Abstract
The chronic myeloproliferative disorders (MPDs) include the spectrum of clonal hematopoietic stem cell disorders whose phenotype derive from the primary cell expanded in a proliferative state. The MPDs (which include polycythemia vera (PV), essential thrombocythemia (ET), chronic eosinophilic leukemia (CEL), primary myelofibrosis (PMF), chronic myelomonocytic leukemia (CMML), and systemic mast cell disease (SMCD)) exclude chronic myeloid leukemia (CML) because of the pathognomic importance of the BCR-ABL translocation for the diagnosis and treatment of this disorder with imatinib mesylate. Empiric use of imatinib mesylate against the spectrum of BCR-ABL negative MPDs has had mixed results. Significant benefits were obtained when empiric use of imatinib in CEL and CMML led to significant clinical benefit and the discovery of the role of rearrangements of the platelet derived growth factor receptor -alpha (PDGFRa-FIP1L1 in CEL and SMCD) and -beta (PDGFRb through TEL-PDGFRb) for CMML). Empiric use of imatinib in PMF has been disappointing, and in PV quite modest. Although next generation Abelson kinase inhibitors such as dasatinib or nilotinib may expand the role for these agents in MPDs, targeted inhibition of the mutant kinase JAK2V617F is more likely to make significant therapeutic gains in the classic MPDs of PV, ET, and PMF.
Keywords: myeloproliferative diseases, essential thrombocythemia, polycythemia vera, myelofibrosis, therapy
The chronic BCR-ABL negative myeloproliferative disorders (MPDs)
The myeloproliferative disorders are a group of interrelated clonal disorders of the myeloid lineages all felt to arise from aberrations in the hematopoietic stem cell. Their understanding and classification have largely derived from their individual phenotypic manifestations and the individual cell over-represented in the peripheral blood (Dameshek 1951). Specific lineage associations being the following for MPDs (Tefferi 1998), erythrocytes (polycythemia vera (PV)), platelets (essential thrombocythemia (ET)), granulocytes (chronic myeloid leukemia (CML)), monocytes (chronic myelomonocytic leukemia (CMML)), eosinophils (chronic eosinophilic leukemia (CEL)), mast cells (systemic mast cell disease (SMCD), and the disorder where this myeloid proliferation is also accompanied by intramedullary fibrosis (primary myelofibrosis (PMF) (Mesa et al 2007). The classification, diagnosis, and treatment of these disorders branched long ago with the discovery of the t(9; 22) (q34; q11)(Nowell and Hungerford 1960) (or “Philadelphia Chromosome” for CML, and the subsequent understanding of the role of the tyrosine kinase BCR-ABL in the pathogenetic process for these individuals. Indeed, the role of BCR-ABL in CML led to the development of imatinib mesylate (Druker et al 1996) a tyrosine kinase inhibitor which has made a profound impact on the disease manifestations, progression, and survival amongst CML patients (Druker et al 2001). Indeed, imatinib for CML has become a role model for the potential therapeutic gains of targeted therapies. Given all these latter breakthroughs for CML, let us consider separately the BCR-ABL negative myeloproliferative disorders, their current therapies, and whether given biological similarities do they share a role for the clinical inhibition of tyrosine kinases with either imatinib mesylate or similar agent?
Presentation and initial management of MPD patients
The BCR-ABL negative MPDs (MPDs; when CML is included it will be stipulated specifically). Phenotypically the MPDs have a wide range of manifestations including a variable age of diagnosis (typically around age 60 (Mesa et al 1999), although patients in the third, fourth, and fifth decades of life are common). Clinical presentations vary from incidental peripheral blood abnormalities to overt acute leukemia. Clinically the MPDs share a variable spectrum of symptomatology arising from myeloproliferation (erythrocytosis, leukocytosis, or thrombocytosis) as well as target organ damage from the intramedullary proliferative state (organomegaly (Tefferi et al 2000), vascular complications (Landolfi 1998), skin manifestations (van Genderen and Michiels 1997), liver dysfunction (Tefferi, Jimenez et al 2001), pulmonary hypertension (Dingli et al 2001), etc.). Indeed, initial and subsequent manifestations are influenced by the nature of the cells which are aberrantly increased. Specifically, erythrocytes and platelets when elevated may lead to vascular complications; eosinophils can cause organ damage to the heart, lungs, or target organs; mast cells can cause allergic like manifestations and skin manifestations; immature myeloid cells, granulocytes, and monocytes may cause splenomegaly, and other areas of apparent extramedullary hematopoiesis. Individuals with advancing disease, PMF or advanced ET or PV have worsening cytopenias, constitutional symptoms, risk of leukemic transformation, and risk of premature death.
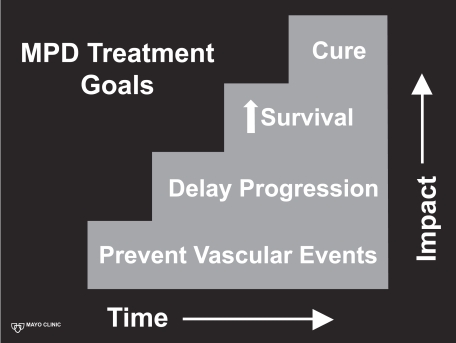
When considering therapy of MPD patients there are both immediate, short term and long term treatment decisions (Figure 1). Initially MPD patients whom present with overt thrombosis or hemorrhage need to be stabilized appropriately. Stabilization includes (1) therapeutic anti-coagulation in those with overt venous or arterial thrombosis, (2) direct intervention upon an arterial thrombosis when appropriate and indicated (such as thrombolysis or thrombectomy), (3) correction of any overt coagulopathy, (4) correction of symptomatic anemia, and (5) rapid correction of thrombocytosis (to the normal range) when appropriate. It is underscored that thrombocytosis can directly lead to hemorrhage (especially with platelet counts in excess of 1500 × 109/L) due to a relative coagulopathy. Rapid correction of thrombocytosis can be achieved by therapeutic platelet apheresis (with a goal of correction to the normal range, ie, <400 × 109/L), concurrently with the use of a cytoreductive agent (described subsequently) to maintain the platelet count in the goal range. Apheresis may be repeated as necessary until the cytoreductive medications have begun to adequately control the thrombocytosis.
