Abstract
It has been close to a century since calcium phosphate materials were first used as bone graft substitutes. Numerous studies conducted in the last two decades have produced a wealth of information on the chemistry, in vitro properties, and biological characteristics of granular calcium phosphates and calcium phosphate cement biomaterials. An in depth analysis of several key areas of calcium phosphate cement properties is presented with the aim of developing strategies that could lead to break-through improvements in the functional efficacies of these materials.
Keywords: Calcium phosphate, hydroxyapatite, bone graft, calcium phosphate bone cement
INTRODUCTION
Studies on the use of calcium phosphates for bone defect repair appeared in the scientific literature as early as in 19201. Ceramic hydroxyapatite (HA) granules for bone defect repair was first reported in early 1950s2, and the first self-hardening calcium phosphate cements (CPC) were developed in late 1980s3. In the last fifteen years, a large number of publications on calcium phosphate materials, especially on CPCs, have appeared in the literature, reflecting a sharp increase in the interest in the research aimed at developing better materials for a broader clinical use. A brief review of the current understanding of these calcium phosphate biomaterials is presented below. The discussion will focus on areas of research that could lead to development of the next generation calcium phosphate cement materials, which would have greater efficacies in a broader range of clinical applications. Because of space limitations, other calcium-containing biomaterials such as calcium sulfate, bioactive glasses, and calcium phosphate-containing polymeric scaffolds are not included in the discussion.
GRANULAR CALCIUM PHOSPHATE BIOMATERIALS
In 1920, Albee and Morrison1 reported the use of “triple calcium phosphate” as stimulus for bone growth. The results indicated that bone fractures, with bone loss, showed a more rapid bone growth and union when triple calcium phosphate was injected into the gap between the bone ends than did the controls without its use. Although this may be the first scientific study on the use of calcium phosphate for bone defects repair, a thin slurry of a calcium phosphate powder (described as a “5 per cent triple calcium phosphate suspension in distilled water”) was used. Thus, it is unclear whether the calcium phosphate used in the study was a precipitated or ceramic material and whether it was in a powder or granular form. In 1951, Ray and Ward2 evaluated the effectiveness of a granular synthetic HA for repairing surgically created defects in the long bones and iliac wings of dogs and bur holes in the crania of cats. It was concluded from these experiments that the synthetic HA granules could be replaced by new bone, but they were not as effective as autogenous bone grafts in repairing the defects. In subsequent years, many attempts have been made to improve the usefulness of calcium phosphate ceramics in bone repair procedures. Notably the use of ceramic β-tricalcium phosphate (β-TCP)4,5, which is bioresorbable because of its greater solubility than HA in physiological environments. Macroporous HA block6 was prepared from coral skeleton in which the inherent macropores are preserved during the hydrothermal conversion of calcium carbonate to HA. More recently, partial conversion of the calcium carbonate to HA was used7 to produce a hybrid of the two phases, which allows a predictable resorption rate of the material in vivo. Similarly, biphasic calcium phosphate is a synthetically prepared hybrid of HA and β-TCP8 that possesses a greater resorption rate than HA alone. The properties and functionality of ceramic calcium phosphate materials currently in use have been extensively reviewed recently9.
As outlined above, nearly all of the granular calcium phosphate materials that have been evaluated for bone defect repair are of ceramic nature. Octacalcium phosphate (OCP), prepared by precipitation methods from aqueous solutions, is the only non-sintered granular calcium phosphate that has been studied extensively as possible bone graft substitutes. Although implantation of dicalcium phosphate anhydrous (DCPA), amorphous calcium phosphate (ACP), and OCP in aggregate forms into the subperiosteal areas of mice were all found to lead to new bone formation more rapidly than HA or Ca-deficient HA, OCP was unique in that fine filaments and granular materials in the newly formed bone matrix were detected around the remnants of OCP particles as short as seven days after implantation10. A subsequent study found that when synthetic OCP was implanted into the critical-sized defects in rat calvaria, bone formation was initiated from the margin of the defect and on the implanted OCP away from the margin of the defect11. These results support the feasibility of using OCP as a possible bone graft substitute. The results may also be applicable to self-hardening OCP-forming cements, described later, in terms of their biological properties.
Granular calcium phosphate materials have well established biocompatibility and osteoconductivity. Their clinical use has been partially replaced by calcium phosphate cement materials because of the advantages associated self-hardening properties of the cements. However, granular materials remain highly useful when used alone or as osteoconductive fillers in polymeric tissue scaffolds or calcium phosphate cement implants.
CALCIUM PHOSPHATE CEMENTS
Calcium phosphate cements (CPC) are materials that consist essentially of only calcium phosphate compounds and are capable of self-setting to a hard mass. Although some literature reports suggest that developments of CPCs stemmed from existing knowledge of calcium silicate or calcium sulfate cements, the discovery of the first CPC3 was in fact a result of decades of basic studies on calcium phosphate solubility behaviors12. Based on solubility phase diagrams13, described in greater detail later, calcium phosphate scientists were aware of the fact that both tetracalcium phosphate (TTCP) and DCPA or dicalcium phosphate dihydrate (DCPD) are much more soluble than HA under neutral pH conditions. Further, a slurry containing appropriate amounts of these compounds can produce continuous HA precipitation while maintaining the solution composition relatively constant14. In early 1980s, studies on the reaction, TTCP + DCPD (or DCPA) → HA, were conducted with the aim of developing slurries for remineralizing carious lesions. It was observed that some of the TTCP + DCPD (or DCPA) aqueous pastes became a hardened mass when left in test tubes for a few hours. Thus, these scientists inadvertently discovered a new type of self-hardening cements that consisted of only calcium phosphates and formed HA as the product12. In subsequent years, results from a series of animal studies15,16,17 demonstrated that because CPC formed biocompatible, precipitated nano-crystalline HA as the product, implanted CPC was gradually replaced by new bone without a loss in volume. This CPC composition received approval by the US Food and Drug Administration in 1996, thus becoming the first commercially available CPC for use in humans.
