Abstract
Background
Protein kinases represent one of the most promising groups of drug targets owing to their involvement in such pathological conditions as cancer, inflammatory diseases, neural disorders, and metabolism problems. In the last few years, numerous pharmaceutical and biotech companies have established kinase high-throughput screening (HTS) programs, and the reagent and service industries for kinase assay platforms, kits, and profiling services have begun to thrive.
Objective
The plethora of different assay formats available today poses a great challenge to scientists who want to select a technology that will be cost efficient, convenient to use, and have low false positive and false negative rates.
Methods
In the current review, we summarize the most commonly used kinase assay methods in the drug discovery process, present the advantages and disadvantages of each of these methods, and discuss the challenges of discovering kinase inhibitors by using these technologies.
Conclusions
The decision of selecting the assay formats for HTS or service platform for profiling should take into account not only the final goals of the screens but also the limitation of resources.
Keywords: drug discovery, drug profiling, drug screening, ELISA, fluorescence polarization, fluorescence resonance energy transfer, kinase assay, kinase profiling, luminescence assays, radioisotope filtration binding assay, time-resolved fluorescence resonance energy transfer
1. Introduction
Protein kinases play an integral role in many cell signaling pathways and comprise one of the largest families of homologous proteins, with ~ 518 members in the human kinome [1]. As such, protein kinases represent the largest ‘druggable’ gene family within the human genome [2]. Overexpression and/or dysregulation of protein kinases result in many diseases, thus providing numerous targets for drug development [3]. Since 2001, the FDA has approved nine kinase inhibitors for oncology targets (Table 1), and many more are now in clinical trials for the treatment of such diseases as cancer and cardiovascular and inflammatory diseases [4]. At present, ~ 24% of all research spending on drug discovery and development is focused on kinases [5]. Given the huge demand for small molecules that target this class of proteins, many technologies and platforms have been developed for discovering kinase inhibitors, including biochemical based functional assays and compound binding competition assays.
Table 1.
Approved kinase drugs: targets and indications.
| Compound | Trade name | Target | Diseases | Company | FDA approved |
|---|---|---|---|---|---|
| Imatinib (STI-571) | Gleevec ™ | Bcr-Abl, PDGFR, c-Kit | Philadelphia chromosome-positive CML [42]; Kit-positive unresectable/metastatic GIST [43] | Novartis | 2001, May |
| Gefitinib (ZD-1839) | Iressa ™ | EGFR | Locally advanced or metastatic NSCLC [44] | AstraZenica | 2003, May |
| Erlotinib (−358774, OSI-774) | Tarceva ™ | EGFR | Locally advanced or metastatic NSCLC [45] | OSI/DNA | 2004, November |
| Sorafenib (BAY-43-9006) | Nexavar ™ | VEGR2, 3; DGFRb; Kit, FLT3, Raf1, bRAF | Advanced RCC [46] | Onyx/Bayer | 2005, December |
| Sunitinib (SU11248) | Sutent ™ | VEGR1, 2, 3; DGFR a,b; Kit, FLT3, RET; CSF1R | RCC [47], imatinib-refractory GIST [48] | Pfizer | 2006, January |
| Dasatinib (BMS-354825) | Sprycel ™ | Src, Abl | CML [49], Philadelphia chromosome-positive acute lymphoblastic leukemia [50] | BMS | 2006, June |
| Lapatinib (GW572016) | Tykerb ™ | EGFR/HER2 | Advanced metastatic breast cancer in conjunction with the chemotherapy drug Xeloda ™ [51] | GSK | 2007, March |
| Temsirolimus (CCI-779) | Torisel ™ | mTOR | Advanced RCC [52] | Wyeth | 2007, May |
| Nilotinib (AMN-107) | Tasigna ™ | Bcr-Abl | Philadelphia chromosome-positive CML [53] | Novartis | 2007, October |
CML: Chronic myeloid leukemia; GIST: Gastrointestinal stromal tumor; GSK: GlaxoSmithKline; NSCLC: Non-small-cell lung cancer; RCC: Renal cell carcinoma.
Protein kinases are phosphoryl transferases that transfer the γ-phosphate of ATP to conserved serine, threonine, or tyrosine residues on specific substrate proteins. Classical methods to assay kinase activity involve the quantification of this phosphoryl transfer by detection of the production of the phosphorylated product or the change in the ratio of ATP to ADP. The traditional biochemical based method used to achieve this goal is the radioisotope filtration binding assay, in which the reactions are performed using radioisotope labeled γ-ATP. The incorporation of this radiolabeled phosphate into the kinase substrate is then assayed after a series of binding and washing steps to remove unincorporated radioisotope. This allows for the detection of kinase phosphoryl transfer activity, which is directly proportional to the amount of phosphorylated substrate. Despite the sensitivity and resolution of this assay, this method is not commonly used in a high-throughput screening (HTS) format owing to the drawbacks associated with the use of radioisotope materials, such as waste disposal, radiation safety, and the cumbersome washing and detection process.
To facilitate the HTS for kinase inhibitors, many convenient and automation friendly ‘mix and read’ assays have been recently developed that use fluorescence emission as a detection method. Technologies such as fluorescence intensity (FI), fluorescence polarization (FP), fluorescence resonance energy transfer (FRET), time-resolved fluorescence (TRF), time-resolved fluorescence resonance energy transfer (TR-FRET), and chemiluminescence offer the advantage of reagents that contain no radioactive materials. However, these new methods also pose new challenges in areas such as assay development, data analysis, and interpretation. In addition, these assays are susceptible to fluorescence interference caused by fluorescent compounds and fluorescently-labeled substrates, antibodies, and tracers [6–8]. More critically, owing to the modified reaction components and the variety of detection methods, assays with different formats for screening the same library and target could create strikingly different sets of inhibitors [9–16]. Therefore, extensive confirmation assays to validate hits are necessary to reduce the false positive and false negative rates. In addition to these common fluorescence and luminescence methods, there exist several special platforms that monitor the production of phosphorylated substrates or the binding of potential chemical inhibitors to target kinases. Such platforms include the scintillation proximity assay, ELISA, mobility shift assay, and protein binding assay. A common advantage of these assays is that there is little to no interference from compound or probe labeling [7,8,14].
The myriad of screening technologies have given scientists the freedom to pick the screening parameters that suit their individual drug discovery platforms or personal preferences. However, the plethora of assay formats also poses a great challenge to scientists who must decide on a technology that will be cost efficient and convenient to use for internal screening, as well as providing low false positive and false negative results. In the sections below, we will discuss the commonly used kinase assay methods along with their pros and cons in general drug screening applications. The common kinase profiling models used at present are also discussed in detail with reference to data reliability and how data obtained from kinase profiling may be used to guide the drug discovery process.
2. Radiometric based assays
Radiometric based assays are the most reliable methods for detecting kinase reactions; therefore, they are the preferred method for kinase profiling [8].
