Abstract
Management of male infertility is always a difficult task, and the pathologic process is often poorly understood. Even though modern assisted reproduction techniques (ART) can help overcome severe male factor infertility, the application of these methods in all infertile couples would definitely represent over-treatment. Several conditions can interfere with spermatogenesis and reduce sperm quality and production. A careful diagnostic work-up is necessary before any andrological treatment can be initiated so that adequate treatment options can be selected for individual patients. Most hormonal imbalances can be readily identified and successfully treated nonsurgically. However, the treatment of men with unexplained idiopathic infertility remains a challenge. In the absence of a correctable etiology, patients are managed with either empirical medical therapy or ART. Empiric medical therapy continues as a viable option. However, physicians and patients must understand that the success rates with any of the pharmacological therapies remain suboptimal.
Keywords: male infertility, oligospermia, drug therapy
Introduction
Approximately 10% of all couples seek fertility assessment. Infertility services are increasingly being utilized due to the later age of first pregnancy and associated reduction in female fertility. Assisted reproductive techniques remain an effective option for infertile couples. However, the cost is considerable, and there is a small but definite risk of ovarian hyperstimulation as well as fetal and maternal consequences associated with multiple gestation and developmental defects. In half of infertility cases, a male factor is involved. Thus, identifying the pathology and treating the male may allow couples to regain fertility and conceive through natural intercourse. The goal of specific medical management of infertility is to diagnose reversible causes of infertility and treat them with appropriate medications to achieve seminal improvement and pregnancy.
In spite of advancements in the diagnostic work-up of infertile men, up to 25% of patients exhibit abnormal semen analyses for which no etiology can be identified (Greenberg et al 1978). This condition is referred to as idiopathic male infertility, and nonspecific treatments are usually applied that are based on theoretical concepts. Empiric therapies may also be used for men with a known but otherwise untreatable cause of infertility or for patients with identified and potentially treatable causes of infertility but for whom previous treatments failed. A variety of empiric medical therapies have been recommended to treat these patients. However, with few exceptions, none of these therapies has been shown to be effective in repeated controlled randomized studies. Such studies are difficult to conduct since effective treatments are available, and few infertile couples are willing to delay treatment.
When empiric pharmacologic therapy is going to be used, treatment should last at least 3 to 6 months to incorporate a full 74-day spermatogenic cycle. The infertile couple should be advised of the inconsistent response to therapy and the low conception rate that may follow when compared with the results of ART. Lack of a significant improvement in seminal parameters or no pregnancy after at least two spermatogenic cycles may be an indication to proceed with ART.
Current medical treatment options for male infertility are discussed in this article. As stated earlier, most of the recommendations are not based on controlled studies.
Specific treatment
Hypogonadotropic hypogonadism
Common causes of hypogonadotropic hypogonadism (HGH) include Kallmann’s syndrome, pituitary tumors, pituitary trauma and anabolic steroid use. HGH is the cause of infertility in a small percentage of patients; the disorder can be classified as congenital or acquired (Liu and Handelsman 2003). One example of congenital HGH is Kallmann syndrome, which is a malformation of the midline cranial structures (Cunningham and Lipshultz 1986). In this syndrome, the defect is at the level of the hypothalamic secretion of gonadotropin-releasing hormone (GnRH) and may be associated with other congenital anomalies such as anosmia, deafness, cleft palate and renal anomalies. Acquired causes of HGH include pituitary tumors, pituitary trauma, panhypopituitarism and anabolic steroid use.
The initial evaluation of patients with suspected HGH may include a pituitary MRI to rule out a pituitary tumor (Gilbaugh and Lipshultz 1994). Pituitary tumors can cause local destruction of the anterior pituitary. A serum prolactin level should be measured, and hyperprolactinemia must be ruled out and treated before gonadotropin replacement.
In patients with acquired HGH, normal spermatogenesis can usually be restored by treatment with exogenous gonadotropins or GnRH. Human chorionic gonadotropin (hCG) therapy, which contains LH-like activity, is the most commonly used treatment in HGH for economic and compliance reasons (Conte et al 1990; Shin and Honig 2002). Human menopausal gonadotropin (hMG), which contains both FSH and LH, also has been used for replacement therapy in these patients. Normally, the treatment involves the subcutaneous administration of hCG 1500–3000 IU three times per week (March and Isidori 2002). However, congenital causes frequently require the addition of follicle-stimulating hormone (FSH). In these cases, after approximately 3 months of hCG therapy, intramuscular injections of FSH at dose of 37.5 to 75 IU are added three times per week. FSH is available in a recombinant form as well as in a highly purified urinary form. Serum testosterone levels and seminal analysis are followed during treatment. On average, it takes approximately 6 to 9 months before spermatozoa appear in the ejaculate (Haidl 2002). However, this period can be much longer (March and Isidori 2002). Once sperm concentrations reach satisfactory levels, FSH can be suspended, and spermatogenesis may be maintained with hCG alone (Siddiq and Sigman 2002). Although patients usually present with abnormal seminal parameters, many of them can initiate a pregnancy even when their sperm concentration is well below the conventional lower limit of 20 × 106/ml (Burris et al 1988). Also, hormone replacement therapy is more cost-effective than sperm retrieval/intracytoplasmic sperm injection (ICSI) in these patients (Shin and Honig 2002).
Patients who do not respond after hCG/FSH combination therapy may respond to GnRH via either intravenous or subcutaneous injections given in a pulsatile fashion with a portable infusion pump. Pulsatile GnRH is an effective treatment of non-pituitary gonadotrophin deficiency, both for inducing androgenization and spermatogenesis (Mortimer et al 1974; Crowley et al 1985). Normally, GnRH 5 to 20 mg/120 min is administered subcutaneously through an indwelling butterfly needle (Liu and Handelsman 2003). Intranasal GnRH can maintain previously induced spermatogenesis (Klingmuller and Schweikert 1985). However, the need for a 2-hour dosing regimen makes this clinically unfeasible. Nevertheless, pulsatile GnRH therapy depends on a intact pituitary gonadotropin response to the exogenous GnRH (Chuang and Howards 1998). Crowley and Whitcomb reported that eight of nine patients with idiopathic HGH desiring induction of fertility were able to father a child with the use of pulsatile GnRH therapy (Crowley and Whitcomb 1990).
Hyperprolactinemia
Hyperprolactinemia is a form of HGH caused by excessive prolactin secretion (Burrows et al 2002). An excess of prolactin inhibits the hypothalamic secretion of GnRH and has been implicated as a cause of reproductive and sexual dysfunction. Routine screening of infertile men for hyperprolactinemia has not been shown to be useful (Eggert-Kruse et al 1991). The condition may be caused by a pituitary tumor (macroadenoma or microadenoma), hypothyroidism, stress, medications such as phenothiazines, tricyclic antidepressants and some antihypertensives, medical illness, and idiopathic factors (Siddiq and Sigman 2002). The most common causes of hyperprolactinemia are prolactin-secreting microadenomas (<10mm) and prolactin-secreting macroadenomas (>10mm) (Jane and Laws 2001).
The level of prolactin elevation provides insight into the type of pathology. Prolactin levels greater than 250 ng/ml, between 100 and 250 ng/ml, between 25 and 100 ng/ml, and from 0 to 25 ng/ml most commonly correspond to macroadenoma, microadenoma, pituitary stalk compression and normal levels, respectively (Burrows et al 2002). Generally, in patients with prolactin-secreting pituitary adenomas, gonadotropin and testosterone levels are suppressed whereas prolactin levels are elevated. All patients with hyperprolactinemia should be evaluated with a pituitary MRI with gadolinium contrast to rule out a pituitary tumor. An elevation in peripheral prolactin should be followed with repeat testing because prolactin levels can vary widely throughout the day and with physical activity.
