Abstract
Chronic myeloid leukemia (CML) is a stem cell disease, in which the BCR/ABL oncoprotein is considered essential for abnormal growth and accumulation of neoplastic cells. During the past 10 years, the BCR/ABL tyrosine kinase inhibitor imatinib (STI571) has successfully been introduced in the treatment of the disease. However, intrinsic as well as acquired resistance against the drug have been described and have been recognized as an emerging problem and challenge in clinical practice, and a key issue in CML research. Most of the respective concepts focus on imatinib-resistant mutants of BCR/ABL that are detectable in a high proportion of cases. However, other factors also contribute to resistance against imatinib, including the genetic background, the biologic features of CML stem cells, gene amplifications, silencing of tumor suppressor genes, and various pharmacologic aspects. In this article, the mechanisms of resistance against imatinib and other BCR/ABL tyrosine kinase inhibitors in CML are discussed together with strategies to overcome and to prevent resistance with available drugs or with novel antileukemic approaches.
Keywords: CML, imatinib, drug-resistance, BCR/ABL mutations, stem cells
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disease characterized by the t(9; 22) and the related oncogene, BCR/ABL (Nowell and Hungerford 1960; Rowley 1973; de Klein et al 1982). The respective fusion gene product, BCR/ABL, is a cytoplasmic 210 kDa protein that is considered essential for growth and survival of leukemic cells (Daley et al 1990; Lugo et al 1990; Gishizky and Witte 1992; Wetzler et al 1993; Biernaux et al 1995; Ren 2005). BCR/ABL displays constitutive tyrosine kinase (TK) activity and triggers a number of downstream signalling molecules including phosphoinositide 3-kinase (PI3K), mitogen-activated protein (MAP) kinase, nuclear factor-κB (NFκB), RAS, and signal transducer of activation and transcription 5 (STAT5) (Pendergast et al 1993; Puil et al 1994; Skorski et al 1997; Sillaber et al 2000; Sattler and Griffin 2003; Melo and Deininger 2004; Van Etten 2007). These signalling molecules and pathways supposedly act together to promote malignant transformation, to enhance genetic instability, and to suppress apoptosis in leukemic cells (Hoover et al 2001; Melo and Deininger 2004; Van Etten 2007).
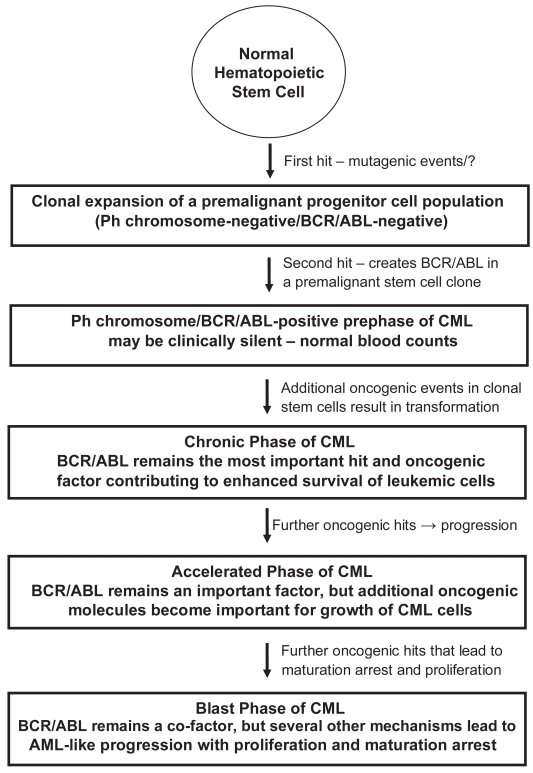
The (natural) clinical course in CML can be divided into a chronic (early) phase (CP), in which cellular differentiation and maturation are largely preserved, an accelerated phase (AP) of the disease, and a terminal (=blast) phase of CML (CML-BP), which resembles acute leukemia (Cortes and Kantarjian 2003; Giles et al 2004; Cortes et al 2006). In addition, based on the detection of BCR/ABL in apparently healthy subjects, a prephase of CML (with normal leukocyte counts), in which clonal BCR/ABL+ stem cells expand and generate subclones (Biernaux et al 1995; Bose et al 1998), has been postulated (Figure 1). What hits drive BCR/ABL-positive cells (subclones) from a prephase into overt CML, remains at present unknown. It also remains uncertain whether a ‘pre-BCR/ABL-phase’ of CML exists, in which monoclonal but preleukemic stem cell clones develop and expand to provide a suitable cellular background for the establishment of a BCR/ABL+ clone (Figure 1). This hypothesis has been based on rare cases of BCR/ABL-negative but apparently monoclonal populations of leukemic cells (subclones) that may develop in CML patients during treatment with imatinib. All in all, BCR/ABL is considered a most critical factor, but may per se not be sufficient for disease-initiation. Also, whereas in CP, BCR/ABL is considered to play a predominant role for leukemia cell survival, additional pro-oncogenic molecules and pathways may become (more) important and contribute to malignant growth and thus disease-progression in advanced CML (AP, BP) (Shet et al 2002; Sattler and Griffin 2003; Calabretta and Perrotti 2004; Melo and Barnes 2007) (Figure 1).
Figure 1.
Evolution of CML with prephases—a proposed hypothesis.
Abbreviations: Ph, Philadelphia chromosome; CML, chronic myeloid leukemia; AML, acute myeloid leukemia.
The leukemic clone in CML is organized hierarchically, with more mature cells that have a limited capacity to divide and to survive, and cells with unlimited capacity to divide and to self-renew, so-called leukemic stem cells (Eaves et al 1993, 1998; Holyoake et al 2000, 2001; Eisterer et al 2005; Elrick et al 2005). Taking this concept into consideration, it seems clear that the clinically relevant portion of MRD and any resulting relapse derives from CML stem cells, and that therapy is curative only when eradicating these cells. During disease evolution and probably even before overt disease is diagnosed (prephase of CML), CML stem cells may acquire multiple (transforming) hits, resulting in subclone-formation (Holyoake et al 2002; Jiang et al 2007a). Therefore, the CML clone supposedly is composed of several different subclones at diagnosis in most (if not all) patients, a hypothesis that explains the ‘occurrence’ of drug-resistant BCR/ABL-mutants during therapy through subclone-selection (Roche-Lestienne et al 2002; Jiang et al 2007a). An unresolved question is why wild type (wt) BCR/ABL-bearing cells have a growth advantage over subclones exhibiting BCR/ABL-mutants. In fact, in most patients, the mutant subclone is only detectable after initiation of BCR/ABL-targeting therapy. A related question is how the disease can suppress growth of normal hematopoietic stem cells. Here, one hypothesis is, that stem cell-derived negative growth-regulators (chalones) such as lipocalin, suppress growth of normal (stem) cells through a specific receptor, whereas CML stem cells are resistant, as they display only low amounts or lack lipocalin-binding sites (Devireddy et al 2005; Lin et al 2005). Whether mutant BCR/ABL-bearing subclones are also suppressed by leukemic cells displaying wt BCR/ABL through chalone-dependent inhibition or other mechanisms, remains unknown.
The BCR/ABL kinase inhibitor imatinib has successfully been introduced in the treatment of CML. Thus, imatinib induces major cytogenetic responses in a majority of all patients with CP CML (Druker et al 2001a; Kantarjian H et al 2002; Barbany et al 2002; O’Brien et al 2003). Responses are also seen in (some) patients with AP or BP (Druker et al 2001b; Talpaz et al 2002; Kantarjian et al 2002; Sawyers et al 2002). However, despite overwhelming initial data and high expectations, little is known about long-term effects of imatinib (Druker et al 2006). An apparent result from follow up studies is that imatinib is unable to eradicate all neoplastic stem cells (subclones) in CML. Rather, many patients develop overt resistance against imatinib during therapy, which is often associated with the outgrowth of subclones bearing mutations in BCR/ABL (Branford et al 2002, 2003; Kantarjian et al 2006a; Hochhaus et al 2007a). For such patients, treatment options are usually limited. In fact, many of them are in AP or BP, and only a subgroup of them are eligible for stem cell transplantation (SCT).
Therefore, a number of attempts have been made to identify new drugs that act antileukemic in imatinib-resistant CML (Shah et al 2004; Weisberg et al 2005, 2007b; O’Hare et al 2005; Martinelli et al 2005). Such drugs are directed against BCR/ABL and its mutants, but may also be directed against other (BCR/ABL-independent) molecules that play a role in malignant transformation (Martinelli et al 2005; Weisberg et al 2007b). Thus, molecular resistance against imatinib may not only be caused by changes in BCR/ABL, but also by other pro-oncogenic molecules (Kantarjian et al 2006a; Hochhaus et al 2007a). Therefore, less specific targeted drugs and combinations of targeted drugs have been proposed, and are currently applied in clinical trials to overcome resistance. Some of the emerging TK inhibitors act on BCR/ABL as well as on other key signalling targets, such as Lyn or/and other Src kinases (Shah et al 2004; Martinelli et al 2005; Kimura et al 2005; Weisberg et al 2007b).
Apart from molecular resistance against imatinib, other mechanisms that cause resistance in CML, have also been described. First, immature leukemic cells (stem cells) may exhibit intrinsic (BCR/ABL-independent) resistance (Barnes and Melo 2006; Jiang et al 2007b). Second, a number of cellular molecules involved in the regulation of drug-uptake, drug-metabolism or drug-efflux, may influence the bio-availability of imatinib (Herweijer et al 1990; Illmer et al 2004; Thomas et al 2004; Wang et al 2007; Brendel et al 2007). Lastly, more and more data suggest that imatinib is not capable of entering all organ-compartments in vivo. Likewise, imatinib is unable to cross the blood-brain barrier in amounts sufficient to reach a pharmacologic drug concentration in the central nervous system (CNS) (Takayama et al 2002; Senior 2003; Dai et al 2003; Wolff et al 2003). Correspondingly, CNS relapses are increasingly described in CML patients treated with imatinib (Abruzzese et al 2003; Leis et al 2004; Bornhäuser et al 2004; Rajappa et al 2004; Rytting and Wierda 2004; Matsuda et al 2005; Pavlu et al 2005; Kim et al 2006; Aichberger et al 2007).
In the following sections, various types of resistance against imatinib are discussed together with possibilities to prevent or to overcome resistance with currently available drugs, combination strategies employing drugs and SCT, or future therapeutic approaches such as siRNA or immunotherapies.
