Abstract
Activation of TLR4 by administration of LPS shortens the survival of skin allografts in mice treated with costimulation blockade through a CD8 T cell-dependent, MyD88-dependent, and type I IFN receptor-dependent pathway. The effect of TLR activation on the establishment of allogeneic hematopoietic chimerism in mice treated with costimulation blockade is not known. Using a costimulation blockade protocol based on a donor-specific transfusion (DST) and a short course of anti-CD154 mAb, we show that LPS administration at the time of DST matures host alloantigen-presenting dendritic cells, prevents the establishment of mixed allogeneic hematopoietic chimerism, and shortens survival of donor-specific skin allografts. LPS mediates its effects via a mechanism that involves both CD4+ and CD8+ T cells and results from signaling through either the MyD88 or the type I IFN receptor pathways. We also document that timing of LPS administration is critical, as injection of LPS 24 h before treatment with DST and anti-CD154 mAb does not prevent hematopoietic engraftment but administration the day after bone marrow transplantation does. We conclude that TLR4 activation prevents the induction of mixed allogeneic hematopoietic chimerism through type I IFN receptor and MyD88-dependent signaling, which leads to the up-regulation of costimulatory molecules on host APCs and the generation of alloreactive T cells. These data suggest that distinct but overlapping cellular and molecular mechanisms control the ability of TLR agonists to block tolerance induction to hematopoietic and skin allografts in mice treated with costimulation blockade.
Induction of allogeneic hematopoietic chimerism using non-cytoreductive conditioning regimens based on costimulation blockade has been shown to be effective for establishing allogeneic hematopoietic chimerism and inducing central tolerance (1–3). These costimulation blockade-based protocols for the establishment of hematopoietic chimerism are promising alternatives to the use of immunosuppression for prolonging allograft survival. We (4) and others (5) have shown that administration of TLR agonists at the time of costimulation blockade shortens the survival of multiple solid organ allografts, including skin, islet, and cardiac grafts. However, the effects of environmental perturbants such as TLR agonists on the establishment of mixed hematopoietic chimerism in mice treated with costimulation blockade have not been reported.
TLRs constitute a family of at least 12 pattern recognition receptors expressed on many cell types including APCs, T cells, and endothelial cells (6). TLRs recognize conserved microbial components known as pathogen-associated molecular patterns (7, 8). Activation of TLRs can lead to the maturation of APCs and the production of proinflammatory cytokines such as IL-6, TNF-α, IL-12, IL-15, and type I IFNs (IFN-α/β) (9, 10). TLR activation in the setting of transplantation engenders a proinflammatory environment (11–17) that makes the recipient less susceptible to the tolerance-promoting effects of costimulation blockade (4, 5, 18–21), in part by signaling through the type I IFN receptor (18). Two cellular mechanisms for the effects of TLR agonists on tolerance induction have been proposed. First, we have shown that TLR agonists given at the time of donor-specific transfusion (DST)4 and anti-CD154 mAb prevent the deletion of alloreactive CD8+ T cells and that CD8 T cell depletion prevents the detrimental effects of TLR agonists on allograft survival (4). Second, Chen et al. (5) have shown that administration of TLR agonists prevents the recruitment of regulatory T cells (Tregs) to the allograft.
In this report, we demonstrate that treatment with the TLR agonists LPS, polyinosinic:polycytidylic acid (poly(I:C)), and Pam3-Cys-Ser-(Lys)4 (Pam3Cys) at the time of costimulation blockade prevents the establishment of allogeneic hematopoietic chimerism and long-term skin allograft survival by activating host APCs, thereby leading to the generation of alloreactive CD8 T cells. These effects are dependent on the presence of both CD4 and CD8 host T cells and can be mediated through both MyD88 and type I IFN receptor signaling pathways.
Materials and Methods
Animals
BALB/c (H2d) and C57BL/6 (H2b) mice were obtained from Charles River Laboratories or The Jackson Laboratory. C57BL/10ScSnJ (H2b abbreviated as C57BL/10), C57BL/10ScNJ-Tlr4lps-del (H2b, abbreviated as C57BL/10.TLR4−/−), and C57BL/6.129S2-Cd8atm1Mak/J (H2b, abbreviated as C57BL/6.CD8α−/−) mice were obtained from The Jackson Laboratory and bred at the University of Massachusetts Medical School. C57BL/6.MyD88−/− (N6, abbreviated as B6.MyD88−/−) (22) and C57BL/6.129S2-Ifnar1tm1At (N12, abbreviated as B6.IFNARI−/−) (23) were a gift from Dr. E. Lien (University of Massachusetts Medical School, Boston, MA), who originally obtained the B6.MyD88−/− mice from Dr. D. Golenbock (University of Massachusetts Medical School) and the B6.IFNARI−/− mice from Dr. J. Sprent (The Scripps Research Institute, La Jolla, CA). B6.MyD88−/− IFNAR1−/− mice were produced by crossing B6.IFNAR1−/− and B6.MyD88−/− mice and then performing an F1 intercross and selecting double knockout mice by PCR typing. All mice were housed in microisolator cages, given ad libitum access to autoclaved food, and maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Abs and flow cytometry
Purified hamster anti-mouse CD154 mAb (clone MR1) was produced at The National Cell Culture Center (Minneapolis, MN) (24). FITC-conjugated anti-H2-Kd (clone SF1-1.1), anti-mouse H2-Kb-PE (clone AF6-88.5), anti-mouse CD8α-PerCP (clone 53-6.7), anti-mouse CD8α-Pacific Blue (clone 53-6.7), anti-mouse CD8β-PE (clone H35-17.2), anti-mouse CD4-PerCP (clone RM4-5), anti-mouse CD4-PE (clone RM4-4), anti-mouse CD3-biotin (clone 145-2C11), anti-mouse IFN-γ-allophycocyanin (clone XMG1.2), anti-mouse IFN-γ-PE (clone XMG1.2), anti-mouse CD11a- FITC (clone 2D7), anti-mouse CD11a-PE-Cy7 (clone 2D7), allophycocyanin-conjugated streptavidin, purified anti-mouse CD16/32 (clone 2.4G2), and corresponding isotype control Abs were purchased from BD Pharmingen. Labeled cells were analyzed with a FACSCalibur or LSRII instrument (BD Biosciences) and FlowJo software (Tree Star).
DST and mAb treatment
Mice 5–10 wk of age were treated with a DST consisting of 10 × 106 BALB/c spleen cells injected i.v. DST was given on day −7 and 0.5 mg of anti-CD154 mAb was injected i.p. on days −7, −4, 0, and +4 relative to bone marrow and/or skin transplantation on day 0. Anti-CD4 mAb (clone GK1.5, 0.5 mg) or anti-CD8α (clone 2.43, 0.5 mg) was injected i.p. on day −10, −9, and −8 relative to transplantation on day 0. We confirmed >95% CD4 and CD8 T cell depletion by flow cytometry using Abs against a noncompeting CD4 (clone RM4-4) epitope and the CD8β chain. Anti-NK1.1 Ab (clone PK136, 0.025 mg) was injected i.p. on day −10 relative to transplantation. We confirmed >95% NK cell depletion by flow cytometry using Abs against CD49b and Ly-49D.
Bone marrow transplantation
Single-cell suspensions of bone marrow were prepared from femurs and tibiae, filtered through sterile 70-µm nylon mesh (BD Biosciences), washed, and counted. Recipient mice received a single i.v. injection of 50 × 106 bone marrow cells in a volume of 0.5 ml, as described previously (1).
Skin transplantation
Full-thickness skin grafts 1–2 cm in diameter were obtained from the trunks of donor mice and transplanted onto the dorsal flanks of recipients as previously described (1). Skin graft survival was evaluated three times a week, and graft rejection was defined as the first day on which the entire graft was necrotic.
Determination of chimerism
Blood samples were obtained from mice for determination of chimerism. The percentage of donor and host cells expressing MHC class I in chimeric mice was determined by dual-labeling with Abs to H2-Kb and H2-Kd as described previously (1). We defined recipients as chimeric if the percentage of donor-origin PBMCs was >0.10% at 8 wk.
IFN-α/β bioassay
IFN-α/β was measured using a standard virus-inhibition bioassay (25). Briefly, whole blood was isolated from mice 8 or 24 h after treatment with DST and anti-CD154 mAb and was then centrifuged to obtain serum. The serum was then diluted 2-fold across a 96-well plate. Wells were seeded with 3 × 104 mouse L-929 cells (NCTC clone 929; American Type Culture Collection) and incubated overnight. The following day each well was infected with 2 × 105 PFUs of vesicular stomatitis virus strain Indiana and incubated for 2 days. The cytopathic effects were evaluated using microscopy. The IFN-α/β titer was determined as the reciprocal of the dilution that provided 50% protection from cytopathic effects (25), and this value was expressed as arbitrary units per ml (U/ml).
Preparation and injection of TLR agonists and recombinant IFN-β
LPS from Escherichia coli 0111:B4 (Sigma-Aldrich) was purified as previously described (26), except that phenol-PBS phase separation was conducted at 2000 × g for 30 min to accommodate larger volumes. Purified LPS was suspended in Dulbecco’s PBS (D-PBS) and stored at 4°C until used, with an assumed 10% loss during purification (26). Poly(I:C) (Sigma-Aldrich) was dissolved in D-PBS at a concentration of 1 mg/ml. Stock was filtered through 0.45-µm sterile nylon mesh (BD Biosciences) and stored at −20°C until used. Pam3Cys (EMC Microcollections) was reconstituted in PBS and was stored at −20°C. Mice were injected i.p. with the indicated ligand and dose in a volume of 0.5 ml of D-PBS. Recombinant mouse IFN-β was obtained from PBL IFN Source and injected i.p. at the indicted dose on the day of DST and the first injection of anti-CD154 mAb.
Alloantibody assay
The generation of donor-specific Abs was determined by flow cytometry. Dilutions (undiluted, 1/10, 1/100) of mouse serum were incubated with BALB/c thymocytes for 20 min at 4°C. Cells were washed and incubated with FITC-conjugated polyclonal anti-mouse Ig (BD Pharmingen) for 20 min. The median fluorescence intensity (MFI) of the samples was determined by flow cytometry.
Intracellular IFN-γ assay
IFN-γ production was assayed in spleen cells and circulating leukocytes as described elsewhere (4). Briefly, RBC from heparinized whole blood or single-cell spleen suspensions were lysed using 0.84% ammonium chloride. Cells were then incubated for 5 h in GolgiPlug (BD Pharmingen) with 10 U/ml rIL-2 (R&D Systems) and 1 µg of purified anti-CD28 mAb (BD Pharmingen) at 37°C in the presence of single-cell suspensions of irradiated, LPS-treated syngeneic (C57BL/6, H2b) or allogeneic (BALB/c, H2d) splenocytes (1 × 106 cells/stimulation). Samples were stained with anti-H2-Kb-PE, anti-CD8α-Pacific Blue, anti-CD4-PerCP, and anti-CD11a-FITC, followed by fixation with BD Biosciences Cytofix/Cytoperm and staining with anti-IFN-γ-allophycocyanin.