Treatment of hyperprolactinemia depends on the cause. Treatment of hypothyroidism or cessation of source medications usually brings serum prolactin levels back into the normal range. Although surgery and radiation therapy have been used in the past to treat patients with prolactin-secreting pituitary tumors, the vast majority of patients with idiopathic hyperprolactinemia or pituitary adenomas do not require surgery, and medical therapy is the initial treatment of choice (Molitch 1999). However, patients with macroadenomas generally require surgical therapy. Bromocriptine, a dopaminergic antagonist, can significantly reduce the serum prolactin levels in oligospermic men with hyperprolactinemia and increase sperm counts to a level that may result in pregnancy (Chuang and Howards 1998). Doses range from 2.5 to 7.5 mg per day and are given two to four times per day to avoid gastrointestinal side effects. Doses can be increased with time (Mancini et al 1984). In most cases, the serum prolactin levels return to normal.
Cabergoline is a new long-acting dopamine agonist that is effective and well tolerated in patients with pathological hyperprolactinemia. Cabergoline has been shown to be as effective as bromocriptine in lowering prolactin levels and reducing tumor size (Verhelst et al 1999). Moreover, cabergoline has the advantage of fewer side effects than bromocriptine and requires less frequent dosing. The median dose of cabergoline at the start of therapy is 1.0 mg/week, but once prolactin secretion is adequately controlled, the dose can be reduced to 0.5 mg/week, which further reduces therapy costs (Verhelst et al 1999). Selected patients with idiopathic hyperprolactinemia can be treated with medication as well, which may be withdrawn yearly to assess for persistent hyperprolactinemia (Dollar and Blackwell 1986; Wang et al 1987) Transsphenoidal surgery remains an option, especially for patients with microadenomas, when medical therapy is ineffective (Molitch 1999).
Immunologic infertility
An immunologic basis for some cases of infertility has been identified in a significant number of infertile men, suggesting that antisperm antibodies (ASA) may have a harmful effect in fertilization (Rumke and Hellinga 1959). Immunologic infertility is characterized by the presence of antibodies against spermatozoa in the serum and/or in the seminal plasma or on the sperm surface. The presence of multiple ASA can lead to the immobilization and/or agglutination of spermatozoa, which blocks sperm-egg interaction. They can also prevent implantation, and/or arrest embryo development (Haas 1986; Koide et al 2000). Common causes of ASA include previous genital tract infection, testicular biopsy, testicular trauma, testicular torsion and vasectomy (Broderick et al 1989; Koide et al 2000; Arap et al 2007).
The real significance of ASA in infertile men is controversial and currently, there are no standardized treatment regimens (Marshburn and Kutteh 1994). Oral corticoids are commonly used to suppress antibody production, but to date, no double-blind, randomized trial has confirmed their efficacy. Studies following different protocols report pregnancy rates between 0 to 44% (De Almeida and Soufir 1977; Shulman and Shulman 1982; Hendry et al 1986; Dondero et al 1993). Studies in which treatment was continued for more than 3 months reported a significant increase in the number of pregnancies amongst those receiving prednisolone compared with placebo (Hendry et al 1990; Omu et al 1996). However, a meta-analysis showed no significant improvement in pregnancy rates with prednisolone therapy (Kamischke and Nieschlag 1999).
ICSI is considered to be the treatment of choice for patients with severe sperm autoimmunity (Check et al 2000). Clarke et al showed no significant differences in fertilization rates (62% versus 58%) or clinical pregnancy rates (19% versus 12%) between sperm antibody-positive and sperm antibody-negative patient groups (Clarke et al 1997). However, recently, higher fertilization rates during in vitro fertilization (IVF) were reported in patients with antisperm antibodies and immunosuppressive therapy compared to IVF alone (Shin et al 1998; Haidl 2002). Thus, treatment of antisperm antibodies using corticosteroids should not be prescribed routinely, but it can be considered in patients with antisperm antibodies and earlier failed fertilization during IVF or ICSI.
Genital tract infection
The prevalence of leukocytospermia (>106 WBC/mL semen) among male infertility patients is approximately 10%–20% (Wolff 1995). Under wet mount microscopy, both leukocytes and immature germ cells have a similar appearance and are properly termed “round cells.” Although many laboratories improperly report all round cells as white blood cells, the clinician must make sure that the two types of cells are differentiated. Leukocytes are difficult to differentiate from immature germ cells without use of traditional cytologic staining and immunohisthochemical techniques (Wolff and Anderson 1988). The World Health Organization considers leukocytospermia to be a condition in which leukocyte levels are equal to or exceed 1 × 106/mL (WHO 1999). However, recent studies reported that leukocyte counts below 1 × 106/mL were significantly correlated with production of seminal reactive oxygen species (ROS) as well as decreased sperm DNA integrity (Sharma et al 2001; Henkel et al 2003; Henkel et al 2005; Athayde et al 2007). All men with elevated seminal white blood cell levels (>1 × 106/mL) should be evaluated for a genital tract infection or inflammation, and a semen culture should be performed. Unexpectedly, approximately 80% of leukocytospermic samples are microbiologically negative (Jennings et al 1986; Wolff 1995).
The significance of WBC in semen is controversial. Most studies found that leukocytospermia is associated with decreased sperm motility and fertilization capacity (Berger et al 1982; Maruyama et al 1985; Wolff et al 1990; Aitken et al 1992; Aitken et al 1994). However, El-Demiry et al reported no association between standard seminal parameters and leukocyte concentration in human semen (El-Demiry et al 1986). This discrepancy may be due to the fact that different techniques were used to determine leukocyte concentration in semen. In addition, the studies differed in regards to the lower leukocyte concentration responsible for sperm damage (Shekarriz et al 1995; Henkel et al 2005; Athayde et al 2007). Infections located in the testis and epididymis produce ROS that are particularly harmful to sperm due to their lack of a pro-oxidant defense system.
The most commonly found gram-positive and gram-negative bacteria are Streptococcus fecalis and Escherichia coli, respectively (March and Isidori 2002). Also, Chlamydia trachomatis and Ureaplasma urealyticum are often involved. Once the responsible microorganism has been identified, antibiotic therapy is initiated. However, culture-negative patients should be treated with anti-inflammatory therapy and frequent ejaculation because empiric antibiotic therapy generally provides no benefit and may be harmful (Comhaire et al 1986; Yanushpolsky et al 1995). In cases of refractory leukocytospermia, sperm washing can be performed before intrauterine insemination to remove the white cells.
According to the microbiological findings the following agents can be used as treatment: Doxycycline 200 mg/day, tetracycline 1.5 to 2 g/day, fluoroquinolones (ofloxacin, norfloxacin, ciprofloxacin, levofloxacin) 0.5 to 1 g/day, cotrimoxazole (sulfamethoxazole 800 mg, trimethoprim 160 mg) or macrolides, eg, erythromycin 1.5 to 2 g/day. These drugs are administered for 2 to 3 weeks (Haidl and Schill 1991). The objectives of treatment are to reduce or eradicate microorganisms in prostatic secretions and semen, normalize inflammatory parameters such as leukocyte levels and biochemical markers such as granulocyte elastase, and improve sperm parameters (Weidner et al 1999). Although antibacterial therapy can reduce inflammatory influences when administered in patients with genital tract infection, there are no available studies on this subject that show improved pregnancy rates (Weidner 1999).
Disorders of ejaculation
Ejaculatory dysfunction includes a variety of disorders with individualized treatments. Although this is a relatively unusual cause of male infertility, its represents an interesting challenge to the treating physician (Schuster and Ohl 2002). Ejaculatory dysfunction should be suspected in any patient with low-volume (<1.0 ml) or absent ejaculate and should be distinguished from anorgasmia. Retrograde ejaculation can be defined as the abnormal backward flow of semen into the bladder with ejaculation; the etiology may be anatomic, neurogenic, pharmacologic or idiopathic. Pharmacologic agents implicated in retrograde ejaculation include neuroleptics, tricyclic antidepressants, alpha-blockers used in the treatment of prostatism and certain antihypertensives (Hendry 1998; Debruyne 2000; Schuster and Ohl 2002). The diagnosis of retrograde ejaculation is made by examining the post-ejaculate urine for sperm. Although exact criteria have not been established for a positive post-ejaculate urinalysis, the finding of greater than 10 to 15 sperm per high-power field confirms the presence of retrograde ejaculation. In contrast, sperm will not be present in the urine of a patient with failure of emission, which must be diagnosed by clinical suspicion.