Leukemic stem cells exhibit ‘intrinsic resistance’
Most patients in CML CP enter a complete cytogenetic response (CCR) during treatment with imatinib (Druker et al 2001a; Kantarjian H et al 2002; Barbany et al 2002; O’Brien et al 2003). In many of these patients, BCR/ABL-transcripts decrease to low or even undetectable levels over time (Barbany et al 2002; Hughes et al 2003; Druker et al 2006). However, discontinuation of imatinib is usually followed by a cytogenetic and hematologic relapse (Breccia et al 2006; Rousselot et al 2007). Based on this observation and other studies, it has been hypothesized that MRD in imatinib-treated patients contains leukemic stem cells (clinically relevant subclones), and that these residual stem cells exhibit imatinib-resistance (Graham et al 2002; Bhatia et al 2003; Michor et al 2005; Goldman and Gordon 2006; Deininger 2007). A remarkable aspect is that the relapsing subclones that reappear after discontinuation of imatinib usually display wt BCR/ABL. Therefore, apart from well known molecular mechanisms (BCR/ABL mutations) leading to resistance, stem cell resistance against imatinib in CML is also considered to result from stem cell-related (intrinsic) mechanisms. The exact molecular basis of intrinsic stem cell resistance against imatinib is not well understood. The different hypotheses that have been raised are summarized in Table 1. Apart from stem cell quiescence and overexpression of BCR/ABL, these cells may also utilize BCR/ABL-independent survival mechanisms (Copland et al 2005; Copland et al 2006; Barnes and Melo 2006; Jiang et al 2007b). In addition, it has been hypothesized that imatinib-resistance in CML stem cells may be associated with reduced drug-uptake and increased drug-efflux. In particular, compared to more mature clonal cells, CML stem cells (CD34+/CD38−/Lin−) apparently display decreased levels of organic cation transporter-1 (OCT-1), a surface transporter involved in the uptake of imatinib, and increased levels of drug-efflux-related surface molecules including the multi-drug resistance protein-1 (MDR-1), known to mediate the efflux of imatinib (Jiang et al 2007b). Efflux mechanisms may also contribute to resistance against other drugs including new BCR/ABL TK inhibitors, such as nilotinib (AMN107) (Brendel et al 2007). More recently, it has been described, that dasatinib may act better on immature CML (stem) cells compared to imatinib, but still may not be capable of killing all leukemic stem cells (subclones) (Copland et al 2006). An interesting approach to measure the response to imatinib on a qualitative basis and to possibly predict the response (and relapse) in progenitor compartments, are recently proposed mathematical models (Michor et al 2005; Roeder et al 2006). It may be an interesting idea to employ such models in forthcoming clinical trials studying new TK inhibitors or combination therapies.
Table 1.
Resistance of CML stem cells against imatinib: Proposed hypotheses*
| Observations | Specific hypothesis/ molecular basis |
|---|---|
| Stem cell quiescence (dormance) | G0 arrest by chalones or by specific cell cycle regulators, quite similar mechanisms may lead to dormance of normal stem cells** |
| Stem cell plasticity | Development of subclones may be facilitated by mechanisms similar to those responsible for differentiation of normal myeloid stem cells into various myeloid lineages** – exact mechanisms are unknown |
| Overexpression of BCR/ABL | BCR/ABL mRNA and protein over- expression – mechanisms unknown |
| Specific BCR/ABL-induced stem cell deregulation | Altered DNA repair; hypermethylation; induction of stem cell subclones. Over time, BCR/ABL may overcome some of the stem cell-protecting mechanisms |
| BCR/ABL-independent survival | Stem cell-specific survival factors; altered survival factors in subclones*** |
| Decreased uptake of imatinib | Decreased expression of OCT-1, a major transporter of imatinib |
| Increased drug efflux | Overexpression of P-glycoprotein(MDR-1) and other efflux pumps**** |
Notes:
Relate all to differences between CML stem cells and more mature CML cells. The following articles refer to these concepts: Copland et al 2005, 2006; Barnes and Melo 2006; Brendel et al 2007; Jiang et al 2007b);
The biology of normal stem cells and CML stem cells may be quite similar;
CML stem cells may even survive after complete deactivation of BCR/ABL;
Normal and neoplastic stem cells may defend their long-term existence against external ‘enemies’ (toxins, drugs) by high toxin/drug efflux.
Abbreviations: OCT-1, organic cation transporter; MDR-1, multidrug resistance gene-1.
As outlined above, stem cell resistance in CML is an emerging issue and major focus in clinical and preclinical research, and although it is difficult to purify CML stem cells for in vitro investigations, the availability of sensitive MRD parameters offers a valuable basis for the design of clinical trials examining the effects of novel drugs and drug-combinations on residual leukemic stem cells. For the near future, one of the most important questions will be whether any of the new TK inhibitors, like dasatinib, nilotinib, INNO-406, or others, can induce long-lasting CCR and consecutive cure through eradication of all relevant CML stem cell subclones in CP. Respective clinical trials employing dasatinib or nilotinib as frontline therapy in CML CP are in progress. These trials should reveal the exact curative potential of these drugs and thus will answer the question as to whether they can overcome ‘intrinsic stem cell resistance’. It should be mentioned here that not all CML stem cell subclones may be of clinical relevance (causing relapse), and that some of these patients may stay in complete hematologic remission even if a BCR/ABL+(sub)clone is detectable (Griswold et al 2006; Khorashad et al 2006; Goldman and Gordon 2006).
The next important question would be whether combinations of targeted drugs may (better) overcome stem cell resistance against imatinib. First, some of these combinations may facilitate the uptake of imatinib or other BCR/ABL TK inhibitors in CML stem cells, or may prevent enhanced drug efflux from these cells (Mahon et al 2003; Thomas et al 2004; Illmer et al 2004). Likewise, a number of MDR-1 blockers (cyclosporin-A, verapamil, others) are available, and it may be an interesting approach to combine such inhibitors with imatinib or other BCR/ABL TK inhibitors to enhance intracellular drug levels in CML stem cells. More recently, it has been described that combinations of TK inhibitors with each other may also enhance intracellular levels of individual drugs and thereby may lead to cooperative (synergistic) antileukemic effects (White et al 2007). Indeed, most of the BCR/ABL TK inhibitors exert synergistic antileukemic effects on CML cells (Weisberg et al 2007a).
Another important aspect is that conventional antileukemic drugs such as interferon-alpha, may have a more pronounced effect on CML progenitor cells compared with imatinib (Angstreich et al 2005; Verbeek et al 2006). Therefore, several trials employ combinations between interferon-alpha and BCR/ABL TK inhibitors. Another important aspect is that many of the novel inhibitors are less specific drugs that do not only recognize BCR/ABL, but also (many) other key kinase-targets (Shah et al 2004; Martinelli et al 2005; Kimura et al 2005; Weisberg et al 2007b). The differential target profiles of TK inhibitors may also explain why several of them, when combined, produce synergistic antileukemic effects (Weisberg et al 2007a). In this regard it may be of great importance to learn which kinase-targets and related pathways play a predominant role in the biology and growth of CML stem cells. An important consideration in this regard is that the biology, function, and target expression profiles of CML stem cells may be similar but not identical to that of normal stem cells, and that the profile may change during disease evolution, ie, progression to AP or BP (Zheng et al 2006; Radich et al 2006; Villuendas et al 2006; Diaz-Blanco et al 2007).
Lastly, it has to be emphasized that the only established stem cell eradicating (=curative) therapy in CML remains SCT, and that SCT may also work in a group of patients with advanced CML (Goldman et al 1986; Silberman 1994; Gratwohl et al 1998; Dutcher and Wiernik 2000; Deininger 2007). It also has been described that pre-transplant therapy with imatinib may be a reasonable approach in advanced CML (Giralt et al 2007; Weisser et al 2007). Moreover, SCT should remain an important option and major decision-point in treatment algorithms in imatinib-resistant CML (Jabbour et al 2006). Depending on the clinical situation and other factors, such therapy (SCT) may be combined with BCR/ABL TK inhibitors (Menzel et al 2007). Likewise, patients with imatinib-resistant CML in AP or BP who are young and have a suitable donor, may benefit from targeted therapy with a second generation BCR/ABL TK inhibitor inducing remission or at least disease-reduction (debulking), followed by allogeneic SCT (Jabbour et al 2007a; Menzel et al 2007). As to whether such patients should also be treated with the same BCR/ABL TK inhibitors after SCT (maintenance, prophylaxis) remains at present unknown. At least for patients with detectable BCR/ABL after SCT, maintenance therapy should be considered. Patients who fail SCT or relapse after SCT may also benefit from new TK inhibitors. Whether such patients may even have a better outcome when receiving combination therapy (drug-combinations or donor lymphocytes plus a TK inhibitor) remains to be defined.
Pharmacologic aspects and pharmacologic resistance
Orally administered imatinib is rapidly (within 1–2 hours) and completely absorbed, with a bioavailability of >95%, and a peak plasma concentration reached after 2–4 hours (Cohen et al 2002; Peng et al 2005). The pharmacologic half-life of the drug is approximately 18 hours (Cohen et al 2002; le Coutre et al 2004; Peng et al 2004a, 2005). At a daily dose of 400 mg, imatinib plasma concentrations peak to about 2–3 μg/mL, with a trough level of approximately 1 μg/mL (le Coutre et al 2004; Peng et al 2004a, 2005), which exceeds imatinib-doses required for complete inhibition of wt BCR/ABL TK activity (0.5 μg/mL = 1 μM). Imatinib is >95% bound to human plasma proteins, mainly albumin and alpha1-acid glycoprotein (Cohen et al 2002; Peng et al 2005). The drug is eliminated predominantly via the bile in form of metabolites (Cohen 2002; Gschwind et al 2005; Peng et al 2005). One of these metabolites, CGP74588, exhibits pharmacological activity comparable to the parent-drug (Cohen et al 2002). Imatinib is metabolized via cytochrome (CYP) P450 isoenzymes, primarily CYP3A4, but also by other CYP 450 species (CYP3A5, CYP2D6). Therefore, imatinib can competitively inhibit the metabolism of drugs that are substrates of CYP P450 isoenzymes (Cohen et al 2002; O’Brien et al 2003). Vice versa, drugs that are metabolized via or are inducers of these CYP enzymes may influence the bioavailability of imatinib, and thus lead to changes (eg, decrease) in imatinib plasma concentrations (Cohen et al 2002; Bolton et al 2004; Dutreix et al 2004; Frye et al 2004). Also, hepatic and renal dysfunction may result in slight changes in imatinib concentrations in biological fluids and tissues (Peng et al 2005; Pappas et al 2005; Eckel et al 2005). However, these changes usually are mild and do not require dose-adjustments. Age, race, sex, and bodyweight have no documented influence on the pharmacokinetics or pharmacodynamics of imatinib (Peng et al 2005).
