Abstract
AIM: To investigate the potential effects of chelidonine, the main alkaloid of Chelidonium majus, on telomerase activity and its regulation in HepG2 cells.
METHODS: Cytotoxicity of chelidonine for HepG2 cells was determined by neutral red assay. A modified polymerase chain reaction (PCR)-based telomerase repeat amplification protocol was used to estimate relative telomerase activity in chelidonine-treated cells in comparison with the untreated control cells. Relative expression level of the catalytic subunit of telomerase (hTERT) gene and P-glycoprotein (pgp) were estimated using semi-quantitative real-time reverse transcription-PCR (RT-PCR). Cell senescence in treated cells was demonstrated using a β-galactosidase test.
RESULTS: Cytotoxicity of chelidonine in HepG2 cells was not dose-dependent and tended to reach plateau immediately after the living cells were reduced in number to slightly higher than 50%. However, 12 μmol/L concentration of chelidonine was considered as LD50, where the maximal attainable effects were realized. Real-time RT-PCR data showed that the expression of pgp increased three-fold in chelidonine treated HepG2 cells in comparison with the untreated controls. Morphologically, treated HepG2 cells showed apoptotic features after 24 h and a small fraction of cells appeared with single blister cell death. The relative expression level of Bcl-2 dropped to less than 50% of control cells at a sub-apoptotic concentration of chelidonine and subsequently increased to higher than 120% at LD50. Telomerase activity was reduced considerably after administration of very low doses of chelidonine, whereas higher concentrations of chelidonine did not remarkably enhance the effect. Real-time RT-PCR experiments indicated a drastic decrease in expression level of hTERT subunit of telomerase under treatment with chelidonine. Repeated treatment of cells with very low doses of chelidonine caused a decline in growth rate by 4 wk and many of the cells appeared to be aged with large volume and dark staining in the β-galactosidase assay.
CONCLUSION: Chelidonine reduces telomerase activity through down-regulation of hTERT expression. Senescence induction might not be directly caused by reducing telomerase activity as it occurs after a few population doublings.
Keywords: Chelidonine, Telomerase, Inhibition, Apoptosis, Senescence
INTRODUCTION
Chelidonium majus (C. majus), the greater celandine (family Papaveraceae), has a long history in phytomedicine for the treatment of many diseases or health disturbances. It contains isoquinoline alkaloids, especially protoberberine and benzophenanthridine alkaloids[1]. Extracts of this plant exhibit interesting antitumor and antiviral activities[2] in addition to hepato-protective and anti-genotoxic effects in induced hepatocarcinogenesis in mice[3]. Ukrain, a semisynthetic derivative of C. majus alkaloids has been reported to be selectively toxic to malignant cells without toxic side effects to normal cells[4,5]. Ukrain has been used in the therapy of several solid tumors. It is known to induce apoptosis in glioblastoma cells[6] and in cell cultures[7]. Data from randomized clinical trials based on numerous pre-clinical and clinical investigations has established Ukrain as an anticancer drug[8].
Chelidonine, the tertiary hexahydro-benzophenanthridine alkaloid, is the major component of Ukrain. It primarily causes apoptosis in primary human uveal melanoma cell lines as shown by cellular morphology studies and flow cytometric analysis[9]. This alkaloid arrests mitosis by inhibition of tubulin polymerisation and activation of the stress activated protein kinase/jun kinase pathway (SAPK/JNK)[10]. A mitochondrial cell death pathway induced by chelidonine in MT-4 cells has also been realized with an increased ratio of cytosolic to mitochondrial cytochrome c and increased percentage of cells with active caspase-3 and pro-apoptotic Bax protein[11].
In this communication telomerase activity has been investigated in HepG2 cells after treatment with chelidonine, using a modified telomerase repeat amplification protocol (TRAP). Telomerase, basically a reverse transcriptase, compensates telomere attrition, which occur in proliferating cells due to incomplete genome replication by DNA polymerases. This enzyme, while detectable, is diminished and shows very low activities in many adult somatic cells but is drastically overexpressed in almost 90% of different kinds of human cancer cells by up to 100-fold over the normal counterpart cells[12], and confers a strong selective advantage for continued growth of malignant cells[13]. This enzyme is considered as a key player in cell immortality[14] as it protects chromosome ends from degradation and also plays an important role in the telomere-capping function[15]. It has been proposed that telomerase inhibitors can be utilized as complementary drugs in cancer therapy[16,17]. Critically shortened telomere(s) induce proliferative senescence, apoptosis or continued proliferation accompanied by genomic instability[18]. Many studies have indicated that inhibition of telomerase can affect the survival of cancer cells through apoptosis induction without long-term treatment of the cells[19,20].
There is currently much interest in the inhibition of telomerase by various strategies such as antisense oligonucleotides[21], small-molecule inhibitors[22], ribozymes[23] and RNA interference[24], which in turn initiate the onset of growth inhibition and antitumor activity.
This study was focused mainly on the effect of chelidonine on telomerase activity and its regulation. The focus on drugs such as natural benzophenanthridines that may possibly inhibit telomerase may help in use of these alkaloids specifically against cancer. The expression levels of the catalytic subunit of telomerase (hTERT) gene and morphological changes were studied as well. Expression of Bcl-2, the related gene to suppress apoptosis, P-glycoprotein (pgp) involved in drug resistance, and β-galactosidase assay as a biomarker to identify cell senescence were also analyzed.