In vivo tracking of DST
BALB/c spleens were harvested, prepared into single-cell suspension by mechanical disruption, washed two times in PBS, and counted. Splenocytes were incubated at 37°C in 5 µM CFSE (Molecular Probes). After 15 min, the cells were washed three times in PBS and counted using trypan blue (Sigma-Aldrich). The viability after CFSE labeling was >80%. The cells were then suspended in PBS at a concentration of 2 × 107 viable cells/ml 0.5 ml was injected into the tail veins of recipients. Sixteen, 24, or 48 h later, recipients were euthanized, the splenocytes were recovered, and flow cytometry analysis was performed for detection of CFSE-labeled cells. Data shown are at 16 h after CFSE-labeled cell transfer as CFSE-labeled cells were only detectable above background levels at this time point. Single-cell suspensions were prepared by mechanical disruption and the cells were washed with PBS two times at room temperature. Samples were stained with LIVE/DEAD blue (Molecular Probes) for 20 min to visualize and exclude dead cells. Cells were then washed with PBS containing 1.0% fetal clone serum and 0.1% sodium azide before incubating with anti-CD16/CD32 and fluorescent Abs as described above.
Statistical methods
Statistical analyses were made using GraphPad Prism Software (version 4.0). Comparisons of three or more means used one-way ANOVA and Bonferroni-adjusted unpaired t tests. Comparisons of two means used unpaired t tests without assuming equal variance. Skin allograft survival curves were generated by the Kaplan-Meier method and compared by the log-rank test. Duration of allograft survival is presented as the median. Values of p < 0.05 were considered statistically significant.
Results
TLR agonists administered at the time of DST and anti-CD154 mAb prevent the establishment of allogeneic hematopoietic chimerism and shorten skin allograft survival
We first investigated the hypothesis that TLR agonists given during tolerance induction with DST and anti-CD154 mAb would prevent the establishment of allogeneic hematopoietic chimerism. To test this, C57BL/6 mice were injected with our standard costimulation blockade protocol consisting of injection of BALB/c DST on day −7 and four injections of anti-CD154 mAb on days −7, −4, 0, and +4 relative to injection with 50 × 106 BALB/c bone marrow cells and transplantation with BALB/c skin on day 0. In group 1, no additional treatment was given, and the majority of these mice (64%) established stable allogeneic hematopoietic chimerism (Table I, group 1). Chimeric mice also exhibited prolonged skin allograft survival, whereas nonchimeric mice exhibited shorter skin allograft survival (Table I). We have previously shown that the skin graft tolerance produced by this protocol is specific to the DST and bone marrow donor, with third-party skin grafts being rapidly rejected (27). Group 2 was given an i.p. injection of the TLR4 agonist LPS on day −7 relative to bone marrow injection and skin transplantation. None of the mice in group 2 became chimeric and skin graft survival was short (Table I, group 2). Similarly, i.p. injection of the TLR3 agonist poly(I:C) or the TLR1/2 agonist Pam3Cys on day −7 prevented the establishment of chimerism and skin graft survival again was short (Table II, groups 1 and 2). Importantly, transplantation of skin allografts was not required for TLR agonists to prevent bone marrow engraftment, as mice conditioned with DST, anti-CD154 mAb, and given BALB/c bone marrow became chimeric at 8 wk (six of seven), while those given DST, anti-CD154 mAb, LPS, and BALB/c bone marrow did not (zero of seven; p < 0.001). These data indicate that TLR agonists prevent the establishment of allogeneic hematopoietic chimerism and shorten skin allograft survival in mice treated with DST and anti-CD154 mAb.
Table I.
Cell subsets important in mediating the effects of LPS on the establishment if hematopoietic chimerism in mice treated with costimulation blockadea
| Group | Host | LPS | Anti-NK | Anti-CD8 | Anti-CD4 | Chimerism Frequency | Donor-Origin PBMCs at 8 wk (%) | MST of Skin Grafts in Transplanted Mice (days) | MST of Skin Grafts in Nonchimeric Mice (days) | MST of Skin Grafts in Chimeric Mice (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C57BL/6 | No | No | No | No | 29/45 (64%) | 1.59 ± 1.81 | 144 | 88 | >260 |
| 2 | C57BL/6 | Yes | No | No | No | 0/23 (0%)b | <0.10 | 11* | 11 | N/A |
| 3 | C57BL/6 | No | Yes | No | No | 11/13 (84.6%) | 2.45 ± 1.16 | >221 | 72 | >221 |
| 4 | C57BL/6 | Yes | Yes | No | No | 0/5 (<0.10%)c | <0.10 | 12** | 12 | N/A |
| 5 | C57BL/6 | No | No | Yes | No | 15/17 (88.2%) | 0.90 ± 1.13 | 144 | 72.5 | 148 |
| 6 | C57BL/6 | Yes | No | Yes | No | 0/16 (<0.10%)d | <0.10 | 44# | 44 | N/A |
| 7 | C57BL/6 | No | Yes | Yes | No | 7/8 (87.5%) | 3.67 ± 1.23 | 81 | 23 | 81 |
| 8 | C57BL/6 | Yes | Yes | Yes | No | 0/9 (<0.10%)e | <0.10 | 28## | 28 | N/A |
| 9 | C57BL/6 | No | No | No | Yes | 1/12 (8.33%) | 0.58 | 46 | 37 | >147 |
| 10 | C57BL/6 | Yes | No | No | Yes | 0/12 (<0.10%)f | <0.10 | 18 | 18 | N/A |
| 11 | C57BL/6 | No | No | Yes | Yes | 4/4 (100%) | 2.42 ± 0.55 | >228 | N/A | >228 |
| 12 | C57BL/6 | Yes | No | Yes | Yes | 4/4 (100%)g | 1.14 ± 0.35 | 228 | N/A | 228 |
| 13 | CD8α−/− | No | No | No | No | 12/17 (70.5%) | 2.29 ± 1.76 | 96 | 51 | 136 |
| 14 | CD8α−/− | Yes | No | No | No | 0/17 (<0.10%)h | <0.10 | 21$ | 21 | N/A |
All mice were treated with a BALB/c DST and anti-CD154 mAb and transplanted with BALB/c bone marrow and skin according to our standard protocol without or with injection of 100 µg of LPS. Groups 3, 4, 7, and 8 were also given an injection of anti-NK1.1 mAb i.p on day −10 relative to bone marrow and skin transplantation. Groups 5, 6, 7, 8, 11, and 12 were given three doses of 0.5 mg of anti-CD8 mAb i.p. on days −10, −9, and −8 relative to bone marrow and skin transplantation. Groups 9, 10, 11, and 12 were given three doses of 0.5 mg of anti-CD4 mAb i.p. on days −10, −9, and −8 relative to bone marrow and skin transplantation. Hematopoietic chimerism was defined as >0.10% donor origin PBMCs 8 wk after bone marrow injection. The percentage of donor origin PBMCs is the mean ± SD percentage of the levels observed in chimeric mice. For groups with no chimeric mice, we used the limit of detection (<0.10) to indicate a lack of chimerism.
p = 0.017 vs group 1
p = 0.0005 vs group 3
p < 0.0001 vs group 5
p = 0.0003 vs group 7
p = NS vs group 9
p = NS vs group 11
p < 0.0001 vs group 13 by χ2 analysis.
p < 0.001 vs group 1
p < 0.0001 vs group 3
p < 0.0001 vs group 5
p < 0.01 vs group 7
p < 0.001 vs group 13 by log-rank analysis.
N/A, Not applicable.
Table II.
TLR4, Type I IFN, and MyD88 are important in the LPS-mediated prevention of costimulation blockade-induced hematopoietic chimerisma
| Group | Host | TLR Agonist | Chimerism Frequency | Donor Origin PBMCs at 8 wk (%) | MST of Skin Grafts (days) | MST of Skin Grafts in Nonchimeric Mice (days) | MST of Skin Grafts in Chimeric Mice (days) |
|---|---|---|---|---|---|---|---|
| 1 | C57BL/6 | Poly(I:C) | 0/20 (0%)b | <0.10 | 9* | 9 | N/A |
| 2 | C57BL/6 | PAM | 0/3 (0%)c | <0.10 | 10* | 10 | N/A |
| 3 | C57BL/10 | None | 3/7 (42.8%) | 1.76 ± 0.34 | 125 | 70 | >176 |
| 4 | C57BL/10 | LPS | 0/12 (0%)d | <0.10 | 11** | 11 | N/A |
| 5 | C57BL/10.TLR4−/− | None | 10/13 (76.9%) | 2.78 ± 1.61 | 176 | 18 | >195 |
| 6 | C57BL/10.TLR4−/− | LPS | 9/10 (90.0%)e | 1.94 ± 1.19 | >195 | 10 | >195 |
| 7 | C57BL/6.MyD88−/− | None | 4/5 (80.0%) | 2.30 ± 2.47 | N/A | N/A | N/A |
| 8 | C57BL/6.MyD88−/− | LPS | 0/9 (0.00%)f | <0.10 | N/A | N/A | N/A |
| 9 | C57BL/6.IFNAR1−/− | None | 13/15 (87.0%) | 3.52 ± 2.14 | >218 | 28 | >218 |
| 10 | C57BL/6.IFNAR1−/− | LPS | 6/17 (35.0%)g | 2.07 ± 1.24 | 80# | 14 | >218 |
| 11 | C57BL/6.IFNAR1−/− | Poly(I:C) | 5/12 (41.7%)h | 2.22 ± 0.97 | 84 | 16 | >218 |
| 12 | C57BL/6.IFNAR1−/− | PAM | 0/4 (0.00%)i | <0.10 | 10$ | 10 | N/A |
| 13 | C57BL/6.MyD88−/−IFNAR1−/− | None | 5/7 (71.4%) | 4.13 ± 0.98 | >140 | 50 | >140 |
| 14 | C57BL/6.MyD88−/−IFNAR1−/− | LPS | 5/8 (62.5%)j | 5.37 ± 1.08 | >140 | 57 | >140 |
All mice were treated with a BALB/c DST, bone marrow, and anti-CD154 mAb according to our standard protocol without or with an i.p. injection of 100 µg of LPS, 50 µg of poly(I:C), or 100 µg of Pam3Cys. Groups 1–6 and 9–16 also received a BALB/c skin graft on day 0, the day of bone marrow transplant. Hematopoietic chimerism was defined as >0.10% donor origin PBMCs 8 wk after bone marrow injection. The percentage of donor origin PBMCs is the mean ± SD percentage of the levels observed in chimeric mice. For groups with no chimeric mice, we used the limit of detection (<0.10) to indicate a lack of chimerism.
p < 0.001 vs Table I, group 1
p < 0.05 vs Table I, group 1
p < 0.05 vs group 3
p = NS vs group 5
p < 0.01 vs group 7
p < 0.01 vs group 1 and p < 0.05 vs group 9
p < 0.001 vs group 9
p = NS vs group 13 by χ2 analysis.
p < 0.001 vs Table I, group 1
p < 0.05 vs group 3
p < 0.05 vs group 9
p < 0.001 vs group 9 by log-rank analysis.