From experience in clinical trials, patients with CML are judged to be imatinib-resistant when response is lost or is not seen with a daily dose of >400 mg imatinib (Kantarjian et al 2003; Baccarani et al 2006). Studies on pharmacokinetics and pharmacodynamics of imatinib in CML suggest that a minimum dose of imatinib of 350–400 mg daily is required to reach a constant effective drug concentration in plasma, that would block wt BCR/ABL (Peng et al 2004b). However, no detailed studies on tissue-concentrations of imatinib in various organs have been presented so far, and some of the tissues and organ-compartments (brain) may not be reached sufficiently by imatinib. In addition, a number of genetic and other factors may influence the bioavailability of the drug (Peng et al 2005; Pappas et al 2005; Eckel et al 2005). Moreover, the expression of drug transporters and drug-efflux pumps, that are expressed in the apical membrane of the small intestine and the bile canalicular membrane, have been implicated in pharmacologic resistance (Burger and Nooter 2004; Burger et al 2005). All in all, a number of factors may influence the plasma- and tissue levels of imatinib, and under certain circumstances, may contribute to pharmacologic resistance.
More recent data suggest that pharmacologic resistance may indeed be of clinical relevance. In fact, it has been described that the trough plasma level of imatinib is associated with the rate of CCR and of major molecular responses (MMR) in patients with CML (Picard et al 2007). In particular, significantly higher trough levels were found in patients with CCR and MMR (often >1 μg/ml) compared to those without CCR or MMR (often <1 μg/ml) (Picard et al 2007). An unresolved question is whether the different trough levels in the two groups of patients resulted from a primary defect(s) in bioavailability (true pharmacologic resistance) or from massive drug-uptake by residual CML cells in less well responding patients. Whatever the reason is, the observation of different trough levels may be of clinical significance, and it seems appropriate to recommend that plasma trough levels are measured in patients with otherwise unexplained suboptimal response (or no response) to imatinib.
A number of different strategies have been proposed to overcome pharmacologic resistance against imatinib. Suspicion for pharmacologic resistance must be raised when cytogenetic (and molecular) response is lost or not achieved, no BCR/ABL mutations and no signs of clonal evolution are found, and trough imatinib levels are low. It is then important to ask for possible drug-interactions, patient’s complience, and concomitant disorders. After having excluded such causes, dose adjustments (increase) should be considered and may lead to a better response (Kantarjian et al 2003). Another potential strategy, that may become subject of future studies, would be to try to increase the imatinib uptake in the intestinal wall (and in other critical target cell populations), and thus bioavailability of the drug, or by imatinib with modulators of transport-proteins (Breedveld et al 2006).
‘Anatomic resistance’ against imatinib
A special problem with imatinib is its marginal accumulation in the central nervous system (CNS) which is caused by low uptake via the blood-brain barrier (Takayama et al 2002; Senior 2003; Dai et al 2003; Wolff et al 2003). The biochemical basis of poor uptake is not well understood. One hypothesis is that the abundant expression of MDR-1 (P-glycoprotein) in cells forming the blood-brain barrier is associated with constant drug-efflux (Dai et al 2003; Breedveld et al 2005; Breedveld et al 2006). Clinically, the poor uptake into the CNS is reflected by CNS relapses that occur in imatinib-treated patients (Abruzzese et al 2003; Leis et al 2004; Bornhäuser et al 2004; Rajappa et al 2004; Rytting and Wierda 2004; Matsuda et al 2005; Pavlu et al 2005; Kim et al 2006). This is a well known problem in lymphoid leukemias and in the lymphoid blast phase of CML. However, more recently, myeloid CNS relapses have also been described (Rytting and Wierda 2004; Aichberger et al 2007). Some of these CNS relapses occur even in patients with CCR (Bornhäuser et al 2004; Aichberger et al 2007).
A number of strategies have been proposed to treat and to prevent CNS relapses in CML. Once diagnosed, local therapy of the CNS relapse with intrathecal cytostatic drugs (cytarabine and others) and/or radiation seems an appropriate therapeutic maneuver (Abruzzese et al 2003; Leis et al 2004; Bornhäuser et al 2004; Rajappa et al 2004; Rytting and Wierda 2004; Matsuda et al 2005; Pavlu et al 2005; Kim et al 2006; Aichberger et al 2007). In those with a concomitant systemic relapse, the additional replacement of imatinib by a second generation BCR/ABL inhibitor must be considered (Abdelhalim et al 2007; Aichberger et al 2007). Interestingly, for some of these emerging drugs (dasatinib, INNO-406), it has been described that they can cross the blood-brain barrier quite effectively in animal models (Wild et al 2004; Yokota et al 2007), and the same may hold true for patients with CML in CNS relapse (Abdelhalim et al 2007; Aichberger et al 2007). Therefore, it seems logic to consider the use of such new TK inhibitors as prophylaxis of CNS relapses as well. In case that the frequency of reported CNS relapses will further increase, such prophylactic therapy must be regarded as a mandatory approach. An alternative approach might be to increase the uptake of imatinib by applying modulators of drug transporters (eg, MDR-1).
BCR/ABL mutations
The predominant molecular defect that causes resistance against imatinib are point mutations in the BCR/ABL oncogene (Gorre et al 2001; von Bubnoff et al 2002; Barthe et al 2002; Branford et al 2002, 2003; Shah et al 2002; Hochhaus et al 2002). The respective BCR/ABL mutants retain their kinase activity and their oncogenic potential, but usually display impaired or absent drug-binding capacity (Roumiantsev et al 2002; Azam et al 2003; Cowan-Jacob et al 2004; Weisberg et al 2007b). Other mutants may be less oncogenic and may not play an important role in disease evolution (Griswold et al 2006; Khorashad et al 2006). Most of the relevant mutations cluster within or in next vicinity to the imatinib-binding site, or are located in BCR/ABL domains critical to the topography and tertiary structure of the imatinib/ATP-binding site, with consecutive steric hindrance of drug-binding (Branford et al 2002, 2003; Shah et al 2002; Hochhaus et al 2002; Roumiantsev et al 2002; Azam et al 2003; Cowan-Jacob et al 2004; O’Hare et al 2005, Weisberg et al 2007b). Examples of BCR/ABL residues that (when derived from mutated genes) directly inhibit imatinib-binding, are Thr315 and Phe317 (Cowan-Jacob et al 2004; Weisberg et al 2007b). Other BCR/ABL mutations destabilize the inactive conformation of the nucleotide-binding loop (P-loop) or the DFG motif that binds to imatinib, thereby reducing imatinib-binding affinity (Roumiantsev et al 2002; Cowan-Jacob et al 2004; Weisberg et al 2007b). Residues affecting imatinib-binding through destabilization of the inactive conformation include Glu255, Tyr253, and Gly250 in the P-loop of ABL (Cowan-Jacob et al 2004; Weisberg et al 2007b).
More than 50 different mutations in BCR/ABL have been described (von Bubnoff et al 2002; Shah et al 2002; Hochhaus et al 2002; Cowan-Jacob et al 2004; O’Hare et al 2005; Weisberg et al 2007b). These mutations cluster in four major regions of the oncogene, namley the phosphate-binding (P-loop) domain (examples: M224V, L248V, G250E/R, Q252R/H, Y253F/H, E255K/V), the imatinib-binding domain (F311L/I, T315I, F317L), the catalytic domain (M351T, E355G/D), and the activation loop domain (V379I, F382L, L387M, H396R/P) (Shah et al 2002; Branford et al 2003; Hochhaus et al 2002). Table 2 shows BCR/ABL mutations frequently detected in patients with imatinib-resistant CML.
Table 2.
BCR/ABL mutations detectable in CML patients treated with imatinib
| Mutant | IC50* imatinib | may benefit from IM-dose-escalation** | IC50* dasatinib | IC50* nilotinib |
|---|---|---|---|---|
| no (wt) | 250–500 | - | 0.8 | 13 |
| M244V | 1,600–3,100 | yes | 1.3 | 38 |
| M244I | 1,400 | yes | nk | nk |
| G250E | >1,000 (>3,000) | no | 1.8 | 48 |
| Q252H | 1,300–2,900 | yes | 3.4 | 70 |
| Y253H | 4,000–17,000 | no | 1.3 | 450 |
| Y253F | 1,800–5,000 | no | 1.4 | 125 |
| E255K | 5,000–12,000 | no | 5.6 | 200 |
| E255V | 6,000–20,000 | no | 11 | 430 |
| F311L | 480–1,300 | no | 1.3 | 23 |
| T315I | >10,000 | no | >200 | >2,000 |
| F317L | 1,000–2,300 | no | 7.4 | 50 |
| M351T | 900–4,900 | yes | 1.1 | 15 |
| M351I | 1,600 | yes | nk | nk |
| F359V | 1,400–1,800 | yes | 2.2 | 175 |
| E355G | 2,000–2,400 | yes | nk | nk |
| V379I | 1,000–1,600 | yes | 0.8 | 51 |
| L387M | 1,000–1,100 | yes | 2 | 49 |
| H396P | 850–4,300 | no | 0.6 | 41 |
| H396R | 1,750–5,400 | no | 1.3 | 41 |
Notes:
IC50 values are given in nM and refer to published data obtained with Ba/F3 cells exhibiting wild type BCR/ABL or various BCR/ABL mutants using cell – proliferation assays (O’Hare et al 2005; Martinelli et al 2005);
recommendations are derived from Martinelli and colleagues (2005).
Abbreviations: IM, imatinib; wt, wild type; nk, not known.
In most CML patients, BCR/ABL mutations may already be present in (stem cell) subclones before imatinib therapy is initiated (Roche-Lestienne et al 2002, 2003; Kreuzer et al 2003; Jiang et al 2007a). However, in some patients, the BCR/ABL mutation may not simply be revealed through selection by drug-therapy, but may represent a newly occurring defect. An unresolved question in this regard is whether treatment with imatinib or other (targeted) drugs can modulate (increase) the BCR/ABL mutation rate (mutagenic potential of drug). The more likely scenario is that the rapid and sustained elimination of all (many) subclones by TK inhibitors is important and should counteract the development of new BCR/ABL mutations, because the size of the target cell population (CML stem cells) in which such mutations can develop, is constantly shrinking over time in responding patients.