MATERIALS AND METHODS
Cell culture
HepG2 cell line (ACC 180 from DSMZ) was maintained in 75 cm2 culture flasks in RPMI-1640 culture medium (Gibco, Invitrogen), supplemented with 10% heat-inactivated fetal bovine serum (Biochrome, Berlin, Germany), 100 000 U/L penicillin, and 100 mg/L streptomycin. Cells were grown in 5% CO2 and 95% air atmosphere at 37°C and 100% humidity. Media were changed every 48 h, and the cells were subcultured after 5-6 d using trypsin-EDTA. Cell viability was evaluated by staining with 0.1% trypan blue exclusion method using a hemocytometer. To estimate the cytotoxicity of chelidonine a neutral red uptake test was employed[25].
For long-term growth of HepG2 cells, chelidonine-treated or untreated cells were seeded into 75 cm2 tissue culture flask at 1 × 106 for 4-5 d until 70%-80% confluence, then trypsinized and counted. Each time 1 × 106 cells were replated into new culture flasks but in each passage the chelidonine series was treated at the desired concentration for 48 h and afterward cells were fed with fresh normal medium. The rest of the cells in each passage were plated for β-galactosidase assay. All the treatments were done in duplicate.
Cytotoxicity test
Exponentially growing HepG2 cells were seeded in 96 well plates with 10 000 cells per well. The medium was changed after 24 h with the fresh medium plus various concentrations of chelidonine. After 48 h the cells were washed with PBS and incubated in fresh medium including 50 mg/L neutral red. The medium was removed after 3 h incubation and 200 μL isopropanol containing 0.4 mol/L hydrochloric acid was added to each well and shaken for 15 min at room temperature. The absorbance of neutral red was measured at 570 nm. The test was repeated four times each in triplicate and the 50% lethal dosage, LD50, values were calculated from dose-response curves.
Assay of telomerase activity
Subconfluent HepG2 cells were seeded in six well plates with 100 000 cells per well and incubated for 24 h at 37°C, in 5% CO2 and 100% humidity. The media were then changed for fresh ones containing various concentrations of chelidonine and followed by 48 h incubation. After trypsinizing and washing with cold PBS, lysis buffer was added to the cell pellet according to Gelmini’s method with small modifications[26]. Cell lysates were incubated on ice for 30 min and then centrifuged at 16 000 g for 30 min. The protein concentration of the supernatant was measured based on the Bradford assay[27]. For each of control and/or treated cells 0.5 μg of extracted total protein was used for TRAP assay based on Gelmini’s method[26]. Telomerase activity was determined for each concentration of chelidonine in at least three repetitions, each one in duplicate. Two controls were used: a positive control using cell extract containing 0.5 μg protein of untreated HepG2 cells, and a negative control which was the same as the positive control but treated with boiling heat and/or RNase A at 37°C for 20 min.
RNA isolation, reverse transcription and real-time RT- PCR
HepG2 cells were seeded in 12-well plate culture dishes with 50 000 cells per well. After 24 h incubation the medium was replaced with fresh medium including various concentrations of chelidonine lower than the LD50 and incubated for another 48 h. For RNA extraction, the cells were harvested and total RNA was isolated using RNeasy Mini Kit (Qiagen; Germany) according to the manufacturer’s instruction and treated with DNaseI (Rnase-Free DNase Set, Qiagen; Germany) and stored at -80°C. Purity and quality of the extracted RNA were checked by gel electrophoresis, and the concentrations were measured using UV spectrophotometry.
First strand cDNA synthesis was performed according to the protocol suggested for the Reverse Transcription System (Promega Corporation; Madison, USA) using oligo(dT)16 primers (Pharmacia Biotech; Uppsala, Sweden). Quantification of mRNA levels of the genes was achieved by quantitative real-time RT-PCR (Light Cycler, Roche Applied Science; Mannheim, Germany) using β2-microglobulin as the housekeeping gene. PCR amplification was carried out in 20 μL reaction volume containing 0.2 μg of cDNA, 1 × Light Cycler Fast Start DNA Master SYBR Green I (Roche Applied Science; Mannheim, Germany), 0.5 μmol/L of each primer set. MgCl2 concentration was 3.25 mmol/L for β2-microglobulin gene and 3-4 mmol/L for other genes. The sequence of the primer sets used was: forward 5' GCTGCTCAGGTCTTTCTTTTATGTC3' and reverse 5'TCAAGTGCTGTCTGATTCCAATG3' for hTERT, forward 5'AGGAAGTGAACATTTCGGTGAC3' and reverse 5'GCTCAGTTCCAGGACCAGGC3' for Bcl-2. Primers used for β2-microglobulin and pgp (MDR1) were chosen as described by Albermann et al[28]. Data analysis was performed using a calibrator and a standard curve for each gene and based on measurements of at least two repetitions. Each repetition includes two measurements for each of the duplicated samples for the defined concentrations of chelidonine.
β-galactosidase assay
After removing medium, control and treated HepG2 cells with chelidonine were washed in PBS, fixed for 3-5 min at room temperature in 2% formaldehyde/0.2% glutaraldehyde. Cells were washed twice with PBS and incubated at 37°C (no CO2) with fresh senescence-associated β-gal (SA-β-gal) stain solution containing 0.4 g/L X-gal, 40 mmol/L citric acid/sodium phosphate, pH 6.0, 5 mmol/L potassium ferrocyanide, 5 mmol/L potassium ferrocyanide, 150 mmol/L NaCl and 2 mmol/L MgCl2 as described by Dimri et al[29]. Staining was achieved in 2-4 h.
