Abstract
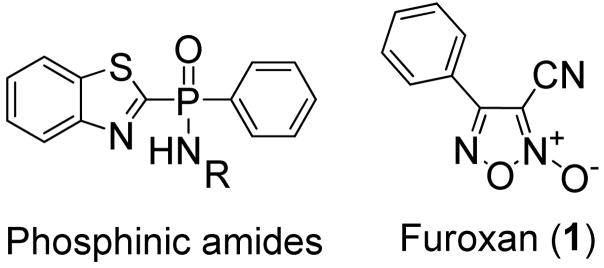
The synthesis of several 1,2,5-oxadiazole-2-oxide (Furoxan) analogues is described herein. These compounds were prepared in an effort to probe the SAR around the phenyl substituent and oxadiazole core for our studies toward thioredoxin-glutathione reductase (TGR) inhibition and anti-schistosomal activity.
As part of our ongoing interest in neglected diseases of third world countries, we recently reported the identification of several novel chemotypes1–2 (Figure 1) for the treatment of schistosomiasis. 3–4 Schistosomiasis is caused by five species of the trematode flatworm, two of which are Schistosoma mansoni (S. mansoni) and Schistosoma haetobium. It has been estimated that more than 200 million people are infected worldwide with an estimated 200,000–280,000 deaths occurring annually in sub-saharan Africa alone as a result of this disease.5 Current treatment of schistosomiasis relies primarily on the use of praziquantel (PZQ) because of its low cost and effectiveness against all schistosome species. However, given its widespread use, drug-resistant parasites may become more prevalent and PZQ-resistant isolates have already been identified.6–7
Figure 1.
Quantitative high-throughput screen (qHTS) hits.1
The majority of eukaryotes contain two native mechanisms by which they detoxify reactive oxygen species, the tripeptide glutathione (GSH) and the 12 kDa protein thioredoxin (Trx).8–9 These systems also play a critical role in several other cellular functions including cell proliferation, redox regulation of gene expression, and xenobiotic metabolism to name a few.9–10 Interestingly, Williams and co-workers found that in S. mansoni both TrxR and GR pathways are absent, and instead rely on a single multifunctional selenocysteine-containing flavoenzyme, thioredoxin-glutathione reductase (TGR).12 As a result, one may expect that inhibition of TGR would present a viable target for the treatment of S. mansoni parasites. Accordingly, we sought out and discovered numerous small molecules with potent in vitro inhibition of TGR, including the oxadiazole-2-oxide chemotype (Furoxan) (1).
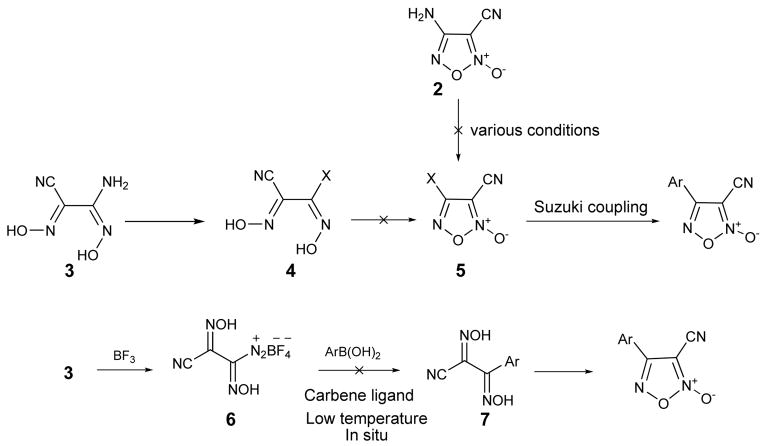
Our initial interest was to explore modification of the phenyl moiety of furoxan (1). Ideally we hoped to develop a convergent synthesis which would allow for late-stage installation of differentially substituted phenyl groups providing rapid access to a library of compounds. Unfortunately, as shown in Scheme 1, these attempts were met with limited success. Synthesis of amidoxime 3 did proceed as planned to haloglyoxime 4 using HBr and NaNO2,13 however this product proved unstable and thus could not be cyclized to halo-furoxan 5. Additionally, direct conversion of 4-amino-3-cyano furoxan (2) using various conditions to compound 5 also failed. Alternatively, in a one-pot procedure, amidoxime 3 was treated with BF3•OEt2 to form the diazonium ion 6 in situ which was then subjected to palladium-imidazolium carbene catalyzed Suzuki-Miyaura coupling conditions.14 As shown in scheme 1, this too failed to produce the desired product 7. In light of these findings, we chose to focus our efforts on more conventional approaches to furoxan analogues.
Scheme 1.
Unsuccessful attempts at a convergent synthesis of furoxan analogues.