Since the development of the first CPCs consisting of TTCP and DCPA or DCPD, many other combinations of different calcium and phosphate-containing compounds have been investigated as potential CPC materials. With the exception of several CPCs that form brushite as the product, most CPCs form apatitic products and have similar in vivo properties despite the different starting compositions. The properties of the various CPCs have been extensively reviewed in several publications18,19,20,21, thus discussions presented below will focus on providing an in depth analysis of specific CPC properties that could lead to break-through improvements for the next-generation CPC materials.
CALCIUM PHOSPHATE CEMENT SETTING CHEMISTRY
Cement setting reaction is perhaps the most important properties of CPC because it not only directly controls cement hardening time and other setting properties but also determines the nature of cement products and therefore most of the physical and biological properties of the hardened cement. CPCs may be divided into the following categories in terms of cement setting reactions:
1. Cement hardening is a result of reactions among calcium phosphate compounds
As a result of extensive research on new CPC compositions, data in the literature indicates that cementation can now occur in mixtures containing a wide variety of calcium phosphate compounds. In fact, nearly all of the compounds listed in Table 1 have been used as cement ingredients, and many of these same compounds are also products formed in other cement systems.
Table 1.
Calcium phosphate compounds and their solubility product constants at 25°C22
| Compound | Formula | Ca/P | Ksp |
|---|---|---|---|
| Monocalcium phosphate monohydrate (MCPM) | Ca(H2PO4)2·H2O | 0.5 | highly soluble |
| Monocalcium phosphate anhydrous (MCPA) | Ca(H2PO4)2 | 0.5 | highly soluble |
| Dicalcium phosphate anhydrous (DCPA) | CaHPO4 | 1.0 | 10−6.90 (23) |
| Dicalcium phosphate dihydrate (DCPD) | CaHPO4·2H2O | 1.0 | 10−6.59 (24) |
| Octacalcium phosphate (OCP) | Ca8H2(PO4)6·5H2O | 1.33 | 10−96.6 (25) |
| α-Tricalcium phosphate (α-TCP) | α-Ca3(PO4)2 | 1.5 | 10−25.5 (26) |
| β-Tricalcium phosphate (β-TCP) | β-Ca3(PO4) 2 | 1.5 | 10−28.9 (27) |
| Amorphous calcium phosphate (ACP) | Ca3(PO4)2 | 1.5 | 10−25.2 to 10−24.8 (28) |
| Hydroxyapatite (HA) | Ca10(PO4)6(OH)2 | 1.67 | 10−116.8 (29) |
| Tetracalcium phosphate (TTCP) | Ca4(PO4)2O | 2.0 | 10−38 (30) |
All reactions between calcium phosphate compounds that occur in an aqueous environment can be characterized as dissolution/re-precipitation reactions. For example, in the TTCP+DCPA cement paste, dissolution of TTCP and DCPA would lead to a solution composition that is highly supersaturated with respect to HA, resulting in HA precipitation. The driving force for such a reaction is the relative solubilities of the reactants and product for a given solution composition. In the above example, the TTCP+DCPA → HA reaction occurs because both TTCP and DCPA are considerably more soluble than HA. Setting reactions mechanisms in other CPC mixtures that contain other calcium phosphate starting materials are in fact quite similar and may be understood by analyzing the solubility behavior of the compounds involved.
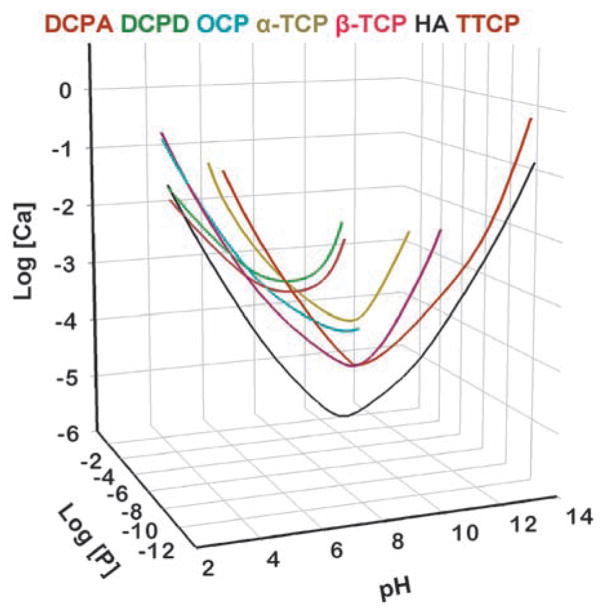
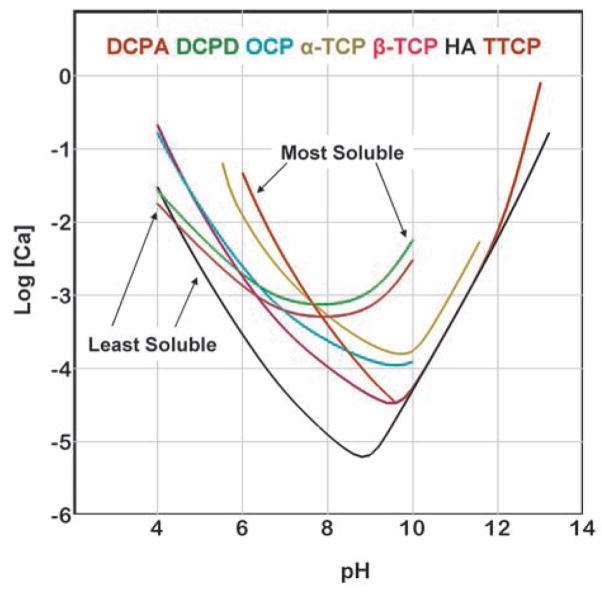
Solubility is conventionally described as the amount of a solid that can dissolve into a unit volume of solution. For calcium phosphates, this quantity can change by several orders of magnitude with changes in pH and concentrations of acids and bases, such as HCl and NaOH. Thus, on the surface, the solubility may appear to be a complex function of these solution parameters. However, a clearer picture of the solubility properties for calcium phosphates can be gained by understanding the basic principles governing the solid-solution equilibrium. Fig. 1 is known as a solubility phase diagram. Each curve in the diagram, known as a solubility isotherm, can be calculated from the solubility product constant (Ksp) of the compound (Table 1). The isotherm describes the solubility of a salt, expressed as the total calcium and total phosphate concentrations of the saturated solution as a function of pH. Because at a fixed temperature and pressure, there exists only one degree of freedom for solutions saturated with respect to a given salt, a solubility isotherm is a single curve rather than a surface in the three dimensional phase diagram. For ease of examination, the phase diagram is shown in a two dimensional graph (Fig. 2), in which a salt whose isotherm lies below that of another salt is less soluble (more stable) than the other salt. The point where two isotherms cross is known as a singular point. The solution at the singular point is simultaneously saturated with respect to both salts.