In the treatment of retrograde ejaculation, an initial trial of pharmacologic therapy is likely to be effective only in patients who do not have bladder neck abnormalities caused by surgery and in patients with failure of emission. The agents commonly utilized are alpha-adrenergic agonists such as ephedrine sulfate (25 to 50 mg q.i.d.), pseudoephedrine (60 mg q.i.d.), and imipramine (25 mg b.i.d.). The drugs may induce ejaculation secondary to an increase in the sympathetic tone of the internal sphincter and vas deferens. Medical therapy for ejaculatory dysfunction is administered on a cyclical basis timed to the female partner’s ovulatory cycle. These medications are more effective if given for a period of at least 7 to 10 days before planned ejaculation, and tolerance may develop if they are administered continuously over several cycles. However, success is unlikely if no effect is observed within 2 weeks of treatment.
If medical therapy fails to restore normal ejaculation, spermatozoa may be retrieved from the postejaculatory urine before intrauterine insemination (Shangold et al 1990). Urine may damage spermatozoa because of its acidity, changes in osmolarity or contamination (Crich and Jequier 1978). Several methods to circumvent these problems have been proposed, including neutralizing the urine pH with oral bicarbonate (650 mg q.i.d. for 2 days) and hydrating the patient before sperm collection. Subsequent to ejaculation, urine is voided and processed for insemination. A more invasive method involves catheterizing the bladder with 30 cc of a buffered medium, which is discarded, and then instilling an additional 30 cc (Suominen et al 1991). After ejaculation, the patient voids or is catheterized to retrieve the specimen. This procedure is also timed to coincide with ovulation in the female partner.
Reactive oxygen species
Spermatozoa produce a small amount of ROS, which is necessary for normal physiologic cell function such as capacitation, hyperactivation and sperm-oocyte fusion (Sies 1993; Lewis et al 1995). Elevated ROS levels have been recognized as an independent marker of male factor infertility, irrespective of whether patients have normal or abnormal semen parameters (Agarwal et al 2006). Although the body employs a number of mechanisms to minimize ROS-induced damage, antioxidants in seminal plasma are the most important form of protection that sperm have against ROS insult (Agarwal and Prabakaran 2005). These findings form the basis for the use of oral antioxidants as supplements to decrease oxidative stress and improve fertility.
The seminal plasma contains two different types of anti-oxidants to minimize free radical-induced damage: enzymatic and non-enzymatic antioxidants. The antioxidant protection mechanisms comprise three levels of defense: prevention, interception and repair. Avoidance of ROS formation is the first line of defense against an oxidative insult. As an example, the binding of metal ions, particularly iron and copper ions, prevents them from initiating a chain reaction (Sies 1993). Once transition metals become freely bound to ROS, they can generate more reactive oxidants, mainly OH− (Halliwell 1990). Free radicals have a predisposition toward activating a chain reaction. The interruption of this reaction to avoid further injury is the process of deactivation, which leads to a nonradical end product formation (Sies 1993). Alpha-tocopherol, a chain-breaking antioxidant, restrains lipid peroxidation by scavenging peroxyl (RO−) and alkoxyl (ROO−radicals. The capability of α-tocopherol to preserve a steady-state rate of peroxyl radical decline in the plasma membrane depends on the recycling of α-tocopherol by external reducing agents such as ascorbate or thiols. In this way, α-tocopherol is capable of functioning as a free radical chain-breaking antioxidant even if its concentration is low (Buettner 1993). In most cases, free-radical induced damage can be repaired. However, spermatozoa plasma membranes contain large quantities of polyunsaturated fatty acids and their cytoplasm contains low concentrations of scavenging enzymes. As a result, spermatozoa are unable to repair damage caused by excessive ROS (Halliwell 1990; Buettner 1993; Ochsendorf 1999; Irshad and Chaudhuri 2002). The pathological levels of ROS detected in the semen of infertile men are more likely to be caused by increased ROS production than by reduced antioxidant capacity of the seminal plasma (Lewis et al 1995).
Current studies report the detection of increased ROS levels in the semen of 25% to 40% of infertile men (de Lamirande and Gagnon 1995; Padron et al 1997). Men classified as having idiopathic infertility usually present with higher seminal ROS levels and lower antioxidant properties than healthy controls (Pasqualotto et al 2001). Given the major role that oxidative stress plays in the pathogenesis of male infertility, reducing seminal oxidative stress levels is necessary for natural as well as assisted reproductive technologies (Agarwal et al 2005). Various clinical trials have demonstrated the beneficial effects of antioxidants in selected cases of male infertility (Lenzi et al 1993, 1994; Kodama et al 1997; Okada et al 1997; Comhaire et al 2000; Vicari and Calogero 2001), whereas others have failed to report similar benefits (Abel et al 1982; Kessopoulou et al 1995; Rolf et al 1999). Pregnancy, the most relevant outcome parameter of fertility, was reported in only a few of these studies (Suleiman et al 1996; Comhaire et al 2000; Vicari and Calogero 2001; Vicari et al 2002; Lenzi et al 2003).
The majority of studies analyzed multiple antioxidant combinations, different doses and durations. Patient selection must also be considered in these studies since oxidative stress may not be the cause of male infertility in all selected patients. Recently, Agarwal et al (2004), in an extensive review of the literature, concluded that the studies suffer from the lack of a placebo-controlled, double-blind design. Without such a study design, the effectiveness of antioxidant supplementation in infertile patients remains inconclusive.
Considering the etiology of infertility in various patients, antioxidants may not be effective (Agarwal et al 2004). Therapeutics directed against each specific etiological cause of elevated ROS should be attempted. Once the primary cause of infertility has been treated, or if no specific etiology can be identified, patients may be advised to take antioxidant supplements.
Nonspecific treatment
Gonadotropin releasing hormone therapy
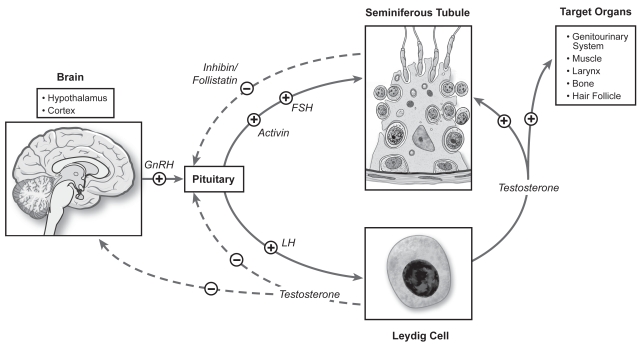
Hormone therapies manipulate the hypothalamic pituitary gonadal (HPG) axis in order to enhance sperm production. The axis is a complex, tightly regulated system of positive and negative feedback loops at numerous levels (Figure 1). However, hormonal manipulations of the normal HPG axis are not very successful due to homeostatic mechanisms that counteract the superimposed perturbation. A possible explanation for any beneficial effects on spermatogenesis of empirical therapy may be due to the presence of a previous “subclinical endocrinopathy.”
Figure 1.
Hypothalamic pituitary gonadal axis.
Exogenous GnRH administration can increase gonadotropin production and, potentially, spermatogenesis. GnRH seems to be a more physiologic agent for increasing the pituitary’s production of FSH and LH, especially when it is administered in a pulsatile fashion in programmable, portable minipumps. Although GnRH therapy is effective in the treatment of patients with HGH, two controlled studies failed to find efficacy of pulsatile GnRH treatment for idiopathic oligo-astheno-teratozoospermia (Badenoch et al 1988; Crottaz et al 1992). The pregnancy rates were similar to those of the controls with no seminal improvement. The lack of controlled studies indicating actual treatment benefits and the high cost of this therapy discourage its routine clinical use of GnRH for idiopathic oligozoospermia.
Gonadotropins
The two gonadotropins, FSH and LH, stimulate spermatogenesis and steroidogenesis, respectively. Exogenous gonadotropin treatments include the use of hCG and hMG. hCG is analogous to LH, and it stimulates the Leydig cell secretion of both testosterone and estradiol and inhibits FSH due to negative feed back. hMG has both LH and FSH activity. The reason for gonadotropin administration in idiopathic oligozoospermia is based on observed efficacy in the treatment of HGH. However, their effectiveness for treating normogonadotropic oligospermia, either alone or in combination, is less clear (Siddiq and Sigman 2002).
Two randomized controlled studies have examined the effect of FSH stimulation alone using either purified hMG (Matorras et al 1997) or recombinant human FSH (Kamischke et al 1998). Neither reported improved pregnancy rates with FSH, although one claimed a benefit from a post-hoc analysis for a selected subpopulation (Matorras et al 1997). Numerous uncontrolled studies have been performed with hCG, but few have assessed the use of hMG in men with idiopathic oligospermia (Siddiq and Sigman 2002). These studies have revealed limited efficacy (Siddiq and Sigman 2002), whereas the only available randomized double-blind, placebo-controlled, crossover study of hCG/hMG treatment of normogonadotropic men with idiopathic oligoasthenoteratospermia failed to demonstrate any beneficial effect on semen parameters or pregnancy rates (Knuth et al 1987). Although the treatment is safe, the side effects include libido changes and acne. As with GnRH, these treatments are very expensive, and given the lack of convincing outcomes with controlled studies, this treatment is not routinely recommended in men without a demonstrable hormonal abnormality. Further research to examine its impact on pregnancy rates in carefully selected infertile men, possibly in combination with ART, is warranted (Liu and Handelsman 2003).
Antiestrogens
Antiestrogens are the most commonly used therapy for idiopathic infertility. The antiestrogens indirectly stimulate the secretion of FSH and LH by blocking estrogen and estosterone receptors in the hypothalamus, which increases the release of GnRH. Two non-steroidal antiestrogens, clomiphene and tamoxifen, have been evaluated for empirical treatment of idiopathic male infertility.
Clomiphene is a synthetic, nonsteroidal drug that is similar in structure to diethylstilbestrol. Although it has a mild estrogenic effect, it functions predominantly as an antiestrogen. Clomiphene citrate is normally prescribed in a 25-mg daily oral dose. Drug doses generally range from 12.5 to 400 mg/day; however higher doses may cause down-regulation of the system (Heller et al 1969). Men treated with clomiphene citrate consistently demonstrate an elevation in serum FSH, LH and testosterone levels. As a result, serum gonadotropins and testosterone must be monitored to ensure that the testosterone level remains within normal limits, because higher levels may negatively influence spermatogenesis. In addition, patients should be cautioned that a small number of patients have suffered a deterioration in semen quality with antiestrogen therapy. Therefore, frequent semen analysis is essential during follow-up (Gilbaugh and Lipshultz 1994). Side effects of clomiphene therapy are usually mild and occur in less than 5% of patients (Siddiq and Sigman 2002). They include nausea, headache, weight gain, alterations in libido, visual field changes, dizziness, gynecomastia and allergic dermatitis.
Many well-designed prospective, randomized, controlled studies of clomiphene citrate failed to identify any efficacy over placebo (Foss et al 1973; Paulson 1979; Ronnberg 1980; Abel et al 1982; Sokol et al 1988). Only two studies revealed a positive effect on both sperm counts and pregnancy rates (Wang et al 1983; Check et al 1989). However, a multicenter WHO study of 190 couples randomized to receive 25 mg clomiphene daily or placebo showed only an 8% pregnancy rate in the treatment arm (WHO 1992).
Tamoxifen citrate is an antiestrogen that exhibits less estrogenic activity than clomiphene citrate and has been used in the treatment of male infertility. Doses range from 10 to 30 mg orally per day. Side effects are similar to those seen with clomiphene citrate but occur with lower frequency because of its weaker estrogenic properties. Although initial uncontrolled studies reported impressive results, including increased sperm densities and pregnancy rates (Vermeulen and Comhaire 1978; Bartsch and Scheiber 1981; Buvat et al 1983), all controlled studies using tamoxifen 10 to 20 mg per day reported negative results (Willis et al 1977; AinMelk et al 1987; Krause et al 1992).
Many trials have reported improvements in all semen parameters and pregnancy rates with the use of antiestrogens in men with idiopathic infertility (Allag and Alexander 1979; Bartsch and Scheiber 1981; Wang et al 1983; Check et al 1989). Given the conflicting data in well-controlled studies, meta-analyses have been performed to resolve these differing conclusions. Kamischke and Nieschlag performed a meta-analysis of antiestrogen therapy (clomiphene and tamoxifen) that included randomized, placebo-controlled trials, and they concluded that treatment with antiestrogens had no significant influence on pregnancy rates in the 459 patients analyzed (odds ratio, 1.33; 95% confidence interval, 0.78–2.28) (Kamischke and Nieschlag 1999). More recently, a Cochrane database review assessed ten studies involving 738 men with idiopathic infertility in which antiestrogen therapy was administered for at least 3 months (Vandekerckhove et al 2000). Only 5 trials specified the randomization protocol. In these studies, the overall analysis showed improved testosterone, but the pregnancy rate was no better than that of the controls.
Antiestrogens are reasonably inexpensive and safe oral medications for the treatment for idiopathic male infertility, which explains their popularity. Nevertheless, their efficacy is in doubt, and prolonged courses of empirical antiestrogen therapy should not be used as a substitute for more effective modes of management.
Aromatase inhibitors
The majority of estrogen production occurs within fat cells, where the enzyme aromatase converts circulating testosterone into estrogen. Hence, markedly obese men may have an excessive endogenous conversion of testosterone into estrogen. In theory, an alteration in the ratios of estrogen and testosterone systemically or within the testis could decrease pituitary levels of LH and FSH and impair sperm production (Kulin and Reiter 1972; Veldhuis et al 1985). Aromatase inhibitors block the conversion of testosterone to estrogen, thereby enhancing spermatogenesis (Ciaccio et al 1978). Additionally, aromatase inhibitors block the inhibitory feedback of testosterone on the hypothalamic pituitary gonadal axis by reducing the amount of testosterone that is converted to the more potent inhibitory signal, estrogen. In the testis, aromatase activity is primarily located in the Leydig and Sertoli cells (Inkster et al 1995).
Aromatase inhibitors are relatively expensive pharmaceutical agents and may be steroidal (testolactoma) or nonsteroidal (anastrozole, letrozole and exemestane). Anastrazole represents the fourth generation of aromatase inhibitors. Although highly potent and specific for the aromatase enzyme, it differs from earlier steroid-based inhibitors in that it is less likely to exhibit agonist or antagonist steroidal properties. The drug is safe and well-tolerated and can be administered orally in men with idiopathic oligozoospermia. One indication for treatment is an abnormal testosterone/estrogen ratio. However, normal ratio ranges have not yet been standardized. Serum testosterone, estrogen concentrations and seminal parameters are followed at regular intervals. In addition, serum liver function tests should be performed because transaminase elevations are common but tend to resolve after therapy is stopped (Siddiq and Sigman 2002).
In older studies, treatment with testosterone aromatase inhibitors produced conflicting results (Haidl and Schill 1991). Most recently, it was shown that in men who are infertile with a low serum testosterone-to-estradiol ratio, treatment with the aromatase inhibitor testolactone 50 to 100 mg twice daily significantly increased sperm count and motility and corrected the hormonal abnormality (Pavlovich et al 2001; Raman and Schlegel 2002). Similar changes were seen after treatment with the more selective aromatase inhibitor anastrozole 1 mg/day (Raman and Schlegel 2002). Aromatase inhibitors may be useful in a subpopulation of subfertile men, especially in those with subnormal testosterone and high estradiol levels. However, the testosterone-to-estradiol ratio remains to be defined in men with normogonadotropic idiopathic infertility. Placebo-controlled, randomized studies are still needed to assess definitively the effect of aromatase inhibitors in patients with idiopathic male infertility.
Miscellaneous treatments
Many nonhormonal treatments for idiopathic infertility are currently being evaluated. Some nonhormonal therapies improve sperm quality by boosting the kallifrein-kinin system or by interfering with the production of prostaglandins. Another growing area of interest centers on the use of anti-oxidants to scavenge excessive seminal ROS, which may be causing direct spermatozoa damage.
A variety of vitamins, nutritional supplements and anti-inflammatory agents have been used in the empirical therapy of male infertility. Thyroxine, arginine, corticosteroids, antibiotics, zinc, methylxanthines, bromocriptine and vitamins A, E and C have all been shown to be of little or no benefit in the treatment of male infertility without evidence of a specific deficiency (Siddiq and Sigman 2002). Controlled studies of kallikrein, indomethacin and glutathione have produced varying results, none of which are sufficient to encourage their use (Barkay et al 1984; Glezerman et al 1993; Lenzi et al 1993).
L-Carnitine is a known component of epididymal secretions and is now available as an over-the-counter nutritional supplement for the treatment of idiopathic male infertility. In human seminal fluid, approximately 50% of total carnitine exists as acetyl-carnitine. The compound plays a critical role in intracellular energy metabolism as well as spermatozoa membrane stabilization. Carnitine also has an antioxidant capacity, and it protects sperm from oxidative damage (Agarwal and Said 2004). However, studies have not shown a direct relationship between semen L-carnitine levels and fertility or that orally administered carnitine increases levels within the epididymis (Soufir et al 1984). Uncontrolled studies demonstrate improvement in semen parameters but not fertility (Costa et al 1994; Vitali et al 1995). Two recent randomized, controlled trials of carnitine and acetyl carnitine for idiopathic infertility (Lenzi et al 2003, 2004) reported statistically significant improvements in seminal parameters, but they have certain drawbacks. Carnitine levels in semen did not change despite therapy. Reported pregnancy rates were only 8% (Lenzi et al 2003) and 13% (Lenzi et al 2004). Although improvements in the motile sperm count were statistically significant, they may not be clinically relevant as it was only 9 million per ml in the treated group and 7.4 million per ml in the controls. At this time, there is little evidence that carnitine therapy has any clinical benefit. Thus, the use of carnitine supplementation in idiopathic male infertility remains questionable.
Take home message
Numerous advances have been made in reproductive medicine in the last few years. Infertile couples who previously were considered untreatable now have a chance at genetic paternity. ART provide a great opportunity to families with infertility, and their used has become routine in the treatment of infertile couples. The increasing use of ICSI as an efficient therapy for cases of male infertility has become an applicable means to overcome multiple sperm deficiencies. Even men with potentially treatable causes of infertility can be treated with ART instead of a specific therapy. However, the potential medical risks such as those of multiple-gestation pregnancies and the associated costs cannot be ignored. Primarily, specific therapeutic therapy directed against the etiological cause of infertility should be attempted. Specific medical management of infertility is based on identifying reversible causes of infertility and treating them with appropriate medications. However, if no specific etiology can be identified, empiric therapy can be introduced in an attempt to improve semen parameters and subsequent fertility potential through natural intercourse. It is mandatory that a treatment timeline and endpoints be established prior to the initiation of empiric therapy. Many of the empiric therapies do hold potential benefit, and as greater understanding is gained of what is now considered idiopathic infertility, a more specific application of these therapies may yield more successful results.
References
- Abel BJ, Carswell G, Elton R, et al. Randomised trial of clomiphene citrate treatment and vitamin C for male infertility. Br J Urol. 1982;54:780–4. doi: 10.1111/j.1464-410x.1982.tb13647.x. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Nallella KP, Allamaneni SS, et al. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963–74. [PubMed] [Google Scholar]
- Agarwal A, Prabakaran SA, Said TM. Prevention of oxidative stress injury to sperm. J Androl. 2005;26:654–60. doi: 10.2164/jandrol.05016. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Said TM. Carnitines and male infertility. Reprod Biomed Online. 2004;8:376–84. doi: 10.1016/s1472-6483(10)60920-0. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Sharma RK, Nallella KP, et al. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–85. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- AinMelk Y, Belisle S, Carmel M, et al. Tamoxifen citrate therapy in male infertility. Fertil Steril. 1987;48:113–17. doi: 10.1016/s0015-0282(16)59299-1. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Buckingham D, West K, et al. Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. J Reprod Fertil. 1992;94:451–62. doi: 10.1530/jrf.0.0940451. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, West K, Buckingham D. Leukocytic infiltration into the human ejaculate and its association with semen quality, oxidative stress, and sperm function. J Androl. 1994;15:343–52. [PubMed] [Google Scholar]
- Allag IS, Alexander NJ. Clomiphene citrate therapy for male infertility. Urology. 1979;14:500–3. doi: 10.1016/0090-4295(79)90184-5. [DOI] [PubMed] [Google Scholar]
- Arap MA, Vicentini FC, Cocuzza M, et al. Late hormonal levels, semen parameters and presence of antisperm antibodies in patients treated for testicular torsion. J Androl. 2007 doi: 10.2164/jandrol.106.002097. [DOI] [PubMed] [Google Scholar]
- Athayde KS, Cocuzza M, Agarwal A, et al. Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl. 2007 doi: 10.2164/jandrol.106.001966. [DOI] [PubMed] [Google Scholar]
- Badenoch DF, Waxman J, Boorman L, et al. Administration of a gonadotropin releasing hormone analogue in oligozoospermic infertile males. Acta Endocrinol Copenh. 1988;117:265–7. doi: 10.1530/acta.0.1170265. [DOI] [PubMed] [Google Scholar]
- Barkay J, Harpaz-Kerpel S, Ben-Ezra S, et al. The prostaglandin inhibitor effect of antiinflammatory drugs in the therapy of male infertility. Fertil Steril. 1984;42:406–11. doi: 10.1016/s0015-0282(16)48081-7. [DOI] [PubMed] [Google Scholar]
- Bartsch G, Scheiber K. Tamoxifen treatment in oligozoospermia. Eur Urol. 1981;7:283–7. doi: 10.1159/000473241. [DOI] [PubMed] [Google Scholar]
- Berger RE, Karp LE, Williamson RA, et al. The relationship of pyospermia and seminal fluid bacteriology to sperm function as reflected in the sperm penetration assay. Fertil Steril. 1982;37:557–64. doi: 10.1016/s0015-0282(16)46166-2. [DOI] [PubMed] [Google Scholar]