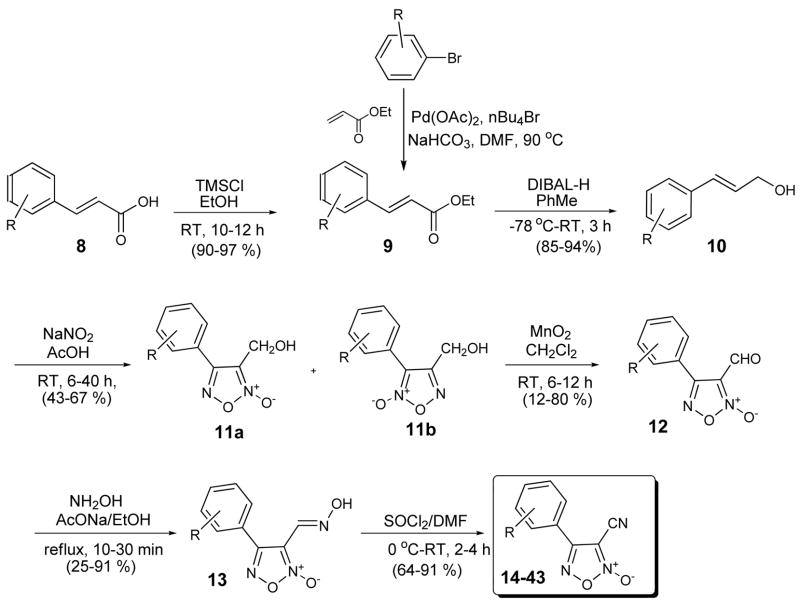
Gasco and co-workers have studied furoxan and related analogues for many years and have published pioneering work towards their syntheses.15–22 The key step typically involves treatment of the requisite cinnamyl alcohol 10 with sodium nitrate and acetic acid at room temperature to form the corresponding 4-phenyl-3-furoxanmethanol derivative 11.(Scheme 2) While Gasco and co-workers often report the use of aqueous sodium nitrate in acetic acid, we found use of glacial acetic acid with anhydrous sodium nitrate worked best for the analogues reported herein. The required cinnamyl alcohols were obtained either through esterification of commercially available cinnamic acid derivatives 8 or via Heck coupling of the corresponding aryl bromides with ethyl acrylate to give 9. Subsequent hydride reduction with DIBAL-H gave alcohols of type 10. Of note, the sodium nitrate mediate cyclization often gave a mixture of two products 11a and 11b, always favoring the desired regioisomer 11a. The structural assignments were based on known differences in 1H and 13C NMR resonances of the methylene group in the 3-position which results from shielding by the N-oxide moiety.15,19 The chromatographic separation of these isomers was typically complex and thus the mixture was carried through and subsequently oxidized using MnO2 to give the corresponding aldehydes 12 in yields ranging from 12–80%. At this stage the mixture of regioisomers were easily separated using flash chromatography. Formation of oxime 13 was achieved using hydroxylamine hydrochloride in the presence of sodium acetate in ethanol. Dehydration was carried out using SOCl2 in DMF to provide to requisite 4-phenyl-3-cyano derivatives 14–42. Using this approach, 28 novel furoxan analogues were synthesized in an effort to define SAR around the phenyl moiety with respect to TGR inhibitory activity.(Table 1)
Scheme 2.
General Scheme for the synthesis of furoxan analogues 14–43.
Table 1.
Phenyl ring analogues synthesized as depicted in Scheme 1.
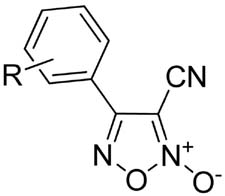 | |
|---|---|
| R = 4-F (14) | R = 3-OMe (28) |
| 4-Cl (15) | 3-OH (29)c |
| 4-Br (16) | 2-OMe (30) |
| 4-CF3 (17) | 4-F, 3-Br (31) |
| 4-NO2 (18) | 4-Cl, 3-NO2 (32) |
| 4-OMe (19) | 3-CF3, 5-CF3 (33) |
| 4-OH22 (20)a | 3,4,5-OMe (34) |
| 4-Me (21) | 3,4-OCH2O (35) |
| 4-Phe (22) | napthyl (36) |
| 4-OCH2CCHb(23) | furan (37) |
| 3-NO2 (24) | thiophene (38) |
| 3-Cl (25) | 2-NO2-furan (39) |
| 3-Br (26) | 3-furoxan (i.e. m-bis-furoxan) (40) |
| 3-CF3 (27) | 4-furoxan (i.e. p-bis-furoxan) (41) |
| 1,4-thiophene-bis-furoxan (42) | |
| 3-F,5-furoxan (i.e. m-F, m-bis-furoxan) (43) | |
was obtained via deprotection of 19 with AlCl3.