Another unresolved question is how the wt BCR/ABL subclone is capable of suppressing (all) BCR/ABL mutants. This phenomenon may be explained by chalone-dependent inhibition or may be related to the different oncogenic potencies of the mutants. Clinically, this phenomenon is of diagnostic importance, as BCR/ABL mutations may not be detectable at diagnosis but only after drug-induced selection of stem cell subclones.
As mentioned above, the various BCR/ABL mutants display different oncogenic (transforming) potential (Griswold et al 2006; Skaggs et al 2006; Weisberg et al 2007a). Taking their in vitro activity into consideration, the following rank order of (oncogenic) potency is found: Y253F = E255K > wt BCR-ABL > T315I > H396P > M351T > others. Thus, certain P loop mutations (Y253F, E255K) and the T315I mutation display a high oncogenic potential, which is consistent with the clinical observation of a poor outcome concerning overall and progression-free survival (Branford et al 2003; Soverini et al 2005; Nicolini et al 2006). However, not all P-loop mutations may be associated with a poor prognosis in CML (Jabbour et al 2006). In particular, several of the BCR/ABL mutations are far less oncogenic, and some of them may not even exhibit a proliferative advantage over normal (stem) cells, and thus may not even cause overt CML (Khorashad et al 2006). These mutations should not count in the evaluation of drug resistance and the consecutive treatment plan in the same way as clinically relevant (oncogenic) mutations.
A number of different strategies have been proposed to treat patients with imatinib resistant CML, in whom BCR/ABL mutations are detected. Treatment in these patients depends on several different factors, including the type (oncogenic potential) of the mutation, phase of disease, presence of other pro-oncogenic disease-features (clonal evolution), age, co-morbidity, overall status of the patient, and availability of a SCT donor in those who are eligible for high-dose therapy. With regard to BCR/ABL mutations, four categories are proposed and related to specific treatment recommendations: a) mutations that do not cause clinically overt resistance (recommendation: wait and watch if possible), b) mutants that have low oncogenic potential and may disappear (at least kept under control) upon dose-escalation (recommendation: increase imatinib from 400 to 600 or 800 mg/day), c) non-T315I-mutants that are not expected to disappear on imatinib dose-escalation (recommendation: switch from imatinib to a second generation BCR/ABL inhibitor: nilotinib or dasatinib), and d) the T315I mutant as well as a few other mutants that are also resistant against dasatinib and nilotinib (recommendation: high-dose chemotherapy or experimental drugs and proceed to SCT if possible). The majority of all imatinib-resistant patients are in group b and c. Therefore, recent efforts have focused on the development of new, more effective BCR/ABL TK inhibitors that can overcome resistance. Among these are nilotinib (AMN107), dasatinib (BMS354825), INNO-406, and several others (Table 3). These drugs act on various imatinib-resistant BCR/ABL mutants and can produce complete hematologic and cytogenetic responses in patients with imatinib-resistant disease (Talpaz et al 2006; Kantarjian et al 2006; Weisberg et al 2006; Quintas-Cardama et al 2007; Hochhaus et al 2007b; Cortes et al 2007; Guilhot et al 2007). Encouraging results have particularly been obtained in CP, but hematologic and sometimes cytogenetic or molecular responses may also be seen in AP or BP. However, as stated above, not all BCR/ABL mutants are responsive to these inhibitors, and the relative potencies vary among drugs. Unfortunately, patients with the T315I mutant of BCR/ABL are clinically resistant against nilotinib and dasatinib, and also against most other available TK inhibitors (Talpaz et al 2006; Kantarjian et al 2006; Weisberg et al 2006). As mentioned above, for these patients, alternative therapies have to be considered. One possibility are novel kinase inhibitors or drugs that act independent of BCR/ABL (Martinelli et al 2005; Gumireddy et al 2005; Tauchi and Ohyashiki 2006; Jabbour et al 2007b) (Table 3). Another option is SCT with or without a second generation BCR/ABL inhibitor (Jabbour et al 2007a; Menzel et al 2007).
Table 3.
Novel pharmacologic inhibitors proposed for imatinib-resistant CML
| Drug name | Drug type class | Known target(s) | active in cells bearing BCR/ABL T315I |
|---|---|---|---|
| Dasatinib (Sprycel) | TKI | Abl, Src, Lyn, Btk, Kit, PDGFR, | no |
| Nilotinib (Dasigna) | TKI | Abl, Kit, PDGFR, ...... | no |
| SKI-606 (Bosutinib) | TKI | Abl, Src, .. | no |
| INNO-406 (NS-187) | TKI | Abl, Lyn, Kit, .. | no |
| AZD0530 | TKI | Abl, Src, .. | no |
| AP23464 | TKI | Abl, Src, .. | no |
| CGP76030 | TKI | Abl, Src, .. | +/−* |
| PP1 | TKI | Abk, Src, .. | +/−* |
| PD166326 | TKI | Abl, Src, .. | no |
| ON012380 | TKI | Abl, PDGFR, Lyn | yes |
| MK-0457 (VX-680), other AuK-I | AuK-I | Aurora-kinase | yes |
| BIRB-796, 43-9006 (sorafenib) | p38-I | p38 MAP kinase, (Abl) | no |
| Kinase-I | multiple kinases, Mcl-1 | yes | |
| WP1130 | - | ? (Jak, Abl-knock-down) | yes |
| various | hypomethylating agents | re-expression of tumor suppressors | +/− |
| various | Hsp-I | Hsp32, Hsp70, Hsp90, … | yes |
| various compounds | FTIs | RAS | +/− |
| various compounds | PI3K-I | PI3-kinase | yes |
| Rapamycin and its derivatives | mTOR-I | mTOR | yes |
Notes:
relatively high drug concentrations needed to block growth of cells.
Abbreviations: TKI, tyrosine kinase inhibitor; PDGFR, platelet derived growth factor receptor; Hsp, heat shock proteins; FTI, farnesyl transferase inhibitor; mTOR, mammalian target of rapamycin.
An interesting aspect is that BCR/ABL TK inhibitors, when applied in combination, may produce antileukemic effects on CML cells exhibiting BCR/ABL T315I, even if leukemic cells are resistant against single agents (White et al 2007; Weisberg et al 2007a). This phenomenon may be explained by additional drug targets expressed in these cells, by cooperative effects at BCR/ABL epitopes, or by increased drug accumulation in target cells (White et al 2007; Weisberg et al 2007a). Whether combinations of TK inhibitors will also induce long lasting remission in (all) patients with TK-inhibitor resistant CML, remains at present unknown. It also remains unknown which of the new drugs, that have been described to counteract in vitro growth of leukemic cells exhibiting BCR/ABL T315I (Tseng et al 2005; Carter et al 2005; Giles et al 2007; Cheetham et al 2007; Rahmani et al 2007), will induce complete (and long lasting) cytogenetic remissions in vivo in these patients.
An important therapeutic consideration is prevention of occurrence (selection) of subclones carrying imatinib-resistant BCR/ABL mutants. One approach may be to combine TK inhibitors in an early phase of disease, similar to the situation in HIV-positive patients, where early intervention is performed using multiple drugs. Another strategy may be to combine novel TK inhibitors with a stem cell-attacking approach, like SCT or high-dose chemotherapy, or with stem cell-suppressing maintenance therapy (eg, interferon-alpha).
Finally, several treatment concepts focus on the mobilization of the immune system, with the ultimate goal to target residual leukemic (stem) cells (MRD) in CML (Li et al 2005; Westermann et al 2007; Volpe et al 2007; Peng et al 2007). In most instances, immunotherapy is combined with a BCR/ABL TK inhibitor. Whether such intervention may lead to the eradication of (all) relevant CML stem cell subclones remains to be elucidated.
Other BCR/ABL defects
Apart from BCR/ABL mutations, other defects in BCR/ABL may also contribute to resistance against imatinib. Such alternative defects include BCR/ABL gene duplications and -amplifications (le Coutre et al 2000; Weisberg and Griffin 2000; Gorre et al 2001; Nguyen Khac et al 2002; Campbell et al 2002; Morel et al 2003; Gargallo et al 2003; Gadzicki et al 2005). These defects may be associated with (multiple) cytogenetic abnormalities (Gadzicki et al 2005; Pienkowski-Grela et al 2007), and several of these patients are in an accelerated phase or blast phase of CML (Tanaka et al 2000; Gadzicki et al 2005; Pienkowski-Grela et al 2007). Therefore, in most cases, the contribution of amplified BCR/ABL in the malignant process (progression) and in drug resistance remains uncertain. Nevertheless, some of these patients respond to elevated doses (or even standard doses) of imatinib, suggesting that the BCR/ABL defect may have pharmacologic and clinical impact.
BCR/ABL-independent molecular resistance
During disease, the CML clone may acquire additional BCR/ABL-independent molecular (genetic) defects and pro-oncogenic hits in stem cell subclones, which may lead to disease-progression. Such clonal evolution is often accompanied by the occurrence of cytogenetic defects. Leukemic cells in these patients are frequently resistant against imatinib, and may exhibit aneuploidy, sometimes in form of a second Ph chromosome or trisomy 8 (+8) (Hochhaus et al 2002). Other cytogenetic defects that have been described in imatinib-resistant CML include trisomy 6 (+6), +9, +12, +18, and monosomy 7 (−7) (Hochhaus et al 2002; Cortes et al 2003; Marktel et al 2003; O’Dwyer et al 2004). Most cytogenetic defects are considered to be of prognostic significance concerning survival in imatinib-treated patients (Cortes et al 2003; Marktel et al 2003; O’Dwyer et al 2004). However, not all cytogenetic defects may lead to imatinib-resistance. Especially isolated chromosome defects may disappear or persist at stable (low) level without loss of hematologic response during therapy. In other patients, resistance may develop within short time.