Figure 1.
Myeloproliferative disorder therapeutic goals.
MPD patients may also have previously had occult hemorrhagic events (ie, gastrointestinal bleeding from varices, low grade hemorrhage from genitourinary sources), or thrombotic events (occult pulmonary emboli, pulmonary hypertension from pulmonary emboli, arterial thrombotic events).
Synopsis of current MPD therapeutic options: short and long term
When establishing a therapeutic plan for a particular patient one quickly realizes that the MPDs carry a variable prognostic outlook. PMF (and post ET/PV MF) carry the worst prognosis with median survival rivaling many lethal solid malignancies. Various prognostic features can help stratify the suspected outcomes in MF patients (Cervantes 2001) including anemia (Dupriez et al 1996), karyotypic abnormalities (Tefferi, Mesa et al 2001), age, the presence of circulating blasts (Dupriez et al 1996), and markedly increased angiogenesis in the marrow (Mesa et al 2000). Patients with ET and PV can potentially have life expectancies as long as age matched controls (Passamonti et al 2004; Wolanskyj et al 2006). However, such longevity amongst these patients is not universal as both short and long term risks of both morbidity and mortality exist.
Currently, no therapy has been shown to be curative or prolonging survival in MPD patients, except allogeneic stem cell transplantation. The concept of using stem cell transplantation for the therapy of PMF is the most attractive given this MPD disorder is the most likely to decrease survival amongst those afflicted. Initial reports have shown that this therapy does have curative potential in PMF patients (Guardiola et al 1997; Guardiola et al 1999). Recent reports describe a 58% 3 year survival in a group of 56 PMF (and post ET/PV MF) patients (age 10–66), with a 32% non-relapse mortality rate (Deeg et al 2003). The significant toxicity of full allogeneic transplant in PMF led to non-myeloablative trials (Devine et al 2002; Kröger et al 2005; Rondelli et al 2005). The latter trials have been encouraging in terms of decreased non-relapse mortality, and increasing ages of those successfully transplanted. However, allogeneic transplant still carries a significant risk of graft versus host disease (at least 33%) and the exact role and benefit depends on the long term prognosis of the patient. There is currently no data on the use of stem cell transplantation in ET or PV patients. Indeed, the significant risks of any of the stem cell transplantation procedures make it difficult to justify this therapy for ET and PV given the overall good prognosis of these patients.
ET and PV both share a risk of thrombosis and hemorrhagic events. The exact mechanisms for the development of thrombohemorrhagic complications in MPD patients are several and clearly include uncorrected erythrocytosis, thrombocytosis, and, as recently described, perhaps leukocytosis too (Falanga et al 2005). Current data on prevention of these events has been somewhat limited. Patients with PV benefit from control of erythrocytosis (by phlebotomy), and the use of low dose aspirin (Landolfi et al 2004). Hydroxyurea was shown in a randomized fashion to aid in the prevention of thrombotic events in patients with high risk ET (Cortelazzo et al 1995). The PT-1 (primary thrombocythemia 1) trial compared in a randomized fashion hydroxyurea and anagrelide (both along with low dose aspirin) for ET patients and found hydroxyurea plus aspirin to be superior in regards to preventing arterial events, hemorrhage, and transformation to post-ET MF (Harrison et al 2005). Therefore, standard therapy for those patients whom require platelet lowering therapy (high risk because of prior vascular events, age or cardiovascular risk factors) is hydroxyurea. However, concerns linger as to whether hydroxyurea accelerates an MPD towards leukemic transformation, although this has never been proven when used as a single agent (Finazzi et al 2005). Additionally, not all patients are able to tolerate, nor achieve adequate control with single agent hydroxyurea.
Both ET and PV have the risk of transformation to post ET/PV MF (Passamonti et al 2004), with a 15 year cumulative incidence of 4% and 6%, respectively. Risk of leukemic transformation in ET and PV at 15 years is 2% and 7%, respectively (increased by radioactive phosphorus and alkylator exposure (Finazzi et al 2005)). No medical therapy (specifically hydroxyurea, anagrelide, or interferon) has definitively helped to decrease these long term risks, pressing the need for development of newer therapies. Indeed, if we look at a stepwise approach to the impact of our therapies for ET and PV (Figure 1) they remain strictly at the first level, prevention of thrombohemorrhagic events only.
Medical therapy for PMF and post ET/PV MF historically can best be described as empirically derived with palliative outcomes at best (Mesa 2003). Cytopenias have improved in subsets of patients with erythropoietin supplementation (Cervantes et al 2004), androgens (Cervantes et al 2000), and/or corticosteroids. Similarly, the use of non-specific myelosuppressive regimens such as oral hydroxyurea (Lofvenberg et al 1990), busulfan (Chang and Gross 1988), melphalan (Petti et al 2002), and cladribine (Faoro et al 2005) have all been reported to provide palliative reduction in painful splenomegaly. However, all of these prior therapies have shared disappointing features of (1) lacking the ability to improve intramedullary manifestations of the disease, (2) no improvement in the disease course, and (3) no prolongation of survival. Therapy of PMF (post ET/PV MF) remain (Figure 1) on a basic palliative level, which heightens the need for more efficacious targeted treatments.
