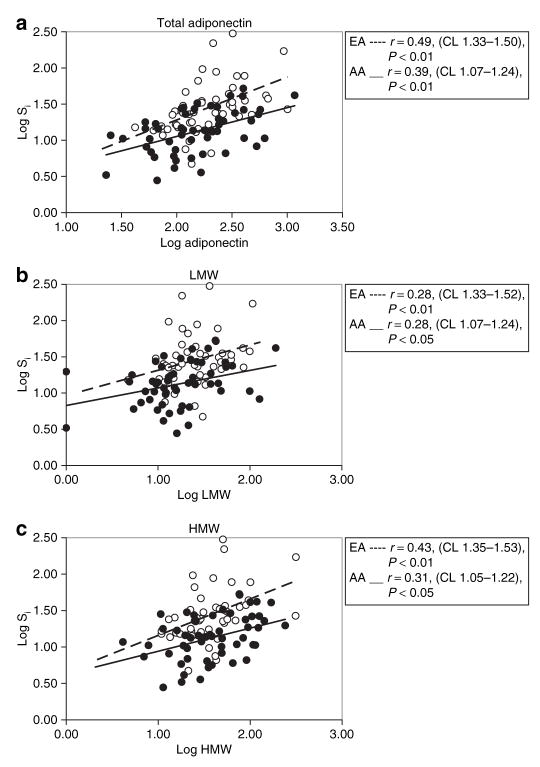
Previous studies (27,28) and the current data (Table 1) indicate that visceral fat mass is markedly reduced at any given level of general adiposity in AAs. Because visceral fat contributes to the production of Ad and is pathophysiologically linked with insulin resistance, it remained possible that lower total Ad, LMW, and trimer in AAs was simply a function of reduced visceral fat and that relative insulin resistance in the AAs could be explained by the reduction in Ad. In this way, race could be altering the interrelationships among visceral fat, Ad, and Si. To examine this issue, it was necessary to examine the relationships between Ad and visceral adiposity, and Ad and Si, over the continuum of values in these two races. As is demonstrated in Figure 3a, at any given level of visceral adiposity, AAs exhibited lower total Ad levels than EAs. This same observation was applicable to LMW in Figure 3b, but not with HMW because this form was not correlated with VAT in AAs (Figure 3c). Similarly, VAT was correlated with trimer in AAs (r = −0.25, P < 0.05) but not in EAs (r = −0.05, P = ns). These data are consistent with the possibility that AAs produce less total Ad and LMW at any given amount of visceral fat. We next assessed the continuous relationship between Si, total Ad, and multimers. The continuous relationship between Si, total Ad, and multimers is shown in Figure 4. As shown in Figure 4a, the rise in Si as a function of total Ad was sharp in EAs whereas in AAs the slope was reduced, and there was a trend toward a significant difference between these two slopes (total Ad vs. race interaction, P = 0.06). Although the slopes of the regression lines for Si and total, HMW, and LMW Ad were similar in both races, AAs exhibited significantly lower Si at similar levels of total, HMW, and LMW Ad as shown in Figure 4b,c.
Figure 3.
Relationship between visceral adipose tissue (VAT) and (a) total adiponectin, (b) LMW, and (c) HMW in AA (filled circles), and EA (empty circles). The regression line is depicted continuous in AA, and discontinuous in EA. AA, African American; CL, 95% confidence limits; EA, European American; HMW, high molecular weight; LMW, low molecular weight; ns, nonsignificant.
Figure 4.
Relationship between insulin sensitivity (Si) and (a) total adiponectin, (b) LMW, and (c) HMW in AA (filled circles), and EA (empty circles). The regression line is depicted continuous in AA, and discontinuous in EA. AA, African American; CL, 95% confidence limits; EA, European American; HMW, high molecular weight; LMW, low molecular weight.