DNA ladder formation
The cells were treated with chelidonine at the specified concentrations for 48 h and harvested; cellular DNA was isolated and subjected to agarose gel electrophoresis followed by visualization of bands.
RESULTS
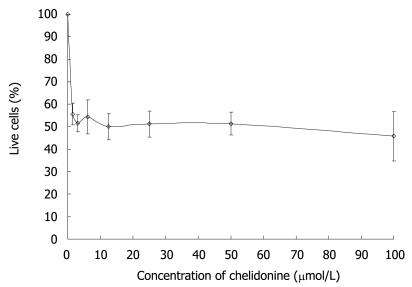
The effect of chelidonine on HepG2 cell survival tested with neutral red uptake assays after 48 h treatment is shown in Figure 1. The cell viability curve of HepG2 cells under chelidonine treatment had two distinct parts with quite different slopes. The first part with a sharp decline indicated its high toxicity, even at very low concentrations. The second part was a plateau. The fraction of live cells treated with 1.5-6 μmol/L chelidonine was slightly more than 50% and the maximal effect of chelidonine in reducing the live cells by 50% was at 12 μmol/L. By increasing the concentration of chelidonine up to 100 μmol/L only a negligible increment in cytotoxicity was seen. This may suggest a potential mechanism involved in drug resistance.
Figure 1.
The effect of chelidonine on the growth of HepG2 cells after 48 h exposure. Viability of cells at each concentration of chelidonine was evaluated by neutral red uptake test and is expressed as a percentage relative to untreated control cells. The values presented are the mean ± SD of six repeats each one in triplicate.
Chelidonine treated HepG2 cells showed apoptotic features after 24 h and a small fraction of cells appeared with single blister cell death[30]. Figure 2 shows the cells at 48 h after treatment with 12 μmol/L chelidonine (LD50). DNA fragmentation due to apoptosis, as shown in Figure 3, represent the ladder formation in apoptotic HepG2 cells at different concentration after 48 h treatment.
Figure 2.
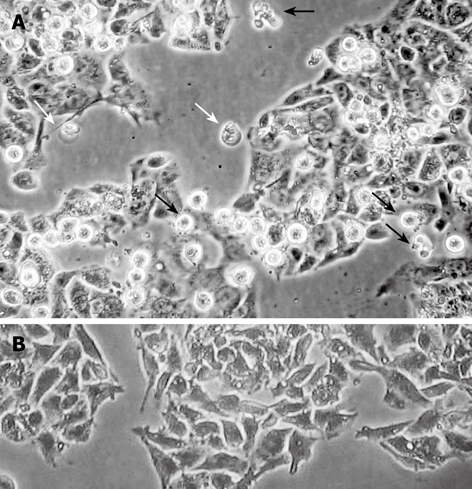
Morphological changes in HepG2 cells after 48 h treatment with 12 μmol/L chelidonine (LD50) visualized by phase contrast microscopy. A: Apoptotic cells (black arrows) and a few cells undergoing blister cell death (white arrows) are clearly seen; B: Untreated control HepG2 cells.
Figure 3.
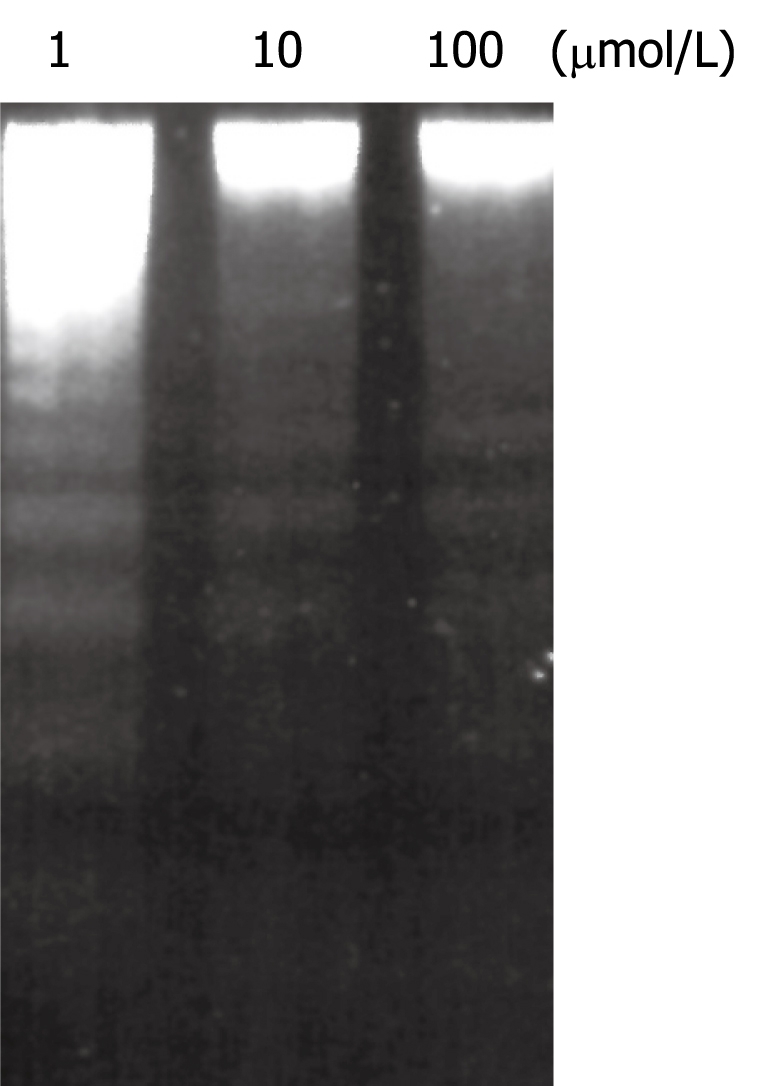
Induction of DNA fragmentation in HepG2 cells after 48 h treatment with different concentrations of chelidonine. The concentration of chelidonine is indicated at the top of each well.