Fig. 1.
Solubility phase diagram.
Fig. 2.
Solubility phase diagram in a two dimensional graph.
It can be seen from Fig. 2 that TTCP and DCPD are the two most soluble calcium phosphates for pH below and above the pH (approximately 7.6) of the TTCP-DCPD singular point, respectively. DCPA, which has a solubility curve similar to that of DCPD except with a slightly lower Ksp value (Table 1), is the second most soluble compound above the pH (approximately 7.8) of the TTCP-DCPA singular point. In contrast, DCPA and HA are the two least soluble calcium phosphates for pH below and above the pH (approximately 4.4) of the DCPA-HA singular point. DCPD, which behaves similarly to DCPA except with a slightly higher Ksp value, is the second least soluble compound below the pH (approximately 4.2) of the DCPD-HA singular point.
Because HA is the most stable (least soluble) compound over a very wide pH range (4.4 and above), most neutral pH CPC formulations would form HA or other closely related apatitic phases, e.g., Ca-deficient HA, carbonated HA, other non-stoichiometric HA, etc., as products. TTCP and DCPD (DCPA) were used as the starting materials in the first HA-forming CPC3 because these are the most soluble salts and thus would provide the greatest driving force for the HA-forming reaction. This may explain why the TTCP+DCPD and TTCP+DCPA CPCs are the only cement formulations known to harden rapidly with water as the cement liquid. Since for this pH range, all non-HA calcium phosphate compounds are more soluble than HA, these compounds can also be used as starting materials for HA-forming cements. In these cases, a smaller driving force for the HA-forming reaction is in effect, and use of a setting accelerator, most commonly a sodium phosphate solution, is required to produce rapid cement hardening. α-TCP and MCPM are among the most commonly used compounds after TTCP, DCPA, and DCPD as starting cement components19,21.
Although several non-HA calcium phosphate phases, such as OCP and whitlockite (a magnesium-containing calcium phosphate having a structure similar to that of β-TCP), are more soluble than HA under neutral pH conditions, they can be the major phase in the cement products. This is because these phases precipitate more rapidly than HA under certain solution conditions. Indeed, OCP-forming cements have been successfully formulated31,32. In view of the unique in vivo properties resulted from studies on granular OCP implants11, OCP-forming CPC might have some unique biological properties that are yet to be more fully understood.
Because DCPD and DCPA are the least soluble calcium phosphates under acidic pH (< pH 4), they are the products formed in acidic CPC formulations. All other calcium phosphate phases, being more soluble under these pH conditions, can be used as the starting materials for the DCPD- or DCPA-forming cements. Despite the acidic pHs, these cements are quite compatible to tissues and several such products have been in successful clinical use19. DCPD was reported to dissolve nearly three times faster than HA in an acidified simulated physiological solution33, explaining why DCPD-forming CPC resorb faster than HA-forming CPCs34. Data in the literature suggest that DCPD-forming cements have lower mechanical strengths than HA-forming CPCs when other parameters, e.g., porosity, being essentially the same.
Formation of different calcium phosphate phases as the major cement product offers an effective means for formulating CPCs with a range of in vivo resorption rates. Different cement systems also have widely different pH conditions depending on the cement setting reaction. In addition to biological response, a CPC with desired pH can be selected in order to gain compatibility with other components, such as osteoinductive factors, antibiotics, etc., to be used with the cement.
2. Hardening of the cement is a result of reaction between calcium and a carboxylic acid
A number of carboxylic acids readily form calcium complexes as well as relatively insoluble and often amorphous Ca-carboxylate compounds. These acids include glycolic, citric, tartaric, malonic, malic, succinic, and maleic acids. Some of these acid solutions, when mixed with a powder containing one or more of calcium phosphate compounds, produce a fast hardening cement35,36,37. After the initial setting, calcium phosphate components in the cement continue to react to form more stable final products. Although most of the reported cements form apatitic final product, DCPD and DCPA could also be formed if the molar Ca/P ratio in the cement formulation is lower than about 1.338. These cements generally have lower mechanical strengths than those HA-forming CPCs that do not use an organic acid solution as the liquid. Because of their good handling properties, e.g., fast setting, cohesive consistency, etc., several commercial CPC products belong to this category19.