2.1 Filtration binding assay
The radiometric based filtration binding assay is considered the ‘gold standard’ to which non-radiometric methods are compared. In this assay format, the kinase reaction is performed in the presence of 32 P-γ-ATP or 33 P-γ-ATP, followed by binding of the final radioisotope labeled products to filters after which unreacted phosphate can be washed away without interfering with the detection of real phosphorylated products. The major advantage of this method is that it is a true universal kinase assay method which can be used for any kinase and substrate without limitations. This method does not require any special substrate labeling or modification, and detection is free from interference from compounds and unreacted radioisotope. However, the use of radioisotopes and the complex washing and separation steps present a major limitation to applying this technology for large-scale HTS. Nevertheless, filtration binding assays represent one of the most favorable assay methods for kinase profiling work, owing to its error-free detection. Both Reaction Biology Corporation’s HotSpotSM [17] and Millipore’s KinaseProfiler [18] use this technology for profiling large kinase panels. The HotSpotSM technology is a miniaturized kinase assay platform which reduces the consumption of radioisotope materials, kinase targets, substrates, and chemical compounds. As such, this technology is easily adaptable for use in HTS.
2.2 Scintillation proximity assay
The separation and washing steps used in the radiometric filtration binding assays limit its application in large-scale screenings. To circumvent the need for separation steps, GE and PerkinElmer have developed the scintillation proximity assay (SPA), which is a mix and read method similar to homogenous fluorescence-based detections. GE’s SPA assay is a homogenous system that uses microscopic beads containing a scintillant that can be stimulated by beta particles or auger electrons to emit light. This stimulation event occurs only when radiolabeled molecules of interest are bound to the surface of the beads, which results in the emission of light that can be detected using standard scintillation counters or with a CCD (charge coupled device) camera-based imaging instrumentation, such as the LEADseeker™ made by GE. Unbound radiolabeled molecules are not in close enough proximity to stimulate the bead to emit light. Therefore, the washing of unbound radiolabeled materials from the assay system is unnecessary. To reduce interference from intrinsic emission and absorption properties of chemical compounds used in the assays, GE has developed beads that emit red-shifted light [19]. PerkinElmer takes a different approach by offering two types of scintillant-coated microtiter plates, Scintiplates® and Flashplates®, for direct assays that eliminate the need to add scintillation cocktail as with bead-based assays. The interior of each well is permanently coated with a thin layer of polystyrene-based scintillant, which provides a platform for non-separation assays using a variety of isotopes (e.g., 3H, 125I, 14C, and 33P)[20]. ProQinase (Freiburg, Germany) uses the FlashPlate® technology for its 33 PanQinase® profiling services [21].
3. Fluorescence-based detection assays
Kinase assay formats that use fluorescence-based detection are the most widely used methods for HTS-based kinase drug discovery because they are automation friendly, easy to use, relatively low cost, and widely available. In this type of assay format, some of the more commonly applied techniques use FP, FRET, and TRF to identify lead compounds.
3.1 Fluorescence Intensity assays
FI is arguably the most common fluorescence-based method for detecting enzyme activity, and is widely used among assays that use protease-based detection reactions. However, to detect the transferase activity of a kinase using this assay, one or more coupling reactions must be used. One such FI-based method is to detect the production of ADP by using linked reactions involving pyruvate kinase, pyruvate oxidase, and horseradish peroxidase. After completion of the kinase reaction, pyruvate kinase is added to convert ADP to ATP and phosphoenolpyruvate to pyruvate. Subsequently, the pyruvate oxidase converts pyruvate to hydrogen peroxide (H2O2), which is then detected by using the fluorescent substrate Amplex Red (10-acetyl-3,7-dihydroxyphenoxazine) and horseradish peroxidase, resulting in the production of highly fluorescent resorufin [22]. This approach is commercially available from DiscoveRx as ADP Hunter™, which is a universal and sensitive assay. However, the multistep reactions involve many enzymes, which can complicate the hit conformation process. Another FI method is Promega’s ProFluor® approach, in which a rhodamine-110 fluorophore is conjugated with a peptide substrate. The non-phosphorylated peptide can be digested by a proprietary endopeptidase to release free fluorescent dye rhodamine-110, but the phosphorylated peptide is resistant to such digestion [23]. We have tested the ProFluor® PKA assay kit using the DisocoveryDot™ chemical microarray technology and found that the assay is very robust and sensitive, with faithful detection attained in a 1 nl reaction volume by using a CCD imager or DNA microarray scanner [24]. However, at present only a few assay kits are available for a few kinases, simply because it is difficult to develop enough peptide substrates that can be used by both the kinases and the special peptidase in the reaction.
3.2 Fluorescence polarization assays
FP, a technique that monitors molecular movement and rotation, is a widely employed detection method used in HTS for kinase inhibitors. The principle of this assay is that when excited with polarized light, a molecule with high molecular weight will have a slower rotational movement compared with a molecule with low molecular weight. Similarly, when a molecule is linked to a fluorescence tracer, the polarized fluorescent signal will be dominated by the size of the molecule. Fluorescence polarization (P) is defined by the following equation: P = (I|| − I⊥)//(I|| + I⊥)[25], where I|| is the emission intensity parallel to the excitation plane and I⊥ is the emission intensity perpendicular to the excitation plane of a fluorophore when excited by polarized light. The polarization value (P) is not dependent on the intensity of the emitted light or on the concentration of the fluorophore. When the fluorophore is free to rotate, the polarization is a smaller number, which is called low FP, and after the fluorophore binds to a larger molecule that limits the rotation of molecule, the polarization becomes a larger number, which is called high FP.
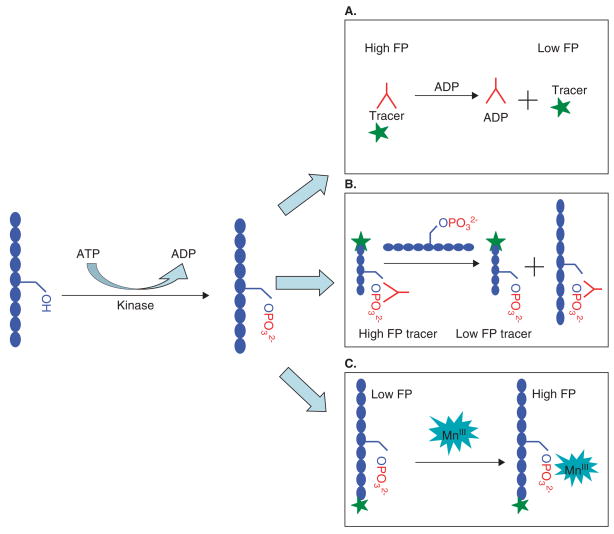
Many companies have developed assay kits that use this method, including BellBrook’s Transcreener™ FP assay, DiscoveRx’s HitHunter™ FP Assay, Invitrogen’s Far-Red PolarScreen™ FP Assay, Millipore’s KinEASE™ FP Assay, and Molecular Devices’ IMAP™ FP assay. This larger group of assays can be further divided into two groups based on the method used to monitor kinase activity. The first method, which is used in the Transcreener™ Kinase FP assay, uses an ADP-specific antibody to detect the conversion of ATP to ADP by the target kinase (Figure 1A). By contrast, the second method detects the production of phosphorylated substrates as in the HitHunter™, PolarScreen™, and KinEASE™ systems, which use substrate-specific antibodies (Figure 1B), and in the IMAP™ assay, which uses the high affinity of trivalent metal ions to phosphate (Figure 1C).