N/A, Not applicable.
Alloantibodies are not generated following the administration of LPS during costimulation blockade
A mechanism to prevent the establishment of allogeneic chimerism by LPS could be the production of alloreactive Abs. B lymphocytes express the LPS receptor TLR4 and can be activated directly by LPS to produce Abs (6). Given that alloantibodies are known to induce allograft rejection (28), we investigated whether alloantibodies were produced in mice treated with costimulation blockade and given LPS. C57BL/6 mice were left untreated, primed with BALB/c DST, or given BALB/c DST, anti-CD154 mAb, and grafted with BALB/c bone marrow without or with LPS treatment. As shown in Fig. 1A, completely untreated (naive) mice had background levels of circulating alloantibodies, whereas mice primed with BALB/c DST developed high levels of alloantibodies. In contrast, mice treated with anti-CD154 mAb at the time of DST injection developed only low levels of alloantibodies, even in LPS-treated mice that had rejected BALB/c bone marrow grafts. These data show that the ability of LPS to prevent the establishment of hematopoietic chimerism is not due to alloantibody production.
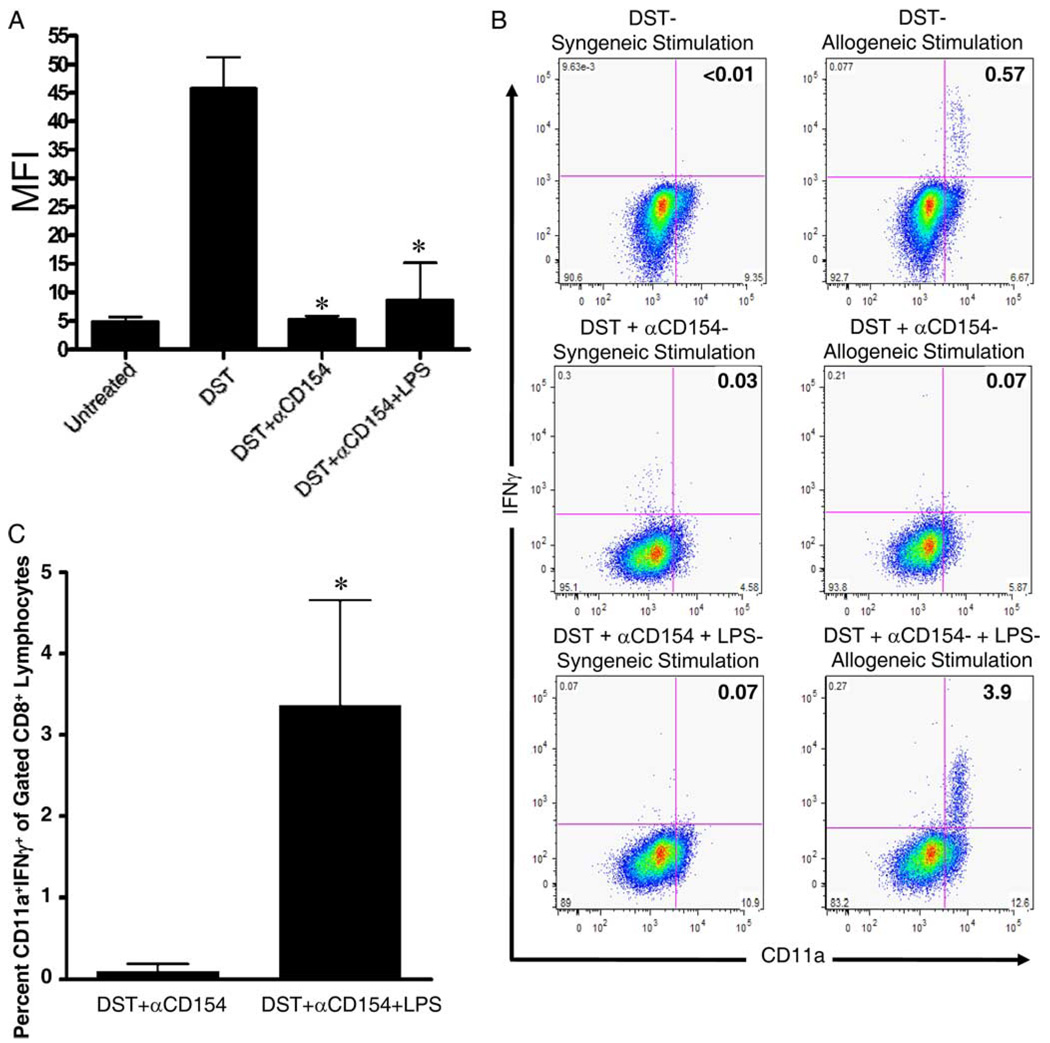
FIGURE 1.
LPS administration at the time of costimulation blockade permits the generation of alloreactive CD8+ T cells but does not induce alloantibody production. A, C57BL/6 mice were treated with BALB/c DST and anti-CD154 mAb according to our standard protocol without or with injection of 100 µg of LPS on day −7 relative to transplantation of BALB/c bone marrow on day 0. All mice were bled 2 wk after transplantation. Serum was analyzed for alloantibody content by flow cytometry. Serum was also taken from untreated mice and mice primed with a single injection of BALB/c splenocytes to serve as negative and positive controls, respectively, for the alloantibody assay. Data are presented as MFI + 1 SD. Data are pooled from two independent experiments with at least four mice per group. *, p < 0.001 vs splenocyte (DST)-injected-only group; p = NS vs untreated group. p = NS for DST + anti-CD154 mAb vs DST + anti-CD154 mAb + LPS. B and C, C57BL/6 mice were treated with BALB/c DST and anti-CD154 mAb according to our standard protocol without or with coinjection of 100 µg of LPS on day −7 relative to transplantation of BALB/c bone marrow and skin on day 0. All mice were bled 2 wk after transplantation. Peripheral blood cells were recovered 2 wk after transplantation and stimulated in vitro for 5 h with either irradiated syngeneic (H2b) or allogeneic (H2d) splenocytes and their production of IFN-γ was quantified by flow cytometry. B, Representative flow cytometry dot plots showing CD11a and IFN-γ expression in gated CD8+ lymphocytes. As a positive control, C57BL/6 mice were injected with BALB/c splenocytes (DST) 7 days before blood cell recovery. C, The mean + 1 SD of the percentage of CD8+ lymphocytes producing IFN-γ. Data are representative of two independent experiments with at least three mice per group. *, p = 0.012.
Cell populations required for LPS-mediated effects in mice treated with DST and anti-CD154 mAb
Based on the absence of alloantibodies in mice treated with DST, anti-CD154 mAb, and LPS, we next investigated the cell populations that are responsible for LPS-mediated effects on the establishment of hematopoietic chimerism and skin allograft survival. NK cells. NK cells are known to be a barrier to the establishment of hematopoietic chimerism (29–31). To test the role of NK cells, we depleted C57BL/6 mice of NK cells and treated them with our standard costimulation blockade protocol without or with coin-jection of LPS at the time of DST. In the absence of LPS, the majority of NK-cell depleted mice became chimeric (11 of 13) and chimeric mice exhibited permanent skin allograft survival (Table I, group 3). In contrast, mice depleted of NK cells and treated with LPS on day −7 failed to become chimeric and skin allograft survival was short (Table I, group 4). These data indicate that host NK cells, although a barrier to hematopoietic chimerism, are not solely responsible for the detrimental effects of LPS on the establishment of chimerism.
CD8+ cells
We have observed that TLR agonists impair the deletion of alloreactive CD8+ T cells in mice treated with DST and anti-CD154 mAb and that host CD8+ T cells are required for LPS to shorten skin allograft survival in these mice (4). To determine whether CD8 T cells are also required for LPS to prevent the establishment of chimerism, C57BL/6.CD8α−/− mice were treated with our standard costimulation blockade protocol without or with injection of LPS at the time of DST. As expected, hematopoietic chimerism and prolonged skin allograft survival was observed in the majority of C57BL/6.CD8α−/− mice treated with DST and anti-CD154 mAb (Table I, group 13). Surprisingly, LPS was able to prevent chimerism in C57BL/6.CD8α−/− mice, and skin survival was short (Table I, group 14).
Given this surprising result, we further tested the role of CD8+ T cells using wild-type C57BL/6 mice depleted of CD8+ T cells using an anti-CD8α mAb. C57BL/6 mice were depleted of CD8+ cells and treated with our standard costimulation blockade protocol without or with LPS at the time of DST injection. C57BL/6 mice depleted of CD8+ cells became chimeric and exhibited prolonged skin allograft survival (Table I, group 5). Again, mice depleted of CD8+ T cells and treated with LPS on day −7 failed to become chimeric and skin allograft survival was short (Table I, group 6). These data indicate that host CD8+ T cells are not required for LPS to prevent the establishment of hematopoietic chimerism in mice treated with costimulation blockade.
CD8+ plus NK cells
Although the detrimental effect of LPS was not dependent on host NK cells or CD8+ cells, it was possible that either population on its own could prevent the establishment of hematopoietic chimerism and shorten skin allograft survival. To test this, mice were depleted of both NK and CD8+ cells and treated with DST and anti-CD154 mAb without or with LPS at the time of DST injection. In the absence of LPS treatment, mice developed chimerism and exhibited prolonged skin allograft survival (Table I, group 7). In contrast, coinjection of LPS at the time of DST in mice depleted of both CD8 and NK cells completely prevented chimerism and shortened skin allograft survival (Table I, group 8).
CD4 plus CD8 cells
We next tested the possibility that host CD4+ T cells were capable of mediating the rejection of BALB/c allografts. However, we have previously shown and confirmed here (Table I, group 9) that CD4+ T cells are required for the establishment of hematopoietic chimerism in mice treated with DST and anti-CD154 mAb, presumably as a requirement for tolerization of alloreactive CD8+ cells (27). As expected, CD4-depleted mice treated with costimulation blockade and treated with LPS failed to become chimeric and exhibited short skin allograft survival (Table I, group 10). Therefore, we depleted both CD4+ and CD8+ cells and then treated the mice with our standard costimulation blockade protocol. Mice treated with both anti-CD4 and anti-CD8 mAb developed chimerism and exhibited permanent skin graft survival (Table I, group 11). Chimerism and prolonged skin graft survival were also observed in CD4 and CD8-depleted mice given LPS at the time of DST (Table I, group 12). These data suggest that LPS can mediate its detrimental effects through either alloreactive CD4+ or CD8+ T cells.
LPS administration leads to the generation of effector/memory alloreactive CD8+ T cells
Given that the removal of host CD8+ T cells is required for the establishment of chimerism, we next investigated whether effector/memory CD8+ T cells were generated in mice treated with LPS, costimulation blockade, and transplanted with allogeneic bone marrow. C57BL/6 mice treated with costimulation blockade and transplanted with BALB/c bone marrow exhibited very low levels of BALB/c-reactive IFN-γ-producing CD8+CD11ahigh T cells 2 wk after bone marrow transplantation. In contrast, mice given LPS injection at the time of DST developed high levels of IFN-γ-producing CD8+CD11ahigh T cells (Fig. 1, B and C).