3. Cement hardening is a result of a reaction between calcium phosphate and an aqueous polymer solution
Calcium phosphate compounds, especially the more alkaline phases such as TTCP and HA, are reactive to aqueous solutions of a variety of polymers. Matsuya et al39. reported properties of cements prepared by mixing aqueous solutions of poly(methyl vinyl ether-maleic acid) and TTCP or a TTCP+DCPA mixture. These cements hardened rapidly and have higher strengths than the CPC control without the polymer. The hardening is a result of acid-base reaction between the carboxyl groups of the polymer and the alkaline calcium phosphate. Chitosan is another polymer that can offer great advantages when used with CPC. A biopolymer derived from chitin, chitosan is soluble in acidic solutions. An increase in pH, brought about by addition of an alkaline calcium phosphate such as TTCP, would cause chitosan precipitation, forming a hardened hydrogel. These natural biopolymers are biocompatible, biodegradable40, and osteoconductive41. Chitosans also appear to be good candidates for formulating CPC with elastomeric properties as described later. Chitosan has been used with granular calcium phosphate compounds42,43,44, producing a moldable and self-hardening scaffold. When used with CPC, chitosan precipitation produces a rapid initial cement hardening, while allowing further reactions among the calcium phosphate components of the cement to form HA as the major final product45,46,47. Incorporation of a small amount of chitosan increases the mechanical properties of CPC, and with higher chitosan content, the material becomes elastomeric.
The cement setting reaction determines the intermediate and the final products that will be formed in a CPC. Consequently, the reaction determines the mechanical and biological properties of the cement as well. The three types of CPC setting reactions described above are not mutually exclusive, and two or more reactions could be employed to produce the desired setting properties and the final cement products for the intended clinical application.
STRENGTH AUGMENTATION
Although compressive strengths of as high as 80 MPa have been achieved, CPCs are brittle and have relatively low bending/flexural strengths. Fortunately, room temperature hardening makes it possible to incorporate various kinds of biocompatible/bioresorbable fibers and meshes into a CPC paste or in prefabricated CPC implants. These should significantly increase the strengths. Xu et al53. reported that incorporation of Vicryl meshes to a CPC increased the flexural strength more than 6 times and work-of-fracture (toughness) by 750 times in 24 h samples. In the same study, chitosan was added to a second CPC group and both the mesh and chitosan were added to a third group of samples. The 24 h flexural strengths in these two groups were 3.6 times and 13.1 times, respectively, that of the control CPC samples. Similarly, the work-of-fracture were 13 times and 1390 times greater, respectively. It is interesting to note that after 84 d of immersion of the samples in a simulated physiological solution, the Vicryl meshes were completely degraded, forming interconnecting macropores, yet both the flexural strength and work-of-fracture of the chitosan+mesh reinforced group were still significantly higher than the control CPC. Additional studies48,49,50 using various other reinforcement approaches also showed significant increases in the mechanical properties of CPC. These results suggest that both the flexural strength and toughness of CPC materials can be increased many fold by incorporating biocompatible and bioresorbable reinforcement additives. Further work in this area is needed to develop CPC materials that are useful for repairing defects in load-bearing bones.
INCORPORATION OF MACROPORES
CPC is a micro porous solid consisting of nanometer sized HA or micrometer sized DCPD crystals. CPC is slowly resorbed and replaced by new bone without the need to have an interconnecting macroporous structure. However, presence of macropores can facilitate ingrowth of bone and accelerate the overall process of replacing the cement by bone. Because CPC hardens in situ at room temperatures, macropores can be easily created by including nontoxic, water-soluble granules such as sodium bicarbonate51, mannitol52, etc. After hardening of the cement, dissolution of the granules produces macropores in the shapes of the granules. Incorporating macropores into the cement has always led to a significant decrease in mechanical strengths as would be expected. As described above, one attempt to partially counteract this shortcoming is to incorporate bioresorbable fibers and meshes into CPC paste, which can provide significant strength augmentation for an intended period and subsequently create interconnecting macropores after degradation of the polymers53. Animal study results54 confirmed that a CPC with macropores formed in situ by mannitol particles led to bone formation in the macropores in advance of the implant-defect interface.
A RANGE OF RESORPTION RATES FOR DIFFERENT APPLICATIONS
An important in vivo characteristic of HA-forming CPC is that it does not dissolve spontaneously in a normal physiological fluid environment, yet is resorbable under cell-mediated acidic conditions. Although DCPD is soluble in normal physiological fluids55, studies have shown that resorption of DCPD-forming CPC was also essentially cell-mediated34,56. Being more soluble, the DCPD-forming CPCs resorb more rapidly than HA-forming CPC34, which is advantageous in some applications. However, under certain clinical situation, rapid resorption was reported to lead to lower quality bone formation34. These observations suggest that in order to achieve optimum clinical results, an appropriate CPC resorption rate is an important parameter to be reckoned with, and this rate is likely to vary with the intended clinical applications. For some applications, such as cranioplasty, rapid implant resorption and replacement by bone is perhaps not an as important factor as implant stability and integrity. For other applications, such as periodontal bone defect repairs, sinus lift, etc., the ability of the implant cement to be replaced quickly by bone is highly desirable. In addition to the nature of the mineral phase of the implanted cement, macroporosity of the implant can play an important role in the overall rate of the graft-to-bone conversion process.
INCORPORATION OF OSTEOINDUCTIVE FACTORS
It is generally accepted that calcium phosphate materials, including CPCs, are osteoconductive but not osteoinductive. CPC materials can be a suitable substrate for the various osteoinductive factors currently under development because its pH can be controlled quite precisely, and it can be made macroporous. CPC also has a large surface area and can act as a stable scaffold. A “growth factor cement (GFC)” was reported in a study57 in which a combination of bone morphogenetic protein-2 (BMP-2), transforming growth factor-beta (TGF-beta), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF) were used in a CPC for treatment of peri-implant defects in a dog model. The findings indicated a significant effect of GFC on increased bone-to-implant contact and amount of bone over a given surface area compared with the cement-only and the no-cement treatment groups. Several other studies58,59,60 have shown that incorporation of bone growth factors into CPCs with or without macroporous structures produced osteoinduction and provided improved bone healing. Thus, incorporation of osteoinductive factor should be one of the most effective ways to improve the efficacy of CPC materials for repairing defects with insufficient available bone surfaces.