Figure 1. The common fluorescence polarization (FP) assays.
A. This approach (e.g., Transcreener™ Kinase FP assay) detects the change of ADP concentration by using a specific ADP antibody labeled with a fluorescence tracer. The ADP produced in the reaction will compete off the antibody from the tracer. B. This approach (e.g., HitHunter™, PolarScreen™, and KinEASE™ assays) requires an antibody that has a different binding affinity towards the real phosphorylated substrate product and the phosphorylated peptide tracer. C. The IMAP™ assay is a non-antibody approach that uses the high affinity of trivalent metal ions to bind the phosphate.
The advantages of the FP assay include a mix and read approach that is automation friendly, low cost of materials, and the availability of many assay formats. However, the cost of developing new assays is high, especially for the methods that use specific antibodies and tracers. Therefore, the approaches adapted by IMAP™ and Transcreener™ are more generally applicable to a variety of kinases. Generally speaking, the Transcreener™ method can also be used for other types of enzymes that generate ADP in the reaction systems.
Similar to other types of fluorescence-based detection methods (described later), FP detection can generate both false positive and false negative results because of the fluorescence interferences from fluorescent tracers, labeled substrates, and colored and fluorescence compounds. For example, Kashem et al. used the competition FP method to screen a 10,280 compound library against the IL-2 inducible T cell kinase and found nine florescent compounds that were false positive, six of which were also colored [16]. In an early study by Beasley et al.[11], many strong inhibitors generated from the FP-based assay were shown as weak inhibitors in the TRF and TR-FRET assays. The authors suggest that a few situations could cause the observed high mP values. The first was the low concentration of phosphorylated substrate and high concentration of antibody–tracer complex used in the assays; the second was the aggregation of fluorescent compounds interfering with the signal from the antibody–tracer complex; the third was that compound aggregation could increase light-scattering during detection or sequester the kinase or tracer. Invitrogen has studied the compound and tracer interference by mixing 10 μM of Sigma’s LOPAC1280 library compounds with 1 nM of green, red, and far-red tracers individually, and found that using green tracer produced the most number of compounds that could interfere with detection, with 1.5% compounds showing > 50% signal intensity. The red tracer followed with 0.7% compounds showing > 50% signal intensity and the far-red tracer had the least problems with 0.2% compounds showing > 50% signal intensity [26]. Based on this information, the Far-Red PolarScreen™ FP Assay may be a good choice for a library with a high incidence of fluorescent compounds.
3.3 Fluorescence resonance energy transfer
The FRET assay is another technology that is amenable to automation. This assay uses ‘donor’ and ‘acceptor’ fluorophores in proximity, with the excited donor fluorophore exhibiting non-radiative energy transfer to an acceptor fluorophore. The commercially available assays include Invitrogen’s Z-Lyte™ assay and Perkin Elmer’s AlphaScreen®.
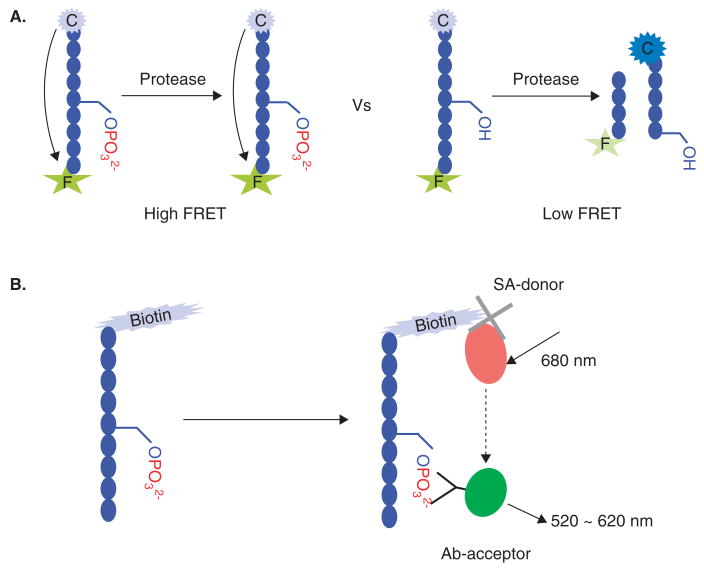
Invitrogen has developed a full panel of synthetic FRET-peptide substrates labeled with a donor (i.e., coumarin) and acceptor (i.e., fluorescein) for its Z-Lyte™ system. In a non-phosphorylated state, these peptides can be digested by a reporter protease, which will disrupt the FRET pair and release fluorescence (Figure 2A). A benefit of the Z-Lyte™ assay is its ratiometric method to quantify kinase reactions by calculating the ratio of donor emissions to acceptor emissions after excitation of the donor fluorophore at 400 nm. This method reduces the well-to-well variations in FRET-peptide concentration and signal intensities.
Figure 2. The common FRET based assays.
A. The Z-Lyte™ assay uses FRET-peptide as the substrate with donor (coumarin) and acceptor (fluorescein) fluorophores. In the reaction, a site-specific protease recognizes and cleaves the non-phosphorylated FRET-peptides to yield a high coumarin fluorescent signal. B. The AlphaScreen™ assay relies on the use of a donor and an acceptor bead that are coated with a layer of hydrogel for conjugating functional groups. The biotinylated substrate and antiphosphate antibody bring the donor and acceptor beads into close proximity (~ 200 nm); when excited by a laser the donor bead converts ambient oxygen to a more excited singlet state, and the singlet state oxygen molecules diffuse across to react with a chemiluminescer in the acceptor bead, which further activates fluorophores contained within the same bead.
Invitrogen uses this technology to provide both kinase profiling services [27] and individual assay kits [28]. We have tested many Z-Lyte™ kits on our DiscoveryDot™ chemical microarray-based assays, and many showed very good activities (HM, unpublished data). However, this method is not without disadvantages. The use of a protease in the detection reaction necessitates that inhibitory compounds be screened against the protease to exclude the possibility that they are protease inhibitors.
AlphaScreen™ uses a different approach by using a biotinylated kinase substrate together with the proprietary latex donor bead coated with streptavidin and acceptor bead linked with antiphosphate antibody. Upon laser excitation (680 nm), a photosensitizer in the donor converts ambient oxygen to the excited singlet state, which diffuses across to react with thioxene in the acceptor generating chemiluminescence at 370 nm. This in turn further activates fluorophores on the same acceptor bead to emit fluorescence signal at 520 – 620 nm (Figure 2B).
FRET assays are difficult to develop because the two fluorophores need to be within a closely defined distance for the energy to transfer. Another specific challenge for the Z-Lyte™ system is designing specific substrate sequences, so Invitrogen uses only a few common substrates for many kinases. Similarly, the production of specific antibodies against phosphorylated substrates, especially for antiphosphorylated serine/threonine peptides as in the AlphaScreen™ method, is very difficult.
3.4 Time-resolved fluorescence and time-resolved fluorescence resonance energy transfer
TRF uses fluorophores with long fluorescence decay time, and the fluorescence is monitored as a function of time after excitation by a flash of light. Lanthanide ions, such as europium, samarium, and terbium, are often used in this technology owing to their longer emission lifetimes (hundreds of microseconds versus several nanoseconds for conventional organic fluorophores). TRF detection, therefore, will reduce background fluorescence from chemical compounds and increase detection sensitivity. Perkin Elmer’s DELFIA® kits are such TRF-based assays; however, this specific assay involves additional substrate binding and separation steps for detection.