Racial genetic admixture, Si, and Ad multimers
Using African admixture estimates rather than categorical self-identified race, we investigated the determinants of Si in the study group as a whole. In simple regression analysis, percent-age of African admixture was negatively related to Si and predicted 11% of the variance in Si (P < 0.001). Furthermore, VAT (P < 0.001), SCAT (P < 0.001), waist circumference (P < 0.001), fat mass (P < 0.01), and fasting insulin (P < 0.001) were negative predictors of Si; and LMW, HMW, and trimer were positive predictors of Si explaining 14, 12, and 11% of the variance in Si, respectively (all P < 0.01). The ability of these variables in predicting Si was further quantified using multiple linear regression analyses. In the final model, African admixture, HMW, and fasting insulin, collectively were independent determinants of Si, and explained 52% of the variance in Si (P < 0.001). On the contrary, LMW, trimer, fat mass, and VAT, were not independent predictors of Si as shown in Table 3.
Table 3.
Multiple linear regression model for the dependent variable log Si (R2 for the model = 0.52)
| Variable | Parameter estimate ± SEE | P |
|---|---|---|
| Intercept | 1.20 ± 0.33 | <0.0001 |
| African admixture (%) | −0.40 ± 0.13 | 0.003 |
| LMWa | −0.07 ± 0.10 | 0.45 |
| Trimera | 0.15 ± 0.09 | 0.12 |
| HMWa | 0.29 ± 0.09 | 0.003 |
| Fasting insulin (μIU/ml) | −0.03 ± 0.00 | <0.001 |
| VAT (cm2) | 0.003 ± 0.00 | 0.85 |
| Fat mass (kg) | 0.00 ± 0.003 | 0.78 |
| Age (years) | 0.00 ± 0.006 | 0.56 |
Significant values in boldface.
VAT, visceral adipose tissue.
Si, insulin sensitivity index; SEE, s.e. of estimate; LMW, low molecular weight adiponectin form (hexamer); HMW, high molecular weight adiponectin.
DISCUSSION
AA adults and children have previously been reported to have lower circulating levels of total immunoreactive Ad compared to EAs (3,29,30). We now report that there are also racial differences in the distribution of Ad multimers, as well as in the relationship between Ad multimers and metabolic traits. We have extensively characterized a biracial sample of premenopausal, nondiabetic women by measuring serum levels of total Ad, HMW, LMW, and trimer, Si using the frequently sampled, intravenous glucose tolerance test, total body fat and abdominal fat distribution using dual-energy X-ray absorptiometry and abdominal computed tomography scanning, and conventional lipid panels. In addition, we have quantified African and European ancestral genetic admixture as a covariate to assess whether genetics contribute to differences in Ad and its role in metabolism. We found that there exist racial differences in the distribution of Ad multimers with AAs having lower levels of total immunoreactive Ad, and reduced levels of the LMW and trimer compared with EAs, whereas HMW complexes were similar in the two racial subgroups. The plasma concentrations of the different Ad oligomers are within the range obtained in previous studies (9,12,26,31), and the bands separated by immunoblotting in our study are consistent with those reported previously by using antiadiponectin affinity chromatography (26). Moreover, there are distinct racial differences in the relationships between Ad and its multimeric forms with multiple anthropomorphic and metabolic traits. In EAs, HMW was consistently and strongly correlated with multiple traits relevant to the metabolic syndrome including BMI, fat mass, waist circumference, VAT, HDL, and Si. In contrast, in AAs, we observed correlations between HMW with HDL and fasting insulin, whereas the HMW multimer was not related to measures of general or regional adiposity. Rather, both LMW and trimers were more broadly associated with metabolic and body composition traits in AAs, including significant correlations with waist circumference, VAT, HDL, fasting insulin, and Si.
We extend previous reports on lower levels of total Ad in AAs. In our study, lower Ad levels in AAs were explained by predominantly lower Ad forms, LMW and trimers. The reasons for the differences in the distribution of circulating multimeric forms are not completely understood. Once the main Ad isoforms appear in circulation they do not interconvert with each other. Therefore, the production and secretion of Ad isoforms from adipocytes has a main influence on the circulating levels of each complex (32,33). Because our study included only overweight or obese, otherwise healthy premenopausal women, our findings cannot be generalized to men, postmenopausal or lean women and thus further studies are necessary to confirm these differences.