Tyrosine kinase inhibition: the rise of imatinib for CML
In summary the MPDs currently have therapeutic options which range from potentially curative but with sobering to prohibitive morbidity and mortality (allo-stem cell transplant), to those which can either decrease vascular events or abrogate disease associate manifestations. Indeed no medical therapies which can delay disease progression in patients with early disease, nor prolong survival in those with advanced disease. This status is not to dissimilar to the pre-imatinib mesylate status of therapy with CML. Indeed prior to that agent, patients either went for allo-transplant or had therapy with non specific agents such as hydroxyurea or busulfan. Although interferon had some positive data in this group, CML was not very different than our current options for PMF, CMML or other advanced MPDs. The development of imatinib mesylate for chronic phase CML was a watershed moment for hematology (Druker et al 2001). The most recent reports of the long-term efficacy and safety of imatinib in CML remain quite encouraging with approximately 70% of patients remaining on imatinib from the initial IRIS trial of imatinib (Roy et al 2006).
MPDs with a clear imatinib target (Table 1)
Table 1.
Synopsis of role of imatinib mesylate in BCR-ABL negative myeloproliferative disorders
| Disease | Targets | Comments | Ref | |
|---|---|---|---|---|
| Imatinib Effective with Identified Molecular Target | Eosinophilia
|
|
|
(Gleich et al 2002; Pardanani et al 2002; Cools et al 2003b; Martinelli et al 2005; Score et al 2006) |
| SMCD |
|
|
(Pardanani, Elliott et al 2003) | |
| CMML |
|
|
(Apperley et al 2002) | |
| Modest to Disap- pointing Results with Imatinib | Myelofibrosis |
|
|
(Tefferi et al 2002; Cortes, Ault, et al 2003) |
| Polcythemia Vera |
|
|
(Borthakur et al 2004; Hasselbalch 2004; Kuriakose and Shurafa 2004; Silver et al 2004) |
Abbreviations: CEL, chronic eosinophilic leukemia; HES, hypereosinophilic syndrome; CMML, chronic myelomonocytic leukemia; Myelofibrosis, Primary myelofibrosis and post essential thrombocythemia/polycythemia vera myelofibrosis
Imatinib mesylate is successful in the therapy of CML because of the inhibition of the BCR-ABL kinase (Druker et al 1996). By definition, the other MPDs lack the BCR-ABL as a therapeutic target. Therefore why would imatinib be expected to provide benefit in any of the other MPDs. The answer is really twofold, first imatinib inhibits a range of known kinases (platelet derived growth factor (PDGF) for example) which might have pathogenetic implications in MPDs, second there may be additional unknown targets for imatinib in MPD patients and given the lack of effective treatments (and the similarities to CML) perhaps these patients might respond. Indeed with this spirit of a combination of targeted therapy, part empiric application of a therapy efficacious for a similar disease imatinib has been tested in a wide range of MPDs. The subsequent discussion of the efficacy and role of imatinib in the MPDs will be divided into those disorders which truly seem to have a sensitive imatinib target and strong clinical efficacy, and those in which the specificity and role are still not as certain.
Eosinophilic disorders
Eosinophilia in the peripheral blood (absolute eosinophil count >0.6 × 109/L) can arise from either familial or acquired origins. Acquired eosinophilia can be either secondary (from infectious and non-infectious etiologies; the scope of these and excluding secondary causes of eosinophilia are outside the scope of this manuscript and I would refer the reader to many excellent reviews by Ayalew Tefferi (Tefferi, Patnaik et al 2006)). Primary eosinophilia is comprised of clonal disorders (eosinophilia associated with an acute myeloid leukemia (ie, FAB M4, with an inversion 16 karyotypic change; or a chronic myeloid disorder (ie, chronic eosinophilic leukemia (CEL; by WHO criteria)), and the diagnosis of exclusion hypereosinophilic syndrome (HES) (Tefferi, Elliott et al 2006).
Therapy of CEL and HES have in the past focused on empiric myelosuppression (in many ways mirroring the therapy of other MPDs) with hydroxyurea, interferon, or steroids (Tefferi, Elliott et al 2006). The discovery of a role for imatinib mesylate in the disorders of eosinophilia is a fascinating story both in terms of clinical efficacy, but how an agent tested for empiric reasons assisted in augmenting the pathogenetic understanding of a disease. Imatinib mesylate was empirically tested in patients with very active hypereosinophilic syndrome (Gleich et al 2002), and the agent was so efficacious in rapidly eliminating eosinophilia from the blood (at even low doses) that the rapid release of the contents of toxic eosinophil granules led to an acute myocardial insult (Pardanani, Reeder et al 2003). These unanticipated sequelae of imatinib fortunately resolved with timely use of corticosteroids, but asked the question why were these patients exquisitely sensitive to the agent? Astute investigators reasoned that an additional, previously un-described, tyrosine kinase target, for imatinib must exist in these patients. Indeed, these investigations led to the description of the an 800 kb interstitial deletion involving chromosome 4q12 leading to a juxtaposition of the PDGFRa (platelet derived growth factor receptor alpha) and the FIP1L1 gene (Cools et al 2003a). This latter fusion leads to constitutively active tyrosine kinase that transforms and is the imatinib sensitive target in these patients. These observations have led to a cascade of similar reports and subsequent clinical trials. However it should be noted that the prevalence of this mutation in un-selected cases of eosinophilia is <20% (Pardanani, Ketterling et al 2006). Individuals whom have the PDGFRA-FIP1L1 deletion have been demonstrated to clearly respond quickly and durably to even low dose imatinib in the range of 100 mg/day, although may require concurrent steroids to decrease the likelihood of acute myocardial stunning by rapid death of eosinophils. Individuals without this mutation may still respond to imatinib at higher doses (400 mg/day), and with a greater delay for response than what is seen for individuals with the mutation (Martinelli et al 2005). Additional imatinib sensitive novel targets have been described in PDGFRA-FIP1L1 negative patients including KIF5B-PDGFRA (Score et al 2006). The use of imatinib mesylate in patients whom do not express a clear sensitive target remains empiric, requires higher doses of the agents (ie, 400 mg/ day), leads most commonly to partial responses, and frequently is a more appropriate second or third line therapy (Tefferi, Patnaik et al 2006).