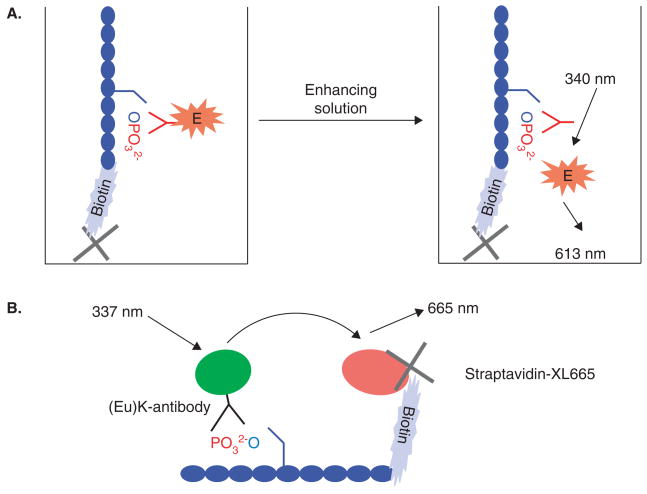
In DELFIA® assays, the kinase reactions are carried out in a homogeneous fashion with a biotinylated substrate, and the reaction mixture is then transferred into the capture plate coated with streptavidin. The plate is washed multiple times before europium-labeled antibody is added to detect phosphorylated substrates. An enhancement solution is then added to dissociate the europium ions into solution, which forms a highly fluorescent chelate with components from the enhancement solution (Figure 3A). The assay is sensitive and free of interference from compound fluorescence or fluorophore labeling. However, it may not be a time- and cost-efficient method owing to the multistep process of transferring, washing, developing, and detecting kinase activity. In addition, antibody selection and development is another issue for serine/threonine kinase assays. To combine TRF and FRET methods, many companies have created TR-FRET assay kits. In such kits, the typical donor is the long lifetime fluorophore europium and the acceptor is the fluorescent protein allophycocyanin (also known as APC or XL-665). Available products include PerkinElmer’s Lance® assay, CisBio’s homogeneous time-resolved fluorescence (HTRF®) KinEASE™, and Invitrogen’s LanthaScreen™.
Figure 3. The common TRF assays.
A. DELFIA® assay is very similar to ELISA-based assays but with a lanthanide-labeled antibody for detection. The lanthanide can be dissociated by a low pH enhancement solution and forms a stable fluorescent chelate inside a protective micelle in the enhancer solution. B. The HTRF assay is a combination of the TRF and FRET assays. The europium cryptate complex can transfer excitation energy to an acceptor molecule, XL665, when they are brought into close proximity with one another. The energy captured by the cryptate europium during excitation is transferred to the acceptor and then released as a FRET signal.
The Lance® assay uses europium chelate as the donor to link with antiphosphorylated biotinylated-substrate antibodies and uses straptavidin–allophycocyanin as the acceptor. In HTRF assays, the donor europium is in the cryptate form instead of chelate form, which contains a europium ion caged within a tris-bipyridine structure to improve reagent stability in acidic media and to avoid potential competing chelating activities from other bioreagents such as Mn2+. The donor and acceptor can be formed by using antibodies against either phosphorylated substrate (Figure 3B) or ADP. Invitrogen has used the same technology and developed the LanthaScreen™ reagent system, in which the terbium chelate replaces the europium chelate as donor and the small molecule fluorescein is used as the acceptor instead of streptavidin conjugated allophycocyanin. In these assays, the peptide is labeled with fluorescein directly. On completion of the kinase reaction, terbium-labeled antiphosphopeptide antibody is added for detection, with no need to add additional acceptor molecules. In this manner, the cost of reagents is reduced, whereas at the same time the speed of the FRET complex formation is increased.
4. Enzyme-linked immunosorbent assay
ELISA-based detection was widely used before fluorescence-based methods gained wide popularity. In this format, the substrate is captured by the membrane and detected by a specific antiphosphorylated substrate antibody. The chemical compounds are washed away before detection; therefore, the final fluorescent signal is not affected by the compound’s fluorescence. The detection can also be very sensitive when using antibodies labeled with highly fluorescent dyes. However, the requirement of separation with multiple washing steps limits ELISA’s use in HTS applications. In addition, the development of a specific antibody that recognizes serine/threonine kinase substrates presents another challenge. Reaction Biology Corporation has developed a chemical microarray-based ELISA method, DiscoveryDot™, for kinase HTS and profiling applications [29,30]. The drawbacks of ELISA assays are minimized or eliminated when HTS is conducted using DiscoveryDot™ technology, which reduces reagent consumption (compound, kinase, substrate, and detector reagents), eliminates compound interference, and allows automation of multiple wash steps [16]. Carna Biosciences (Kobe, Japan) is also offering tyrosine kinase profiling and assay kits with ELISA detection [31].
5. Luminescence detection
Fluorescence detection is clearly the dominant player in HTS; however, luminescence has also been adapted for a variety of assays in drug screening both in biochemical and in cell-based forms, mainly under the strong development by companies such as Promega [32], PerkinElmer, and DiscoveRx. Whereas the calcium sensitive aequorin protein from jelly fish is used widely in HTS of G protein-coupled receptors, the firefly protein luciferase is commonly used in HTS with ATP involved, such as in kinase assays (see the reaction scheme below). This enzyme converts the substrate luciferin into oxiluciferin, which releases a yellow-green photon of light with a spectral maximum of 560 nm. Promega’s Kinase-Glo™ detects kinase activity by measuring the depletion of ATP, making it similar to technologies that measure the production of ADP. This method is a universal biochemical assay that can be used for any combination of kinase and substrate, regardless of the nature of the substrate such as peptide, protein, sugar, and lipids. PerkinElmer’s ATPLite™ method uses the same approach. As observed for other assay formats that use additional enzymatic components for detection, the assays listed above that use luciferase require that inhibition of this reporter enzyme by small molecules must be considered. Reactions in the presence of luciferase inhibitors will produce no light, which can be easily recognized. However, small molecules that act as partial inhibitors for both luciferase and kinase could complicate the interpretation of observed inhibitory activity. The probability of encountering such compounds may be low, as Kashem et al. found that only three compounds in a 10,280 compound library inhibited luciferase activities > 30% [16].
6. Mobility shift assays
The mobility shift assay takes advantage of the fact that the phosphorylated peptide substrate is more negatively charged than the same substrate in an unphosphorylated state. Consequently, when a mixture of these peptides is introduced to electrophoresis, they have different mobilities.
Caliper Life Sciences has developed one of the early microfluidic chip-based assays by using this approach. In this approach, kinase reactions directly take place in the chip, and the reaction mixtures are then subjected to electrophoretic separation and detection (on-chip assay). Caliper has also introduced a second method that allows the reactions to be performed in conventional microtiter plates after which the reaction mixtures are sipped into the microfluidic chip for separation and detection (off-chip assay). In general, the on-chip format requires a high conversion rate (~ 20%) within a short reaction time, which may make the off-chip approach a better choice for the observation of enzyme kinetics within the linear range. One advantage of the off-chip assay is that the reaction mixture can be sipped and separated at different time points to give a real-time kinetic measurement. Similarly, Nanostream has developed a multichannel chromatography chip that can be used for separation and detection of kinase reactions [33].