Despite having much higher prevalence rates of type 2 diabetes, it is a conundrum that fewer AA men than EA men satisfy the criteria for metabolic syndrome, a major risk factor for type 2 diabetes, and that AA and EA women are equally likely to have the metabolic syndrome (34). Because Ad has been proposed as a key marker of the metabolic syndrome (1,2,35), we analyzed the relationships between Ad multimeric forms and the various constituent components of the syndrome. We found that total Ad was correlated with abdominal adiposity, HDL, and Si in both AAs and EAs. However, racial differences were noted in these relationships when we examined Ad multimers. In EAs, HMW was widely correlated with components of the syndrome including measures of generalized and abdominal adiposity, HDL, and Si, whereas in AAs, LMW rather than HMW Ad was the best correlate of metabolic and body composition traits. The findings in EAs are in agreement with previous studies showing a strong correlation of HMW with metabolic traits in different populations of predominant European or Asian origin (4–7,12,36–38), as well as extends previous observations of ethnic variation in adiponectin isoform distribution (39). Despite the fact that the strength of the associations between total and HMW Ad, and metabolic traits were higher in EAs compared to AAs, these differences did not achieve statistical significance. The current study is the first to demonstrate that AAs are unique in that HMW is not superior in predicting metabolic variables. Although lower molecular weight Ad forms have been shown to correlate with different metabolic parameters (6,9,16), albeit to a lesser degree than HMW, it was the LMW multimer that was most broadly correlated with multiple metabolic traits in AAs. Overall, these findings are in agreement with functional studies indicating that full length Ad and the different forms including HMW, LMW, and trimers exert receptor activation of metabolic pathways, however, evidence has indicated that it is the HMW multimer that exerts more potent biological effects in vitro (26,40). It is remarkable that our data indicate that, in the context of the metabolic syndrome, HMW might not be of predominant relevance in AAs.
Because increased abdominal fat accumulation is one of the key pathophysiological features of the metabolic syndrome, we analyzed the relationships between different abdominal fat compartments and Ad multimers. VAT but not SCAT was correlated with Ad multimers in both races, supporting previous studies that indicated a strong relationship between Ad and VAT in nondiabetic women (41). Because VAT mass was most closely related to LMW in AAs, it was possible that the lower levels of VAT in AAs could explain reduced circulating concentrations of LMW. However, at every level of visceral fat, AAs reveal significantly lower total Ad, and LMW than EAs (Figure 3). Similarly, when the relationship between Ad multimers and Si was analyzed, the correlation coefficients between Si and either total Ad or HMW, although not significantly different, tend to be lower in AAs. In addition, at every level of total, HMW and LMW Ad, AAs exhibited lower Si compared to EAs, suggesting that AAs may require higher Ad levels, particularly the more bioactive forms HMW and LMW, to attain similar degrees of Si compared to EAs, as shown in Figure 4a–c. Taken together these findings lead us to speculate that potential intrinsic biological differences in Ad regulation, set point or Ad resistance operates in AAs. AAs were observed to have reduced LMW, trimer, and total Ad concentrations for any given level of BMI or VAT mass, which would compound Ad resistance with lower Ad concentrations resulting in reduced Ad biological effect. However, because of the cross-sectional nature of this study, no evidence of causality can be inferred regarding Ad and any component of the metabolic syndrome.
Results from this study confirm previous findings that total Ad is an independent determinant of Si (i.e., Si) in children, adolescents, and adults (3,42). In our sample of biracial premenopausal women ~50% of the variance in Si is explained collectively and independently by a model including genetic admixture, HMW, and fasting insulin. Measures of African admixture yield some insight into the influence of genetic vs. environmental factors on ethnic differences in physiologic outcomes. In this study, African admixture independently predicted Si, similar to previous reports on youth (20), supporting the role of genetic factors in explaining relative insulin resistance in individuals of predominant African descent. Moreover, regression modeling showed that HMW is a strong and independent predictor of Si, on the contrary LMW and trimer Ad were no longer significant after adjusting for African admixture. This observation is consistent with the idea that genetic factors influence the distribution of Ad multimers and the relationship between Ad multimers and metabolic traits. However, all previous genetic studies of Ad in AAs have focused on total Ad rather than on the relative distribution of adiponectin multimers, and thus further research is necessary to address this issue.