LPS up-regulates costimulatory molecules on cells in the DST in mice treated with anti-CD154 mAb
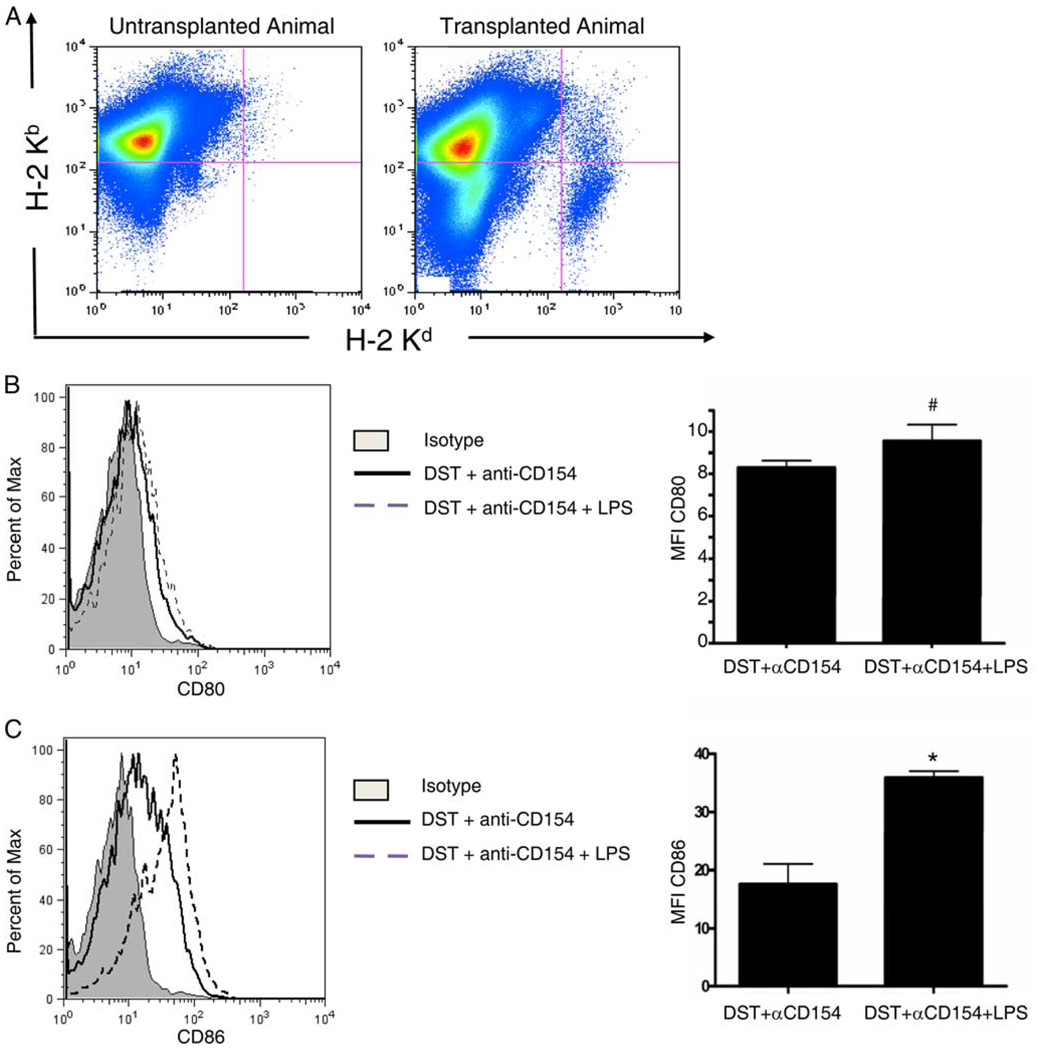
We have previously shown that anti-CD154 mAb prevents up-regulation of CD80 on APCs in the DST (32). To investigate the activation status of cells in the DST following administration of LPS treatment, spleen cells from C57BL/6 mice were analyzed by flow cytometry 15 h after treatment with BALB/c DST, anti-CD154 mAb, without or with LPS injected at the time of DST. Gating on donor (H2-Kb −2-Kd+) cells (Fig. 2A), the DST of mice treated with anti-CD154 mAb and LPS had a modest, but statistically significant increase in the expression of CD80 (MFI = 9.6 ± 0.8, n = 4) compared with mice treated only with anti-CD154 (MFI = 8.3 ± 0.4; n = 4; p = 0.025; Fig. 2B). Expression of CD86 was enhanced 2-fold on the DST of mice treated with anti-CD154 mAb and LPS (MFI = 36.0 ± 1.1, n = 4) compared with mice treated only with anti-CD154 mAb (MFI = 17.6 ± 3.4; n = 4; p < 0.0001; Fig. 2C). These data suggest that LPS increases the expression of costimulatory molecules on cells in the DST.
FIGURE 2.
LPS administration induces up-regulation of costimulatory molecules on cells in the DST in mice treated with costimulation blockade. C57BL/6 mice were injected with 10 × 106 BALB/c DST and 0.5 mg of anti-CD154 mAb without or with an i.p. injection of LPS. Fifteen hours later, splenocytes were recovered and stained with Abs to H2-Kb (host), H2-Kd (donor), and CD80 or CD86 and analyzed by flow cytometry. A, Representative flow cytometry dot plots showing host H2-Kb and donor H2-Kd staining. A group of nontransplanted mice were used as negative controls for H2-Kd staining. B and C, Left panels show a representative histogram of the MFI of CD80 (B) and CD86 (C) expression on the H2-Kb −2-Kd+ cells that are seen in A. These cells represent cells in the BALB/c DST. The right panels are histograms that summarize the MFI + 1 SD of CD80 (B) and CD86 (C) expression. Data contain four mice per group. #, p = 0.025; *, p < 0.0001.
LPS up-regulates expression of costimulatory molecules on host alloantigen-presenting dendritic cells (DCs)
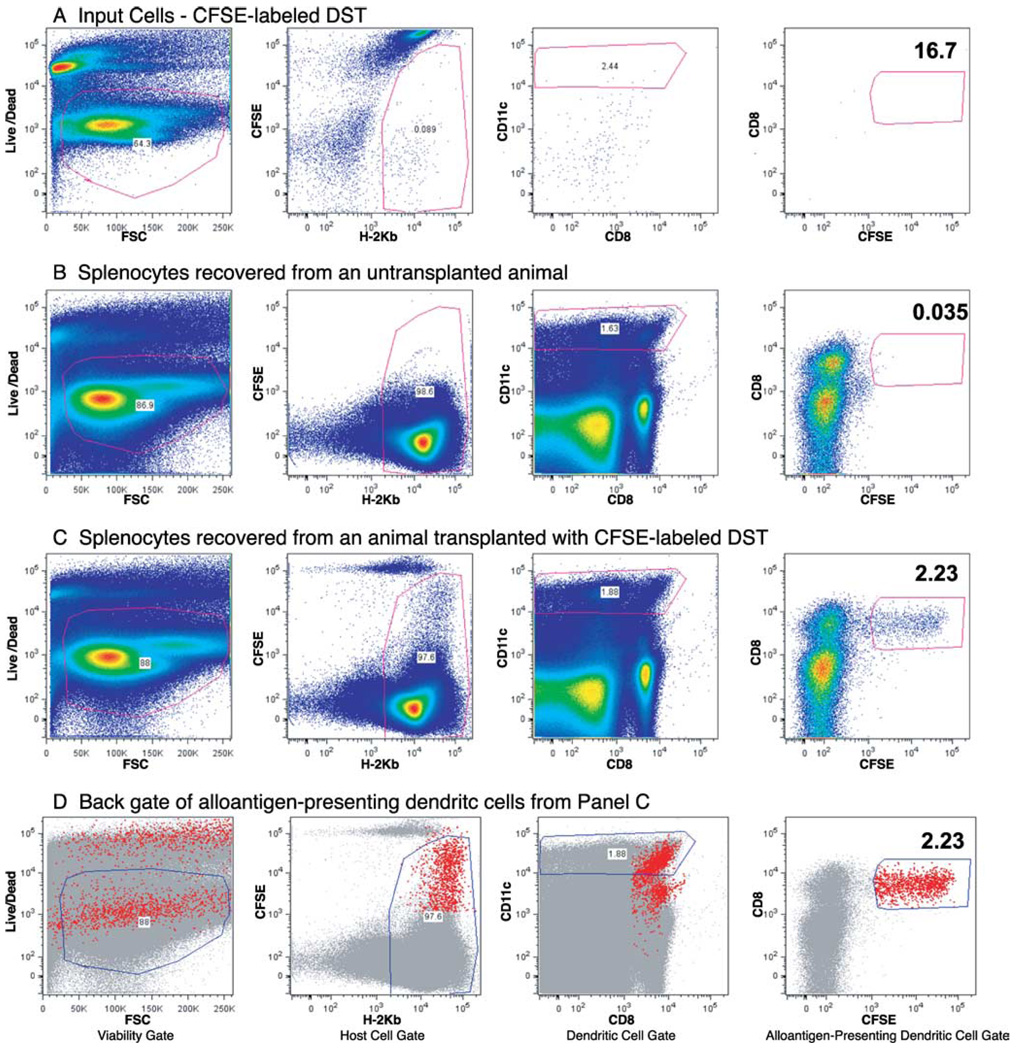
Although LPS treatment appears to increase expression of CD80/86 on cells in the DST, we have observed that CD80/86 expression on the DST is not required to rescue alloreactive CD8+ T cells from costimulation blockade-induced deletion in the presence of LPS (18). Therefore, we next examined the effect of LPS on host APC maturation. C57BL/6 mice were injected with CFSE-labeled BALB/c DST and treated with anti-CD154 mAb without or with injection of LPS at the time of DST. Host DCs that have phagocytosed the cells in the DST can be detected by gating on H2-Kb+CFSE+ DCs. Fig. 3 represents the gating strategy used to identify alloantigen-presenting host DCs.
FIGURE 3.
Gating scheme for in vivo tracking of phagocytosed DST. C57BL/6 mice were injected with 10 × 106 CFSE-labeled BALB/c DST and 0.5 mg of anti-CD154 mAb without or with an i.p. injection of LPS. Sixteen hours later, splenocytes were harvested, stained with Live/Dead blue, Abs to H2-Kb (host), CD8α, CD11c, and CD86, and analyzed by flow cytometry. For each panel, the dot plot on the left shows the cells that were considered “viable” based on Live/Dead blue staining. The dot plot second from the left shows the cells that were considered of host origin based on staining with H-2Kb. The dot plot second from the right shows the cells that were considered DCs based on staining with CD11c. The dot plot on the right shows DCs that have engulfed CFSE+ DST (the alloantigen-presenting DCs or “apDCs”). A, Representative flow cytometry dot plots of the input cell, i.e., CFSE-labeled BALB/c splenocytes, before injection. B, Representative flow cytometry dot plots of splenocytes from an uninjected C57BL/6 mouse. These splenocytes do not contain a CFSE+ population. C, Representative flow cytometry dot plots of splenocytes from a C57BL/6 mouse injected with CFSE-labeled BALB/c DST. This panel demonstrates that the spleen of animals injected with CFSE-labeled DST contain a population of host DCs that have phagocytosed CFSE-labeled DST. D, A “back gate” of the CFSE+CD11c+H2-Kb+ cells from C (i.e., host DCs that have phagocytosed DST). This panel indicates that the host alloantigen-presenting DCs are found in the mid-to-upper right of the Host Cell Gate (second panel from the left). That population is only present in the spleen of animals transfused with DST.