INJECTABILITY
Injectability of CPC is of crucial importance for surgical procedures utilizing minimally invasive procedures such as in vertebraplasty and kyphoplasty or for delivery of the cement into a very narrow space as in root canal obturation. Two excellent recent articles61,62 provide extensive treatise and discussion on factors that influence the injectability of CPC materials. In addition to a large number of parameters relating to CPC composition, e.g., particle size and shape, particle size distribution, powder/liquid ratio, liquid viscosity, etc., the injectability of a setting cement obviously depends strongly on the post-mixing time interval relative to the cement setting time. In this regard, premixed CPCs, described later, that do not harden until being placed into the defect would be of advantage in that the injectability would remain essentially constant with time.
ELASTORMERIC PROPERTIES
Like other brittle ceramics, CPC materials fracture catastrophically at a relatively small strain. It is desirable to have CPC in a non-rigid form that can sustain significant strains without fracture. Chitosan and its derivatives are good candidates for forming non-rigid elastomeric matrices. In a recent study46 incorporation of chitosan lactate (up to mass fraction of 20%) into CPC increased the CPC strength by 4 to 6 times, and work-of-fracture (toughness) by an order of magnitude. CPC-chitosan composite can be made totally flexible with higher chitosan content. Chitosan is an acid soluble, biocompatible and biodegradable polymer, with the resorption rate affected by the degree of deacetylation and molecular weight. Thus, CPC-chitosan composites are bioresorbable with a controllable resorption rate. Non-rigid CPC implant can provide the needed compliance for micro-motion of the defect walls without fracturing or dislodging of the implant.
PREMIXED FORMULATIONS
Most of the presently available CPCs are in the form of a powder and a liquid that are mixed immediately before use. In the clinical situation, the ability of the surgeon to properly mix the cement and then place the cement paste in the defect within the prescribed time is a crucial factor in achieving optimum results. Thus, it would be desirable to have a premixed cement paste, prepared in advance using a well controlled process, that is stable in the package and hardens only after being placed in the defect. Such a paste can be devised by combining CPC powders with a non-aqueous but water miscible liquid. In this way, after the CPC is placed in the defect, diffusion of water from the tissues into the CPC would cause the cement to harden. One such premixed CPC was formulated by combining water-free glycerin with a TTCP+DCPA CPC powder, a sodium phosphate setting accelerator, and cellulose gelling agent63. While this premixed CPC has the advantage of infinite work time, especially desirable when used as an injectable CPC, it hardened too slowly for certain clinical applications. Several rapid hardening premixed CPC were subsequently developed that have setting times below 10 min, and had non-cytotoxicity matching the conventional CPC64. However, these formulations have a relatively short shelf life because of the high reactivity with moisture. Further, the interior of a large implant would not harden for a long time due to slow water diffusion. Recently, dual-paste premixed cement formulations were developed65. One of the pastes is aqueous to provide the water needed for the setting reaction to occur, while the other paste is non-aqueous and contains cement components that are highly reactive to water. The setting time and mechanical properties of the dual-paste CPC were similar to those of a conventional powder/liquid CPC of similar chemical composition. While the individual pastes were completely stable in the package, by supplying the needed water, the dual-paste premixed CPC hardens more uniformly and rapidly than the single-paste premixed CPCs. In general, premixed CPC have lower mechanical properties probably related to the volume initially taken up by the non-aqueous liquid. Additional work is needed to overcome this shortcoming.
CPC USE IN DENTAL APPLICATIONS
Since the first FDA’s approval of CPC for human use in 1996, a large number of CPC products have been approved in different countries or regions for a wide range of clinical applications including cranio-maxillofacial, trauma, and orthopedic indications. In comparison, clinical use of CPC for dental and intraoral applications has been substantially lacking. Similarly, scientific research focused on CPC’s use for intraoral applications is also relatively sparse. Research conducted by Sugawara et al over the years have shown that due to CPCs self-hardening properties, high osteoconductivity, excellent interface to bone defect surfaces, and gradual replacement by new bone, these materials can be used with outstanding results in a number of dental applications, including ridge augmentation, implant grouting, periodontal pocket fill, sinus lift, and cleft palate defect repair. Some of the literature references and animal and clinical data on the use of CPC for various dental applications can be found in a series of recent publications66,67,68.
CONCLUDING REMARKS
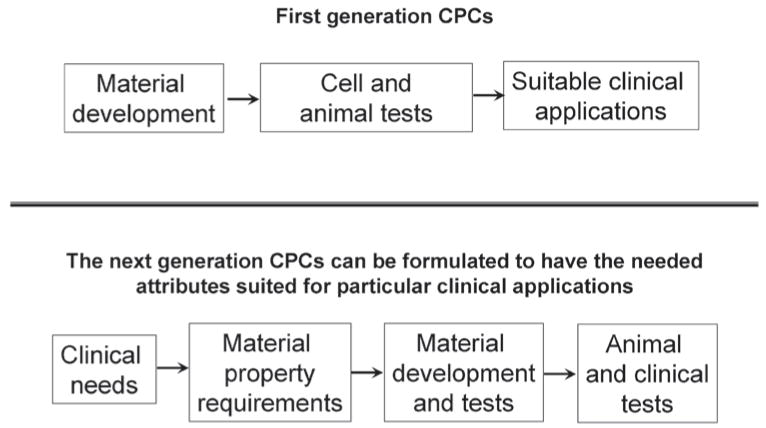
Two decades ago when CPC was in the early years of development, research was driven primarily by scientific curiosity and interests in material science. Often a new formulation was developed and the material properties determined before much thoughts were given concerning the clinical applications for which the material might be useful. As briefly discussed in this article, substantial understandings of CPCs in terms of their setting chemistry, mechanical properties, in vivo tissue reactions, etc., have been gained through numerous studies conducted since then. However, research efforts on CPC have been somewhat unfocused so that despite a wealth of knowledge gained, clinical uses of CPC remain restricted to a relatively narrow area. In seeking a break-through in the clinical use of CPC, it may be necessary to adopt a strategy that may appear to be in the reverse order of the earlier approach (Fig. 3). Perhaps the material property requirements for a specific clinical application should first be thoroughly analyzed and well understood. With this input, researchers can utilize the existing knowledge on CPC together with new innovative research to develop CPC materials that can fully meet the requirements. Animal and clinical studies can then be conducted to evaluate the functional efficacy of the material for the specific application. Information in the literature clearly indicates that the requirements of the CPC material vary significantly for different clinical applications. Thus, it should not be anticipated that a CPC formulation can be universally efficacious. It would seem a realistic goal that in the foreseeable future CPC type materials with the necessary attributes can replace many of the autografts currently in use.