was obtained from reaction of 20 with propargyl bromide. (see supporting information for details)
was obtained via deprotection of 28
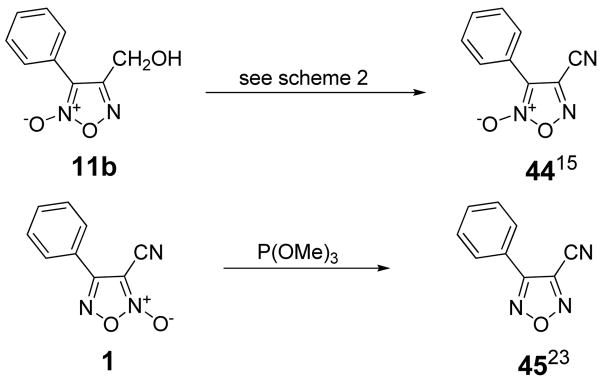
Another primary interest was the importance of the N-oxide moiety. As such, we sought to synthesize both 3-phenyl-4-cyano furoxan 44 and the 1,2,5-oxadiazole derivative 45.23 (Scheme 3) As mentioned previously, cyclization of 10 to 11 gives a mixture of isomers, with the minor being 3-phenyl-4-cyano-furoxan 11b (see Scheme 2). However, Gasco showed that heating 11a in refluxing toluene leads to partial isomerization to 11b,15 allowing access to ample material of the minor isomer to be collected. Carrying through the remaining steps as shown in scheme 2 gave 3-phenyl-4-cyano-furoxan 44. Compound 45 was prepared by treatment of furoxan 1 with neat P(OMe)3 as previously reported for a similar system.23 The removal of the N-oxide moiety was confirmed by IR in which the common absorbance for the N-oxide at 1590–1620 cm−1 is absent.
Scheme 3.
Synthesis of furoxan analogues 44 and 45.
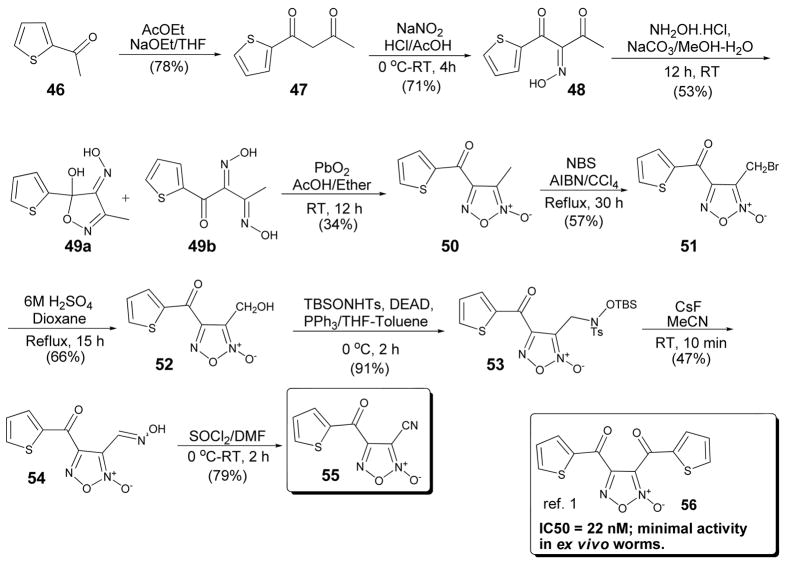
Finally, we set out to synthesize the 4-(2-thienoyl)-3-cyano furoxan derivative 55. (Scheme 4) We reported previously that the bis-thienoyl furoxan 56 had quite potent in vitro TGR inhibitory activity as compared to 1 (20 nM and 8 μM respectively) yet lacked appreciable activity against ex vivo worms.1 Structurally similar dibenzoyl furoxans have recently been shown by Nirode and co-workers to be effective NO-donors through nucleophilic attack of thiols.24 Thus, we reasoned 55 may retain some of the desirable potency while the presence of the nitrile may provide the structural requirements necessary for ex vivo worm killing activity.
Scheme 4.
Synthesis of 4-thienoyl-3-cyano furoxan (55).