In conclusion, our study provides evidence for differences in Ad multimer distribution in AAs and EAs. In both races, serum Ad is associated with increased Si, reduced abdominal fat, and a more cardioprotective lipid profile. These relationships are primarily driven by HMW quantity in EAs but not AAs in whom the lower molecular weight forms, particularly LMW, correlate more extensively with metabolic traits. HMW might be a less relevant factor in explaining the metabolic syndrome trait cluster in populations of predominant African background, and the data are indicative of relative Ad resistance in AAs.
Acknowledgments
We thank our sources of support: the NIH grants DK 49779, DK51684, DK-38764, and PO1 HL-55782, the UAB GCRC M01-RR00032, the CNRU (P30-DK56336), the T32 grant (HL-007457) (PI: S. Oparil), and the Merit Review program of the Department of Veterans Affairs. We also acknowledge support from Nestlé Food Co. for providing Stouffer’s Lean Cuisine entrées.
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- 1.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 3.Bush NC, Darnell BE, Oster RA, Goran MI, Gower BA. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54:2772–2778. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli J, Li W, Kishore P, et al. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53:1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- 5.Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 6.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 7.Fisher FF, Trujillo ME, Hanif W, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 8.Bodles A, Banga A, Rasouli N, et al. Pioglitazone increases secretion of high molecular weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006;291:E1100–E1105. doi: 10.1152/ajpendo.00187.2006. [DOI] [PubMed] [Google Scholar]
- 9.Bobbert T, Rochlitz H, Wegewitz U, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 10.Hammarstedt A, Sopasakis VR, Gogg S, Jansson PA, Smith U. Improved insulin sensitivity and adipose tissue dysregulation after short-term treatment with pioglitazone in non-diabetic, insulin-resistant subjects. Diabetologia. 2005;48:96–104. doi: 10.1007/s00125-004-1612-3. [DOI] [PubMed] [Google Scholar]
- 11.Halperin F, Beckman JA, Patti ME, et al. The role of total and high-molecular-weight complex of adiponectin in vascular function in offspring whose parents both had type 2 diabetes. Diabetologia. 2005;48:2147–2154. doi: 10.1007/s00125-005-1901-5. [DOI] [PubMed] [Google Scholar]
- 12.Sinha MK, Songer T, Xiao Q, et al. Analytical validation and biological evaluation of a high molecular-weight adiponectin ELISA. Clin Chem. 2007;53:2144–2151. doi: 10.1373/clinchem.2007.090670. [DOI] [PubMed] [Google Scholar]
- 13.Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi H, Ouchi N, Kihara S, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Schober F, Neumeier M, Weigert J, et al. Low molecular weight adiponectin negatively correlates with the waist circumference and monocytic IL-6 release. Biochem Biophys Res Commun. 2007;361:968–973. doi: 10.1016/j.bbrc.2007.07.106. [DOI] [PubMed] [Google Scholar]
- 17.van der Merwe MT, Crowther NJ, Schlaphoff GP, et al. Evidence for insulin resistance in black women from South Africa. Int J Obes Relat Metab Disord. 2000;24:1340–1346. doi: 10.1038/sj.ijo.0801416. [DOI] [PubMed] [Google Scholar]
- 18.Stein E, Kushner H, Gidding S, Falkner B. Plasma lipid concentrations in nondiabetic African American adults: associations with insulin resistance and the metabolic syndrome. Metabolism. 2007;56:954–960. doi: 10.1016/j.metabol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández JR, Shiver MD. Using genetic admixture to study the biology of obesity traits and to map genes in admixed populations. Nutr Rev. 2004;62:S69–S74. doi: 10.1111/j.1753-4887.2004.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 20.Gower BA, Fernández JR, Beasley TM, Shriver MD, Goran MI. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes. 2003;52:1047–1051. doi: 10.2337/diabetes.52.4.1047. [DOI] [PubMed] [Google Scholar]