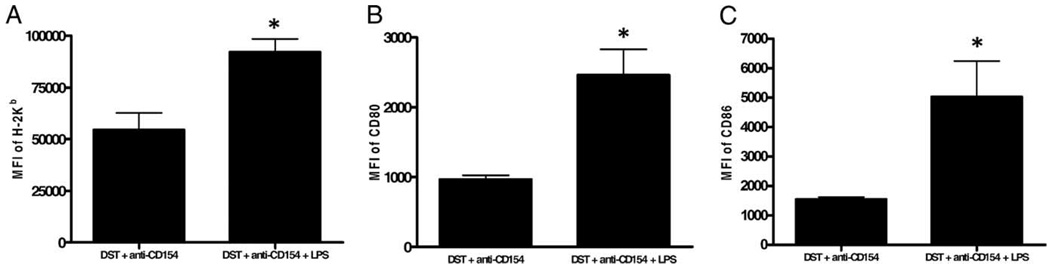
Fifteen hours after injection of DST, spleen cells were recovered and analyzed by flow cytometry for the expression of CFSE, MHC class I, CD80 and CD86. Similar to the findings of Iyoda et al. (33), we observed that essentially all of the alloantigen-containing host DCs were CD8α+. These APCs have been shown to be presenting alloantigen by the indirect Ag presentation pathway (33). Gating on H2-Kb+CD11c+CD8α+CFSE+ cells, we observed that LPS leads to marked up-regulation of MHC class I (MFI = 92,104 ± 6,419; n = 4) and both CD80 (MFI = 2,461 ± 371; n = 3) and CD86 (MFI = 5,032 ± 1,210; n = 3) compared with non-LPS-treated controls (H-2Kb MFI = 54,565 ± 8,207; n = 4, p < 0.001, Fig. 4A; CD80 MFI = 966 ± 61; n = 3; p < 0.01, Fig. 4B; CD86 MFI = 1,547 ± 76; n = 3; p < 0.01, Fig. 4C). These data demonstrate that LPS increases the maturation state of host DCs that have phagocytosed alloantigen.
FIGURE 4.
TLR activation leads to up-regulation of costimulatory molecules on alloantigen-presenting DCs in mice treated with costimulation blockade. C57BL/6 mice were injected with 10 × 106 CFSE-labeled BALB/c DST and 0.5 mg of anti-CD154 mAb without or with an i.p. injection of 100 µg of LPS. Sixteen hours later, splenocytes were harvested, stained with Live/Dead blue, and Abs to H2-Kb (host), CD8α, CD11c, and CD80 or CD86 and analyzed by flow cytometry. Data are representative of at least two independent experiments with at least three mice per group. A, Histogram of the MFI of the class I molecule H2-Kb. *, p < 0.01 vs DST + anti-CD154. B, Histogram of the MFI of CD80. *, p < 0.01 vs DST + anti-CD154. C, Histogram of the MFI of the class I molecule CD86. *, p < 0.01 vs DST + anti-CD154.
TLR4 expression is required on host cells for the effects of LPS on the establishment of hematopoietic chimerism in mice treated with costimulation blockade
Previously, we have shown that LPS administration mediates its effects on skin allograft survival in mice treated with costimulation by engaging TLR4 on cells of the host (4). Therefore, to test whether engagement of TLR4 on host cells was also required for LPS to prevent the induction of hematopoietic chimerism, C57BL/10 and C56BL/10.TLR4−/− mice were treated with BALB/c DST, anti-CD154 mAb, and transplanted with BALB/c bone marrow and skin allografts without or with injection of LPS at the time of costimulation blockade.
Treatment of wild-type C57BL/10 mice with costimulation blockade led to chimerism (three of seven) and prolonged skin graft survival (median survival time (MST) = 125 days; Table II, group 3), which were both prevented by administration of LPS (0 of 12 became chimeric; skin graft survival MST = 11 days; p < 0.05; Table II, group 4). In contrast, C57BL/10.TLR4−/− mice treated with DST and anti-CD154 mAb in the absence (10 of 13) or presence (9 of 10) of LPS also became chimeric (p = NS; Table II, groups 5 and 6) and exhibited prolonged skin graft survival (MST >195 days for LPS-treated mice vs MST = 176 days for control mice; p = NS; Table II, groups 5 and 6). These data suggest that TLR4 expression on host cells is required for the effects of LPS on the establishment of hematopoietic chimerism in mice treated with costimulation blockade.
The effect of LPS on the establishment of hematopoietic chimerism in mice treated with costimulation blockade is not dependent through the MyD88-dependent pathway
LPS can signal through two distinct pathways following ligation of TLR4, the MyD88-Toll/IL-1 receptor domain-containing adaptor protein (TIRAP) pathway, and the TRIF-related adaptor molecule (TRAM)-Toll/IL-1 receptor domain-containing adaptor protein inducing IFN-β (TRIF) pathway (7, 9). Therefore, we next examined whether the MyD88-TIRAP pathway was required for LPS to prevent the establishment of hematopoietic chimerism.
To test this, B6.MyD88−/− mice were treated with our standard costimulation blockade protocol without or with an injection of LPS at the time of DST and transplanted with BALB/c bone marrow. B6.MyD88−/− mice treated with DST and anti-CD154 mAb became chimeric (four of five); however, mice treated with LPS did not (zero of nine, p = 0.0015; Table II, groups 7 and 8). These data indicate that signaling through the MyD88 adaptor molecule is not required for the effects of LPS on the establishment of hematopoietic chimerism in mice treated with costimulation blockade.
LPS treatment induces the production of type I IFN, which is sufficient to prevent the establishment of hematopoietic chimerism in mice treated with costimulation blockade
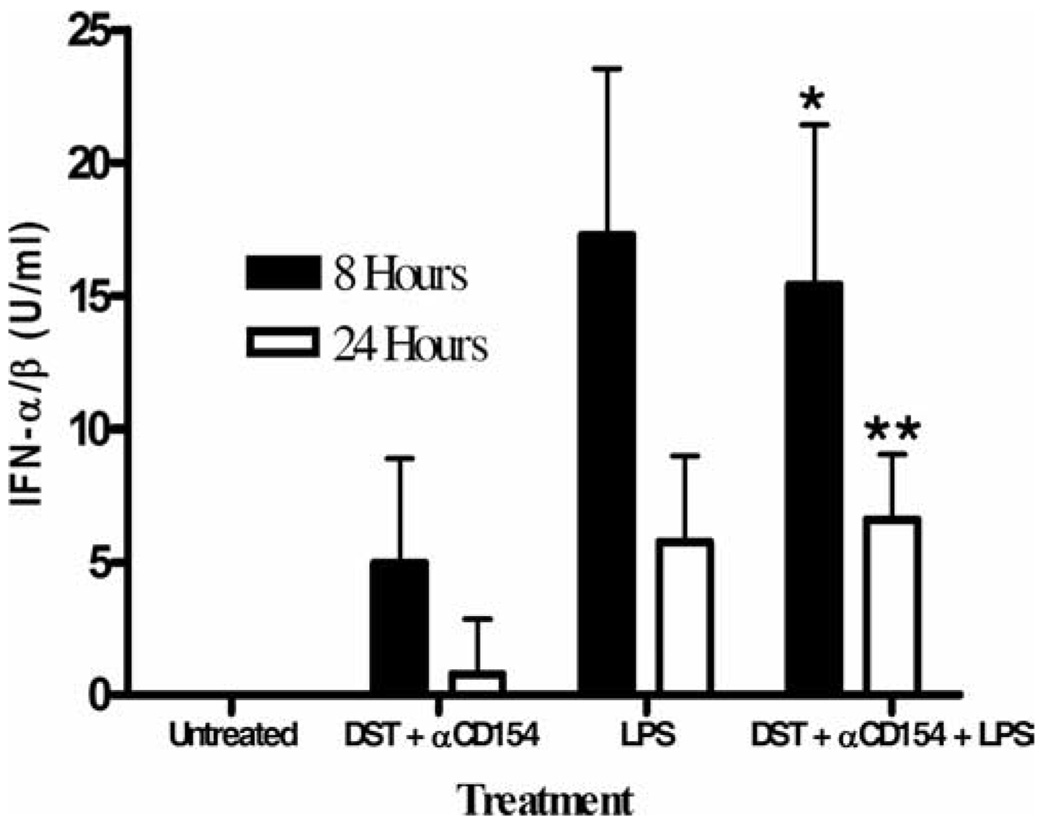
Because the MyD88-TIRAP pathway was not required for LPS to prevent chimerism, we hypothesized that the MyD88-independent TRAM-TRIF pathway may be important. TLR4 signaling through the TRAM-TRIF pathway leads to the up-regulation of type I IFNs and it has been hypothesized that TLR4-dependent induction of type I IFN is entirely mediated through a MyD88-independent pathway (9). We first quantified the levels of IFN-α/β in the serum of wild-type C57BL/6 mice treated with costimulation blockade at various times after LPS administration using a standard IFN-α/β bioassay (25). IFN-α/β was not detected in untreated mice and only low levels were detected in mice treated with DST and anti-CD154 mAb at 8 h (5.0 ± 3.9 U/ml; Fig. 5). These low levels dropped to nearly undetectable levels 24 h after treatment (0.8 ± 2.1 U/ml). In contrast, mice treated with LPS had 3-fold higher levels of IFN-α/β 8 h after treatment with DST, anti-CD154 mAb, and LPS (15.4 ± 6.0 U/ml, p < 0.05; Fig. 5). We also observed that levels of IFN-α/β at 24 h in mice treated with costimulation blockade and LPS were comparable to those in mice treated with DST and anti-CD154 mAb at 8 h (p = NS; Fig. 5). IFN-α/β was also detected at 24 h in the serum of B6.MyD88−/− mice treated with DST, anti-CD154 mAb, and LPS but not in B6.MyD88−/− mice treated with only DST and anti-CD154 mAb (22.5 ± 12 and 2.5 ± 2.9 U/ml, respectively, p < 0.05, n = 4), suggesting that LPS will induce IFN-α/β production independently of MyD88 signaling.
FIGURE 5.
LPS administration leads to a transient production of type I IFN. Sera were collected from C57BL/6 mice 8 and 24 h after indicated treatment. The IFN-α/β titer was determined as the reciprocal of the dilution that protected L-929 cells from cytopathic effect by vesicular stomatitis virus infection. Sera from untreated mice did not exhibit any protection. Data are presented as mean + SD and each group contains at least three mice. *, p < 0.05 vs DST and anti-CD154 mAb at 8 h; **, p < 0.01 vs DST and anti-CD154 mAb at 24 h.