The molecular defects that accompany cytogenetic abnormalities and may contribute to resistance against imatinib, have not been defined yet. Therefore, at present, it is difficult to predict the clinical impact of isolated cytogenetic defects for imatinib-treated patients. A special situation is the occurrence of cytogenetic defects in Ph-negative subclones during imatinib therapy (Medina et al 2003; Terre et al 2004; Loriaux and Deininger 2004; Lin et al 2006; Navarro et al 2007; Jabbour et al 2007). One hypothesis is that these subclones derive from a very immature progenitor that was involved in a pre-Ph (pre-BCR/ABL) phase of CML (Figure 1), and under certain circumstances can be activated (by additional hits) to transform into a secondary Ph-negative (but still monoclonal) neoplasm. Indeed, some of these patients may develop overt secondary disease (that may resemble a myelodysplastic syndrome, MDS or acute myeloid leukemia, AML), even if the Ph-positive (sub)clones are completely suppressed (Loriaux and Deininger 2004; Lin et al 2006; Navarro et al 2007; Jabbour et al 2007). The subclone hypothesis is supported by HUMARA analysis as well as the fact, that the karyotype abnormalities are the same as those detectable in Ph-positive subclones (Terre et al 2004; Navarro et al 2007; Jabbour et al 2007). An alternative hypothesis is that Ph-negative clones develop independent of the primary disease (unrelated clone). Such hypothesis would pose the question as to whether imatinib exhibits a substantial mutagenic potential and can attack normal stem cells similar to conventional cytostatic drugs. So far, no clear evidence for such hypothesis has been presented, although single case reports have suggested that even transplanted normal stem cells may undergo transformation and accumulate cytogenetic defects during treatment with imatinib (Agis et al 2004). However, again, such additional clones may not be relevant clinically (in all patients), and these patients may still stay in a complete hematologic remission with normal blood counts over time (Agis et al 2004).
As mentioned above, little is known so far about specific molecular defects and mechanisms underlying BCR/ABL-independent resistance to imatinib in CML, and especially about defects that can lead to malignant transformation in subclones. In fact, although an extensive number of (potentially deregulated) molecules and numerous mechanisms have been discussed, no specific recurrent gene defects that would explain transformation of CML into AP or BP have been identified (Calabretta and Perrotti 2004; Melo and Barnes 2007). General pathogenetic factors that have been discussed as being involved in disease progression in CML include activation of (mutation-induced) signal transduction molecules (by mutations or oncogene activation), differentiation arrest, genomic instability (deficiency of DNA repair, mutator phenotype), telomer shortening, and loss of tumor suppressor function (Calabretta and Perrotti 2004; Melo and Barnes 2007). Some of these defects may be triggered in part also by BCR/ABL. Likewise, BCR/ABL has been implicated in hypermethylation of the genome, in deactivation of tumor suppressors, and in the hypermutation-status of leukemic cells (Neviani et al 2006; Melo and Barnes 2007). However, most of the secondary transforming hits in CML may be BCR/ABL-independent events. The (numerous) candidate genes potentially involved in disease progression in CML, and their function, have been reviewed elsewhere (Calabretta and Perrotti 2004; Melo and Barnes 2007).
So far, it remains unknown which of these defects and deregulated molecules may contribute to resistance against imatinib in CML. Respective preclinical and clinical studies are in progress and hopefully will reveal new important therapeutic targets in the near future. Such studies focus primarily on genes involved in the differentiation block, in abnormal signalling, in abnormal DNA repair, and in the deactivation of tumor suppressors (Martinelli et al 2005; Melo and Barnes 2007). It is the hope for the future that these studies will lead to the development of new treatment strategies aimed at preventing disease-progression in CML. A likely scenario is that such novel therapies will then be combined with most effective BCR/ABL TK inhibitors.
Intolerance and side effects
An important aspect in the treatment of CML with imatinib or other BCR/ABL TK inhibitors, are side effects that may lead to dose-reductions and thus may predispose for the development of resistance. For imatinib, only a few major side effects have been reported, including transient edema formation and mild myelosuppression (Druker et al 2001a; Kantarjian H et al 2002; Barbany et al 2002; Talpaz et al 2002; Sawyers et al 2002; O’Brien et al 2003). Other side effects such as hepatic dysfunction or cardiac problems are uncommon. However, some of these side effects may lead to dose reductions or even to drug withdrawal. Nilotinib (AMN107) also exhibits a favorable toxicity profile, although rare adverse side effects such as an elevation in pancreatic enzymes, have been reported (Kantarjian et al 2006). With regard to dasatinib, a number of side effects have been reported using the proposed standard dose of 2 × 70 mg per os daily. These side effects include pleural and pericardial effusions and myelosuppression (Talpaz et al 2006; Hochhaus et al 2007). Based on first observations in clinical trials and unpublished data, the frequency of side effects may be lower when the dose of dasatinib is reduced, which points to the question as to whether the standard dose should be reconsidered. Notably, dasatinib is a most potent inhibitor of leukemic cell growth in CML, and in many patients, the drug may still work at reduced dose levels (Talpaz et al 2006; Hochhaus et al 2007). Some of the side effects may also be less frequent when the drug is administered once daily (1 × 140 mg instead of 2 × 70 mg).
For most other TK inhibitors, side effect profiles in CML patients remain to be established.
Clinical practice: Algorithm
Definitions for ‘suboptimal response’ and ‘drug-resistance’ in CML patients treated with imatinib are well established (Baccarani et al 2006). It is also well established, that patients with drug resistance should undergo restaging and BCR/ABL mutation analysis. In addition, the availability of a SCT donor should be (re)explored. The final treatment plan will be based on a number of different variables, including disease-specific factors (phase of disease, presence and type of BCR/ABL mutation, presence and type of additional chromosomal abnormalities, extramedullary involvement, lymphoid versus myeloid blasts), patient-related factors (age, fitness, comorbidity, patients attitude, availability of a donor), and the overall situation in each case. After having collected all necessary information (including BCR/ABL mutations, and if required an imatinib trough level), a straight forward approach may be to estimate chances for long term disease-free survival (cure in young patients) with each therapeutic approach, and to weigh treatment-associated mortality and morbidity against the chances for cure (long term disease-free survival). Depending on mutations and other features of the clone, some patients may benefit from imatinib dose-escalation. In other cases, treatment has to be switched to dasatinib or nilotinib. Both drugs are registered and approved for treatment of imatinib-resistant CML. The decision to introduce such therapy should be based on a thorough investigation for BCR/ABL mutations, as treatment will fail when CML cells display the T315I mutant. For these patients, alternative treatment approaches have to be considered. In younger patients with a suitable donor who display BCR/ABL T315I or other highly resistant mutants, allogeneic SCT should be considered. When no donor is available or the patient is not considered fit enough for SCT, new experimental drugs, some of them known to target BCR/ABL T315I, or drug combinations, should be offered in clinical trials.
Summary and future perspectives
Resistance against imatinib is an emerging problem in the treatment of CML. Dose-adjustments, new BCR/ABL-targeting drugs, and other antileukemic approaches may be sufficient to overcome resistance in many cases. A specific challenge remains the T315I mutant of BCR/ABL that is resistant against most available TK inhibitors. Other specific challenges are the intrinsic resistance of CML stem cells, clonal evolution, involvement of BCR/ABL-independent signalling pathways, and poor accumulation of imatinib in the central nervous system. For the future, new more effective BCR/ABL TK inhibitors, drug combinations, and drugs entering the blood–brain barrier, may be straightforward approaches to improve anti-CML therapy. Such approaches will also aim at preventing the occurrence of drug resistance in an early phase of CML. For those patients who fail drug therapy and are eligible, allogeneic stem cell transplantation with or without additional TK inhibitors, will remain an alternative option of treatment. The value of new future treatment strategies (immunotherapies, siRNA) remains at present unknown.
References
- Abruzzese E, Cantonetti M, Morino L, et al. CNS and cutaneous involvement in patients with chronic myeloid leukemia treated with imatinib in hematologic complete remission: two case reports. J Clin Oncol. 2003;21:4256–8. doi: 10.1200/JCO.2003.99.170. [DOI] [PubMed] [Google Scholar]
- Abdelhalim A, Barcos M, Block AW, et al. Remission of Philadelphia chromosome-positive central nervous system leukemia after dasatinib therapy. Leuk Lymphoma. 2007;48:1053–6. doi: 10.1080/10428190701258370. [DOI] [PubMed] [Google Scholar]
- Agis H, Mannhalter C, Sperr WR, et al. Detection of trisomy 8 in donor-derived Ph- cells in a patient with Ph+ chronic myeloid leukemia successfully treated with Imatinib (STI571) in relapse after allogeneic transplantation. Leuk Lymphoma. 2004;45:1453–8. doi: 10.1080/10428190410001670637. [DOI] [PubMed] [Google Scholar]
- Angstreich GR, Matsui W, Huff CA, et al. Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors. Br J Haematol. 2005;130:373–81. doi: 10.1111/j.1365-2141.2005.05606.x. [DOI] [PubMed] [Google Scholar]
- Aichberger KJ, Herndlhofer S, Agis H, et al. Liposomal cytarabine for treatment of myeloid central nervous system relapse in chronic myeloid leukemia occurring during imatinib therapy. Eur J Clin Invest. 2007;37:808–13. doi: 10.1111/j.1365-2362.2007.01859.x. [DOI] [PubMed] [Google Scholar]
- Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–43. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- Barbany G, Hoglund M, Simonsson B Swedish CML Group. Complete molecular remission in chronic myelogenous leukemia after imatinib therapy. N Engl J Med. 2002;347:539–40. doi: 10.1056/NEJM200208153470719. [DOI] [PubMed] [Google Scholar]
- Barnes DJ, Melo JV. Primitive, quiescent and difficult to kill: the role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle. 2006;5:2862–6. doi: 10.4161/cc.5.24.3573. [DOI] [PubMed] [Google Scholar]