Systemic mast cell disease
Systemic mastocytosis (SMCD) is characterized by clonal proliferation of mast cells leading to skin manifestations (urticaria pigmentosa), mast cell mediator release symptoms (pruritus, diarrhea, syncope), bone lesions, organomegaly, or cytopenias (Tefferi, Elliott et al 2006). Conventional therapy of SMCD has included antihistamines, topical steroids, and cromolyn sodium for mediator release symptoms. Aggressive systemic symptoms have been treated with empiric use of myelosuppressive agents as is the case with other MPDs. Two key set of mutations may have pathogenetic roles in SMCD including c-KIT and PDGFRA. Individuals with mutations in PDGFRA, or some of the c-KIT mutations (V560G; but not the more common D816V) have shown response to imatinib mesylate (Pardanani, Elliott et al 2003). Current use of imatinib for SMCD is wisely limited to those with a clear target PDGFRA-FIP1L1 or c-KITV560G at doses beginning at 100 mg/day, therapy outside these settings in individuals lacking associated eosinophilia is unlikely to be efficacious (Tefferi, Elliott et al 2006).
Chronic myelomonocytic leukemia (CMML)
CMML, has a phenotype and severity, and impact on survival on prognosis that easily rivals that of PMF and CML. Additionally these patients are prone to ascites and serositis from direct tissue injury from the monocytosis. Therapy of CMML has traditionally fallen in the same frustrating trap of these other entities. Specifically, allogeneic stem cell transplant in appropriate candidates, or empiric mildly efficacious myelosuppressive therapies with hydroxyurea or infusional chemotherapy. A subset of patients with monocytosis, whom clinically appear to have a CMML like MPD have an imatinib sensitive rearrangement of PDGFRB arising from chromosomal translocations involving 5q33; such as t(5; 12)(q33; p13) or t(5; 7)(q33; q11.2)(Ross et al 1998). These individuals can have a dramatic response to imatinib mesylate (Apperley et al 2002), although the incidence of CMML patients with this mutation is uncommon. Indeed empiric use of imatinib mesylate in CMML is discouraged in those lacking the mutation based upon the potential for toxicity (Elliott et al 2002).
Empiric trials of imatinib in MPDS (Table 1)
Primary and post PV/ET myelofibrosis
Primary myelofibrosis (and the clinically indistinguishable post ET/PV MF) were a diseased of interest for imatinib mesylate for 2 reasons. The first is that the clinical phenotype of PMF can resemble closely that of CML, indeed in some patients only the presence or absence of the BCR-ABL is a reliable marker to distinguish these entities. The second is the fact that Imatinib mesylate is an inhibitor of the tyrosine kinase activity of not only Abl but also PDGFR, kit and arg (Kantarjian et al 2002). The employment of imatinib for the therapy of patients with PMF was based on its inhibitory activity against PDGF-mediated signaling and the reduction of bone marrow fibrosis and microvessel density observed in patients with chronic myeloid leukemia (CML), for which imatinib is a standard therapy (Kvasnicka and Thiele 2004). Additionally, imatinib has been shown to increase the number of clonogenic megakaryocytic progenitors in bone marrow, indicating that imatinib may restore mega-karyocyte differentiation and be effective for the treatment of thrombocytopenia in PMF (le Bousse-Kerdiles 2005). However, despite this encouraging pre-clinical potential for efficacy, results from all the phase II trials of imatinib in patients with PMF reported to date are modest (Tefferi et al 2002; Cortes, Ault et al 2003). Only one study showed significant improvement in splenomegaly, including 4 (29%) patients who had normalization of spleen span (Cortes, Giles et al 2003). Imatinib was administered at doses ranging from 200 to 800 mg daily but dropout rates were in excess of 50% across all trials due to adverse events (Tefferi et al 2002; Cortes, Ault et al 2003). Indeed, even splenic rupture has been described with the use of this agents for PMF (Elliott et al 2002), a rare event for these patients. Imatinib should not be considered as part of the therapeutic armamentarium for PMF patients.
Polycythemia vera
Based upon the potential for imatinib mesylate to inhibit the tyrosine kinase phosphorylation of c-Kit (the stem cell factor receptor essential for erythroid progenitor cell proliferation) imatinib mesylate was tested in a variety of trials for PV. These trials initially reported in 2004 included a total of 49 patients (Borthakur et al 2004; Hasselbalch 2004; Kuriakose and Shurafa 2004; Silver et al 2004). Although the methodology was slightly different in these reports patients were allowed to continue standard care for their PV (phlebotomy and low dose aspirin) with ranges of imatinib mesylate from 100 mg to 800 mg/day. The largest, multi-center trial (Silver et al 2004) reported 13 responders (48%) with responses ranging from both resolution of peripheral myeloproliferation and splenomegaly to erythroid only control, in no patient did the agent control thrombocytosis. Toxicity described was dermatologic, gastrointestinal and bone pain. The other trials mirrored the findings of Silver and colleagues with the agent mainly decreased erythrocytosis and phlebotomy requirements (Borthakur et al 2004; Hasselbalch 2004; Kuriakose and Shurafa 2004). The logical question arose whether the imatinib was truly benefitting the PV patients clinically in any way that could not be attributable to non-specific myelosuppression with traditional therapies such as hydroxyurea, busulfan, or even interferon. Indeed several patients on these trials experienced progression of their disease to post PV MF, suggesting limited or no ability of this therapy to alter the natural history of PV.