We next investigated whether administration of recombinant mouse IFN-β would recapitulate the effects of LPS administration. To test this, we transplanted C57BL/6 mice with BALB/c bone marrow and skin grafts using our standard costimulation blockade protocol without (group 1) or with 5.0 × 104 U (group 2) or 7.5 × 104 U (group 3) of IFN-β on day −7, the day of DST. Only one of three mice treated with 5.0 × 104 U of IFN-β became chimeric, but two of three mice did exhibit prolonged skin graft survival (MST = >252 days; Table III). Mice treated with 7.5 × 104 U of IFN-β on day −7 uniformly failed to become chimeric (zero of four; p < 0.05 vs group 1) and skin survival was short (MST = 36 days, p < 0.05 vs group 1; Table III). These data suggest that IFN-β by itself is sufficient to recapitulate the effects of LPS on the establishment of chimerism and prolongation of skin allograft survival when given at the time of tolerance induction.
Table III.
Recombinant IFN-β is sufficient to prevent the establishment of hematopoietic chimerism and transplantation tolerance in mice treated with costimulation blockade
| Group | IFN-β | Chimerism Frequency | Donor origin PBMCs at 8 wk (%) | MST of Skin Grafts in Transplanted Mice (days) | MST of Skin Grafts in Nonchimeric Mice (days) | MST of Skin Grafts in Chimeric Mice (days) |
|---|---|---|---|---|---|---|
| 1 | None | 6/10 (60.0%) | 1.98 ± 2.34 | >252 | 35 | >252 |
| 2 | 5.0 × 104 U | 1/3 (33.3%) | 2.65 | >252 | 181 | >252 |
| 3 | 7.5 × 104 U | 0/4 (0.00%)* | <0.10 | 36# | 36 | N/A |
C57BL/6 mice were treated with BALB/c DST, anti-CD154 mAb, and transplanted with BALB/c bone marrow and skin according to our standard protocol. Mice treated with rIFN-β were given an i.p. injection of the indicated amount on day −7 relative to bone marrow cell and skin transplantation. Hematopoietic chimerism was defined as >0.10% donor origin PBMCs 7–8 wk after bone marrow injection. The percentage of donor origin PBMCs is the mean ± SD percentage of the levels observed in chimeric mice. For groups with no chimeric mice, we used the limit of detection (<0.10) to indicate a lack of chimerism.
p < 0.05 vs group 1 by unpaired t test
p < 0.05 vs group 1 by log-rank analysis.
N/A, not applicable.
The TLR4→type I IFN pathways are important for LPS-mediated up-regulation of costimulatory molecules on host alloantigen-presenting DCs and for priming of host alloreactive CD8+ T cells
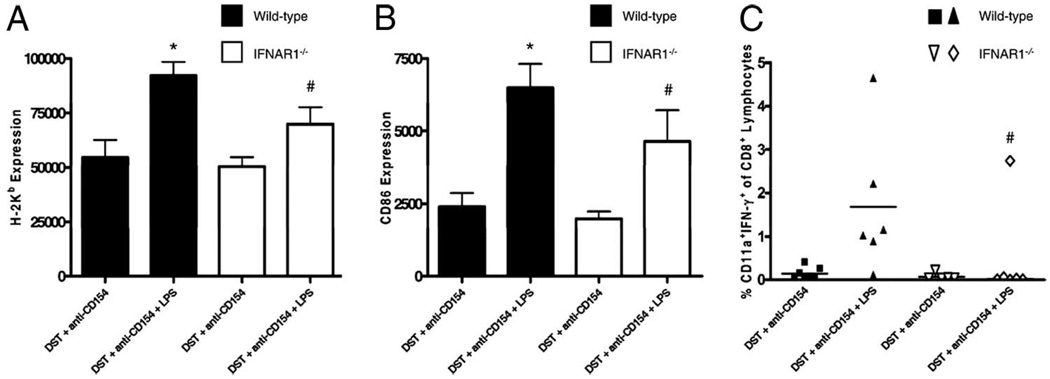
Type I IFN has been reported to enhance DC maturation (34) and is important in the priming of naive T cells (35). We next hypothesized that induction of type I IFN by TLR4 activation may be critical for the up-regulation of costimulatory molecules on host alloantigen-presenting cells. To test this, wild-type C57BL/6 and B6.IFNARI−/− mice were injected with CFSE-labeled BALB/c splenocytes and treated with anti-CD154 mAb without or with LPS injection. Fifteen hours later, spleen cells were recovered and analyzed for the expression of MHC class I and CD86. Alloantigen-presenting DCs from mice lacking the type I IFN receptor exhibited a significantly lower expression of MHC class I (H2-Kb MFI = 69,793 ± 7,852, p < 0.01; Fig. 6A) and the costimulatory molecule CD86 (CD86 MFI = 4,650 ± 1,066, p < 0.01; Fig. 6B) after injection of LPS compared with alloantigen-presenting DCs from wild-type mice (92,104 ± 6,419 and 6,487 ± 829, respectively). The expression of both molecules, however, was higher in B6.IFNAR1−/− mice treated with LPS when compared with their non-LPS-treated controls (p < 0.05), indicating that other factors may be involved in the maturation of host alloantigen-presenting DCs.
FIGURE 6.
Signaling through the type I IFN receptor is important for the maturation of host alloantigen-presenting DCs and the priming of alloreactive CD8+ T cells. A and B, C57BL/6 and C57BL/6.IFNARI−/− mice were injected with 10 × 106 CFSE-labeled BALB/c DST and 0.5 mg of anti-CD154 mAb without or with an i.p. injection of LPS. Fifteen hours later, splenocytes were harvested, stained with Abs to H2-Kb (host), CD8α, CD11c, and CD86 and analyzed by flow cytometry. Data are representative of two independent experiments with at least three mice per group. A, MFI of the MHC class I molecule H2-Kb. *, p < 0.01 vs all other groups. #, p = NS vs wild-type mice treated with DST and anti-CD154 mAb; p < 0.01 vs wild-type mice treated with DST, anti-CD154 mAb, and LPS; p < 0.05 vs IFNAR1−/− mice treated with DST and anti-CD154 mAb. B, MFI of CD86. *, p < 0.05 vs IFNAR1−/− mice treated with DST, anti-CD154 mAb, and LPS; p < 0.001 vs both IFNAR1−/− and wild-type mice treated with DST and anti-CD154 mAb. #, p < 0.01 vs both IFNAR1−/− and wild-type mice treated with DST and anti-CD154 mAb. C, All mice were treated with a BALB/c DST, bone marrow, and anti-CD154 mAb according to our standard protocol without or with an i.p. injection of 100 µg of LPS. Splenocytes were harvested 1 wk after bone marrow transplantation, stimulated in vitro with either irradiated syngeneic (H2b) or allogeneic (H2d) splenocytes, and analyzed by flow cytometry for intracellular IFN-γ production. The percentage of CD8+ lymphocytes producing IFN-γ is shown (bar represents the mean). Data are pooled from two independent experiments. #, p < 0.05 vs wild-type C57BL/6 mice treated with DST, anti-CD154 mAb, and LPS. p = NS vs IFNARI−/− treated with DST and anti-CD154 mAb.
We next hypothesized that decreased DC activation in the mice lacking the type I IFN receptor may prevent the generation of alloreactive CD8+ effector/memory T cells. To test this, we performed intracellular flow cytometry for IFN-γ on CD8+ splenocytes isolated from C57BL/6 and B6.IFNARI−/− mice 1 wk after treatment with our standard costimulation blockade protocol and transplantation of BALB/c bone marrow without or with LPS. Interestingly, we observed that mice lacking the type I IFN receptor did not develop effector/memory CD8+ T cells when exposed to LPS, whereas wild-type C57BL/6 mice did (Fig. 6C). These data suggest that type I IFNs are required for DC induction of alloreactive CD8+ effector/memory T cells.
Type I IFN signaling is important for the effects of LPS on the establishment of hematopoietic chimerism in mice treated with costimulation blockade
The observation that type I IFN was critical for the full maturation of host alloantigen-presenting DCs and for the priming of alloreactive CD8+ T cells prompted us to hypothesize that signaling through the type I IFN receptor would be required for the effects of LPS on chimerism in mice treated with costimulation blockade. To test this, B6.IFNARI−/− mice were treated with DST and anti-CD154 mAb and transplanted with BALB/c bone marrow and skin without or with injection of LPS at the time of DST administration. As expected, B6.IFNARI−/− not treated with LPS became chimeric (13 of 15) and chimeric mice exhibited permanent skin graft survival (MST = >218 days; Table II, group 9). Interestingly, some B6.IFNARI−/− mice treated with LPS became chimeric (6 of 17; p = 0.0005 vs wild-type animals treated with LPS), and chimeric mice again exhibited permanent skin graft survival (MST = >218 days; Table II, group 10). However, the percentage of B6.IFNARI−/− mice treated with LPS that became chimeric was lower than that achieved in B6.IFNARI−/− not treated with LPS (p = 0.008; Table II, groups 9 and 10). These data suggest that although signaling through the type I IFN receptor is important, other factors may be involved.
In support of this interpretation, B6.IFNARI−/− mice were also partially protected from the effects of poly(I:C), as some of the mice conditioned with costimulation blockade and transplanted with BALB/c bone marrow and skin became chimeric (5 of 12; p < 0.01 vs B6.IFNARI−/− not treated with poly(I:C)) and chimeric mice also exhibited permanent skin graft survival (MST = >218 days; Table II, group 11). Interestingly, mice deficient in signaling through the type I IFN receptor were not protected from the effects of Pam3Cys (zero of four chimeric, MST = 10; Table II, group 12). These data indicate that signaling through the type I IFN receptor is important for the ability of some, but not all, TLR agonists to prevent the establishment of hematopoietic chimerism in mice treated with costimulation blockade.
Type I IFN signaling and MyD88 act synergistically to mediate the effects of LPS on the establishment of hematopoietic chimerism in mice treated with costimulation blockade
The observation that B6.IFNARI−/− mice treated with LPS became chimeric at a frequency that was significantly lower than B6.IFNARI−/− mice not treated with LPS (Table II) led us to conclude that other molecules in the TLR4 pathway were important. We hypothesized that in the absence of IFN-α/β signaling downstream mediators of the TLR4→MyD88 axis might be sufficient to prevent the establishment of hematopoietic chimerism. To test this, we treated B6.MyD88−/− IFNAR1−/− mice with our standard costimulation blockade protocol without or with coinjection of LPS and transplanted them with BALB/c bone marrow and skin. The majority of the B6.MyD88−/− IFNAR1−/− mice transplanted in the absence of LPS developed hematopoietic chimerism (five of seven) and exhibited prolonged skin graft survival (MST = >140 days; Table II, group 13). Similar to TLR4−/− mice, B6.MyD88−/−IFNAR1−/− mice treated with LPS also developed hematopoietic chimerism (five of eight; p < 0.001 vs Table I, group 2; p = NS vs Table II, group 13) and displayed prolonged skin allograft survival (MST = >140 days; Table II, group 14). Importantly, in the absence of both MyD88 and IFNAR1, mice not conditioned with costimulation blockade did not become chimeric (zero of three) and they exhibited short skin allografts survival (MST = 11 days). These data suggest that signaling through the type I IFN receptor and mediators downstream of MyD88 are important for LPS-mediated effects in mice treated with costimulation blockade.