The synthesis commenced with condensation of commercially available 2-acetylthiophene (46) with ethyl acetate in the presence of sodium ethoxide to give 1,3-diketone 47 in 78% yield. Treatment with sodium nitrate in HCl/AcOH provided 48 in 71%. Oxime formation was achieved with hydroxyl amine hydrochloride to give the desired bisoxime 49b in 53% yield along with a minor amount of undesired 49a. Cyclization was accomplished with lead dioxide to give the requisite 4-ketothiophene-3-methyl furoxan 50 in 34% yield. Allylic bromination followed by hydrolysis gave 4-ketothiophene-3-furoxanmethanol 52 in 38% yield over two steps. Interestingly, typical oxidation conditions that were utilized in previous analogues (i.e. MnO2) failed to give the desired product. Recently, Fukuyama and co-workers reported a mild two-step synthesis of oximes from alcohols via Mitsunobu reaction with N-(tert-butyldimethylsilyloxy)benzenesulfonamide followed by treatment cesium fluoride.25 Gratifyingly, the reaction worked well on our system providing oxime 54 in 42% yield over two steps. Subsequent treatment with SOCl2 in DMF gave the target compound 55 in 79% yield.
In summary, the preparation of numerous novel furoxan analogues is described. While the convergent strategy was not successful, a combination of new and established chemistry provided access to the desired analogues for biology testing against a medicinally relevant target. Detailed evaluation of the biological activity against both the TGR enzyme in vitro and ex vivo worms (S. mansoni) is ongoing and will be reported in due course.
Supplementary Material
Experimental procedures and NMR data for all final compounds can be found in the Supporting Information.
Acknowledgments
The authors thank Jeremy Smith and Paul Shinn for assistance with compound management. This research was supported by the Molecular Libraries Initiative of the National Institutes of Health Roadmap for Medical Research.
References and notes
- 1.Sayed AA, Simeonov A, Thomas CJ, Inglese J, Austin CP, William DL. Nature Medicine. 2008;14:407. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simeonov A, Jadhav A, Sayed AA, Wang Y, Nelson ME, Thomas CJ, Inglese J, Williams DL, Austin CP. PLos Negl Trop Dis. 2008;2:e127. doi: 10.1371/journal.pntd.0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Proc Natl Acad Sci USA. 2006;103:11473. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin CP, Brady LS, Insel TR, Collins FS. Science. 2004;306:1138. doi: 10.1126/science.1105511. [DOI] [PubMed] [Google Scholar]
- 5.Van der Werf MJ, De Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. 2003;86:125. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 6.Herwaldt BL, Tao LF, van Pelt W, Tsang VC, Bruce JI. Clin Infect Dis. 1995;20:309. doi: 10.1093/clinids/20.2.309. [DOI] [PubMed] [Google Scholar]
- 7.Murray-Smith SQ, Scott BJ, Barton DP, Weinstein PA. Med J Aust. 1996;165:458. doi: 10.5694/j.1326-5377.1996.tb138598.x. [DOI] [PubMed] [Google Scholar]
- 8.Townsend DM, Tew KD, Tapiero H. Biomed Pharmacother. 2003;57:145. [Google Scholar]
- 9.Lillig CH, Holmgren A. Antioxid Redox Signal. 2007;9:25. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 10.Townsend DM, Tew KD, Tapiero H. Biomed Pharmacother. 2003;57:145. [Google Scholar]
- 11.Gromer S, Urig S, Becker K. Med Res Rev. 2004;24:40. doi: 10.1002/med.10051. [DOI] [PubMed] [Google Scholar]
- 12.Alger HM, Williams DL. Mol Biochem Parasitol. 2002;121:129. doi: 10.1016/s0166-6851(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 13.Andrianov V, Eremeev A. Synth Commun. 1992;22:453. [Google Scholar]
- 14.Andrus MB, Song C. Org Lett. 2001;3:3761. doi: 10.1021/ol016724c. [DOI] [PubMed] [Google Scholar]
- 15.Gasco AM, Fruttero R, Sorba G, Gasco A. Liebigs Ann Chem. 1991:1211. [Google Scholar]
- 16.Gasco AM, Di Stilo A, Fruttero R, Sorba G, Gasco A, Sabatino P. Liebigs Ann Chem. 1993:441. [Google Scholar]
- 17.Fruttero R, Ferrarotti B, Serafino A, Gasco A. Liebigs Ann Chem. 1990:335. [Google Scholar]
- 18.Gasco AM, Boschi D, Gasco A. J Heterocycl Chem. 1995;32:1211. [Google Scholar]
- 19.Gasco A, Boulton J. Adv Heterocycl Chem. 1981;29:251. [Google Scholar]
- 20.Medana C, Ermondi G, Fruttero R, Stilo DA, Ferretti C, Gasco A. J Med Chem. 1994;37:4412. doi: 10.1021/jm00051a020. [DOI] [PubMed] [Google Scholar]
- 21.Gasco AM, Cena C, Stilo AD, Ermondi G, Medana C, Gasco A. Helvetica Chimica Acta. 1996;79:1803. [Google Scholar]
- 22.Tosco P, Bertinaria M, Di Stilo A, Cena C, Sorba G, Fruttero R, