Abstract
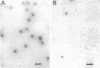
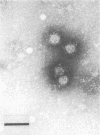
The solid-phase immune electron microscopy-double-antibody technique, which takes less than 1 h to perform, was applied as a rapid, sensitive, and specific diagnostic tool in the demonstration of papovavirus particles. BK virus propagated in 82C human skin fibroblasts and a monospecific high-titer immune serum to BK virus were used to establish the test procedure. When Formvar-carbon-coated grids were treated with appropriately diluted antibody, a 28-fold increase of virus particles per square micrometer was observed. Viewing of the virus particles was facilitated by the addition of a second "decorator" antibody. BK virus preparations at concentrations of 10(2) to 10(3) PFU/ml could be detected by this technique. There was no cross-reaction with mouse polyomavirus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Almeida J. D., Stannard L. M., Shersby A. S. A new phenomenon (SMOG) associated with solid phase immune electron microscopy. J Virol Methods. 1980;1(6):325–330. doi: 10.1016/0166-0934(80)90049-x. [DOI] [PubMed] [Google Scholar]
- Beth E., Cikes M., Schloen L., di Mayorca G., Giraldo G. Interspecies-, species- and type-specific T antigenic determinants of human papovaviruses (JC and BK) and of Simian virus 40. Int J Cancer. 1977 Oct 15;20(4):551–559. doi: 10.1002/ijc.2910200412. [DOI] [PubMed] [Google Scholar]
- Coleman D. V., Wolfendale M. R., Daniel R. A., Dhanjal N. K., Gardner S. D., Gibson P. E., Field A. M. A prospective study of human polyomavirus infection in pregnancy. J Infect Dis. 1980 Jul;142(1):1–8. doi: 10.1093/infdis/142.1.1. [DOI] [PubMed] [Google Scholar]
- Derrick K. S. Quantitative assay for plant viruses using serologically specific electron microscopy. Virology. 1973 Dec;56(2):652–653. doi: 10.1016/0042-6822(73)90068-8. [DOI] [PubMed] [Google Scholar]
- Doane F. W., Anderson N., Zbitnew A., Rhodes A. J. Application of electron microscopy to the diagnosis of virus infections. Can Med Assoc J. 1969 Jun 14;100(22):1043–1049. [PMC free article] [PubMed] [Google Scholar]
- Dougherty R. M., DiStefano H. S. Isolation and characterization of a papovavirus from human urine. Proc Soc Exp Biol Med. 1974 Jun;146(2):481–487. doi: 10.3181/00379727-146-38131. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gardner S. D. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973 Jan 13;1(5845):77–78. doi: 10.1136/bmj.1.5845.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Hogan T. F., Padgett B. L., Walker D. L., Borden E. C., McBain J. A. Rapid detection and identification of JC virus and BK virus in human urine by using immunofluorescence microscopy. J Clin Microbiol. 1980 Feb;11(2):178–183. doi: 10.1128/jcm.11.2.178-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan A. V., Coleman D. V., Koss L. G. Activation of human polyomavirus infection-detection by cytologic technics. Am J Clin Pathol. 1980 Sep;74(3):326–332. doi: 10.1093/ajcp/74.3.326. [DOI] [PubMed] [Google Scholar]
- Katz D., Straussman Y., Shahar A., Kohn A. Solid-phase immune electron microscopy (SPIEM) for rapid viral diagnosis. J Immunol Methods. 1980;38(1-2):171–174. doi: 10.1016/0022-1759(80)90341-5. [DOI] [PubMed] [Google Scholar]
- Lecatsas G., Boes E. G., Horsthemke E. Intermediate size papovavirus particles in pregnancy urine. J Gen Virol. 1981 Feb;52(Pt 2):359–362. doi: 10.1099/0022-1317-52-2-359. [DOI] [PubMed] [Google Scholar]
- Milne R. G., Luisoni E. Rapid high-resolution immune electron microscopy of plant viruses. Virology. 1975 Nov;68(1):270–274. doi: 10.1016/0042-6822(75)90169-5. [DOI] [PubMed] [Google Scholar]
- Nicolaieff A., Obert G., van Regenmortel M. H. Detection of rotavirus by serological trapping on antibody-coated electron microscope grids. J Clin Microbiol. 1980 Jul;12(1):101–104. doi: 10.1128/jcm.12.1.101-104.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Weiner L. P., Herndon R. M., Narayan O., Johnson R. T. Virions from progressive multifocal leukoencephalopathy: rapid serological identification by electron microscopy. Science. 1972 Oct 6;178(4056):60–62. doi: 10.1126/science.178.4056.60. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Studies of mouse polyoma virus infection. 1. Procedures for quantitation and detection of virus. J Exp Med. 1959 Apr 1;109(4):379–391. doi: 10.1084/jem.109.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K., Daniel R., Madden D., Stagno S. Serological investigation of BK papovavirus infection in pregnant women and their offspring. Infect Immun. 1980 Oct;30(1):29–35. doi: 10.1128/iai.30.1.29-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike K. Studies on DNA from low-density particles of SV40. I. Heterogeneous defective virions produced by successive undiluted passages. Virology. 1968 Mar;34(3):391–401. doi: 10.1016/0042-6822(68)90059-7. [DOI] [PubMed] [Google Scholar]