Fig. 3.
A strategy for the development of next generation CPCs.
Acknowledgments
This work was supported by ADAF, NIST, and NIH grant DE11789.
References
- 1.Albee F, Morrison H. Studies in bone growth: triple calcium phosphate as a stimulus to osteogenesis. Ann Surg. 1920;71:32–38. doi: 10.1097/00000658-192001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray RD, Ward AA., Jr . Surgical Forum, American College of Surgeons, 1951. WB Saunders Co; Philadelphia: 1952. A preliminary report on studies of basic calcium phosphate in bone replacement; pp. 429–434. [PubMed] [Google Scholar]
- 3.Brown, Chow . A new calcium phosphate, water-setting cement. In: Brown PW, editor. Cements Research Progress 1986. American Ceramic Society; Westerville, Ohio: 1987. pp. 352–379. [Google Scholar]
- 4.Lange TA, Zerwekh JE, Peek RD, Mooney V, Harrison BH. Granular tricalcium phosphate in large cancellous defects. Ann Clin Lab Sci. 1986;16:467–472. [PubMed] [Google Scholar]
- 5.Le Huec JC, Lesprit E, Delavigne C, Clement D, Chauveaux D, Le Rebeller A. Tricalcium phosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis: comparative clinical results at four years. Acta Orthopedica Belgica. 1997;63:202–211. [PubMed] [Google Scholar]
- 6.White E, Shores EC. Biomaterial aspects of interpore-200 porous hydroxyapatite. Dental Clin North Am. 1986;30:49–67. [PubMed] [Google Scholar]
- 7.Shore EC. Coralline bone graft substitutes. Orthop Clin North Am. 1999;30:599–613. doi: 10.1016/s0030-5898(05)70113-9. [DOI] [PubMed] [Google Scholar]
- 8.Fujibayashi S, Jitsuhiko S, Tanaka C, Matsushita M, Nakamura T. Lumbar posterolateral fusion with biphasic calcium phosphate ceramic. J Spinal Discord. 2001;14:214–221. doi: 10.1097/00002517-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Tadic D, Epple M. A thorough physicochemical characterization of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25:987–994. doi: 10.1016/s0142-9612(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki O, Nakamura M, Miyasaka Y, Kagayama M, Sakurai M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J Exp Med. 1991;164:37–50. doi: 10.1620/tjem.164.37. [DOI] [PubMed] [Google Scholar]
- 11.Kamakura S, Sasano Y, Homma H, Suzuki O, Kagayama M, Motegi K. Implantation of octacalcium phosphate nucleates isolated bone formation in rat skull defects. Oral Dis. 2001;7:259–265. [PubMed] [Google Scholar]
- 12.Chow LC, Takagi S. A natural bone cement – A laboratory novelty led to the development of revolutionary new biomaterials. J Res Natl Inst Stand Technol. 2001;106:1029–1033. doi: 10.6028/jres.106.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown WE. Environmental Phosphorus Handbook. John Wiley & Sons; New York: 1973. Solubilities of phosphates and other sparingly soluble compounds, Chapter 10; pp. 203–239. [Google Scholar]
- 14.Chow LC. Development of Self-setting Calcium Phosphate Cements. J Ceramic Soc Japan. 1991;99:954–964. [Google Scholar]
- 15.Costantino PD, Friedman CD, Jones K, Chow LC, Pelzer HJ, Sisson GA., Sr Hydroxyapatite Cement. I. Basic Chemistry and Histologic Properties. Arch Otolaryngol Head Neck Surg. 1991;117:379–384. doi: 10.1001/archotol.1991.01870160033004. [DOI] [PubMed] [Google Scholar]
- 16.Friedman CD, Costantino PDK, Chow LC, Pelzer HJ, Sisson GA., Sr Hydroxyapatite Cement. II. Obliteration and Reconstruction of the Cat Frontal Sinus. Arch Otolaryngol Head Neck Surg. 1991;117:385–389. doi: 10.1001/archotol.1991.01870160039005. [DOI] [PubMed] [Google Scholar]
- 17.Costantino PD, Friedman CD, Jones K, Chow LC, Sisson GA., Sr Experimental Hydroxyapatite Cement. Cranioplasty Plast Reconstr Sugr. 1992;90:174–191. [PubMed] [Google Scholar]
- 18.Ambard AJ, Mueninghoff L. Calcium phosphate cements: Review of mechanical and biological properties. Prostodont. 2006;15:321–328. doi: 10.1111/j.1532-849X.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 19.Bohner M, Gbureck M, Barralet JE. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials. 2005;26:6423–6429. doi: 10.1016/j.biomaterials.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Lewis G. Injectable bone cements for use in vertebroplasty and kyphoplasty: State-of-the-art review. J Biomed Mater Res Part B: Appl Biomater. 2006;76B:456–468. doi: 10.1002/jbm.b.30398. [DOI] [PubMed] [Google Scholar]
- 21.Chow LC, Markovic M, Takagi S. In: Calcium phosphate cements Cem Res Prog. Struble LJ, editor. The American Ceramic Society; Westerville, Ohio: 1998. pp. 215–238. [Google Scholar]
- 22.Chow LC. Solubility of calcium phosphates. In: Chow LC, Eanes ED, editors. Monographs in Oral Science – Vol. 18: Octacalcium Phosphate. Karger; Basel: 2001. pp. 94–111. [DOI] [PubMed] [Google Scholar]
- 23.McDowell H, Brown WE, Sutter JR. Solubility study of calcium hydrogen phosphate: Ion pair formation. Inorganic Chem. 1971;10:1638–1643. [Google Scholar]