Timing of LPS administration influences its ability to prevent the establishment of hematopoietic chimerism in mice treated with costimulation
To determine the kinetic relationship between administration of TLR agonists and bone marrow transplantation, four cohorts of C57BL/6 mice were treated with DST, anti-CD154 mAb, and given BALB/c bone marrow. One cohort was not treated further (group 1), one cohort was given LPS on day −8 (group 2, 24 h before DST and anti-CD154 mAb), another cohort was given LPS on day −7 (group 3), and the last cohort was given LPS on day +1 relative to bone marrow transplantation on day 0 (group 4). As expected, mice not treated with LPS became chimeric (six of seven), while those given LPS on day −7 did not (zero of seven; Table IV, groups 1 and 3, respectively). Mice treated with LPS on day −8 (group 2) became chimeric at the same frequency (six of seven) as mice not treated with LPS (group 1). Mice treated the day after bone marrow transplantation did not become chimeric (zero of seven; Table IV, group 4). These data indicate that the effect of TLR activation on the establishment of hematopoietic chimerism in mice treated with costimulation blockade is dependent on the timing of administration of the TLR agonist.
Table IV.
Effects of LPS on the establishment of hematopoietic chimerism are dependent on the time of administrationa
| Group | TLR Agonist | Chimerism Frequency (%) | Donor Origin PBMCs at 8 wk (%) |
|---|---|---|---|
| 1 | None | 6/7 (85.7%) | 3.20 ± 1.84 |
| 2 | LPS (given on day −8) | 6/7 (85.7%) | 4.93 ± 2.04 |
| 3 | LPS (given on day −7) | 0/7 (0)b | <0.10 |
| 4 | LPS (given on day +1) | 0/7 (0%)b | <0.10 |
C57BL/6 mice were treated with BALB/c DST, anti-CD154 mAb, and transplanted with BALB/c bone marrow according to our standard protocol without (group 1) or with an i.p. injection of 100 µg of LPS 24 h before DST and anti-CD154 mAb (group 2), on the day of DST and anti-CD154mAb (group 3) or 24 h after bone marrow transplantation (group 4). Hematopoietic chimerism was defined as >0.10% donor origin PBMCs 8 wk after bone marrow injection. Percent donor origin bone marrow cells is the mean ± SD chimerism levels in chimeric mice. For groups with no chimeric mice, we used the limit of detection (<0.10) to indicate a lack of chimerism. Data are pooled from two independent experiments.
p = 0.0012 vs groups 1 and 2.
Discussion
In this report, we have shown that TLR activation at the time of costimulation blockade prevents the establishment of mixed allogeneic hematopoietic chimerism and shortens skin allograft survival. Investigation of the cellular mechanisms responsible for this effect revealed that both CD4 and CD8 T cells, but not NK cells, were involved and that LPS administration matured host alloantigen presenting DCs, leading to the generation of alloreactive effector/memory CD8 T cells. Investigation of the molecular mechanisms revealed that LPS effects could be mediated through either the MyD88 pathway or the type I IFN receptor pathway.
Our working hypothesis for costimulation blockade-induced graft tolerance is that conditioning with a transfusion of donor cells in the presence of anti-CD154 mAb leads to a state of hyporesponsiveness in the donor-specific host alloreactive T cell pool before the transplantation of the allograft. This state of donor-specific hyporesponsiveness is thought to be due to an early abortive expansion that leads to apoptosis and deletion of the majority of the alloreactive T cell pool while rendering the remaining host alloreactive T cells nonresponsive (36–39). This process could be initiated by the presentation of allopeptides via the direct or indirect Ag presentation pathways (40). The direct presentation hypothesis postulates that in the presence of anti-CD154 mAb, recognition of immature APCs in the DST leads to host T cell hyporesponsiveness. Work from our laboratory supports this hypothesis, as we have shown that failure to up-regulate the costimulatory molecule CD80 on cells in the DST was associated with costimulation blockade-induced tolerance to islet allografts (32). In other studies using a transgenic model in which B cells of the DST expressed CD80 under the control of the IgM promoter, it was shown that recipients of allogeneic islets could not be tolerized when cells of the DST expressed high levels of CD80. In contrast, others have provided evidence for a role of indirect alloantigen presentation in tolerance induction (40, 41). This hypothesis is based on the observation that cells of the DST are quickly rendered apoptotic and phagocytosed by immature host DCs. Coadministration of anti-CD154 mAb prevents the maturation of the host alloantigen-presenting DCs and, consequently, alloreactive T cells are tolerized by a mechanism that closely mimics those used to maintain peripheral self-tolerance (42). TLR activation at the time of DST transfer could be interfering with either, or both, of these mechanisms.
We observed that APCs in the DST up-regulated the costimulatory molecules CD80 and CD86 in mice treated with costimulation blockade plus LPS. Therefore, it is possible that TLR activation increases the immunogenicity of the DST, leading to alloreactive T cell priming and rescue from costimulation blockade-induced apoptosis. TLR-mediated maturation of APCs within the DST could result from direct engagement of TLRs expressed on the donor cells or through the secretion of cytokines by TLR-stimulated host cells. Our results show that hematopoietic chimerism and long-term skin allograft survival were achieved in the presence of costimulation blockade and LPS only when the host was deficient in TLR4. This would suggest that activation of APCs within the DST by direct ligand-receptor interaction is not sufficient to prevent tolerance induction. Moreover, maturation of APCs in the DST is likely not required for the effects of LPS on naive allospecific T cells, as we have shown that LPS still prevented the deletion of alloreactive CD8+ T cells in mice treated with costimulation blockade even when the DST was obtained from CD80/86 knockout donors (18). Our results, however, do not exclude the possibility that the production of cytokines by LPS-stimulated host cells could activate APCs in the skin allograft, which may contribute to the rejection process. Taken together, our data suggest that 1) there are alternative costimulatory molecules expressed by APCs in the DST that prime donor-specific alloreactive CD8 T cells or 2) Ag presentation by host APCs matured by direct TLR stimulation is playing an important role in preventing the deletion of alloreactive CD8 T cells.
To address the effects of TLR activation on host APCs presenting donor alloantigen, we used a CFSE-labeling system that allowed us to examine the phenotype of host DCs that had engulfed the transferred cells in the DST. We found that activation of TLR4 on the day of DST administration led to a marked up-regulation of MHC class I and costimulatory molecules within 24 h on host DCs that had phagocytosed CFSE-labeled DST. Therefore, we speculate that TLR activation on the day of tolerance induction significantly alters the context in which allopeptides are presented to the alloreactive T cell compartment. Instead of mimicking the mechanism of cross-tolerance to self-Ags and inducing transplantation tolerance, TLR-licensed APCs deliver an immunogenic signal that leads to the generation of cytotoxic effector/memory alloreactive CD8 T cells and graft rejection.
Data from experiments investigating the molecular mechanism by which LPS prevents the establishment of chimerism in mice treated with costimulation blockade also supports a role for host APCs. We observed that signaling through TLR4 and induction of IFNα/β were more important than signaling through MyD88. These data support the in vitro experiments by Hoebe et al. (34) showing that LPS-induced expression of costimulatory molecules on APCs was independent of MyD88 signaling and solely dependent on TLR4 signaling and the induction of type I IFN. Supporting this interpretation, we observed that host alloantigen-presenting DCs isolated from LPS-treated animals lacking the type I IFN receptor expressed lower levels of costimulatory molecules compared with similarly treated wild-type controls. Based on these data, we hypothesize that mice deficient in the type I IFN receptor are more resistant to the effects of LPS due to the incomplete maturation of their alloantigen-presenting DCs. Decreased DC maturation also correlates with the impaired ability of these mice to generate alloreactive CD8+ T cells.
Interestingly, we observed that although mice deficient in the type I IFN receptor became chimeric when exposed to LPS, the frequency of chimerism was lower than that achieved in type I IFN receptor knockout mice not given LPS. This suggested that an additional as yet unidentified mediator(s) might exist. We further confirmed that these factors were MyD88 dependent. One potential MyD88-dependent candidate is IL-6. We have previously shown that Tregs are critical for the induction of hematopoietic chimerism (27). IL-6 has been shown to prevent the induction of Tregs, generate proinflammatory TH17 cells (43), and render effector T cells refractory to Treg suppression (44). Therefore, induction of both IL-6 and type I IFN by LPS may contribute synergistically to prevent hematopoietic chimerism through the generation of effector T cells and the disruption of Tregs, a possibility our laboratory is currently investigating.
We also observed that Pam3Cys but not poly(I:C) prevented the establishment of hematopoietic chimerism in type I IFN receptor knockout mice. This observation is perhaps not surprising, since TLR1/2 signaling by Pam3Cys does not induce type I IFN production; however, it highlights the fact that overlapping, but distinct, immune responses are triggered during an infection, and these may act synergistically to affect tolerance induction in a clinical setting. For example, direct triggering of TLR2, but not TLR4, on Tregs has been shown to decrease the ability of regulatory cells to suppress the activity of effector T cells (45, 46). Differential effects on regulatory cells could explain why TLR agonists like LPS and poly(I:C) depend on type I IFN for their full effects on chimerism induction, whereas Pam3Cys does not.
It has long been appreciated that different tissues vary in their susceptibility to tolerance induction by costimulation blockade. For example, survival of cardiac and islet allografts can be significantly prolonged with anti-CD154 mAb monotherapy, whereas skin allografts cannot (47–49). Data also suggest that the mechanisms by which TLR agonists shorten allograft survival in mice treated with costimulation blockade may differ between tissues as well (4, 5). For example, LPS-shortened skin allograft survival in mice treated with costimulation blockade was solely dependent on host CD8+ cells (4), while CpG-mediated rejection of cardiac allografts was solely dependent on host CD4+ T cells (5). In this study, we show that neither population is the sole mediator of the effects of LPS, as either subset is sufficient to prevent the establishment of hematopoietic chimerism. Furthermore, we have previously shown that skin allograft survival could be prolonged with costimulation blockade when LPS was coadministered on the day of DST and anti-CD154 mAb if the host was deficient in either MyD88 or the type I IFN receptor (18). This indicates that a synergy between the MyD88-dependent and the MyD88-independent pathways are required to shorten skin allograft survival. In contrast, activation of either signaling pathway impairs the establishment of hematopoietic chimerism.
Reasons for these differences are not known, but could include differential susceptibility of the graft to effectors such as NK cells, which are potent killers of allogeneic hematopoietic cells but are not effectively cytolytic to solid organ allografts (15). It could also reflect the fact that hematopoietic grafts, unlike solid organ grafts, must not only evade the host’s immune system, but also home to specific niches in the recipient’s bone marrow in order for long-term hematopoietic chimerism to develop. Recent work has shown that migration of hematopoietic stem cells is affected by fluctuations in soluble mediators such as CXCL12, which can be modulated by stressors such as LPS (50).