- Barthe C, Gharbi MJ, Lagarde V, et al. Mutation in the ATP-binding site of BCR-ABL in a patient with chronic myeloid leukaemia with increasing resistance to STI571. Br J Haematol. 2002;119:109–11. doi: 10.1046/j.1365-2141.2002.03708.x. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 101:4701–7. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- Biernaux C, Loos M, Sels A, Huez G, Stryckmans P, et al. Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood. 1995;86:3118–22. [PubMed] [Google Scholar]
- Bolton AE, Peng B, Hubert M, et al. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol. 2004;53:102–6. doi: 10.1007/s00280-003-0722-9. [DOI] [PubMed] [Google Scholar]
- Bornhäuser M, Jenke A, Freiberg-Richter J, et al. CNS blast crisis of chronic myelogenous leukemia in a patient with a major cytogenetic response in bone marrow associated with low levels of imatinib mesylate and its N-desmethylated metabolite in cerebral spinal fluid. Ann Hematol. 2004;83:401–2. doi: 10.1007/s00277-003-0829-4. [DOI] [PubMed] [Google Scholar]
- Bose S, Deininger M, Gora-Tybor J, et al. The presence of typical and atypical BCR-ABL fusion genes in leukocytes of normal individuals: biologic significance and implications for the assessment of minimal residual disease. Blood. 1998;92:3362–7. [PubMed] [Google Scholar]
- Branford S, Rudzki Z, Walsh S, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002;99:3472–5. doi: 10.1182/blood.v99.9.3472. [DOI] [PubMed] [Google Scholar]
- Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–83. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Breedveld P, Pluim D, Cipriani G, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–82. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- Breccia M, Diverio D, Pane F, et al. Discontinuation of imatinib therapy after achievement of complete molecular response in a Ph(+) CML patient treated while in long lasting complete cytogenetic remission (CCR) induced by interferon. Leuk Res. 2006;30:1577–9. doi: 10.1016/j.leukres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Brendel C, Scharenberg C, Dohse M, et al. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267–75. doi: 10.1038/sj.leu.2404638. 2007. [DOI] [PubMed] [Google Scholar]
- Burger H, Nooter K. Pharmacokinetic resistance to imatinib mesylate: role of the ABC drug pumps ABCG2 (BCRP) and ABCB1 (MDR1) in the oral bioavailability of imatinib. Cell Cycle. 2004;3:1502–5. doi: 10.4161/cc.3.12.1331. [DOI] [PubMed] [Google Scholar]
- Burger H, van Tol H, Brok M, et al. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005;4:747–52. doi: 10.4161/cbt.4.7.1826. [DOI] [PubMed] [Google Scholar]
- Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103:4010–22. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- Campbell LJ, Patsouris C, Rayeroux KC, et al. BCR/ABL amplification in chronic myelocytic leukemia blast crisis following imatinib mesylate administration. Cancer Genet Cytogenet. 2002;139:30–3. doi: 10.1016/s0165-4608(02)00615-5. [DOI] [PubMed] [Google Scholar]
- Carter TA, Wodicka LM, Shah NP, et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci USA. 2005;102:11011–6. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham GM, Charlton PA, Golec JM, et al. Structural basis for potent inhibition of the Aurora kinases and a T315I multi-drug resistant mutant form of Abl kinase by VX-680. Cancer Lett. 2007;251:323–9. doi: 10.1016/j.canlet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Williams G, Johnson JR, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8:935–42. [PubMed] [Google Scholar]
- Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–9. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- Copland M, Jorgensen HG, Holyoake TL. Evolving molecular therapy for chronic myeloid leukaemia – are we on target. Hematology. 2005;10:349–59. doi: 10.1080/10245330500234195. [DOI] [PubMed] [Google Scholar]
- Cortes J, Kantarjian H. Advanced-phase chronic myeloid leukemia. Semin Hematol. 2003;40:79–86. doi: 10.1053/shem.2003.50005. [DOI] [PubMed] [Google Scholar]
- Cortes JE, Talpaz M, Giles F, et al. Prognostic significance of cytoge-netic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003;101:3794–800. doi: 10.1182/blood-2002-09-2790. [DOI] [PubMed] [Google Scholar]
- Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109:3207–13. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306–15. doi: 10.1002/cncr.21756. [DOI] [PubMed] [Google Scholar]
- Cowan-Jacob SW, Guez V, Fendrich G, et al. Imatinib (STI571) resistance in chronic myelogenous leukemia: molecular basis of the underlying mechanisms and potential strategies for treatment. Mini Rev Med Chem. 2004;4:285–99. doi: 10.2174/1389557043487321. [DOI] [PubMed] [Google Scholar]
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–30. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- de Klein A, van Kessel AG, Grosveld G, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–7. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- Dai H, Marbach P, Lemaire M, et al. Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther. 2003;304:1085–92. doi: 10.1124/jpet.102.045260. [DOI] [PubMed] [Google Scholar]
- Deininger MW. Optimizing therapy of chronic myeloid leukemia. Exp Hematol. 2007;35(S1):144–54. doi: 10.1016/j.exphem.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Diaz-Blanco E, Bruns I, Neumann F, et al. Molecular signature of CD34(+) hematopoietic stem and progenitor cells of patients with CML in chronic phase. Leukemia. 2007;21:494–504. doi: 10.1038/sj.leu.2404549. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–42. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Dutcher JP, Wiernik PH. Accelerated and blastic phase of chronic myeloid leukemia. Curr Treat Options Oncol. 2000;1:51–62. doi: 10.1007/s11864-000-0015-z. [DOI] [PubMed] [Google Scholar]
- Dutreix C, Peng B, Mehring G, et al. Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol. 2004;54:290–4. doi: 10.1007/s00280-004-0832-z. [DOI] [PubMed] [Google Scholar]
- Eaves AC, Barnett MJ, Ponchio L, Cashman JD, Petzer AL, Eaves CJ, et al. Differences between normal and CML stem cells: potential targets for clinical exploitation. Stem Cells. 1998;16(S1):77–83. doi: 10.1002/stem.5530160809. [DOI] [PubMed] [Google Scholar]
- Eaves C, Udomsakdi C, Cashman J, et al. The biology of normal and neoplastic stem cells in CML. Leuk Lymphoma. 1993;11S1:245–53. doi: 10.3109/10428199309047894. [DOI] [PubMed] [Google Scholar]
- Eckel F, von Delius S, Mayr M, et al. Pharmacokinetic and clinical phase II trial of imatinib in patients with impaired liver function and advanced hepatocellular carcinoma. Oncology. 2005;69:363–71. doi: 10.1159/000089990. [DOI] [PubMed] [Google Scholar]
- Eisterer W, Jiang X, Christ O, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia. 2005;19:435–41. doi: 10.1038/sj.leu.2403649. [DOI] [PubMed] [Google Scholar]
- Elrick LJ, Jorgensen HG, Mountford JC, Holyoake TL. Punish the parent not the progeny. Blood. 2005;105:1862–6. doi: 10.1182/blood-2004-08-3373. [DOI] [PubMed] [Google Scholar]
- Frye RF, Fitzgerald SM, Lagattuta TF, et al. Effect of St John’s wort on imatinib mesylate pharmacokinetics. Clin Pharmacol Ther. 2004;76:323–9. doi: 10.1016/j.clpt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Gadzicki D, von Neuhoff N, Steinemann D, et al. BCR-ABL gene amplification and overexpression in a patient with chronic myeloid leukemia treated with imatinib. Cancer Genet Cytogenet. 2005;159:164–7. doi: 10.1016/j.cancergencyto.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gargallo PM, Cuello MT, Aranguren PN, et al. Amplification of the BCR/ABL fusion gene clustered on a masked Philadelphia chromosome in a patient with myeloblastic crisis of chronic myelocytic leukemia. Cancer Genet Cytogenet. 2003;143:140–4. doi: 10.1016/s0165-4608(02)00854-3. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Cortes JE, Kantarjian HM, O’Brien SM. Accelerated and blastic phases of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:753–74. doi: 10.1016/j.hoc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Cortes J, Jones D, et al. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007;109:500–2. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- Giralt SA, Arora M, Goldman JM, et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol. 2007;137:461–7. doi: 10.1111/j.1365-2141.2007.06582.x. [DOI] [PubMed] [Google Scholar]
- Gishizky ML, Witte ON. Initiation of deregulated growth of multipotent progenitor cells by bcr-abl in vitro. Science. 1992;256:836–9. doi: 10.1126/science.1375394. [DOI] [PubMed] [Google Scholar]
- Goldman J, Gordon M. Why do chronic myelogenous leukemia stem cells survive allogeneic stem cell transplantation or imatinib: does it really matter. Leuk Lymphoma. 2006;47:1–7. doi: 10.1080/10428190500407996. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Apperley JF, Jones L, et al. Bone marrow transplantation for patients with chronic myeloid leukemia. N Engl J Med. 1986;314:202–7. doi: 10.1056/NEJM198601233140403. [DOI] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–25. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Hermans J, Goldman JM, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087–92. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- Griswold IJ, MacPartlin M, Bumm T, et al. Kinase domain mutants of Bcr-Abl exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol. 2006;26:6082–93. doi: 10.1128/MCB.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind HP, Pfaar U, Waldmeier F, Pokorny R, Seiberling M, Ben-Am M, Peng B, Gross G, et al. Metabolism and disposition of imatinib mesylate in healthy volunteers. Drug Metab Dispos. 2005;33:1503–12. doi: 10.1124/dmd.105.004283. [DOI] [PubMed] [Google Scholar]
- Guilhot F, Apperley J, Kim DW, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–50. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- Gumireddy K, Baker SJ, Cosenza SC, et al. A non-ATP-competitive inhibitor of BCR-ABL overrides imatinib resistance. Proc Natl Acad Sci USA. 2005;102:1992–7. doi: 10.1073/pnas.0408283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herweijer H, Sonneveld P, Baas F, Nooter K. Expression of mdr1 and mdr3 multidrug-resistance genes in human acute and chronic leukemias and association with stimulation of drug accumulation by cyclosporine. J Natl Cancer Inst. 1990;82:1133–40. doi: 10.1093/jnci/82.13.1133. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Erben P, Ernst T, Mueller MC. Resistance to targeted therapy in chronic myelogenous leukemia. Semin Hematol. 2007;44:S15–24. doi: 10.1053/j.seminhematol.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–9. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–6. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- Holyoake TL, Horrocks C, Thomas T, et al. Cell separation improves the sensitivity of detecting rare human normal and leukemic hematopoietic cells in vivo in NOD/SCID mice. Cytotherapy. 2000;2:411–21. doi: 10.1080/146532400539350. [DOI] [PubMed] [Google Scholar]