Mobility shift detection is heavily dependent on the charge difference between the substrate and the product. As a result, assay development is focused on the sequence and size of the substrate used in the reaction. Kinase assays using proteins as substrate are difficult to apply. Dunne et al. performed a comparison study for the on-chip assay and off-chip assay. Using commercially available inhibitors and multiple kinases such as PKA, GSK3β, AKT1, they noticed that the two methods can identify inhibitors with a 70% overlap but the inhibition value for the on-chip assay was lower than that of the off-chip assay [34]. These results indicate that when running a HTS campaign with an on-chip-based method, a different inhibition cutoff for the follow-up assay may have to be considered to avoid losing too many potential hits. Similarly, the 30% difference in hit identification and confirmation could pose a great challenge for profiling work using this method, especially for compounds with solubility problems.
7. Ligand–kinase binding assay
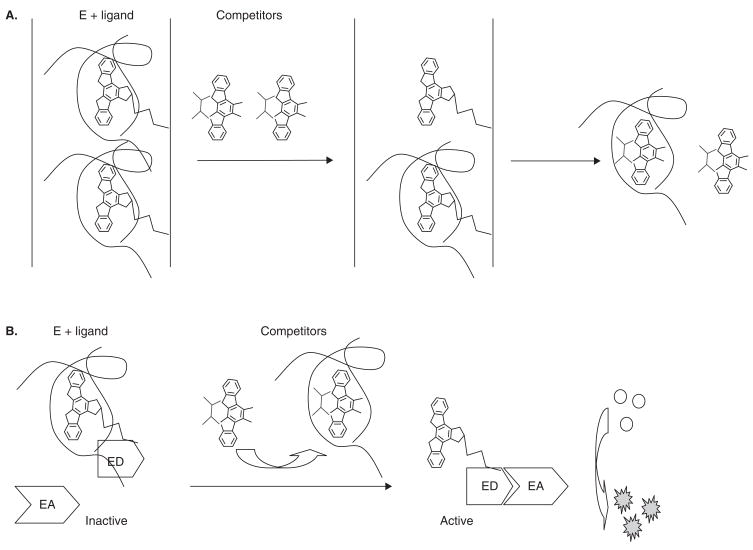
The functional based kinase assay using purified active enzymes is the dominant assay form for HTS and profiling work. However, assays that monitor the binding of kinase to substrate serve an important role for the identification and prediction of lead compounds, especially for purified kinases with low basal activity or no known biochemical function. The KinomeScan™ technology used by Ambit (San Diego, CA) is one of the most popular binding assay platforms [35], due in part to the large number of kinases offered in the service (over 350). The platform uses a competition-based assay (Figure 4A)[36,37] in which standard kinase inhibitors have been biotinylated and immobilized with Streptavidin-coated magnetic beads. The assay is then carried out by combining DNA-tagged kinases produced (either displayed on the surface of modified T7 phage or in cell culture), test compounds, and binding buffers. A test compound with a high binding affinity for the target kinase will compete the kinase away from the bead-bound biotinylated ligands, which will then be eluted and quantified by real-time quantitative PCR. A group of common inhibitors and drugs in clinical trials or approved by FDA have been tested using this platform, and their inhibition profiles are found to be similar to those other publications form [36,37].
Figure 4. The ligand–kinase binding assay.
A. In the KinomeScan™ binding assay, the kinase is first incubated with a standard kinase inhibitor that is biotinylated and immobilized with a streptavidin-coated magnetic bead. The new test compound is then added into this mixture, and it will replace the biotinylated kinase inhibitor if it is a stronger competitor. The displaced kinases can be then quantified by RT-PCR or other technologies. B. In the enzyme fragment complementation assay, the small peptide fragment (enzyme donor (ED)) is conjugated with a known ligand that binds with the kinase, a stronger kinase binder can displace the ligand. The free ED will then rapidly bind with the large protein fragment (enzyme acceptor (EA)) to form active β-galactosidase enzyme that hydrolyzes the substrate to produce an easily detectable chemiluminescent or fluorescent signal. The compound structures in this image are for illustration only.
Other than the KinomeScan™ technology, FP-based detection has also been used to screen inhibitors towards kinases, such as the HitHunter™ binding assay kit developed by DiscoveRx with its proprietary β-galactosidase enzyme fragment complementation technology. In this approach, standard kinase inhibitors (probes) are conjugated with an enzyme donor (ED), which retains its ability to form active β-galactosidase enzyme when complemented to the enzyme acceptor (EA) that catalyzes its substrate to produce an amplified chemiluminescent signal. In the presence of test compounds that bind tightly to the target kinase, the ED-probes bound with targets would be displaced from the kinase and complement with EA to form active β-galactosidase enzyme (Figure 4B). In a GSK3α assay, Vainshtein et al. demonstrated that the IC50 values generated with the HitHunter™ binding assay were comparable with the values produced using a radioisotope-based assay [38].
8. Expert opinion
The basic criteria for selecting an assay platform for HTS and profiling in kinase drug discovery processes is biased. For example, in HTS applications, the assay is optimized for a specific target kinase and as such should be robust, reliable, amenable for high-throughput, and low cost for that specific target. By contrast, kinase profiling work seeks to determine the specificity of a compound towards its targets by assaying the activity of the compound against a large panel of diverse kinases. As such, a profiling assay format should be acceptable for all kinases within the panel, and immune from interferences both from detection and compound. Therefore, of the assay formats discussed above, the homogeneous fluorescence-based and luminescent platforms are more suitable for HTS, whereas the radioisotope-based filtration binding assay becomes the choice for kinase profiling assays. The vast availability of fluorescence-based assays poses a great challenge for scientists who are trying to start a new program of drug screening because each assay has different requirements and may produce different sets of inhibitors from the same library with low overlap [9–16]. Therefore, a few platforms may have to be evaluated in parallel during the process of developing or selecting an assay for HTS. Kashem et al. have used three formats (DELFIA®, FP, and Kinase-Glo™ luminescence) to screen a kinase inhibitor focused library with 10,208 compounds for the IL-2 inducible T cell kinase and found that the three formats produced less than 60% common hits, with the Kinase-Glo™ method yielding the largest number of unique hits [16]. Even less hit overlap was observed when a 30,000 compound library was screened using three technologies (SPA, TR-FRET, and FP) by Sills et al.[9]. The reasons for the discrepancy among assays are also hard to determine because the capability to find hits involves many factors such as substrate sequence, the labeling of fluorescent probes, the conjugation of biotin/streptavidin beads, buffer composition, pH, enzyme quality, library quality, compound background, detection sensitivity, excitation range, reaction steps. Based on others’ and our own experiences, we strongly recommend that scientists test a few different assay formats for assay development and validation before making a final decision. For example, we have tested both FP (Echelon Biosciences, Inc., UT) and TR-FRET (Millipore, CA) assay kits for PI3K reactions and small-scale HTS and found that although both assays produced consistent IC50 values for control inhibitors such as wortmannin, PI3-Kγ Inhibitor (5-quinoxalin-6-ylmethylene-thiazolidine-2,4-dione), and PI-103 (3-(4-(4-morpholinyl) pyrido [3′,2′:4,5] furo [3,2-d] pyrimidin-2-yl) phenol), the TR-FRET kits produced more consistent data in day-to-day operation [39].