- Broderick GA, Tom R, McClure RD. Immunological status of patients before and after vasovasostomy as determined by the immunobead antisperm antibody test. J Urol. 1989;142:752–5. doi: 10.1016/s0022-5347(17)38877-8. [DOI] [PubMed] [Google Scholar]
- Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- Burris AS, Clark RV, Vantman DJ, et al. A low sperm concentration does not preclude fertility in men with isolated hypogonadotropic hypogonadism after gonadotropin therapy. Fertil Steril. 1988;50:343–7. doi: 10.1016/s0015-0282(16)60084-5. [DOI] [PubMed] [Google Scholar]
- Burrows PJ, Schrepferman CG, Lipshultz LI. Comprehensive office evaluation in the new millennium. Urol Clin North Am. 2002;29:873–94. doi: 10.1016/s0094-0143(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Buvat J, Ardaens K, Lemaire A, et al. Increased sperm count in 25 cases of idiopathic normogonadotropic oligospermia following treatment with tamoxifen. Fertil Steril. 1983;39:700–3. doi: 10.1016/s0015-0282(16)47069-x. [DOI] [PubMed] [Google Scholar]
- Check JH, Chase JS, Nowroozi K, et al. Empirical therapy of the male with clomiphene in couples with unexplained infertility. Int J Fertil. 1989;34:120–2. [PubMed] [Google Scholar]
- Check ML, Check JH, Katsoff D, et al. ICSI as an effective therapy for male factor with antisperm antibodies. Arch Androl. 2000;45:125–30. doi: 10.1080/01485010050193887. [DOI] [PubMed] [Google Scholar]
- Chuang AT, Howards SS. Male infertility. Evaluation and nonsurgical therapy. Urol Clin North Am. 1998;25:703–13. doi: 10.1016/s0094-0143(05)70058-6. [DOI] [PubMed] [Google Scholar]
- Ciaccio LA, Joseph AA, Kincl FA. Direct inhibition of testicular function in rats by estriol and progesterone. J Steroid Biochem. 1978;9:1257–9. doi: 10.1016/0022-4731(78)90022-5. [DOI] [PubMed] [Google Scholar]
- Clarke GN, Bourne H, Baker HW. Intracytoplasmic sperm injection for treating infertility associated with sperm autoimmunity. Fertil Steril. 1997;68:112–17. doi: 10.1016/s0015-0282(97)81485-9. [DOI] [PubMed] [Google Scholar]
- Comhaire FH, Christophe AB, Zalata AA, et al. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids. 2000;63:159–65. doi: 10.1054/plef.2000.0174. [DOI] [PubMed] [Google Scholar]
- Comhaire FH, Rowe PJ, Farley TM. The effect of doxycycline in infertile couples with male accessory gland infection: a double blind prospective study. Int J Androl. 1986;9:91–8. doi: 10.1111/j.1365-2605.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Conte D, Romanelli F, Isidori A. Treatment of male idiopathic sterility with gonadotropin. Minerva Endocrinol. 1990;15:91–4. [PubMed] [Google Scholar]
- Costa M, Canale D, Filicori M, et al. L-carnitine in idiopathic asthenozoospermia: a multicenter study Italian Study Group on Carnitine and Male Infertility. Andrologia. 1994;26:155–9. [PubMed] [Google Scholar]
- Crich JP, Jequier AM. Infertility in men with retrograde ejaculation: the action of urine on sperm motility, and a simple method for achieving antegrade ejaculation. Fertil Steril. 1978;30:572–6. doi: 10.1016/s0015-0282(16)43640-x. [DOI] [PubMed] [Google Scholar]
- Crottaz B, Senn A, Reymond MJ, et al. Follicle-stimulating hormone bioactivity in idiopathic normogonadotropic oligoasthenozoospermia: double-blind trial with gonadotropin-releasing hormone. Fertil Steril. 1992;57:1034–43. doi: 10.1016/s0015-0282(16)55022-5. [DOI] [PubMed] [Google Scholar]
- Crowley WF, Jr, Filicori M, Spratt DI, et al. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473–531. doi: 10.1016/b978-0-12-571141-8.50015-9. [DOI] [PubMed] [Google Scholar]
- Crowley WF, Whitcomb RW. Gonadotropin-releasing hormone deficiency in men: diagnosis and treatment with exogenous gonadotropin-releasing hormone. Am J Obstet Gynecol. 1990;163:1752–8. doi: 10.1016/0002-9378(90)91440-n. [DOI] [PubMed] [Google Scholar]
- Cunningham GR, Lipshultz LI. Diseases of the testes and male sex organs. In: Kohler PO, editor. Basic clinical endocrinology. New York: John Wiley and Sons; 1986. pp. 263–78. [Google Scholar]
- De Almeida M, Soufir JC. Corticosteroid therapy for male autoimmune infertility. Lancet. 1977;2:815–16. doi: 10.1016/s0140-6736(77)90742-5. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod. 1995;10(Suppl 1):15–21. doi: 10.1093/humrep/10.suppl_1.15. [DOI] [PubMed] [Google Scholar]
- Debruyne FM. Alpha blockers: are all created equal. Urology. 2000;56:20–2. doi: 10.1016/s0090-4295(00)00744-5. [DOI] [PubMed] [Google Scholar]
- Dollar JR, Blackwell RE. Diagnosis and management of prolactinomas. Cancer Metastasis Rev. 1986;5:125–38. doi: 10.1007/BF00046427. [DOI] [PubMed] [Google Scholar]
- Dondero F, Lenzi A, Gandini L, et al. Immunological infertility in humans. Exp Clin Immunogenet. 1993;10:65–72. [PubMed] [Google Scholar]
- Eggert-Kruse W, Schwalbach B, Gerhard I, et al. Influence of serum prolactin on semen characteristics and sperm function. Int J Fertil. 1991;36:243–51. [PubMed] [Google Scholar]
- el-Demiry MI, Young H, Elton RA, et al. Leucocytes in the ejaculate from fertile and infertile men. Br J Urol. 1986;58:715–20. doi: 10.1111/j.1464-410x.1986.tb05919.x. [DOI] [PubMed] [Google Scholar]
- Foss GL, Tindall VR, Birkett JP. The treatment of subfertile men with clomiphene citrate. J Reprod Fertil. 1973;32:167–70. doi: 10.1530/jrf.0.0320167. [DOI] [PubMed] [Google Scholar]
- Gilbaugh JH, 3rd, Lipshultz LI. Nonsurgical treatment of male infertility. An update. Urol Clin North Am. 1994;21:531–48. [PubMed] [Google Scholar]
- Glezerman M, Lunenfeld E, Potashnik G, et al. Efficacy of kallikrein in the treatment of oligozoospermia and asthenozoospermia: a double-blind trial. Fertil Steril. 1993;60:1052–6. [PubMed] [Google Scholar]
- Greenberg SH, Lipshultz LI, Wein AJ. Experience with 425 subfertile male patients. J Urol. 1978;119:507–10. doi: 10.1016/s0022-5347(17)57531-x. [DOI] [PubMed] [Google Scholar]
- Haas GG., Jr The inhibitory effect of sperm-associated immunoglobulins on cervical mucus penetration. Fertil Steril. 1986;46:334–7. doi: 10.1016/s0015-0282(16)49538-5. [DOI] [PubMed] [Google Scholar]
- Haidl G. Management strategies for male factor infertility. Drugs. 2002;62:1741–53. doi: 10.2165/00003495-200262120-00004. [DOI] [PubMed] [Google Scholar]
- Haidl G, Schill WB. Guidelines for drug treatment of male infertility. Drugs. 1991;41:60–8. doi: 10.2165/00003495-199141010-00006. [DOI] [PubMed] [Google Scholar]
- Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- Heller CG, Rowley MJ, Heller GV. Clomiphene citrate: a correlation of its effect on sperm concentration and morphology, total gonadotropins, ICSH, estrogen and testosterone excretion, and testicular cytology in normal men. J Clin Endocrinol Metab. 1969;29:638–49. doi: 10.1210/jcem-29-5-638. [DOI] [PubMed] [Google Scholar]
- Hendry WF. Disorders of ejaculation: congenital, acquired and functional. Br J Urol. 1998;82:331–41. doi: 10.1046/j.1464-410x.1998.00758.x. [DOI] [PubMed] [Google Scholar]
- Hendry WF, Hughes L, Scammell G, et al. Comparison of prednisolone and placebo in subfertile men with antibodies to spermatozoa. Lancet. 1990;335:85–8. doi: 10.1016/0140-6736(90)90548-j. [DOI] [PubMed] [Google Scholar]
- Hendry WF, Treehuba K, Hughes L, et al. Cyclic prednisolone therapy for male infertility associated with autoantibodies to spermatozoa. Fertil Steril. 1986;45:249–54. doi: 10.1016/s0015-0282(16)49163-6. [DOI] [PubMed] [Google Scholar]