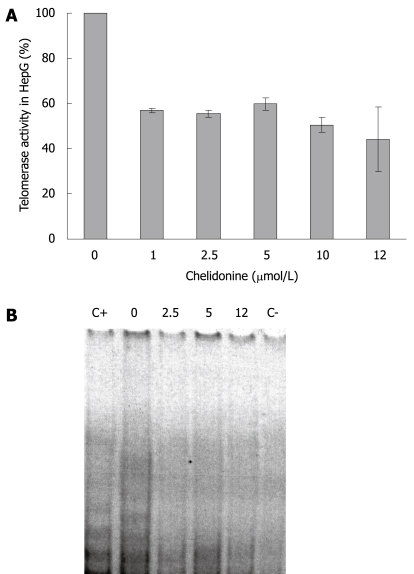
Telomerase activity in HepG2 cells (control and cells treated with different concentration of chelidonine for 48 h) is illustrated in Figure 4, which shows the resolving TRAP assay products on a native polyacrylamide gel electrophoresis. It clearly indicated decreased activity of telomerase in treated cells compared to untreated control cells. Considerable reduction of telomerase activity occurred at very low concentrations of chelidonine, whereas increasing the chelidonine concentration did not strengthen the effect significantly. The inhibitory effect of chelidonine on telomerase activity was not dose dependent. Moreover the 50% activity of telomerase in treated cells compared to the untreated control cells occurred almost at the same concentration ranges of chelidonine as LD50 in cytotoxicity tests.
Figure 4.
Telomerase activity using TRAP assay in HepG2 control and 48 h treated cells with different concentration of chelidonine up to LD50. A: The relative activity of treated cells against untreated control cells are indicated as mean ± SD; B: TRAP products resolved on non-denaturing PAGE and stained with SYBR Green. The concentration of chelidonine is indicated on top of each well in μmol/L. C+: Positive control; C-: Negative control.
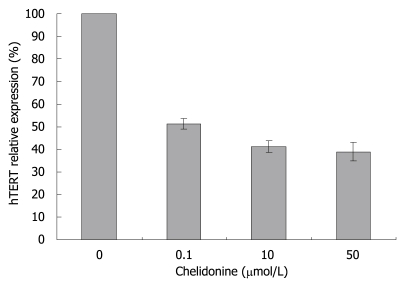
Real-time RT-PCR experiments showed a drastic decrease in the expression level of the hTERT subunit of telomerase by 0.1 μmol/L chelidonine. Again higher concentrations of chelidonine (even up to 50 μmol/L) showed only slight increments of the effect (Figure 5).
Figure 5.
HepG2 cells were treated for 48 h with medium supplemented with solvent (ethanol, not exceeding 0.1%) or 0.1, 10 and 50 μmol/L chelidonine. Relative hTERT gene expression as measured by real-time RT-PCR which was calibrated with the constitutive expression of β2-microglobulin gene. hTERT level in control samples was considered as 100%. The values indicated as mean ± SD (n ≥ 6).
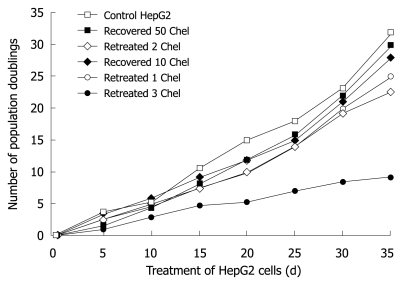
Long-term treatment of HepG2 cells with 0.1 μmol/L chelidonine for 48 h in every passage showed effective growth inhibition at very low concentrations of chelidonine. Figure 6 illustrates the mean number of cell population doubling in relation to different modes of treatments. The growth rate of cells that had been treated four times with 0.1 μmol/L chelidonine for 48 h per passage (re-treated 3 in Figure 6) showed a decline after 12 d and the growth rate tended to reach a plateau by 4 wk in comparison with untreated controls. Morphologically, after 28 d many of the cells appeared to be aged with a large cell volume and high cytoplasmic to nuclear ratio, which could be stained in β-galactosidase assay (Figure 7). The long-term treated cells showed distinctive morphological features associated with senescence of aged human cells. In contrast to control cells, the treated cells became enlarged and vacuolated in the cytoplasm.
Figure 6.
Number of population doublings after long-term treatment with a sub-apoptotic dose of chelidonine (0.1 μmol/L) or recovered cells after 48 h exposure to LD50 concentration of chelidonine in comparison with control HepG2 cells. In re-treatment experiments (long-term treatment) HepG2 cells were exposed to 0.1 μmol/L only 48 h in each passage and followed by recovery with normal medium devoid of chelidonine. Each point indicates the mean value of a duplicated experiment.
Figure 7.
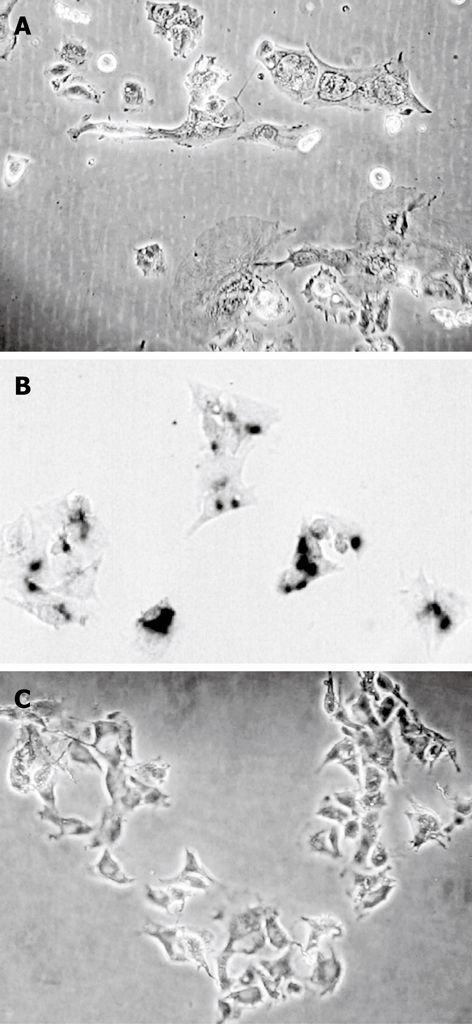
The morphological changes and induction of senescence in HepG2 cells in a long-term treatment experiment. A: HepG2 cells after treatment five times with 0.1 μmol/L chelidonine; B: Cells retreated five times stained for β-galactosidase activity; C: Stained control cells.
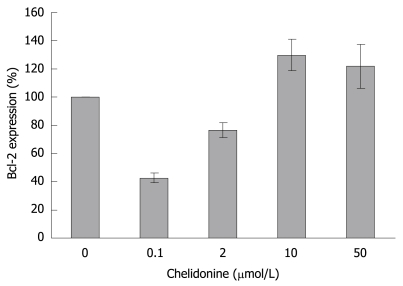
The relative expression level of Bcl-2 in HepG2 treated cells as compared to controls dropped to less than 50% at 0.1 μmol/L and subsequently increased to higher than 120% in LD50 values of chelidonine concentration (Figure 8).
Figure 8.
Relative expression of Bcl-2 gene in HepG2 cells after 48 h treatment with different concentrations of chelidonine in relation to untreated control cells assessed by real-time RT-PCR and calibrated with β2-microglobulin gene expression. The expression level of Bcl-2 in control cells was considered as 100%. mean ± SD are indicated (n = 3).
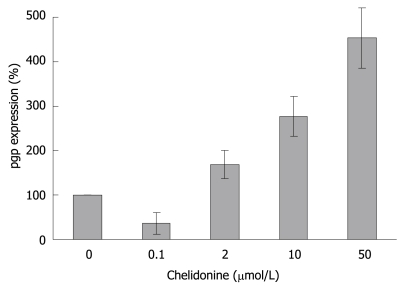
Furthermore real-time RT-PCR data showed (Figure 9) that the expression of pgp which is involved in general drug resistance increased 3-fold in chelidonine treated HepG2 cells in comparison with untreated control cells. However it is interesting that at 0.1 μmol/L chelidonine expression of pgp was reduced by 40%.
Figure 9.
Relative expression level of P-glycoprotein in HepG2 cells 48 h after treatment with different concentrations of chelidonine. Each bar indicates the mean ± SD (n = 3).
DISCUSSION
Several potent natural products, mainly secondary plant metabolites, have so far been reported as anticancer agents. Among alkaloids, derivatives of Ukrain are used in chemotherapy[31]. An inhibition of telomerase by berberine, an alkaloid related to chelidonine, has already been reported[32]. Here for chelidonine, the main compound of Ukrain, the mechanism of action in growth inhibition and its potential effect on telomerase inhibition and senescence induction has been analysed. Cytotoxicity of chelidonine in HepG2 cells is not dose-dependent within the range examined and tends to reach a plateau immediately after reduction of live cells to a point slightly higher than 50%. Therefore several cytotoxicity methods were tested to gain the best curve. The best results were attained from the neutral red uptake assay. Even with six repeats each in triplicate, it is hard to accept 12 μmol/L chelidonine as the LD50 in this cell line as cell viability does not change considerably above this concentration. However, this dose was considered to be LD50, where the maximal attainable effects of chelidonine were realized. The negligible increment of cytotoxicity of chelidonine at higher doses could be due to stimulation of a drug resistance mechanism in this cell line. Therefore pgp as a protein involved in the general mechanism of drug resistance was analyzed. Up-regulation of the gene tested using quantitative real-time RT-PCR in comparison with the β2-microglobulin gene was seen at 2, 10 and 50 μmol/L after 48 h treatment. Enhancement of the expression of pgp looks to be dose-dependent at concentrations above 2 μmol/L. This cell response could be important in dose adjustment of the alkaloid in cancer therapy to avoid chemoresistance as well as side effects.