- 24.Gregory TM, Moreno EC, Brown WE. Solubility of CaHPO4·2H2O in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25 and 37.5°C. J Res Nat Bur Stand. 1968;74(A):773–782. doi: 10.6028/jres.074A.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tung MS, Eidelman N, Sieck B, Brown WE. Octacalcium phosphate solubility product from 4 to 37°C. J Res Nat Bur Stand. 1988;93:613–624. [Google Scholar]
- 26.Fowler BO, Kuroda S. Changes in heated and in laser-irradiated human tooth enamel and their probable effects on solubility. Calcif Tissue Int. 1986;38:197–208. doi: 10.1007/BF02556711. [DOI] [PubMed] [Google Scholar]
- 27.Gregory TM, Moreno EC, Patel JM, Brown WE. Solubility of β-Ca3(PO4)2 in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37°C. J Res Nat Bur Stand. 1974;78(A):667–674. doi: 10.6028/jres.078A.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer JL, Eanes ED. A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif Tissue Res. 1978;25:59–68. doi: 10.1007/BF02010752. [DOI] [PubMed] [Google Scholar]
- 29.McDowell H, Gregory TM, Brown WE. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25 and 37°C. J Res of NBS (Phys and Chem) 1977;81A:273–281. [Google Scholar]
- 30.Matsuya S, Takagi S, Chow LC. Hydrolysis of tetracalcium phosphate in H3PO4 and KH2PO4. J Mater Sci. 1996;31:3263–3269. [Google Scholar]
- 31.Nakano Y, Ohgaki M, Nakamura S, Takagi Y, Yamashita K. In vitro and in vivo characterization and mechanical properties of α-TCP/OCP settings. Bioceramics. 1999;19:315–318. [Google Scholar]
- 32.Bermudez O, Boltong MG, Driessens FCM, Planell JA. Development of an octacalcium phosphate cement. J Mater Sci: Mater Med. 1994;5:144–146. [Google Scholar]
- 33.Chow LC, Markovic M, Takagi S. A dual constant-composition titration system as an in vitro resorption model for comparing dissolution rates of calcium phosphate biomaterials. J Biomed Mater Res Part B: Appl Biomater. 2003;65B:245–251. doi: 10.1002/jbm.b.10009. [DOI] [PubMed] [Google Scholar]
- 34.Kuemmerle JM, Oberle A, Oechslin C, Bohner M, Frei C, Boecken I, von Rechenberg B. Assessment of the suitability of a new brushite calcium phosphate cement for cranioplasty - an experimental study in sheep. J Craniomaxillofac Surg. 2005;33:37–44. doi: 10.1016/j.jcms.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa M, Terada Y, Toda T. Setting time and sealing ability of alpha-tricalcium phosphate cement containing titanic oxide. J Osaka Dent Univ. 1998;32:67–70. [PubMed] [Google Scholar]
- 36.Doi Y, Shimizu Y, Moriwaki Y, Aga M, Iwanaga H, Shibutani T, Yamamoto K, Iwayama Y. Development of a new calcium phosphate cement that contains sodium calcium phosphate. Biomaterials. 2001;22:847–854. doi: 10.1016/s0142-9612(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama A, Yamamoto S, Kawasaki T, Kohgo T, Nakasu M. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials. 2002;23:1091–1101. doi: 10.1016/s0142-9612(01)00221-6. [DOI] [PubMed] [Google Scholar]
- 38.Unpublished data
- 39.Matsuya Y, Antonucci JM, Matsuya S, Takagi S, Chow LC. Polymeric calcium phosphate cements derived from poly(methyl vinyl ether-maleic acid) Dent Mater. 1996;12:2–7. doi: 10.1016/S0109-5641(96)80056-X. [DOI] [PubMed] [Google Scholar]
- 40.Machida Y, Nagai T, Abe M, Sannan T. Use of chitosan and hydroxypropylchitosan in drug formulations to effect sustained release. Drug Dis Deliv. 1986;1:119–130. [PubMed] [Google Scholar]
- 41.Muzzarelli RAA, Biagini G, Bellardini M, Simonelli L, Castaldini C, Fraatto G. Osteoconduction exerted by methylpyrrolidinone chitosan used in dental surgery. Biomaterials. 1993;14:39–43. doi: 10.1016/0142-9612(93)90073-b. [DOI] [PubMed] [Google Scholar]
- 42.Maruyama M, Ito M. In vitro properties of a chitosan-bonded self-hardening paste with hydroxyapatite granules. J Biomed Mater Res. 1996;32:527–532. doi: 10.1002/(SICI)1097-4636(199612)32:4<527::AID-JBM5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 43.Ito M, Miyazaki A, Yamagishi T, Yagasaki H, Hashem A, Oshida Y. Experimental development of a chitosan-bonded beta-tricalcium phosphate bone filling paste. Biomed Mater Eng. 1994;4:439–449. [PubMed] [Google Scholar]
- 44.Ito M, Yamagishi T, Yagasaki H, Kafrawy AH. In vitro properties of a chitosan-bonded bone-filling paste: studies on solubility of calcium phosphate compounds. J Biomed Mater Res. 1996;32:95–98. doi: 10.1002/(SICI)1097-4636(199609)32:1<95::AID-JBM11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 45.Takagi S, Chow LC, Hirayama S, Eichmiller FC. Properties of Elastomeric Calcium Phosphate Cement – Chitosan Composites. Dental Materials. 2003;18:797–804. doi: 10.1016/s0109-5641(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 46.Xu HHK, Quinn JB, Takagi S, Chow LC. Processing and properties of strong and non-rigid calcium phosphate cement. J Dent Res. 2002;81:219–224. [PubMed] [Google Scholar]