- Henkel R, Kierspel E, Stalf T, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–42. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Henkel R, Maass G, Hajimohammad M, et al. Urogenital inflammation: changes of leucocytes and ROS. Andrologia. 2003;35:309–13. [PubMed] [Google Scholar]
- Inkster S, Yue W, Brodie A. Human testicular aromatase: immunocytochemical and biochemical studies. J Clin Endocrinol Metab. 1995;80:1941–7. doi: 10.1210/jcem.80.6.7539819. [DOI] [PubMed] [Google Scholar]
- Irshad M, Chaudhuri PS. Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol. 2002;40:1233–9. [PubMed] [Google Scholar]
- Jane JA, Jr, Laws ER., Jr The surgical management of pituitary adenomas in a series of 3,093 patients. J Am Coll Surg. 2001;193:651–9. doi: 10.1016/s1072-7515(01)01101-2. [DOI] [PubMed] [Google Scholar]
- Jennings MG, McGowan MP, Baker HW. Is conventional bacteriology useful in the management of male infertility. Clin Reprod Fertil. 1986;4:359–66. [PubMed] [Google Scholar]
- Kamischke A, Behre HM, Bergmann M, et al. Recombinant human follicle stimulating hormone for treatment of male idiopathic infertility: a randomized, double-blind, placebo-controlled, clinical trial. Hum Reprod. 1998;13:596–603. doi: 10.1093/humrep/13.3.596. [DOI] [PubMed] [Google Scholar]
- Kamischke A, Nieschlag E. Analysis of medical treatment of male infertility. Hum Reprod. 1999;14(Suppl 1):1–23. doi: 10.1093/humrep/14.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- Kessopoulou E, Powers HJ, Sharma KK, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64:825–31. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- Klingmuller D, Schweikert HU. Maintenance of spermatogenesis by intranasal administration of gonadotropin-releasing hormone in patients with hypothalamic hypogonadism. J Clin Endocrinol Metab. 1985;61:868–72. doi: 10.1210/jcem-61-5-868. [DOI] [PubMed] [Google Scholar]
- Knuth UA, Honigl W, Bals-Pratsch M, et al. Treatment of severe oligospermia with human chorionic gonadotropin/human menopausal gonadotropin: a placebo-controlled, double blind trial. J Clin Endocrinol Metab. 1987;65:1081–7. doi: 10.1210/jcem-65-6-1081. [DOI] [PubMed] [Google Scholar]
- Kodama H, Yamaguchi R, Fukuda J, et al. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997;68:519–24. doi: 10.1016/s0015-0282(97)00236-7. [DOI] [PubMed] [Google Scholar]
- Koide SS, Wang L, Kamada M. Antisperm antibodies associated with infertility: properties and encoding genes of target antigens. Proc Soc Exp Biol Med. 2000;224:123–32. doi: 10.1046/j.1525-1373.2000.22410.x. [DOI] [PubMed] [Google Scholar]
- Krause W, Holland-Moritz H, Schramm P. Treatment of idiopathic oligozoospermia with tamoxifen – a randomized controlled study. Int J Androl. 1992;15:14–18. doi: 10.1111/j.1365-2605.1992.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Kulin HE, Reiter EO. Gonadotropin suppression by low dose estrogen in men: evidence for differential effects upon FSH and LH. J Clin Endocrinol Metab. 1972;35:836–9. doi: 10.1210/jcem-35-6-836. [DOI] [PubMed] [Google Scholar]
- Lenzi A, Culasso F, Gandini L, et al. Placebo-controlled, double-blind, cross-over trial of glutathione therapy in male infertility. Hum Reprod. 1993;8:1657–62. doi: 10.1093/oxfordjournals.humrep.a137909. [DOI] [PubMed] [Google Scholar]
- Lenzi A, Lombardo F, Sgro P, et al. Use of carnitine therapy in selected cases of male factor infertility: a double-blind crossover trial. Fertil Steril. 2003;79:292–300. doi: 10.1016/s0015-0282(02)04679-4. [DOI] [PubMed] [Google Scholar]
- Lenzi A, Picardo M, Gandini L, et al. Glutathione treatment of dyspermia: effect on the lipoperoxidation process. Hum Reprod. 1994;9:2044–50. doi: 10.1093/oxfordjournals.humrep.a138391. [DOI] [PubMed] [Google Scholar]
- Lenzi A, Sgro P, Salacone P, et al. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril. 2004;81:1578–84. doi: 10.1016/j.fertnstert.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Boyle PM, McKinney KA, et al. Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril. 1995;64:868–70. doi: 10.1016/s0015-0282(16)57870-4. [DOI] [PubMed] [Google Scholar]
- Liu PY, Handelsman DJ. The present and future state of hormonal treatment for male infertility. Hum Reprod Update. 2003;9:9–23. doi: 10.1093/humupd/dmg002. [DOI] [PubMed] [Google Scholar]
- Mancini A, Guitelman A, Levalle O, et al. Bromocriptine in the management of infertile men after surgery of prolactin secreting adenomas. J Androl. 1984;5:294–6. doi: 10.1002/j.1939-4640.1984.tb00791.x. [DOI] [PubMed] [Google Scholar]
- March MR, Isidori A. New frontiers in the treatment of male sterility. Contraception. 2002;65:279–81. doi: 10.1016/s0010-7824(02)00296-2. [DOI] [PubMed] [Google Scholar]
- Marshburn PB, Kutteh WH. The role of antisperm antibodies in infertility. Fertil Steril. 1994;61:799–811. doi: 10.1016/s0015-0282(16)56687-4. [DOI] [PubMed] [Google Scholar]
- Maruyama DK, Jr, Hale RW, Rogers BJ. Effects of white blood cells on the in vitro penetration of zona-free hamster eggs by human spermatozoa. J Androl. 1985;6:127–35. doi: 10.1002/j.1939-4640.1985.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Matorras R, Perez C, Corcostegui B, et al. Treatment of the male with follicle-stimulating hormone in intrauterine insemination with husband’s spermatozoa: a randomized study. Hum Reprod. 1997;12:24–8. doi: 10.1093/humrep/12.1.24. [DOI] [PubMed] [Google Scholar]
- Molitch ME. Medical treatment of prolactinomas. Endocrinol Metab Clin North Am. 1999;28:143–69. vii. doi: 10.1016/s0889-8529(05)70061-x. [DOI] [PubMed] [Google Scholar]
- Mortimer CH, McNeilly AS, Fisher RA, et al. Gonadotrophin-releasing hormone therapy in hypogonadal males with hypothalamic or pituitary dysfunction. Br Med J. 1974;4:617–21. doi: 10.1136/bmj.4.5945.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsendorf FR. Infections in the male genital tract and reactive oxygen species. Hum Reprod Update. 1999;5:399–420. doi: 10.1093/humupd/5.5.399. [DOI] [PubMed] [Google Scholar]
- Okada H, Tatsumi N, Kanzaki M, et al. Formation of reactive oxygen species by spermatozoa from asthenospermic patients: response to treatment with pentoxifylline. J Urol. 1997;157:2140–6. [PubMed] [Google Scholar]
- Omu AE, al-Qattan F, Abdul Hamada B. Effect of low dose continuous corticosteroid therapy in men with antisperm antibodies on spermatozoal quality and conception rate. Eur J Obstet Gynecol Reprod Biol. 1996;69:129–34. doi: 10.1016/0301-2115(95)02539-1. [DOI] [PubMed] [Google Scholar]
- Padron OF, Brackett NL, Sharma RK, et al. Seminal reactive oxygen species and sperm motility and morphology in men with spinal cord injury. Fertil Steril. 1997;67:1115–20. doi: 10.1016/s0015-0282(97)81448-3. [DOI] [PubMed] [Google Scholar]
- Pasqualotto FF, Sharma RK, Kobayashi H, et al. Oxidative stress in normospermic men undergoing infertility evaluation. J Androl. 2001;22:316–22. [PubMed] [Google Scholar]
- Paulson DF. Cortisone acetate versus clomiphene citrate in per-germinal idiopathic oligospermia. J Urol. 1979;121:432–4. doi: 10.1016/s0022-5347(17)56813-5. [DOI] [PubMed] [Google Scholar]