Chelidonine showed a very strong inhibitory effect on telomerase activity in HepG2 cells after 48 h incubation. Several mechanisms need to be discussed. Some of them could include alteration of transcription, interaction with the telomerase subunits and probably interaction with telomere structure or telomere binding proteins. The DNA binding capacity (intercalation) of chelidonine was already shown to be negligible[33]. Here, we focused on the expression of hTERT as the most effective component of telomerase in activity and gene regulation. As changed patterns in expression level of hTERT are very similar to TRAP assay results, it seems that chelidonine modulates telomerase activity, at least partially, by down-regulation of the transcription of its catalytic subunit gene. Surprisingly expression of P-glycoprotein in 0.1 μmol/L chelidonine treated cells was reduced by 40%, indicating that it may sensitize cells to chelidonine toxicity, while higher concentrations close to LD50 boost the drug resistance response. This could be a possible explanation of the plateau shape of the cell viability curve attained in cytotoxicity tests as well. A very sharp reduction of the survived cell number in very low concentration of chelidonine is accompanied with a lower level of pgp whereas increased concentrations of chelidonine probably enforce drug resistance through up regulation of pgp and reduce cell death via apoptosis.
Obvious morphological changes toward cell death appeared after 24 h treatment with chelidonine by at least two different modalities of cell death; apoptosis and blister cell death. In this cell line the classical morphology of apoptosis manifests at 1 μmol/L chelidonine while single blister formation or blister cell death is also visible in a smaller number of cells. These data are in good concordance with several reports concerning the involvement of programmed cell death via the mitochondrial pathway induced by C. majus and its alkaloids in cancer cells[34].
It seems that primary down regulation of the anti-apoptotic gene Bcl-2 in the cells treated by a lower concentration of chelidonine (almost 40% in comparison with untreated controls) support apoptosis, while its return to normal expression level and even higher (about 120% in comparison with control) in LD50 concentration restrains apoptosis. This could in part explain the stronger toxicity of chelidonine at relatively lower concentrations.
Our data demonstrate apoptotic cell death in very low concentrations of chelidonine (1 μmol/L) and down-regulation of the hTERT subunit of telomerase in sub-apoptotic concentrations (0.1 μmol/L). Both of these effects seem to be in much lower concentrations of mitotic arrest of chelidonine through inhibition of tubulin polymerization, which has a LD50 of 24 μmol/L in different cells including HeLa[9]. It is also noteworthy that the antimitotic effect of low doses of alkaloids of C. majus is reversible but the effect at higher doses is not[35].
On the other hand long-term treatment of HepG2 cells with sub-apoptotic concentrations of chelidonine is also accompanied by induction of senescence which was confirmed by β-galactosidase activity, a commonly used biomarker in such studies. The cells treated repeatedly with 0.1 μmol/L showed a cell growth plateau, which appeared at day 28 in comparison with control untreated cells that corresponded to the failure of replating the treated cells. At this time, growth of treated cells shows an almost 60% decline in population doubling without affecting cell viability, suggesting an increase in cell cycle duration. Few apoptotic cells are visible in long-term treatments with 0.1 μmol/L chelidonine, whereas aged cells according to the β-galactosidase test are remarkably increased. In long-term treatment there are signs of differentiation visible in viable cells as well; some features of cell shape similar to fibroblasts, including long extensions attached to the substratum and a flat relatively large cytoplasm.
In conclusion, chelidonine shows a promising effect on proliferation inhibition of cancer cells, compatible with a recent suggestion of the Chelidonium majus extract as an anti-tumor and hepatoprotective agent against induced hepatocarcinoma in mice[3]. Our data showed that chelidonine inhibits telomerase in tumor cells strongly and may provide a basis for the development of more specific anticancer agents. Although possible selectivity effects of chelidonine on cancer cells needs further investigation, in a recent study on primary mouse spleen cells and mouse lymphocytic leukemia cells, L1210, chelidonine completely arrested L1210 cells with minimal cytotoxicity[36].
COMMENTS
Background
Telomerase, the key enzyme in most cancer cells for overcoming the proliferation limitation, is a promising target for chemotherapy. Among other strategies including PNA, antisense, ribozymes and RNA interference, chemotherapy using natural bio-products could be helpful in inhibition/down-regulation of telomerase. Several other plant secondary metabolites and their derivatives have been introduced in this proposal. Chelidonium majus (C. majus), which has been used as anti-tumor agent, in traditional medicine has been considered here and the main alkaloid of this plant was investigated for telomerase inhibitory potencies.
Research frontiers
C. majus and its derivative Ukrain have been used for cancer therapy, but little is known about the mechanism of action. Whether it has telomerase inhibitory effects is the main subject of this research. This is the first published data concerned with alteration of telomerase activity in cancer cells after treatment with chelidonine. Senescence induction is another aspect of chelidonine effects on the cancer cell culture model.
Innovations and breakthroughs
The molecular mechanism of chelidonine’s anti-proliferative effect on cancer cells was investigated by measuring telomerase activity and the transcription level of the catalytic subunit of the enzyme. Meanwhile population doubling and senescence induction was under consideration. All the data and viewpoints are original and never been published elsewhere.