- 21.Saad MF, Steil GM, Riad-Gabriel M, et al. Method of insulin administration has no effect on insulin sensitivity estimates from the insulin-modified minimal model protocol. Diabetes. 1997;46:2044–2048. doi: 10.2337/diab.46.12.2044. [DOI] [PubMed] [Google Scholar]
- 22.Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990;71:1508–1518. doi: 10.1210/jcem-71-6-1508. [DOI] [PubMed] [Google Scholar]
- 23.Akey JM, Sosnoski D, Parra E, et al. Melting curve analysis of SNPs (McSNP): a gel-free and inexpensive approach for SNP genotyping. Biotechniques. 2001;30:358–362. 364, 366–367. doi: 10.2144/01302tt05. [DOI] [PubMed] [Google Scholar]
- 24.Higgins PB, Fernández JR, Goran MI, Gower BA. Early ethnic difference in insulin-like growth factor-1 is associated with African genetic admixture. Pediatr Res. 2005;58:850–854. doi: 10.1203/01.PDR.0000182583.92130.08. [DOI] [PubMed] [Google Scholar]
- 25.Hanis CL, Chakraborty R, Ferrell RE, Schull WJ. Individual admixture estimates: disease associations and individual risk of diabetes and gallbladder disease among Mexican-Americans in Starr County, Texas. Am J Phys Anthropol. 1986;70:433–441. doi: 10.1002/ajpa.1330700404. [DOI] [PubMed] [Google Scholar]
- 26.Hada Y, Yamauchi T, Waki H, et al. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun. 2007;356:487–493. doi: 10.1016/j.bbrc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 28.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 29.Degawa-Yamauchi M, Dilts JR, Bovenkerk JE, et al. Lower serum adiponectin levels in African-American boys. Obes Res. 2003;11:1384–1390. doi: 10.1038/oby.2003.187. [DOI] [PubMed] [Google Scholar]
- 30.Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin levels. Metabolism. 2004;53:1–3. doi: 10.1016/j.metabol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Retnakaran R, Hanley A, et al. Total and High molecular weight (HMW) but not trimeric or hexameric forms of adiponectin correlate with markers of the metabolic syndrome and liver injury in Thai subjects. J Clin Endocrinol Metab. 2007;92:4313–4318. doi: 10.1210/jc.2007-0890. [DOI] [PubMed] [Google Scholar]
- 32.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149:2270–2282. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 34.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryo M, Nakamura T, Kihara S, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68:975–981. doi: 10.1253/circj.68.975. [DOI] [PubMed] [Google Scholar]
- 36.Swarbrick MM, Austrheim-Smith IT, Stanhope KL, et al. Circulating concentrations of high-molecular-weight adiponectin are increased following Roux-en-Y gastric bypass surgery. Diabetologia. 2006;49:2552–2558. doi: 10.1007/s00125-006-0452-8. [DOI] [PubMed] [Google Scholar]
- 37.Hara K, Horikoshi M, Yamauchi T, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 38.Nakashima R, Kamei N, Yamane K, et al. Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab. 2006;91:3873–3877. doi: 10.1210/jc.2006-1158. [DOI] [PubMed] [Google Scholar]
- 39.Retnakaran R, Hanley AJ, Connelly PW, et al. Low serum levels of high-molecular weight adiponectin in Indo-Asian women during pregnancy: evidence of ethnic variation in adiponectin isoform distribution. Diabetes Care. 2006;29:1377–1379. doi: 10.2337/dc06-0413. [DOI] [PubMed] [Google Scholar]
- 40.Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park KG, Park KS, Kim MJ, et al. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63:135–142. doi: 10.1016/j.diabres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Tschritter O, Fritsche A, Thamer C, et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]