Finally, we observed that the effects of LPS on the establishment of chimerism are temporally dependent. Administration of LPS on the day of DST and anti-CD154 mAb administration uniformly prevented the establishment of chimerism. In contrast, LPS injected 1 day before the initiation of tolerance induction did not. We speculate that treatment with LPS on the day before DST and anti-CD154 mAb administration does not prevent chimerism because the levels of proinflammatory cytokines such as type I IFN subside by the time that alloantigen (DST) is injected. Therefore, host DCs, or APCs in the DST, are not triggered to mature and present alloantigen in an immunogenic context and, consequently, naive alloreactive T cells are tolerized. Interestingly, injection of LPS the day after bone marrow transplantation prevents the establishment of chimerism. Given that alloreactive CD8+ T cells are largely deleted within the first several days of tolerance induction (38), it is likely that TLR4 activation 1 day after transplantation prevents chimerism independent of its effects on the deletion of alloreactive T cells. This is supported by preliminary observations in mice treated with costimulation blockade and transplanted simultaneously with allogeneic bone marrow and skin and injected 1 day later with LPS. In these mice, hematopoietic chimerism is prevented, but skin allografts are not acutely rejected (data not shown). This suggests that mechanisms that control the establishment of hematopoietic chimerism are not the same as those that control solid organ graft survival when LPS is administrated after transplantation. One possible difference is that NK cells express various TLRs and can be stimulated directly by TLR agonists (51, 52). Thus, it is possible that NK cells may be acting as a barrier to hematopoietic engraftment when LPS is administered at this later time point.
In summary, we have demonstrated that the establishment of hematopoietic chimerism in mice treated with costimulation blockade can be prevented by the administration of TLR agonists. We recognize that the use of individual TLR agonists, such as LPS, poly(I:C), or Pam3Cys, does not mimic all aspects of an actual infection. However, this approach does permit the identification of mechanisms involved in a specific aspect (i.e., TLR engagement or dsRNA pathway activation) of a more complex infection (i.e., viral, bacterial). By dissecting the individual components of the innate immune response, we can more clearly identify the mechanisms by which an infection impairs transplantation tolerance. In this investigation, we found that the ability of LPS to prevent chimerism is dependent on the time of administration and is mediated by CD4+ and CD8+ lymphocytes when injected on the first day of tolerance induction. LPS appears to mediate this effect by enhancing the maturation of alloantigen-presenting DCs, a process dependent on the production of type I IFNs and MyD88-dependent factors. These findings highlight the complex interactions between host immunity and environmental perturbations during the establishment of hematopoietic chimerism and tolerance. This is particularly relevant because data from preclinical (1–3, 53) and clinical studies (54, 55) have demonstrated the ability of concurrent bone marrow transplantation to induce prolonged survival of allogeneic solid organs in the absence of maintenance immunosuppressive therapy. Therefore, understanding the mechanisms that underlie disruption of hematopoietic chimerism may yield novel strategies that circumvent this barrier, and lead to more effective transplantation regimens.
Acknowledgments
We thank Amy Cuthbert, Cindy Bell, Kapil Bahl, and Linda Paquin for their excellent technical assistance.
Footnotes
This work was supported in part by National Institutes of Health Research Grant AI42669, American Diabetes Association Grant 7-05-PST-02, the Juvenile Diabetes Research Foundation, International, and Diabetes Endocrinology Center Research Grant DK32520 from the National Institutes of Health.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this paper: DST, donor-specific transfusion; DC, dendritic cell; MFI, median fluorescence intensity; poly(I:C), polyinosinic: polycytidylic acid; Pam3Cys, Pam3-Cys-Ser-(Lys)4; TIRAP, Toll/IL-1 receptor domain-containing adaptor protein; TRAM, TRIF-related adaptor molecule; Treg, regulatory T cell; TRIF, TIR-domain-containing adaptor protein inducing IFN-β; MST, median survival time.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Seung E, Mordes JP, Rossini AA, Greiner DL. Hematopoietic chimerism and central tolerance created by peripheral-tolerance induction without myeloablative conditioning. J. Clin. Invest. 2003;112:795–808. doi: 10.1172/JCI18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, Shaffer J, Sayegh MH, Sykes M. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat. Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 3.Durham MM, Bingaman AW, Adams AB, Ha J, Waitze SY, Pearson TC, Larsen CP. Cutting edge: administration of anti-CD40 ligand and donor bone marrow leads to hemopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J. Immunol. 2000;165:1–4. doi: 10.4049/jimmunol.165.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini AA, Greiner DL. TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. J. Immunol. 2006;176:1561–1570. doi: 10.4049/jimmunol.176.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, Molinero L, Nozaki T, Phillips T, Uematsu S, et al. TLR engagement prevents transplantation tolerance. Am. J. Transplant. 2006;6:2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 7.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol. Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 10.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DR. Toll-like receptors and other links between innate and acquired alloimmunity. Curr. Opin. Immunol. 2004;16:538–544. doi: 10.1016/j.coi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DR. Toll like receptors and acute allograft rejection. Transplant. Immunol. 2006;17:11–15. doi: 10.1016/j.trim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DR, Palmer SM. Role of Toll-like receptor-driven innate immunity in thoracic organ transplantation. J. Heart Lung Transplant. 2005;24:1721–1729. doi: 10.1016/j.healun.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J. Clin. Invest. 2003;111:1571–1578. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaRosa DF, Rahman AH, Turka LA. The innate immune system in allograft rejection and tolerance. J. Immunol. 2007;178:7503–7509. doi: 10.4049/jimmunol.178.12.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obhrai J, Goldstein DR. The role of toll-like receptors in solid organ transplantation. Transplantation. 2006;81:497–502. doi: 10.1097/01.tp.0000188124.42726.d8. [DOI] [PubMed] [Google Scholar]
- 17.Andrade CF, Waddell TK, Keshavjee S, Liu M. Innate immunity and organ transplantation: the potential role of Toll-like receptors. Am. J. Transplant. 2005;5:969–975. doi: 10.1111/j.1600-6143.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- 18.Thornley TB, Phillips NE, Beaudette-Zlatanova BC, Markees TG, Bahl K, Brehm MA, Shultz LD, Kurt-Jones EA, Mordes JP, Welsh RM, et al. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J. Immunol. 2007;179:6620–6629. doi: 10.4049/jimmunol.179.10.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker WE, Nasr IW, Camirand G, Tesar BM, Booth CJ, Goldstein DR. Absence of innate MyD88 signaling promotes inducible allograft acceptance. J. Immunol. 2006;177:5307–5316. doi: 10.4049/jimmunol.177.8.5307. [DOI] [PubMed] [Google Scholar]
- 20.Tesar BM, Goldstein DR. Toll-like receptors and their role in transplantation. Front. Biosci. 2007;1:4221–4238. doi: 10.2741/2382. [DOI] [PubMed] [Google Scholar]
- 21.Porrett PM, Yuan X, LaRosa DF, Walsh PT, Yang J, Gao W, Li P, Zhang J, Ansari JM, Hancock WW, et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J. Immunol. 2008;181:1692–1699. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 23.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 24.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc. Natl. Acad. Sci. USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein S, Familletti PC, Pestka S. Convenient assay for interferons. J. Virol. 1981;37:755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki M, Pearson T, Brehm MA, Miller DM, Mangada JA, Markees TG, Shultz LD, Mordes JP, Rossini AA, Greiner DL. Different mechanisms control peripheral and central tolerance in hematopoietic chimeric mice. Am. J. Transplant. 2007;7:1710–1721. doi: 10.1111/j.1600-6143.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- 28.Rifle G, Mousson C, Martin L, Guignier F, Hajji K. Donor-specific antibodies in allograft rejection: clinical and experimental data. Transplantation. 2005;79:S14–S18. doi: 10.1097/01.tp.0000153292.49621.60. [DOI] [PubMed] [Google Scholar]
- 29.Kean LS, Hamby K, Koehn B, Lee E, Coley S, Stempora L, Adams AB, Heiss E, Pearson TC, Larsen CP. NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am. J. Transplant. 2006;6:292–304. doi: 10.1111/j.1600-6143.2005.01172.x. [DOI] [PubMed] [Google Scholar]
- 30.Westerhuis G, Maas WG, Willemze R, Toes RE, Fibbe WE. Long-term mixed chimerism after immunologic conditioning and MHC-mismatched stem-cell transplantation is dependent on NK-cell tolerance. Blood. 2005;106:2215–2220. doi: 10.1182/blood-2005-04-1391. [DOI] [PubMed] [Google Scholar]
- 31.Yu YY, Kumar V, Bennett M. Murine natural killer cells and marrow graft rejection. Annu. Rev. Immunol. 1992;10:189–213. doi: 10.1146/annurev.iy.10.040192.001201. [DOI] [PubMed] [Google Scholar]
- 32.Phillips NE, Markees TG, Mordes JP, Greiner DL, Rossini AA. Blockade of CD40-mediated signaling is sufficient for inducing islet but not skin transplantation tolerance. J. Immunol. 2003;170:3015–3023. doi: 10.4049/jimmunol.170.6.3015. [DOI] [PubMed] [Google Scholar]
- 33.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat. Immuno. 2003;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 35.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markees TG, Phillips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4+ T cells, interferon-γ, and CTLA4. J. Clin. Invest. 1998;101:2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J. Immunol. 2000;164:512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 38.Iwakoshi NN, Markees TG, Turgeon N, Thornley T, Cuthbert A, Leif J, Phillips NE, Mordes JP, Greiner DL, Rossini AA. Skin allograft maintenance in a new synchimeric model system of tolerance. J. Immunol. 2001;167:6623–6630. doi: 10.4049/jimmunol.167.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips NE, Greiner DL, Mordes JP, Rossini AA. Costimulatory blockade induces hyporesponsiveness in T cells that recognize alloantigen via indirect antigen presentation. Transplantation. 2006;82:1085–1092. doi: 10.1097/01.tp.0000235521.83772.29. [DOI] [PubMed] [Google Scholar]
- 40.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 41.Quezada SA, Fuller B, Jarvinen LZ, Gonzalez M, Blazar BR, Rudensky AY, Strom TB, Noelle RJ. Mechanisms of donor-specific transfusion tolerance: preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003;102:1920–1926. doi: 10.1182/blood-2003-02-0586. [DOI] [PubMed] [Google Scholar]
- 42.Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J. Immunol. 2005;175:771–779. doi: 10.4049/jimmunol.175.2.771. [DOI] [PubMed] [Google Scholar]
- 43.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 44.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 45.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen CP, Alexander DZ, Hollenbaugh D, Elwood ET, Ritchie SC, Aruffo A, Hendrix R, Pearson TC. CD40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation. 1996;61:4–9. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 48.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, Rossini AA. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl. Acad. Sci. USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64:329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 50.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, Wang F, Godowski PJ. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J. Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 52.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 53.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transplant. Immunol. 2004;13:117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]