Applications
Chelidonine shows strong inhibitory effects on telomerase activity and induces senescence in a cell culture model, so that it could be a promising agent in development of effective anticancer agents with higher specificities.
Terminology
Telomerase: A special kind of reverse transcriptase which extends telomeres in chromosomes using its own RNA components as the template. It is almost completely switched off in most normal somatic cells, while it is up-regulated in more than 90% of different cancers. Chelidonine: A benzophenanthridine alkaloid of Chelidonium majus - a well known plant with anti-tumor effects in traditional medicine.
Peer review
This article shows the effect of chelidonine on telomerase, which has therapeutic potential against cancer. The research topic is interesting and identifies the molecular target of chelidonine.
Acknowledgments
We would like to thank all the people who assisted us in this research; Dr. Maren Möller and Dr. Holger Schäfer. Also special gratitude to Dr. Meike Hoemme for kind help in designing some of the primers for real-time RT-PCR.
Peer reviewer: Sharon DeMorrow, Assistant Professor, Division of Research and Education, Scott and White Hospital and The Texas A&M University System, Health Science Center College of Medicine, Temple, Texas 76504, United States
S- Editor Li LF L- Editor O’Neill M E- Editor Ma WH
References
- 1.Niu CQ, He LY. Determination of isoquinoline alkaloids in Chelidonium majus L. by ion-pair high-performance liquid chromatography. J Chromatogr. 1991;542:193–199. [Google Scholar]
- 2.Colombo ML, Bosisio E. Pharmacological activities of Chelidonium majus L. (Papaveraceae) Pharmacol Res. 1996;33:127–134. doi: 10.1006/phrs.1996.0019. [DOI] [PubMed] [Google Scholar]
- 3.Biswas SJ, Bhattacharjee N, Khuda-Bukhsh AR. Efficacy of a plant extract (Chelidonium majus L.) in combating induced hepatocarcinogenesis in mice. Food Chem Toxicol. 2008;46:1474–1487. doi: 10.1016/j.fct.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Hohenwarter O, Strutzenberger K, Katinger H, Liepins A, Nowicky JW. Selective inhibition of in vitro cell growth by the anti-tumour drug Ukrain. Drugs Exp Clin Res. 1992;18 Suppl:1–4. [PubMed] [Google Scholar]
- 5.Liepins A, Nowicky JW. Ukrain is selectively cytostatic and/ or cytotoxic to human tumor and HIV-infected cells, but not to normal human cells. In: D Adam, T Buchner, E Rubinstein., editors. Recent Advances in Chemotherapy. Futuramed Publishers: Munich; 1992. pp. 2660–2661. [Google Scholar]
- 6.Gagliano N, Moscheni C, Torri C, Donetti E, Magnani I, Costa F, Nowicky W, Gioia M. Ukrain modulates glial fibrillary acidic protein, but not connexin 43 expression, and induces apoptosis in human cultured glioblastoma cells. Anticancer Drugs. 2007;18:669–676. doi: 10.1097/CAD.0b013e32808bf9ec. [DOI] [PubMed] [Google Scholar]
- 7.Rosenkranz V, Wink M. Induction of apoptosis by alkaloids, non-protein amino acids, and cardiac glycosides in human promyelotic HL-60 cells. Z Naturforsch C. 2007;62:458–466. doi: 10.1515/znc-2007-5-621. [DOI] [PubMed] [Google Scholar]
- 8.Ernst E, Schmidt K. Ukrain - a new cancer cure? A systematic review of randomised clinical trials. BMC Cancer. 2005;5:69. doi: 10.1186/1471-2407-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemeny-Beke A, Aradi J, Damjanovich J, Beck Z, Facsko A, Berta A, Bodnar A. Apoptotic response of uveal melanoma cells upon treatment with chelidonine, sanguinarine and chelerythrine. Cancer Lett. 2006;237:67–75. doi: 10.1016/j.canlet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Panzer A, Joubert AM, Bianchi PC, Hamel E, Seegers JC. The effects of chelidonine on tubulin polymerisation, cell cycle progression and selected signal transmission pathways. Eur J Cell Biol. 2001;80:111–118. doi: 10.1078/0171-9335-00135. [DOI] [PubMed] [Google Scholar]
- 11.Philchenkov A, Kaminskyy V, Zavelevich M, Stoika R. Apoptogenic activity of two benzophenanthridine alkaloids from Chelidonium majus L. does not correlate with their DNA damaging effects. Toxicol In Vitro. 2008;22:287–295. doi: 10.1016/j.tiv.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Hiyama E, Yokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW, Matsuura Y. Telomerase activity in gastric cancer. Cancer Res. 1995;55:3258–3262. [PubMed] [Google Scholar]
- 13.Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol. 2003;5:474–479. doi: 10.1038/ncb985. [DOI] [PubMed] [Google Scholar]