For biochemical functional based drug profiling and assay validation, radiometric based assays represent the preferred approach because they produce less false positives and false negatives. Hubert et al.[40] reported a 95% overlap of inhibitors after screening 30,000 compounds by using the radiometric filtration binding assay and antibody-based competition FP assay against the Rho-associated kinase II (ROCK-II). Similarly, Ahsen et al. compared the SPA and TR-FRET assays with a 300,000 compound library HTS against a tyrosine kinase and found that the correlation of the two technologies for hit discovery has an R2 value of more than 0.85. However, the TR-FRET assay had a 10% false positive rate compared with the 2.5% false positive rate in the SPA assay [14].
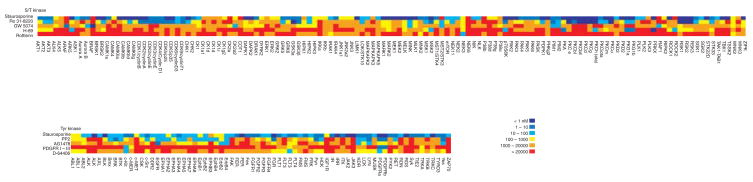
In addition to HTS to identify hits, kinase profiling to select specific inhibitors is another key application for kinase assay development. Because the ATP binding site is highly conserved across the human kinome, and most existing kinase inhibitors are ATP competitive inhibitors, it is essential to profile the lead drugs against a large panel of kinases to avoid any potential off-target activities. Indeed, screening to determine compound specificity has become a key component of the kinase drug discovery process. For this application, radiometric filtration binding assays become the choice for activity-based assays because it is universally applicable to all kinases, and as such is often referred to as the ‘gold standard’. However, even the direct detection of kinase activity assayed using radiometric methods does not guarantee the production of reliable data unless these assays are performed with carefully optimized assay conditions developed for each kinase. For example, the components and concentration of detergent, bovine serum albumin (BSA), and MnCl2 can all interfere with certain chemical scaffolds, and substrate selection can dramatically alter the inhibition profiles. The use of standard control inhibitors in IC50 mode for each kinase can help to reduce the discrepancy created by day-to-day operations; therefore, we have developed each kinase assay by running a group of common control inhibitors and have posted the results as a reference (Figure 5). The values from these control reactions will allow for the assessment of the quality of the data obtained for each target in each experimental run. Based on our experiences with profiling work, we believe that multi-layer controls such as these in each assay are essential.
Figure 5. The IC50 heatmap of common control kinase inhibitors against over 200 kinases.
All assays were performed with 1 μM ATP.
Other important considerations when choosing kinase profiling assays is the mode in which the profiling will be performed and the ATP concentration at which the reaction will be carried out. When testing a small number of compounds, we suggest that the IC50 mode should be the first choice, as this method yields more useful and reliable data. By contrast, if a large panel of compounds is to be tested, researchers may prefer to use a single dose (replicate) mode so as to manage profiling costs. When considering the ATP concentration at which to perform kinase profiling work, it is important to remember that assays performed at the Km value of ATP for a given kinase or a fixed value of ATP can both yield a valid indication of the potency of a group of compounds. However, the interpretation of data obtained using different ATP concentrations will require knowledge of the inhibition mode of a given class of compounds. For example, commonly studied ATP competitive inhibitors have an IC50 value equal to Ki(1+[S]/Km). As such, the IC50 value will increase with increasing ATP concentration; however, the Ki value should not change. Knowledge of the mode of inhibition of test compounds combined with the use of such equations makes it easy to interpret the inhibition data obtained from profiling performed at any ATP concentration. ATP concentration in cells is in the low millimolar range, yet most kinases assays are performed in the low micromolar range for finding inhibitors. In general, we suggest a relatively low ATP concentration when ‘casting a large net’ to identify unwanted off-target effects or when seeking a new potential target for some known compounds. Conversely, a relatively high ATP concentration would be used when seeking to determine the specificity of a given compound towards a more focused group of targets. Ligand binding-based assays may not be affected by ATP concentrations; however, it should be noted that the binding affinity data generated from binding assays and IC50 values generated from functional based assays may be distinct from each other because of the nature of the assay platforms [41]. Therefore, the Kd values and IC50 values using these approaches should not be compared directly without understanding the reaction conditions in each assay.
The overall cost of profiling lead compounds against a large number of kinases is a driving factor in the decision making process of when to profile and with which set of compounds. It is our opinion that if the profiling is performed in the early stage of structure–activity relationship (SAR) studies, the lead optimization process could be moved forward at a faster rate than performing the profiling in the later SAR stage, which is the major practice for most companies. Researchers considering kinase profiling should also note that advances in the techniques discussed in this review make it possible to profile a very large number of compounds (over 10,000) using low-volume fluorescence-based assays such as HTRF and FRET or the low-cost radioisotope filtration binding assays such as the HotSpotSM technology. The ability to profile such a large number of compounds can greatly aid SAR studies and speed up the lead optimization process.
In conclusion, the major forms of kinase assay methods and technologies along with their advantages and challenges are discussed and further summarized in Table 2. Although there is no single technology that is sufficient to satisfy all kinase drug discovery needs, the good news is that it should always be possible to find a suitable assay format for specific needs and targets in the drug development process.
Table 2.
Summary of the common assays.