- Holyoake TL, Jiang X, Drummond MW, et al. Elucidating critical mechanisms of deregulated stem cell turnover in the chronic phase of chronic myeloid leukemia. Leukemia. 2002;16:549–58. doi: 10.1038/sj.leu.2402444. [DOI] [PubMed] [Google Scholar]
- Holyoake TL, Jiang X, Jorgensen HG, et al. Primitive quiescent leukemic cells from patients with chronic myeloid leukemia spontaneously initiate factor-independent growth in vitro in association with up-regulation of expression of interleukin-3. Blood. 2001;97:720–8. doi: 10.1182/blood.v97.3.720. [DOI] [PubMed] [Google Scholar]
- Hoover RR, Gerlach MJ, Koh EY, Daley GQ. Cooperative and redundant effects of STAT5 and Ras signaling in BCR/ABL transformed hematopoietic cells. Oncogene. 2001;20:5826–35. doi: 10.1038/sj.onc.1204549. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- Illmer T, Schaich M, Platzbecker U, et al. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia. 2004;18:401–8. doi: 10.1038/sj.leu.2403257. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Cortes J, Kantarjian HM, et al. Allogeneic stem cell transplantation for patients with chronic myeloid leukemia and acute lymphocytic leukemia after Bcr-Abl kinase mutation-related imatinib failure. Blood. 2006;108:1421–3. doi: 10.1182/blood-2006-02-001933. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Cortes J, Kantarjian H, et al. Novel tyrosine kinase inhibitor therapy before allogeneic stem cell transplantation in patients with chronic myeloid leukemia: no evidence for increased transplant-related toxicity. Cancer. 2007;110:340–44. doi: 10.1002/cncr.22778. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Cortes J, O’Brien S, et al. New targeted therapies for chronic myelogenous leukemia: opportunities to overcome imatinib resistance. Semin Hematol. 2007;44(S1):S25–31. doi: 10.1053/j.seminhematol.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Kantarjian HM, Abruzzo L, et al. Chromosomal abnormalities in Philadelphia chromosome-negative metaphases appearing during imatinib mesylate therapy in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood. 2007 doi: 10.1182/blood-2007-01-070045. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–73. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- Jiang X, Saw KM, Eaves A, Eaves C. Instability of BCR-ABL gene in primary and cultured chronic myeloid leukemia stem cells. J Natl Cancer Inst. 2007;99:680–93. doi: 10.1093/jnci/djk150. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhao Y, Smith C, et al. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007;21:926–35. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Cortes J, O’Brien S, et al. Imatinib mesylate (STI571) therapy for Philadelphia chromosome-positive chronic myelogenous leukemia in blast phase. Blood. 2002;99:3547–53. doi: 10.1182/blood.v99.10.3547. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Talpaz M, Giles F, et al. New insights into the patho-physiology of chronic myeloid leukemia and imatinib resistance. Ann Intern Med. 2006;145:913–23. doi: 10.7326/0003-4819-145-12-200612190-00008. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Talpaz M, O’Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–5. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- Khorashad JS, Anand M, Marin D, et al. The presence of a BCR-ABL mutant allele in CML does not always explain clinical resistance to imatinib. Leukemia. 2006;20:658–63. doi: 10.1038/sj.leu.2404137. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jung CW, Kim K, et al. Isolated blast crisis in CNS in a patient with chronic myelogenous leukaemia maintaining major cytogenetic response after imatinib. J Clin Oncol. 2006;24:4028–29. doi: 10.1200/JCO.2006.05.5608. [DOI] [PubMed] [Google Scholar]
- Kimura S, Naito H, Segawa H, et al. NS-187, a potent and selective dual Bcr-Abl/Lyn tyrosine kinase inhibitor, is a novel agent for imatinib-resistant leukemia. Blood. 2005;106:3948–54. doi: 10.1182/blood-2005-06-2209. [DOI] [PubMed] [Google Scholar]
- Kreuzer KA, Le Coutre P, Landt O, et al. Preexistence and evolution of imatinib mesylate-resistant clones in chronic myelogenous leukemia detected by a PNA-based PCR clamping technique. Ann Hematol. 2003;82:284–9. doi: 10.1007/s00277-003-0644-y. [DOI] [PubMed] [Google Scholar]
- le Coutre P, Kreuzer KA, Pursche S, et al. Pharmacokinetics and cellular uptake of imatinib and its main metabolite CGP74588. Cancer Chemother Pharmacol. 2004;53:313–23. doi: 10.1007/s00280-003-0741-6. [DOI] [PubMed] [Google Scholar]
- le Coutre P, Tassi E, Varella-Garcia M, et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood. 2000;95:1758–66. [PubMed] [Google Scholar]
- Leis JF, Stepan DE, Curtin PT, et al. Central nervous system failure in patients with chronic myelogenous leukemia lymphoid blast crisis and Philadelphia chromosome positive acute lymphoblastic leukemia treated with imatinib (STI-571) Leuk Lymphoma. 2004;45:695–8. doi: 10.1080/10428190310001625728. [DOI] [PubMed] [Google Scholar]
- Li Z, Qiao Y, Liu B, et al. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin Cancer Res. 2005;11:4460–8. doi: 10.1158/1078-0432.CCR-05-0250. [DOI] [PubMed] [Google Scholar]
- Lin H, Monaco G, Sun T, et al. Bcr-Abl-mediated suppression of normal hematopoiesis in leukemia. Oncogene. 2005;24:3246–56. doi: 10.1038/sj.onc.1208500. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bruyere H, Horsman DE, et al. Philadelphia-negative clonal hematopoiesis following imatinib therapy in patients with chronic myeloid leukemia: a report of nine cases and analysis of predictive factors. Cancer Genet Cytogenet. 2006;170:16–23. doi: 10.1016/j.cancergencyto.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Loriaux M, Deininger M. Clonal cytogenetic abnormalities in Philadelphia chromosome negative cells in chronic myeloid leukemia patients treated with imatinib. Leuk Lymphoma. 2004;45:2197–203. doi: 10.1080/10428190410001723278. [DOI] [PubMed] [Google Scholar]
- Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–82. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- Mahon FX, Belloc F, Lagarde V, et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101:2368–73. doi: 10.1182/blood.V101.6.2368. [DOI] [PubMed] [Google Scholar]
- Marktel S, Marin D, Foot N, et al. Chronic myeloid leukemia in chronic phase responding to imatinib: the occurrence of additional cytogenetic abnormalities predicts disease progression. Haematologica. 2003;88:260–7. [PubMed] [Google Scholar]
- Martinelli G, Soverini S, Rosti G, et al. New tyrosine kinase inhibitors in chronic myeloid leukemia. Haematologica. 2005;90:534–41. [PubMed] [Google Scholar]
- Matsuda M, Morita Y, Shimada T, et al. Extramedullary blast crisis derived from 2 different clones in the central nervous system and neck during complete cytogenetic remission of chronic myelogenous leukemia treated with imatinib mesylate. Int J Hematol. 2005;81:307–9. doi: 10.1532/IJH97.04188. [DOI] [PubMed] [Google Scholar]
- Medina J, Kantarjian H, Talpaz M, et al. Chromosomal abnormalities in Philadelphia chromosome-negative metaphases appearing during imatinib mesylate therapy in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase. Cancer. 2003;98:1905–11. doi: 10.1002/cncr.11729. [DOI] [PubMed] [Google Scholar]
- Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441–53. doi: 10.1038/nrc2147. [DOI] [PubMed] [Google Scholar]
- Melo JV, Deininger MW. Biology of chronic myelogenous leukemia – signaling pathways of initiation and transformation. Hematol Oncol Clin North Am. 2004;18:545–68. doi: 10.1016/j.hoc.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Menzel H, von Bubnoff N, Hochhaus A, et al. Successful allogeneic stem cell transplantation in second chronic-phase CML induced by the tyrosine kinase inhibitor nilotinib (AMN107) after blast crisis under imatinib. Bone Marrow Transplant. 2007;40:83–4. doi: 10.1038/sj.bmt.1705683. [DOI] [PubMed] [Google Scholar]
- Michor F, Hughes TP, Iwasa Y, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–70. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- Morel F, Bris MJ, Herry A, et al. Double minutes containing amplified bcr-abl fusion gene in a case of chronic myeloid leukemia treated by imatinib. Eur J Haematol. 2003;70:235–9. doi: 10.1034/j.1600-0609.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Navarro JT, Feliu E, Grau J, et al. Monosomy 7 with severe myelodysplasia developing during imatinib treatment of Philadelphia-positive chronic myeloid leukemia: Two cases with a different outcome. Am J Hematol. 2007;82:849–51. doi: 10.1002/ajh.20859. [DOI] [PubMed] [Google Scholar]
- Neviani P, Santhanam R, Trotta R, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2006;5:355–68. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Nguyen Khac F, Waill MC, Romana SP, et al. Identical abnormality of the short arm of chromosome 18 in two Philadelphia-positive chronic myelocytic leukemia patients with erythroblastic transformation, resulting in duplication of BCR-ABL1 fusion. Cancer Genet Cytogenet. 2002;138:22–6. doi: 10.1016/s0165-4608(02)00574-5. [DOI] [PubMed] [Google Scholar]
- Nicolini FE, Corm S, Le QH, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukemia patients: a retrospective analysis from the French intergroup of CML (Fi(phi)-LMC GROUP) Leukemia. 2006;20:1061–6. doi: 10.1038/sj.leu.2404236. [DOI] [PubMed] [Google Scholar]
- Nowell PC, Hungerford DA. A minute chromosome in human granulocytic leukemia. Science. 1960;132:1497. [Google Scholar]
- O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- O’Brien SG, Meinhardt P, Bond E, et al. Effects of imatinib mesylate (STI571, Glivec) on the pharmacokinetics of simvastatin, a cytochrome p450 3A4 substrate, in patients with chronic myeloid leukaemia. Br J Cancer. 2003;89:1855–9. doi: 10.1038/sj.bjc.6601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer ME, Mauro MJ, Blasdel C, et al. Clonal evolution and lack of cytogenetic response are adverse prognostic factors for hematologic relapse of chronic phase CML patients treated with imatinib mesylate. Blood. 2004;103:451–5. doi: 10.1182/blood-2003-02-0371. [DOI] [PubMed] [Google Scholar]
- O’Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, Cowan-Jacob SW, Lee FY, Heinrich MC, Deininger MW, Druker BJ. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–5. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- Pappas P, Karavasilis V, Briasoulis E, et al. Pharmacokinetics of imatinib mesylate in end stage renal disease. A case study. Cancer Chemother Pharmacol. 2005;56:358–60. doi: 10.1007/s00280-005-1031-2. [DOI] [PubMed] [Google Scholar]
- Pavlu J, Czepulkowski B, Kaczmarski R, Jan-Mohamed R. Early blastic transformation with CNS infiltration in a patient with chronic myeloid leukaemia treated with imatinib. Lancet Oncol. 2005;6:128. doi: 10.1016/S1470-2045(05)01744-4. [DOI] [PubMed] [Google Scholar]
- Pendergast AM, Quilliam LA, Cripe LD, et al. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 1993;75:175–85. [PubMed] [Google Scholar]