- 14.Huffman KE, Levene SD, Tesmer VM, Shay JW, Wright WE. Telomere shortening is proportional to the size of the G-rich telomeric 3’-overhang. J Biol Chem. 2000;275:19719–19722. doi: 10.1074/jbc.M002843200. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 16.Shay JW, Wright WE. Telomerase: a target for cancer therapeutics. Cancer Cell. 2002;2:257–265. doi: 10.1016/s1535-6108(02)00159-9. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig A, Saretzki G, Holm PS, Tiemann F, Lorenz M, Emrich T, Harley CB, von Zglinicki T. Ribozyme cleavage of telomerase mRNA sensitizes breast epithelial cells to inhibitors of topoisomerase. Cancer Res. 2001;61:3053–3061. [PubMed] [Google Scholar]
- 18.Stewart SA, Weinberg RA. Telomerase and human tumorigenesis. Semin Cancer Biol. 2000;10:399–406. doi: 10.1006/scbi.2000.0339. [DOI] [PubMed] [Google Scholar]
- 19.Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, Beijersbergen RL, Knoll JH, Meyerson M, Weinberg RA. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 20.Hsu YH, Lin JJ. Telomere and telomerase as targets for anti-cancer and regeneration therapies. Acta Pharmacol Sin. 2005;26:513–518. doi: 10.1111/j.1745-7254.2005.00098.x. [DOI] [PubMed] [Google Scholar]
- 21.Kondo S, Kondo Y, Li G, Silverman RH, Cowell JK. Targeted therapy of human malignant glioma in a mouse model by 2-5A antisense directed against telomerase RNA. Oncogene. 1998;16:3323–3330. doi: 10.1038/sj.onc.1201885. [DOI] [PubMed] [Google Scholar]
- 22.Damm K, Hemmann U, Garin-Chesa P, Hauel N, Kauffmann I, Priepke H, Niestroj C, Daiber C, Enenkel B, Guilliard B, et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 2001;20:6958–6968. doi: 10.1093/emboj/20.24.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nosrati M, Li S, Bagheri S, Ginzinger D, Blackburn EH, Debs RJ, Kashani-Sabet M. Antitumor activity of systemically delivered ribozymes targeting murine telomerase RNA. Clin Cancer Res. 2004;10:4983–4990. doi: 10.1158/1078-0432.CCR-04-0134. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Rosenberg JE, Donjacour AA, Botchkina IL, Hom YK, Cunha GR, Blackburn EH. Rapid inhibition of cancer cell growth induced by lentiviral delivery and expression of mutant-template telomerase RNA and anti-telomerase short-interfering RNA. Cancer Res. 2004;64:4833–4840. doi: 10.1158/0008-5472.CAN-04-0953. [DOI] [PubMed] [Google Scholar]
- 25.Borenfreund E, Babich H, Martin-Alguacil N. Rapid chemosensitivity assay with human normal and tumor cells in vitro. In Vitro Cell Dev Biol. 1990;26:1030–1034. doi: 10.1007/BF02624436. [DOI] [PubMed] [Google Scholar]
- 26.Gelmini S, Caldini A, Becherini L, Capaccioli S, Pazzagli M, Orlando C. Rapid, quantitative nonisotopic assay for telomerase activity in human tumors. Clin Chem. 1998;44:2133–2138. [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Albermann N, Schmitz-Winnenthal FH, Z’graggen K, Volk C, Hoffmann MM, Haefeli WE, Weiss J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol. 2005;70:949–958. doi: 10.1016/j.bcp.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liepins A, Nowicky JW, Bustamante JO, Lam E. Induction of bimodal programmed cell death in malignant cells by the derivative Ukrain (NSC-631570) Drugs Exp Clin Res. 1996;22:73–79. [PubMed] [Google Scholar]
- 31.Uglyanitsa KN, Nefyodov LI, Doroshenko YM, Nowicky JW, Volchek IV, Brzosko WJ, Hodysh YJ. Ukrain: a novel antitumor drug. Drugs Exp Clin Res. 2000;26:341–356. [PubMed] [Google Scholar]
- 32.Wu HL, Hsu CY, Liu WH, Yung BY. Berberine-induced apoptosis of human leukemia HL-60 cells is associated with down-regulation of nucleophosmin/B23 and telomerase activity. Int J Cancer. 1999;81:923–929. doi: 10.1002/(sici)1097-0215(19990611)81:6<923::aid-ijc14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Kaminskyy VO, Lootsik MD, Stoika RS. Correlation of the cytotoxic activity of four different alkaloids, from Chelidonium majus (greater celandine), with their DNA intercalating properties and ability to induce breaks in the DNA of NK/Ly murine lymphoma cells. Central European J Biology. 2006;1:2–15. [Google Scholar]
- 34.Habermehl D, Kammerer B, Handrick R, Eldh T, Gruber C, Cordes N, Daniel PT, Plasswilm L, Bamberg M, Belka C, et al. Proapoptotic activity of Ukrain is based on Chelidonium majus L. alkaloids and mediated via a mitochondrial death pathway. BMC Cancer. 2006;6:14. doi: 10.1186/1471-2407-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panzer A, Joubert AM, Bianchi PC, Seegers JC. The antimitotic effects of Ukrain, a Chelidonium majus alkaloid derivative, are reversible in vitro. Cancer Lett. 2000;150:85–92. doi: 10.1016/s0304-3835(99)00375-4. [DOI] [PubMed] [Google Scholar]
- 36.Kaminskyy V, Lin KW, Filyak Y, Stoika R. Differential effect of sanguinarine, chelerythrine and chelidonine on DNA damage and cell viability in primary mouse spleen cells and mouse leukemic cells. Cell Biol Int. 2008;32:271–277. doi: 10.1016/j.cellbi.2007.09.004. [DOI] [PubMed] [Google Scholar]