| Assay platform | Assay name | Mechanism of detection | Advantages | Limitations |
|---|---|---|---|---|
| Biochemical assays | ||||
| Radioisotope-based assay | ||||
| Filtration binding | Reaction Biology Corp.’s HotSpot SM and Millipore’s KinaseProfiler ™ |
Direct measurement of phosphorylated substrate | The ‘gold standard’ of detection kinase activity HotSpot SM is a miniaturized format to reduce cost |
Radioisotope materials Multi-washing steps |
| SPA | GE’s bead-based SPA assay | Streptavidin conjugated scintillant-SPA bead | No interference from compounds and reagents Homogeneous assay |
Radioisotope materials Indirect measurement of phosphorylated substrate |
| PerkinElmer’s Flashplates ® SPA assay |
Scintillant-coated plate | No interference from compounds and reagents No need to add scintillant cocktail |
Radioisotope materials Indirect measurement of phosphorylated substrate |
|
| FI | ||||
| Promega ProFluor ® | Peptidase-coupled detection | Automation friendly Instrument friendly |
Not universal Hard to design the substrate for the endoprotease Interference from peptidase inhibitors |
|
| DiscoveRx’s ADP Hunter ™ | Pyruvate kinase/pyruvate oxidase converting ADP to hydrogen peroxide, which is detected using a fluorescent substrate, Amplex Red and peroxidase, by producing resorufin fluor | Universal assay to detect ADP production Automation friendly Instrument friendly |
Multistep assays involving many additional enzymes for detection Possible inhibitor for other enzymes |
|
| FP | ||||
| BellBrook’s Transcreener ™ | ADP competes with fluorescence tracer labeled anti-ADP antibody | Universal assay to detect ADP production |
ADP competition assay Not suitable for high ATP concentration Multi-control reactions |
|
| Molecular Devices’ IMAP ™ | Using high affinity of trivalent metal ions to binding phosphate | Mix and read approach No antibody needed |
Requires specifically designed substrate ATP concentration needs to be optimized Needs more than 5% conversion rate |
|
| DiscoveRx’s HitHunter ™ | Antibody binding the phosphorylated substrate | Mix and read approach Red-shift tracer to reduce compound interference |
Requires special antibody pair Requires extended antibody incubation for detection Not suitable for high ATP concentration |
|
| Invitrogen’s Far-Red PolarScreen ™ |
Antibody binding the phosphorylated substrate | Mix and read approach Red-shift tracer to reduce compound interference |
Requires special antibody pair Requires extended antibody incubation for detection |
|
| Millipore’s KinEASE ™ | Antibody binding the phosphorylated substrate | Mix and read approach Red-shift tracer to reduce compound interference |
Requires special antibody pair Requires extended antibody incubation for detection |
|
| FRET | ||||
| Invitrogen’s Z-Lyte ™ | Protease-coupled detection | Widely available kits for many kinases Robust reactions |
Two-step assay May have to use pseudo-substrate Possible inhibition of proteases |
|
| Perkin Elmer’s AlphaScreen ® | Antiphosphate antibody linked acceptor bead Biotinylated substrate and streptavidin-coated latex donor beads |
Mix and read approach Long excitation and shorter emission reduces inner filter effect with low background |
Requires special antibody pair Requires extended antibody incubation for detection |
|
| TRF | ||||
| Perkin Elmer’s DELFIA ® | Biotin substrate Europium-labeled antibody Streptavidin-coated plate |
Sensitive assay Lees or no interference from compound |
Requires separation and washing steps | |
| TR-FRET and HTRF | ||||
| PerkinElmer’s Lance ® | Europium chelate-donor linked with anti-phosphate antibody Biotinylated substrate Streptavidin–allophycocyanin as acceptor |
Sensitive assay Less interference from compound |
Requires special antibody Chelated donor is less stable in low pH condition and sensitive to Mn 2+ |
|
| Invitrogen’s LanthaScreen ™ | Terbium-chelated donor with anti-phosphate antibody Fluorescein-conjugated substrate as acceptor |
Less reagent is used Faster FRET formation Detection friendly |
Requires special antibody | |
| BellBrook’s Transcreener ™ | Lanthanide donor conjugated with anti-ADP antibody Small molecule fluorescein as acceptor (ADP FAM tracer) |
Universal assay by detection of ADP production Used as real-time assay or end-point assay |
Not suitable for high ATP concentration Multi-control reactions |
|
| CisBio’s HTRF ® | Europium-cryptated donor Anti-phosphate or anti-ADP antibodies |
Europium-cryptated donor is very stable Assay is less affected by reaction condition or reagents |
Requires special antibody | |
| Luminescence | ||||
| Promega’s Kinase-Glo ™ | Measures the depletion of ATP by using luciferase as detection enzyme | Sensitive No fluorescence interference Mix and read |
Two-step assay Possible inhibition of luciferase |
|
| DiscoveRx’s HitHunter ™ | Enzyme fragment complementation assay using anti-phosphate antibody | Sensitive No fluorescence interference |
Needs special antibody Extended incubation required for detection Not suitable for high ATP concentration |
|
| Mobility shift assays | ||||
| Caliper chip-based platform | On-chip- or off-chip-based assay Chip-based separation |
Accurate detection Automation friendly |
Needs special substrate development Relatively expensive |
|
| Nanostream’s LD system | Off-chip based assay, parallel micro-liquid chromatography separation | Accurate detection Automated separation |
A separation and detection tool Not an assay system |
|
| ELISA | ||||
| Self-assemble | Anti-phosphate antibody | No compound interference Reaction product detection |
Requires special antibody Multi-washing and detection steps |
|
| Binding-based assay (non-biochemical based assays) | ||||
| Ambit’s KinomeScan ™ | Ligand competition assay | With broad kinases available | Requires special conjugate ligands Only competes with known ligands As a service product only |
|
| DiscoveRx’s HitHunter ™ | Ligand competition assay | Relatively simple to perform | Only a few assays available Hard to create binding probe to fit into the enzyme fragment complementation format |
|
FI: Fluorescence intensity; FP: Fluorescence polarization; FRET: Fluorescence resonance energy transfer; HTRF: Homogeneous time-resolved fluorescence; SPA: Scintillation proximity assay; TRF: Time-resolved fluorescence; TR-FRET: Time-resolved fluorescence resonance energy transfer.
Acknowledgments
Declaration of interest
This work is partially supported by NIH grants RO1HG003818, R44CA114995 and R44DE017485 to HM.
The authors thank Y Wang, S Liang, W Xu, D Barninger for performing the kinases assays, and MM Eason for the preparation of Figure 5.
Bibliography
- 1.Manning G, Whyte DB, Martinez R, et al. Protein kinase complement of the human genome. Sciences. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Disc. 2002;9:727–30. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 3.Cohen P. Protein kinases: the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–15. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 4.Vieth M, Sutherland JJ, Robertson DH, Campbell RM. Kinomics: characterizing the therapeutically validated kinase space. Drug Discov Today. 2005;10:839–46. doi: 10.1016/S1359-6446(05)03477-X. [DOI] [PubMed] [Google Scholar]
- 5.Fox S, editor. High-throughput screening: new strategies and technologies. Moraga, CA: High-Tech Business Decisions; 2002. [Google Scholar]
- 6.Hemmila IA, Hurskainen P. Novel detection stratagies for drug discovery. Drug Discov Today. 2002;7:150–6. doi: 10.1016/s1359-6446(02)02390-5. [DOI] [PubMed] [Google Scholar]
- 7.Olive DM. Quantitative methods for the analysis of protein phosphorylation in drug development. Expert Rev Proteomics. 2004;1:327–41. doi: 10.1586/14789450.1.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Comely J. Kinase screening and profiling – spoilt for choice. Available from: http://www.ddw-online.com/data/pdfs/2006kinase%20screening.pdf.