Attempts to correlate clinical response to imatinib in PV patients to objective molecular improvements in JAK2V617F burden showed only modest decreases in mutation levels even in those patients with the most robust response (Jones et al 2005; Hyjek et al 2006). Additionally, there was some decrease in the burden of karyotypic abnormalities of del(5q) and −21, but none on abnormalities of chromosome 9 (Najfeld et al 2004). In aggregate, there seems inadequate evidence at this juncture to suggest imatinib makes a significant impact upon the clinical manifestations and natural history of this disorder to recommend this agent in a non-clinical trial setting. Indeed given the expense alone, the ability of this agent to achieve a decrease in phlebotomy seems insufficient efficacy to justify its use. It would be anticipate that tyrosine kinase inhibition against targets more central to the pathology of PV (such as mutations of JAK2V617F, or exon 12) would hopefully yield more promising results.
Next generation tyrosine kinase inhibition
Dasatinib (BMS-354825) is a dual Src- and Abl-kinase inhibitor, 300-fold more potent Abl kinase inhibitor than imatinib, and FDA approved for CML (Lombardo et al 2004). In addition, dasatinib effectively inhibits PDGFR-β (IC50 28 nM) (Lombardo et al 2004). Based on these preclinical data and on the results obtained with imatinib, results from an ongoing clinical trial of dasatinib in patients with MPDs either responsive to imatinib, or not sensitive to imatinib are eagerly awaited. Additional efforts with other kinases inhibitors such as nilotinib (Stover et al 2005; Weisberg et al 2006) are of interest to be explored for a role in the MPDs.
Targeting the JAK2V617F mutation and associated signal transduction pathways
The next generation of targeted therapy for the MPDs, without a clear imatinib target (the majority), will move beyond agents targeting the stromal reaction, or cytokines, to agents aimed at the aberrant clone and constitutively active proliferative stimuli. The discovery of the activating point mutation in the auto-inhibitory pseudokinase domain of JAK2 (the JAK2V617F) was a watershed moment in the understanding of the pathogenesis of the BCR-ABL negative MPDs (Baxter et al 2005; James et al 2005; Kralovics et al 2005; Levine et al 2005). JAK2V617F is present in only about half of PMF and post-ET MF patients, but in the vast majority of post-PV MF patients (Tefferi et al 2005). Recent additional discoveries such as the c-MPLW515L/K (Pardanani, Levine et al 2006; Pikman et al 2006), and the role of additional mutations in exon 12 of JAK2 gene in JAK2V617F negative PV patients (Scott et al 2007) have provided further insight into intrinsic myeloproliferative drive in these patients. Although the currently identified molecular defects do not fully explain many issues of MPD pathogenesis, they provide an exciting and hopefully more fruitful therapeutic target. There have already been multiple reports of agents in development which have demonstrated the ability to inhibit the aberrant JAK2V617F, along with the wild type JAK2, such as TG101209 (Pardanani, Hood et al 2006), Go6976 (Grandage et al 2006), erlotinib (Li et al 2006), MK0457 (Giles et al 2006), CEP-701 (Dobrzanski et al 2006) (now in a clinical study), and Z3 (Sayyah et al 2006). Intriguingly, in primary cells from wild type JAK2 patients, whom have the c-MPLW515L/K, growth inhibition can similarly be accomplished by JAK2 inhibitors, such as TG101209 (Pardanani, Hood et al 2006). These latter observations suggest the possibility that even in JAK2 wild type MPD patients, a growth dependence on the JAK-STAT pathway may exist and agents targeting this pathway may be active regardless of the JAK2 mutation status. This hypothesis is further supported by the continual discovery of aberrations in this pathway, as in exon 12 of JAK2 gene in JAK2V617F negative PV patients (Scott et al 2007).
Challenges as we enter the era of JAK2 inhibitors for PMF and MPD patients are several. First, whom are the most appropriate candidates for agents with uncertain safety profiles given that there are many patients with JAK2V617F positive ET and PV, with a good natural history (Passamonti et al 2004), that will be seeking these agents? Next, although there are many agents that may have ability to inhibit the wild type JAK2, we must proceed with caution as few have desired specificity for the JAK2 itself, let alone the JAK2V617F. This opens up the possibility of many undesired toxicities related to the inhibition of additional tyrosine kinases. Additional challenges are inherent to the process of clinical research: how to effectively choose which candidate agents from pre-clinical testing truly merit clinical testing, and how to properly design trials to truly test optimum dose and schedule of these agents. Indeed, concern that significant potential for type II error exists for agents in which we are uncertain as to how to assess their response, and that we could prematurely discard beneficial agents by having incorrect assumptions regarding the chronology or rapidity of response.
What effects can we expect to observe from the inhibition of JAK2 (no medication is likely to strictly inhibit the JAK2V617F)? As we look at MPD therapeutic goals (Figure 1), will inhibition of the JAK-STAT pathway decrease myelo-proliferation? The current published in vitro and murine data would suggest a positive answer. Will JAK-STAT inhibition decrease the development of thrombohemorrhagic events, given the multifactorial nature of their origin? What about the major therapeutic endpoints desired by clinicians and patients, namely delaying or preventing disease progression, or increasing survival? These latter goals are highly desired but uncertainty exist as to whether the inhibition of JAK2 will accomplish these goals since the exact role of the JAK2V617F mutation in disease progression or development of PMF-BP remains unclear (Mesa et al 2006). The observation that JAK2 mutant MPD patients have the potential to develop acute leukemia from a JAK2 wild type clone (Theocharides et al 2006) questions the role of this mutation in disease progression. Therefore, what impact JAK2 inhibition will have on disease progression (ie, none, decrease, or increase) is quite uncertain and will require close long-term monitoring of patients taking JAK2 inhibitors, to be certain no adverse impact arises from the use of these agents. Finally, if beneficial, will the JAK2 inhibitors lead to the cure? Unlikely, but an outcome that parallels the efficacy of imatinib for CML (Druker et al 2001) in terms of short and long term control of the disease would be greatly welcomed by physician and patient alike.