- 47.Xu HHK, Quinn JB, Takagi S, Chow LC. Fast-setting calcium phosphate scaffold with tailored macropore formation rates for bone regeneration. J Biomed Mater Res. 2004;68A:725–734. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Xu HH. Effects of synergistic reinforcement and absorbable fiber strength on hydroxyapatite bone cement. J Biomed Mater Res A. 2005;75:832–840. doi: 10.1002/jbm.a.30461. [DOI] [PubMed] [Google Scholar]
- 49.Xu HH, Burguera EF, Carey LE. Strong, macroporous, and in situ-setting calcium phosphate cement-layered structures. Biomaterials. 2007;28:3786–96. doi: 10.1016/j.biomaterials.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun L, Xu HH, Takagi S, Chow LC. Fast setting calcium phosphate cement-chitosan composite: mechanical properties and dissolution rates. J Biomater Appl. 2007;21:299–315. doi: 10.1177/0885328206063687. [DOI] [PubMed] [Google Scholar]
- 51.Takagi S, Chow LC. Formation of macropores in calcium phosphate cement implants. J Mater Sci: Mater in Medicine. 2001;12:135–139. doi: 10.1023/a:1008917910468. [DOI] [PubMed] [Google Scholar]
- 52.Markovic M, Takagi S, Chow LC. Formation of macropores in calcium phosphate cement through the use of mannitol crystals. Key Engineering Materials. 2001;192–195:773–776. [Google Scholar]
- 53.Xu HH, Quinn JB, Takagi S, Chow LC. Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials. 2004;25:1029–1037. doi: 10.1016/s0142-9612(03)00608-2. [DOI] [PubMed] [Google Scholar]
- 54.Sugawara A, Fujikawa K, Hirayama S, Mori T, Ikemi T, Takagi S, Chow LC. Histopathological reactions of macroporous premixed calcium phosphates cement for bone defects repair. 2004 (Abstract 2068) IADR. [Google Scholar]
- 55.Eidelman N, Chow L, Brown WE. Calcium Phosphate Saturation Levels in Ultrafiltered Serum. Calcif Tiss Int. 1987;40:71–78. doi: 10.1007/BF02555708. [DOI] [PubMed] [Google Scholar]
- 56.Flautre B, Lemaître J, Maynou C, Van Landuyt P, Hardouin P. Influence of polymeric additives on the biological properties of brushite cements: an experimental study in rabbit. J Biomed Mater Res A. 2003;66:214–223. doi: 10.1002/jbm.a.10539. [DOI] [PubMed] [Google Scholar]
- 57.Meraw SJ, Reeve CM, Lohse CM, Sioussat TM. Treatment of Peri-Implant Defects with Combination Growth Factor Cement. J Periodont. 2000;71:8–13. doi: 10.1902/jop.2000.71.1.8. [DOI] [PubMed] [Google Scholar]
- 58.Kroese-Deutman HC, Ruhé PQ, Spauwen PH, Jansen JA. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants inserted at an ectopic site in rabbits. Biomaterials. 2005;26:1131–1138. doi: 10.1016/j.biomaterials.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 59.Edwards RB, 3rd, Seeherman HJ, Bogdanske JJ, Devitt J, Vanderby R, Jr, Markel MD. Percutaneous injection of recombinant human bone morphogenetic protein-2 in a calcium phosphate paste accelerates healing of a canine tibial osteotomy. J Bone Joint Surg Am. 2004;86-A:1425–1438. doi: 10.2106/00004623-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Ruhé PQ, Kroese-Deutman HC, Wolke JG, Spauwen PH, Jansen JA. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants in cranial defects in rabbits. Biomaterials. 2004;25:2123–2132. doi: 10.1016/j.biomaterials.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Bohner M, Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–1563. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Lewis G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. J Biomed Mater Res B Appl Biomater. 2006;76:456–468. doi: 10.1002/jbm.b.30398. [DOI] [PubMed] [Google Scholar]
- 63.Takagi S, Chow LC, Hirayama S, Sugawara A. Premixed calcium phosphate cement pastes. J Biomed Mater Res Part B Biomater. 2003;67B:689–696. doi: 10.1002/jbm.b.10065. [DOI] [PubMed] [Google Scholar]
- 64.Carey LE, Xu HH, Simon CG, Jr, Takagi S, Chow LC. Premixed rapid-setting calcium phosphate composites for bone repair. Biomaterials. 2005;26:5002–5014. doi: 10.1016/j.biomaterials.2005.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chow LC, Takagi S. Dual-paste cement precursor systems for bone repair. US patent application No. 2007/0092580. [Google Scholar]
- 66.Sugawara A. New generation of bone graft material. Part 1. Requirements for Bone Regeneration. The Journal of Oral Implants (Japanese) 2006;26:55–74. [Google Scholar]
- 67.Sugawara A. New generation of bone graft material. Part 2. Tactics for Bone Regeneration. The Journal of Oral Implants (Japanese) 2006;27:31–56. [Google Scholar]
- 68.Sugawara A. New generation of bone graft material. Part 3. Animal Studies and Clinical Applications. The Journal of Oral Implants (Japanese) 2006;28:47–84. [Google Scholar]