- Pavlovich CP, King P, Goldstein M, et al. Evidence of a treatable endocrinopathy in infertile men. J Urol. 2001;165:837–41. [PubMed] [Google Scholar]
- Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167:624–9. doi: 10.1016/S0022-5347(01)69099-2. [DOI] [PubMed] [Google Scholar]
- Rolf C, Cooper TG, Yeung CH, et al. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod. 1999;14:1028–33. doi: 10.1093/humrep/14.4.1028. [DOI] [PubMed] [Google Scholar]
- Ronnberg L. The effect of clomiphene citrate on different sperm parameters and serum hormone levels in preselected infertile men: a controlled double-blind cross-over study. Int J Androl. 1980;3:479–86. doi: 10.1111/j.1365-2605.1980.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Rumke P, Hellinga G. Autoantibodies against spermatozoa in sterile men. Am J Clin Pathol. 1959;32:357–63. doi: 10.1093/ajcp/32.4.357. [DOI] [PubMed] [Google Scholar]
- Schuster TG, Ohl DA. Diagnosis and treatment of ejaculatory dysfunction. Urol Clin North Am. 2002;29:939–48. doi: 10.1016/s0094-0143(02)00080-0. [DOI] [PubMed] [Google Scholar]
- Shangold GA, Cantor B, Schreiber JR. Treatment of infertility due to retrograde ejaculation: a simple, cost-effective method. Fertil Steril. 1990;54:175–7. doi: 10.1016/s0015-0282(16)53660-7. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Pasqualotto AE, Nelson DR, et al. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J Androl. 2001;22:575–83. [PubMed] [Google Scholar]
- Shekarriz M, Sharma RK, Thomas AJ, Jr, et al. Positive myeloperoxidase staining (Endtz test) as an indicator of excessive reactive oxygen species formation in semen. J Assist Reprod Genet. 1995;12:70–4. doi: 10.1007/BF02211372. [DOI] [PubMed] [Google Scholar]
- Shin D, Honig SC. Economics of treatments for male infertility. Urol Clin North Am. 2002;29:841–53. doi: 10.1016/s0094-0143(02)00078-2. [DOI] [PubMed] [Google Scholar]
- Shin D, Palermo GD, Goldstein M, et al. Indications for corticosteroids prior to epididymal sperm retrieval. Int J Fertil Womens Med. 1998;43:165–70. [PubMed] [Google Scholar]
- Shulman JF, Shulman S. Methylprednisolone treatment of immunologic infertility in male. Fertil Steril. 1982;38:591–9. doi: 10.1016/s0015-0282(16)46640-9. [DOI] [PubMed] [Google Scholar]
- Siddiq FM, Sigman M. A new look at the medical management of infertility. Urol Clin North Am. 2002;29:949–63. doi: 10.1016/s0094-0143(02)00085-x. [DOI] [PubMed] [Google Scholar]
- Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–19. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- Sokol RZ, Steiner BS, Bustillo M, et al. A controlled comparison of the efficacy of clomiphene citrate in male infertility. Fertil Steril. 1988;49:865–70. doi: 10.1016/s0015-0282(16)59898-7. [DOI] [PubMed] [Google Scholar]
- Soufir JC, Ducot B, Marson J, et al. Levels of seminal free L(−) carnitine in fertile and infertile men. Int J Androl. 1984;7:188–97. doi: 10.1111/j.1365-2605.1984.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Suleiman SA, Ali ME, Zaki ZM, et al. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996;17:530–7. [PubMed] [Google Scholar]
- Suominen JJ, Kilkku PP, Taina EJ, et al. Successful treatment of infertility due to retrograde ejaculation by instillation of serum-containing medium into the bladder A case report. Int J Androl. 1991;14:87–90. doi: 10.1111/j.1365-2605.1991.tb01069.x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove P, Lilford R, Vail A, et al. Clomiphene or tamoxifen for idiopathic oligo/asthenospermia. Cochrane Database Syst Rev. 2000:CD000151. doi: 10.1002/14651858.CD000151. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Sowers JR, Rogol AD, et al. Pathophysiology of male hypogonadism associated with endogenous hyperestrogenism. Evidence for dual defects in the gonadal axis. N Engl J Med. 1985;312:1371–5. doi: 10.1056/NEJM198505233122107. [DOI] [PubMed] [Google Scholar]
- Verhelst J, Abs R, Maiter D, et al. Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab. 1999;84:2518–22. doi: 10.1210/jcem.84.7.5810. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Comhaire F. Hormonal effects of an antiestrogen, tamoxifen, in normal and oligospermic men. Fertil Steril. 1978;29:320–7. doi: 10.1016/s0015-0282(16)43160-2. [DOI] [PubMed] [Google Scholar]
- Vicari E, Calogero AE. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum Reprod. 2001;16:2338–42. doi: 10.1093/humrep/16.11.2338. [DOI] [PubMed] [Google Scholar]
- Vicari E, La Vignera S, Calogero AE. Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti-inflammatory compounds. Fertil Steril. 2002;78:1203–8. doi: 10.1016/s0015-0282(02)04350-9. [DOI] [PubMed] [Google Scholar]
- Vitali G, Parente R, Melotti C. Carnitine supplementation in human idiopathic asthenospermia: clinical results. Drugs Exp Clin Res. 1995;21:157–9. [PubMed] [Google Scholar]
- Wang C, Chan CW, Wong KK, et al. Comparison of the effectiveness of placebo, clomiphene citrate, mesterolone, pentoxifylline, and testosterone rebound therapy for the treatment of idiopathic oligospermia. Fertil Steril. 1983;40:358–65. doi: 10.1016/s0015-0282(16)47300-0. [DOI] [PubMed] [Google Scholar]
- Wang C, Lam KS, Ma JT, et al. Long-term treatment of hyperprolactinaemia with bromocriptine: effect of drug withdrawal. Clin Endocrinol (Oxf) 1987;27:363–71. doi: 10.1111/j.1365-2265.1987.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Weidner W. Which efforts towards conservative treatment of male infertility will be successful? Antibiotic therapy. Andrologia. 1999;31:297. doi: 10.1046/j.1439-0272.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- Weidner W, Krause W, Ludwig M. Relevance of male accessory gland infection for subsequent fertility with special focus on prostatitis. Hum Reprod Update. 1999;5:421–32. doi: 10.1093/humupd/5.5.421. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization. A double-blind trial of clomiphene citrate for the treatment of idiopathic male infertility World Health Organization. Int J Androl. 1992;15:299–307. doi: 10.1111/j.1365-2605.1992.tb01129.x. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization. WHO Laboratory manual for the examination of human semen and sperm-cervical mucus interaction . New York: Cambridge University Press; 1999. [Google Scholar]
- Willis KJ, London DR, Bevis MA, et al. Hormonal effects of tamoxifen in oligospermic men. J Endocrinol. 1977;73:171–8. doi: 10.1677/joe.0.0730171. [DOI] [PubMed] [Google Scholar]
- Wolff H. The biologic significance of white blood cells in semen. Fertil Steril. 1995;63:1143–57. doi: 10.1016/s0015-0282(16)57588-8. [DOI] [PubMed] [Google Scholar]
- Wolff H, Anderson DJ. Immunohistologic characterization and quantitation of leukocyte subpopulations in human semen. Fertil Steril. 1988;49:497–504. [PubMed] [Google Scholar]
- Wolff H, Politch JA, Martinez A, et al. Leukocytospermia is associated with poor semen quality. Fertil Steril. 1990;53:528–36. [PubMed] [Google Scholar]
- Yanushpolsky EH, Politch JA, Hill JA, et al. Antibiotic therapy and leukocytospermia: a prospective, randomized, controlled study. Fertil Steril. 1995;63:142–7. [PubMed] [Google Scholar]