Conclusion
The development of novel therapies for MPD patients has been historically hampered by limited progress regarding the molecular pathogenesis of this disease. Empiric therapeutic use of imatinib mesylate has helped identify several pathogenetically relevant mutations in MPDs, and led to effective therapy for subsets of patients with CEL, SMCD, and CMML. However, empiric use of imatinib has been of much more limited efficacy in the balance of MPD patients. Great strides have been made in this regard over the last decade, culminated by the recent discovery of the gain-of-function JAK2V617F mutation. A challenge for the near future will be the development of targeted agents with acceptable toxicity profiles able to interfere with the JAK-STAT signaling pathway.
References
- Apperley JF, Gardembas M, Melo JV, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med. 2002;347:481–7. doi: 10.1056/NEJMoa020150. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Borthakur G, Kantarjian H, Verstovsek S, et al. Imatinib mesylate therapy for patients (pts) with polycythemia vera (PV) ASH Annual Meeting Abstracts. 2004;104:1527. [Google Scholar]
- Cervantes F. Prognostic factors and current practice in treatment of myelofibrosis with myeloid metaplasia: an update anno 2000. Pathol Biol (Paris) 2001;49:148–52. doi: 10.1016/s0369-8114(00)00020-1. [DOI] [PubMed] [Google Scholar]
- Cervantes F, Alvarez-Larran A, Hernandez-Boluda JC, et al. Erythropoietin treatment of the anaemia of myelofibrosis with myeloid metaplasia: results in 20 patients and review of the literature. Br J Haematol. 2004;127:399–403. doi: 10.1111/j.1365-2141.2004.05229.x. [DOI] [PubMed] [Google Scholar]
- Cervantes F, Hernandez-Boluda JC, Alvarez A, et al. Danazol treatment of idiopathic myelofibrosis with severe anemia. Haematologica. 2000;85:595–9. [PubMed] [Google Scholar]
- Chang JC, Gross HM. Remission of chronic idiopathic myelofibrosis to busulfan treatment. American Journal of the Medical Sciences. 1988;295:472–6. doi: 10.1097/00000441-198805000-00011. [DOI] [PubMed] [Google Scholar]
- Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003a;348:1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. [comment] New England Journal of Medicine. 2003b;348:1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332:1132–6. doi: 10.1056/NEJM199504273321704. [DOI] [PubMed] [Google Scholar]
- Cortes J, Ault P, Koller C, et al. Efficacy of imatinib mesylate in the treatment of idiopathic hypereosinophilic syndrome. Blood. 2003;101:4714–16. doi: 10.1182/blood-2003-01-0081. [DOI] [PubMed] [Google Scholar]
- Cortes J, Giles F, O’Brien S, et al. Results of imatinib mesylate therapy in patients with refractory or recurrent acute myeloid leukemia, high-risk myelodysplastic syndrome, and myeloproliferative disorders. Cancer. 2003;97:2760–6. doi: 10.1002/cncr.11416. [DOI] [PubMed] [Google Scholar]
- Dameshek W. Some speculations on the myeloproliferative syndrome. Blood. 1951;6:372–5. [PubMed] [Google Scholar]
- Deeg HJ, Gooley TA, Flowers ME, et al. Allogeneic hematopoietic stem cell transplantation for myelofibrosis. Blood. 2003;102:3912–18. doi: 10.1182/blood-2003-06-1856. [DOI] [PubMed] [Google Scholar]
- Devine SM, Hoffman R, Verma A, et al. Allogeneic blood cell transplantation following reduced-intensity conditioning is effective therapy for older patients with myelofibrosis with myeloid metaplasia. Blood. 2002;99:2255–8. doi: 10.1182/blood.v99.6.2255. [DOI] [PubMed] [Google Scholar]
- Dingli D, Utz JP, Krowka MJ, et al. Unexplained pulmonary hypertension in chronic myeloproliferative disorders. Chest. 2001;120:801–8. doi: 10.1378/chest.120.3.801. [DOI] [PubMed] [Google Scholar]
- Dobrzanski P, Hexner E, Serdikoff C, et al. CEP-701 is a JAK2 inhibitor which attenuates JAK2/STAT5 signaling pathway and the proliferation of primary cells from patients with myeloproliferative disorders. ASH Annual Meeting Abstracts. 2006;108:3594. [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–6. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Dupriez B, Morel P, Demory JL, et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system [see comments] Blood. 1996;88:1013–18. [PubMed] [Google Scholar]
- Elliott MA, Mesa RA, Tefferi A. Adverse events after imatinib mesylate therapy. N Engl J Med. 2002;346:712–13. doi: 10.1056/NEJM200202283460919. [DOI] [PubMed] [Google Scholar]
- Falanga A, Marchetti M, Vignoli A, et al. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol. 2005;33:523–30. doi: 10.1016/j.exphem.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Faoro LN, Tefferi A, Mesa RA. Long-term analysis of the palliative benefit of 2-chlorodeoxyadenosine for myelofibrosis with myeloid metaplasia. Eur J Haematol. 2005;74:117–20. doi: 10.1111/j.1600-0609.2004.00370.x. [DOI] [PubMed] [Google Scholar]
- Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in poly-cythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105:2664–70. doi: 10.1182/blood-2004-09-3426. [DOI] [PubMed] [Google Scholar]
- Giles F, Freedman SJ, Xiao A, et al. MK-0457, a novel multikinase inhibitor, has activity in refractory AML, including transformed JAK2 positive myeloproliferative disease (MPD), and in Philadelphia-positive ALL. ASH Annual Meeting Abstracts. 2006;1967;108 [Google Scholar]
- Gleich GJ, Leiferman KM, Pardanani A, et al. Treatment of hypereosinophilic syndrome with imatinib mesilate. Lancet. 2002;359:1577–8. doi: 10.1016/S0140-6736(02)08505-7. [DOI] [PubMed] [Google Scholar]