- Peng B, Dutreix C, Mehring G, et al. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004;44:158–62. doi: 10.1177/0091270003262101. [DOI] [PubMed] [Google Scholar]
- Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22:935–42. doi: 10.1200/JCO.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Peng C, Brain J, Hu Y, et al. Inhibition of heat shock protein 90 prolongs survival of mice with BCR-ABL-T315I-induced leukemia and suppresses leukemic stem cells. Blood. 2007;110:678–85. doi: 10.1182/blood-2006-10-054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44:879–94. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- Picard S, Titier K, Etienne G, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–9. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- Pienkowska-Grela B, Woroniecka R, Solarska I, et al. Complete cytogenetic and molecular response after imatinib treatment for chronic myeloid leukemia in a patient with atypical karyotype and BCR-ABL b2a3 transcript. Cancer Genet Cytogenet. 2007;174:111–5. doi: 10.1016/j.cancergencyto.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Puil L, Liu J, Gish G, et al. Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. EMBO J. 1994;13:764–73. doi: 10.1002/j.1460-2075.1994.tb06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintas-Cardama A, Kantarjian H, Jones D, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 2007;109:497–9. doi: 10.1182/blood-2006-07-035493. [DOI] [PubMed] [Google Scholar]
- Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2006;103:2794–9. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani M, Nguyen TK, Dent P, et al. The multikinase inhibitor sorafenib induces apoptosis in highly imatinib mesylate-resistant BCR/ABL+ human leukemia cells in association with STAT5 inhibition and MCL-1 downregulation. Mol Pharmacol. 2007;72:788–95. doi: 10.1124/mol.106.033308. [DOI] [PubMed] [Google Scholar]
- Rajappa S, Uppin SG, Raghunadharao D, et al. Isolated central nervous system blast crisis in chronic myeloid leukaemia. Hematol Oncol. 2004;22:179–81. doi: 10.1002/hon.737. [DOI] [PubMed] [Google Scholar]
- Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–83. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- Roche-Lestienne C, Preudhomme C. Mutations in the ABL kinase domain pre-exist the onset of imatinib treatment. Semin Hematol. 2003;40(S2):80–2. doi: 10.1053/shem.2003.50046. [DOI] [PubMed] [Google Scholar]
- Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100:1014–8. doi: 10.1182/blood.v100.3.1014. [DOI] [PubMed] [Google Scholar]
- Roeder I, Horn M, Glauche I, Hochhaus A, Mueller MC, Loeffler M. Dynamic modeling of imatinib-treated chronic myeloid leukemia: functional insights and clinical implications. Nat Med. 2006;12:1181–4. doi: 10.1038/nm1487. [DOI] [PubMed] [Google Scholar]
- Roumiantsev S, Shah NP, Gorre ME, et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci USA. 2002;99:10700–5. doi: 10.1073/pnas.162140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–3. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rytting ME, Wierda WG. Central nervous system relapse in two patients with chronic myelogenous leukemia in myeloid blastic phase on imatinib mesylate therapy. Leuk Lymphoma. 2004;45:1623–6. doi: 10.1080/10428190410001667703. [DOI] [PubMed] [Google Scholar]
- Sattler M, Griffin JD. Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin Hematol. 2003;40:4–10. doi: 10.1053/shem.2003.50034. [DOI] [PubMed] [Google Scholar]
- Sawyers CL, Hochhaus A, Feldman E, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–9. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- Senior K. Gleevec does not cross blood-brain barrier. Lancet Oncol. 2003;4:198. doi: 10.1016/s1470-2045(03)01050-7. [DOI] [PubMed] [Google Scholar]
- Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–25. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- Shet AS, Jahagirdar BN, Verfaillie CM. Chronic myelogenous leukemia: mechanisms underlying disease progression. Leukemia. 2002;16:1402–11. doi: 10.1038/sj.leu.2402577. [DOI] [PubMed] [Google Scholar]
- Silberman G, Crosse MG, Peterson EA, et al. Availability and appropriateness of allogeneic bone marrow transplantation for chronic myeloid leukemia in 10 countries. N Engl J Med. 1994;331:1063–7. doi: 10.1056/NEJM199410203311606. [DOI] [PubMed] [Google Scholar]
- Sillaber C, Gesbert F, Frank DA, et al. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood. 2000;95:2118–25. [PubMed] [Google Scholar]
- Skaggs BJ, Gorre ME, Ryvkin A, et al. Phosphorylation of the ATP-binding loop directs oncogenicity of drug-resistant BCR-ABL mutants. Proc Natl Acad Sci USA. 2006;103:19466–71. doi: 10.1073/pnas.0609239103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorski T, Bellacosa A, Nieborowska-Skorska M, et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 1997;16:6151–61. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–9. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- Takayama N, Sato N, O’Brien SG, et al. Imatinib mesylate has limited activity against the central nervous system involvement of Philadelphia chromosome-positive acute lymphoblastic leukaemia due to poor penetration into cerebrospinal fluid. Br J Haematol. 2002;119:106–8. doi: 10.1046/j.1365-2141.2002.03881.x. [DOI] [PubMed] [Google Scholar]
- Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928–37. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Arif M, Kyo T, et al. Transposition of duplicated chromosomal segment involving fused BCR-ABL gene or ABL oncogene alone in chronic myelocytic leukemia and Ph chromosome-positive acute leukemia with complex karyotypes. Cancer Genet Cytogenet. 2000;119:8–14. doi: 10.1016/s0165-4608(99)00206-x. [DOI] [PubMed] [Google Scholar]
- Tauchi T, Ohyashiki K. The second generation of BCR-ABL tyrosine kinase inhibitors. Int J Hematol. 2006;83:294–300. doi: 10.1532/IJH97.06025. [DOI] [PubMed] [Google Scholar]
- Terre C, Eclache V, Rousselot P, et al. Report of 34 patients with clonal chromosomal abnormalities in Philadelphia-negative cells during imatinib treatment of Philadelphia-positive chronic myeloid leukemia. Leukemia. 2004;18:1340–6. doi: 10.1038/sj.leu.2403399. [DOI] [PubMed] [Google Scholar]
- Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104:3739–45. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- Tseng PH, Lin HP, Zhu J, et al. Synergistic interactions between imatinib mesylate and the novel phosphoinositide-dependent kinase-1 inhibitor OSU-03012 in overcoming imatinib mesylate resistance. Blood. 2005;105:4021–7. doi: 10.1182/blood-2004-07-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten RA. Oncogenic signaling: new insights and controversies from chronic myeloid leukemia. J Exp Med. 2007;204:461–5. doi: 10.1084/jem.20062335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek W, Konig H, Boehm J, et al. Continuous complete hematological and cytogenetic remission with molecular minimal residual disease 9 years after discontinuation of interferon-alpha in a patient with Philadelphia chromosome-positive chronic myeloid leukemia. Acta Haematol. 2006;115:109–12. doi: 10.1159/000089476. [DOI] [PubMed] [Google Scholar]
- Villuendas R, Steegmann JL, Pollan M, et al. Identification of genes involved in imatinib resistance in CML: a gene-expression profiling approach. Leukemia. 2006;20:1047–54. doi: 10.1038/sj.leu.2404197. [DOI] [PubMed] [Google Scholar]
- Volpe G, Cignetti A, Panuzzo C, et al. Alternative BCR/ABL splice variants in Philadelphia chromosome-positive leukemias result in novel tumor-specific fusion proteins that may represent potential targets for immunotherapy approaches. Cancer Res. 2007;67:5300–7. doi: 10.1158/0008-5472.CAN-06-3737. [DOI] [PubMed] [Google Scholar]
- von Bubnoff N, Schneller F, Peschel C, et al. BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet. 2002;359:487–91. doi: 10.1016/S0140-6736(02)07679-1. [DOI] [PubMed] [Google Scholar]
- Wang L, Giannoudis A, Lane S, et al. Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther. 2007 doi: 10.1038/sj.clpt.6100268. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–41. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Manley P, Mestan J, et al. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br J Cancer. 2006;94:1765–9. doi: 10.1038/sj.bjc.6603170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg E, Catley L, Wright RD, et al. Beneficial effects of combining nilotinib and imatinib in preclinical models of BCR-ABL+ leukemias. Blood. 2007;109:2112–20. doi: 10.1182/blood-2006-06-026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg E, Griffin JD. Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood. 2000;95:3498–505. [PubMed] [Google Scholar]
- Weisberg E, Manley PW, Cowan-Jacob SW, et al. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7:345–56. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- Weisser M, Schleuning M, Haferlach C, et al. Allogeneic stem-cell transplantation provides excellent results in advanced stage chronic myeloid leukemia with major cytogenetic response to pre-transplant imatinib therapy. Leuk Lymphoma. 2007;48:295–301. doi: 10.1080/10428190601078464. [DOI] [PubMed] [Google Scholar]
- Westermann J, Kopp J, van Lessen A, et al. Vaccination with autologous non-irradiated dendritic cells in patients with bcr/abl+ chronic myeloid leukaemia. Br J Haematol. 2007;137:297–306. doi: 10.1111/j.1365-2141.2007.06547.x. [DOI] [PubMed] [Google Scholar]
- Wetzler M, Talpaz M, Van Etten RA, et al. Subcellular localization of Bcr, Abl, and Bcr-Abl proteins in normal and leukemic cells and correlation of expression with myeloid differentiation. J Clin Invest. 1993;92:1925–39. doi: 10.1172/JCI116786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DL, Saunders VA, Quinn SR, et al. Imatinib increases the intracellular concentration of nilotinib, which may explain the observed synergy between these drugs. Blood. 2007;109:3609–10. doi: 10.1182/blood-2006-11-058032. [DOI] [PubMed] [Google Scholar]
- Wild R, Castaneda S, Flefleh C, et al. BMS-354825, a dual SRC/ABL kinase inhibitor, displays potent anti-tumor activity in a model of intracranial CML growth. Blood. 2004;104:549a. [Google Scholar]
- Wolff NC, Richardson JA, Egorin M, Ilaria RL. The CNS is a sanctuary for leukemic cells in mice receiving imatinib mesylate for Bcr/Abl-induced leukemia. Blood. 2003;101:5010–3. doi: 10.1182/blood-2002-10-3059. [DOI] [PubMed] [Google Scholar]
- Yokota A, Kimura S, Masuda S, et al. INNO-406, a novel BCR-ABL/Lyn dual tyrosine kinase inhibitor, suppresses the growth of Ph+ leukemia cells in the central nervous system, and cyclosporine A augments its in vivo activity. Blood. 2007;109:306–14. doi: 10.1182/blood-2006-03-013250. [DOI] [PubMed] [Google Scholar]
- Zheng C, Li L, Haak M, et al. Gene expression profiling of CD34+ cells identifies a molecular signature of chronic myeloid leukemia blast crisis. Leukemia. 2006;20:1028–34. doi: 10.1038/sj.leu.2404227. [DOI] [PubMed] [Google Scholar]