- 9.Sills MA, Weiss D, Pham Q, et al. Comparison of assays technologies for a tyrosine kinase assay generates different results in high throughput screening. J Biomol Screen. 2002;7:191–9. doi: 10.1177/108705710200700304. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Glickman JF, Bowen BR, Sills MA. Comparison of assay technologies for a nuclear receptor assay screen reveals differences in the sets of identified functional antagonists. J Biomol Screen. 2003;8:381–92. doi: 10.1177/1087057103256466. [DOI] [PubMed] [Google Scholar]
- 11.Beasley JR, McCoy PM, Walker TL, Dunn DA. Miniaturization, ultra-high throughput screening of tyrosine kinase using homogeneous, competitive fluorescence immunoassays. Assay Drug Dev Technol. 2004;2:141–52. doi: 10.1089/154065804323056486. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JH, Wu X, Sills MA. Probing the primary screening efficiency by multiple replicate testing: a quantitative analysis of hit confirmation and false screening results of a biochemical assay. J Biomol Screen. 2005;10:695–704. doi: 10.1177/1087057105279149. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Sills MA, Zhang JH. Further comparison of primary hit identification by different assay technologies and effects of assay measurement variability. J Biomol Screen. 2005;9:581–9. doi: 10.1177/1087057105275628. [DOI] [PubMed] [Google Scholar]
- 14.Ahsen OV, Schmidt A, Klotz M, Parczyk K. Assay concordance between SPA and TR-FRET in high-throughput screening. J Biomol Screen. 2006;11:606–16. doi: 10.1177/1087057106288183. [DOI] [PubMed] [Google Scholar]
- 15.Quercia AK, Lamarr WA, Myung J, et al. High-throughput screening by mass spectrometry: comparison with the scintillation proximity assay with a focused-file screen of AKT1/PKB. J Biomol Screen. 2007;12:473–80. doi: 10.1177/1087057107300647. [DOI] [PubMed] [Google Scholar]
- 16.Kashem MA, Nelson RM, Yingling JD, et al. Three mechanistically distinct kinase assay compared: measurement of intrinsic ATPase activity identified the most comprehensives set of ITK inhibitors. J Biomol Screen. 2007;12:70–83. doi: 10.1177/1087057106296047. [DOI] [PubMed] [Google Scholar]
- 17.Available from: http://reactionbiology.com/pages/kinase.htm
- 18.Available from: http://www.millipore.com/drugdiscovery/dd2/kinasetarget
- 19.Available from: http://www.rcxg.mdlc.com/aptrix/upp01077.nsf/Content/Products?OpenDocument&parentid=658513&moduleid=166937
- 20.Available from: http://las.perkinelmer.com/Catalog/default.htm?CategoryID=Scintillation+Proximity+Assay+%5BSPA%5D
- 21.Available from: http://www.proqinase.com/pages/service/p2_1_4.html
- 22.Charter NW, Kauffman L, Singh R, Eglen RM. A generic, homogenous method for measuring kinase and inhibitor activity via adenosine 5′-diphosphate accumulation. J Biomol Screen. 2006;11(4):390–9. doi: 10.1177/1087057106286829. [Epub 28 Apr 2006] [DOI] [PubMed] [Google Scholar]
- 23.Available from: http://www.promega.com/paguide/chap7.pdf
- 24.Ma H, Horiuchi KY, Wang Y, et al. Nanoliter homogeneous ultra-high throughput screening microarray for lead discoveries and IC50 profiling. Assay Drug Dev Technol. 2005;3:177–87. doi: 10.1089/adt.2005.3.177. [DOI] [PubMed] [Google Scholar]
- 25.Engelman JA, Luo J, Cantley LC. The evolution of Phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 26.Available from: http://www.invitrogen.com/downloads/Redder_formated_poster.pdf
- 27.Available from: http://www.invitrogen.com/content.cfm?pageid=10413#selection
- 28.Available from: http://www.invitrogen.com/content.cfm?pageid=9866
- 29.Horiuchi KY, Wang Y, Diamond SL, Ma H. Microarrays for the functional analysis of the chemical kinase interactome. J Biomol Screen. 2006;11:48–56. doi: 10.1177/1087057105282097. [DOI] [PubMed] [Google Scholar]
- 30.Ma H, Horiuchi KY. Chemical microarray: a new tool for drug screening and discovery. Drug Discov Today. 2006;11:661–8. doi: 10.1016/j.drudis.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Available from: http://www.carnabio.com/english/product/assay.html
- 32.Fan F, Wood KV. Bioluminescent assays for high-throughput screening. Assay Drug Dev Technol. 2007;5:127–36. doi: 10.1089/adt.2006.053. [DOI] [PubMed] [Google Scholar]
- 33.Available from: http://www.nanostream.com/support/downloads.html
- 34.Dunne J, Reardon H, Trinh V, et al. Comparison of on-chip and off-chip microfluidic kinase assay formats. Assay Drug Dev Technol. 2004;2:121–9. doi: 10.1089/154065804323056468. [DOI] [PubMed] [Google Scholar]
- 35.Available from: http://www.ambitbio.com/technology/
- 36.Fabian MA, Biggs WH, III, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–36. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 37.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitors selectivity. Nat Biotechnol. 2007;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 38.Vainshtein I, Silveria S, Kaul P, et al. A high-throughput, nonisotopic, competitive binding assay For kinases using nonselective inhibitor probes (ED-NSIP™) J Biomol Screen. 2002;6:497–504. doi: 10.1177/1087057102238624. [DOI] [PubMed] [Google Scholar]
- 39.Horiuchi KY, Ma H. Fluorescence polarization and time-resolved fluorescence resonance energy transfer techniques for PI3K assays. In: Roque Ana Cecilia., editor. Ligand-macromolecule interactions in drug discovery. The Humana Press, Inc.; 2008. [Google Scholar]
- 40.Hubert CL, Sherling SE, Johnston PA, Stancato LF. Data concordance from a comparison between filter binding and fluorescence polarization assay formats for identification of ROCK-II inhibitors. J Biomol Screen. 2003;8:399–409. doi: 10.1177/1087057103255071. [DOI] [PubMed] [Google Scholar]
- 41.Buolamwini JK, Kamath S. Molecular-kinase interaction map, Opinion and comment section. Nat Biotechnol. 2005;11:1346–8. doi: 10.1038/nbt1105-1346a. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 43.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–6. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 44.Cella D, Herbst RS, Lynch TJ, et al. Clinically meaningful improvement in symptoms and quality of life for patients with non-small cell lung cancer receiving gefitinib in a randomized controlled trial. J Clin Oncol. 2005;23:2946–54. doi: 10.1200/JCO.2005.05.153. [DOI] [PubMed] [Google Scholar]
- 45.Shepherd FA, Pereira J, Ciuleanu TE, et al. A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) trial. 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) J Clin Oncol. 2004;22(14S Suppl):7022. [Google Scholar]
- 46.Ratain MJ, Flaherty KT, Stadler WM, et al. Preliminary antitumor activity of BAY 43–9006 in metastatic renal cell carcinomaand other advanced refractory solid tumors in a phase II randomized discontinuation trial (RDT). ASCO Proceedings. J Clin Oncol. 2004;23:A4501. [Google Scholar]
- 47.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 48.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 49.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant philadelphia chromosome–positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 50.Shah N, Sawyers CL, Kantarjian HM, et al. Correlation of clinical response to BMS-354825 with BCR-ABL mutation status in imatinib-resistant patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-associated acute lymphoblastic leukemia (Ph+ ALL). 2005 ASCO Annual Meeting Proceedings. J Clin Oncol. 2005;23(16S Suppl Pt I of II):565s . [Abstract 6521] [Google Scholar]
- 51.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 52.Fazio N, Dettori M, Lorizzo K, et al. Temsirolimus for advanced renal-cell carcinoma. N Engl J Med. 2007;357:1050–10. doi: 10.1056/NEJMc071868. [DOI] [PubMed] [Google Scholar]
- 53.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive AL. N Engl J Med. 354:2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]