- Grandage VL, Everington T, Linch DC, et al. Go6976 is a potent inhibitor of the JAK2 and FLT3 tyrosine kinases with significant activity in primary acute myeloid leukaemia cells. Br J Haematol. 2006;135:303–16. doi: 10.1111/j.1365-2141.2006.06291.x. [DOI] [PubMed] [Google Scholar]
- Guardiola P, Anderson JE, Bandini G, et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Societe Francaise de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood. 1999;93:2831–8. [PubMed] [Google Scholar]
- Guardiola P, Esperou H, Cazalshatem D, et al. Allogeneic bone marrow transplantation for agnogenic myeloid metaplasia. British Journal of Haematology. 1997;98:1004–9. doi: 10.1046/j.1365-2141.1997.3073124.x. [DOI] [PubMed] [Google Scholar]
- Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- Hasselbalch H. Imatinib mesylate in polycythemia vera. A heterogeneous response pattern but a consistent reduction in phlebotomy requirements. ASH Annual Meeting Abstracts. 2004;104:4747. [Google Scholar]
- Hyjek E, Chadburn A, Cross NCP, et al. Correlation of clinical and molecular response to imatinib in polycythemia vera (PV) patients with bone marrow morphologic and immunophenotypic changes. ASH Annual Meeting Abstracts. 2006;108:4914. [Google Scholar]
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Jones AV, Higley K, Curtis C, et al. No significant molecular response in polycythemia vera patients treated with imatinib or interferon alpha. ASH Annual Meeting Abstracts. 2005;106:373. [Google Scholar]
- Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Kröger N, Zabelina T, Schieder H, et al. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofibrosis. British Journal of Haematology. 2005 doi: 10.1111/j.1365-2141.2005.05373.x. Published On-Line. [DOI] [PubMed] [Google Scholar]
- Kuriakose P, Shurafa MS. A phase II trial of imatinib mesylate (Gleevec(R)) in myeloproliferative disorders other than CML. ASH Annual Meeting Abstracts. 2004;104:4765. [Google Scholar]
- Kvasnicka HM, Thiele J. Bone marrow angiogenesis: methods of quantification and changes evolving in chronic myeloproliferative disorders. Histol Histopathol. 2004;19:1245–60. doi: 10.14670/HH-19.1245. [DOI] [PubMed] [Google Scholar]
- Landolfi R. Bleeding and thrombosis in myeloproliferative disorders. Current Opinion in Hematology. 1998;5:327–31. doi: 10.1097/00062752-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114–24. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- le Bousse-Kerdiles M, Desteke C, Guerton B, et al. Glivec/STI571 treatment stimulates megakaryopoiesis and normalizes PDGF receptor beta kinase expression in thrombocytopenic patients with myeloid metaplasia with myelofibrosis. Blood. 2005;106 (abstr 2599) [Google Scholar]
- Levine RL, Wadleigh M, Cools J. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myelofibrosis with myeloid metaplasia. Cancer Cell. 2005 doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu M, Xing S, et al. Erlotinib effectively inhibits JAK2V617F activity and polycythemia vera cell growth. J Biol Chem. 2006 doi: 10.1074/jbc.C600277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofvenberg E, Wahlin A, Roos G, et al. Reversal of myelofibrosis by hydroxyurea. European Journal of Haematology. 1990;44:33–8. doi: 10.1111/j.1600-0609.1990.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methyl-pyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–61. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Martinelli G, Cilloni D, Rondoni M, et al. Imatinib mesylate can induce molecular complete remission in idiopathic hypereosinophilic syndrome (HES). A phase II multicentric Italian clinical trial. ASH Annual Meeting Abstracts. 2005;106:375. [Google Scholar]
- Mesa RA. Myelofibrosis with myeloid metaplasia: therapeutic options in 2003. Curr Hematol Rep. 2003;2:264–70. [PubMed] [Google Scholar]
- Mesa RA, Hanson CA, Rajkumar SV, et al. Evaluation and clinical correlations of bone marrow angiogenesis in myelofibrosis with myeloid metaplasia. Blood. 2000;96:3374–80. [PubMed] [Google Scholar]
- Mesa RA, Powell H, Lasho T, et al. JAK2(V617F) and leukemic transformation in myelofibrosis with myeloid metaplasia. Leuk Res. 2006;30:1457–60. doi: 10.1016/j.leukres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Silverstein MN, Jacobsen SJ, et al. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. Am J Hematol. 1999;61:10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT) Leuk Res. 2007 doi: 10.1016/j.leukres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Najfeld V, Silver RT, Hoffman R, et al. Pretreatment cytogenetic abnormalities in polycythemia vera (PV) determines the effectivnes of imatinib : studies from a multi-institutional trial. ASH Annual Meeting Abstracts. 2004;104:2431. [Google Scholar]
- Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. [PubMed] [Google Scholar]
- Pardanani A, Elliott M, Reeder T, et al. Imatinib for systemic mast-cell disease. Lancet. 2003;362:535–6. doi: 10.1016/s0140-6736(03)14115-3. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Hood J, Lasho T, et al. TG101209, a selective JAK2 kinase inhibitor, suppresses endogenous and cytokine-supported colony formation from hematopoietic progenitors carrying JAK2V617F or MPLW515K/L mutations. ASH Annual Meeting Abstracts. 2006;108:2680. [Google Scholar]
- Pardanani A, Ketterling RP, Li CY, et al. FIP1L1-PDGFRA in eosinophilic disorders: prevalence in routine clinical practice, long-term experience with imatinib therapy, and a critical review of the literature. Leuk Res. 2006;30:965–70. doi: 10.1016/j.leukres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Reeder T, Porrata LF, et al. Imatinib therapy for hypereosinophilic syndrome and other eosinophilic disorders. Blood. 2003;101:3391–7. doi: 10.1182/blood-2002-10-3103. [DOI] [PubMed] [Google Scholar]
- Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006 doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- Pardanani AD, Reeder TL, Porrata LF, et al. Imatinib therapy for hypereosinophilic syndrome and other eosinophilic disorders. Blood. 2002;27:27. doi: 10.1182/blood-2002-10-3103. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–61. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Petti MC, Latagliata R, Spadea T, et al. Melphalan treatment in patients with myelofibrosis with myeloid metaplasia. Br J Haematol. 2002;116:576–81. doi: 10.1046/j.0007-1048.2001.03331.x. [DOI] [PubMed] [Google Scholar]
- Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondelli D, Barosi G, Bacigalupo A, et al. Allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in intermediate or high risk patients with myelofibrosis with myeloid metaplasia. Blood. 2005 doi: 10.1182/blood-2004-11-4299. [DOI] [PubMed] [Google Scholar]
- Ross TS, Bernard OA, Berger R, et al. Fusion of Huntingtin interacting protein 1 to platelet-derived growth factor beta receptor (PDGFbetaR) in chronic myelomonocytic leukemia with t(5; 7)(q33; q11.2) Blood. 1998;91:4419–26. [PubMed] [Google Scholar]
- Roy L, Guilhot J, Krahnke T, et al. Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between two phase 3 trials. Blood. 2006;108:1478–84. doi: 10.1182/blood-2006-02-001495. [DOI] [PubMed] [Google Scholar]
- Sayyah J, Ostrov D, Sayeski P. Identification and characterization of a novel Jak2 tyrosine kinase inhibitor. ASH Annual Meeting Abstracts. 2006;108:3604. [Google Scholar]
- Score J, Curtis C, Waghorn K, et al. Identification of a novel imatinib responsive KIF5B-PDGFRA fusion gene following screening for PDGFRA overexpression in patients with hypereosinophilia. Leukemia. 2006;20:827–32. doi: 10.1038/sj.leu.2404154. [DOI] [PubMed] [Google Scholar]
- Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–68. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RT, Fruchtman SM, Feldman EJ, et al. Imatinib mesylate (GLEEVEC(R)) is effective in the treatment of polycythemia vera: a multi-institutional clinical trial. ASH Annual Meeting Abstracts. 2004;104:656. [Google Scholar]
- Stover EH, Chen J, Lee BH, et al. The small molecule tyrosine kinase inhibitor AMN107 inhibits TEL-PDGFRbeta and FIP1L1-PDGFRalpha in vitro and in vivo. Blood. 2005;106:3206–13. doi: 10.1182/blood-2005-05-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A. The Philadelphia chromosome negative chronic myeloproliferative disorders: a practical overview. Mayo Clinic Proceedings. 1998;73:1177–84. doi: 10.4065/73.12.1177. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Elliott MA, Pardanani A. Atypical myeloproliferative disorders: diagnosis and management. Mayo Clin Proc. 2006;81:553–63. doi: 10.4065/81.4.553. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Jimenez T, Gray LA, et al. Radiation therapy for symptomatic hepatomegaly in myelofibrosis with myeloid metaplasia. Eur J Haematol. 2001;66:37–42. doi: 10.1034/j.1600-0609.2001.00342.x. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Schwager SM, et al. The JAK2(V617F) tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: lineage specificity and clinical correlates. Br J Haematol. 2005;131:320–8. doi: 10.1111/j.1365-2141.2005.05776.x. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Mesa RA, Gray LA, et al. Phase 2 trial of imatinib mesylate in myelofibrosis with myeloid metaplasia. Blood. 2002;99:3854–6. doi: 10.1182/blood-2001-12-0154. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Mesa RA, Nagorney DM, et al. Splenectomy in myelofibrosis with myeloid metaplasia: a single-institution experience with 223 patients. Blood. 2000;95:2226–33. [PubMed] [Google Scholar]
- Tefferi A, Mesa RA, Schroeder G, et al. Cytogenetic findings and their clinical relevance in myelofibrosis with myeloid metaplasia. Br J Haematol. 2001;113:763–71. doi: 10.1046/j.1365-2141.2001.02796.x. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133:468–92. doi: 10.1111/j.1365-2141.2006.06038.x. [DOI] [PubMed] [Google Scholar]
- Theocharides A, Boissinot M, Garand R, et al. Myeloid blasts in transformed JAK2-V617F positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. ASH Annual Meeting Abstracts. 2006;108:375. doi: 10.1182/blood-2006-12-062125. [DOI] [PubMed] [Google Scholar]
- van Genderen PJ, Michiels JJ. Erythromelalgia: a pathognomonic microvascular thrombotic complication in essential thrombocythemia and polycythemia vera. Seminars in Thrombosis and Hemostasis. 1997;23:357–63. doi: 10.1055/s-2007-996109. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Manley P, Mestan J, et al. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br J Cancer. 2006;94:1765–9. doi: 10.1038/sj.bjc.6603170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolanskyj AP, Schwager SM, McClure RF, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc. 2006;81:159–66. doi: 10.4065/81.2.159. [DOI] [PubMed] [Google Scholar]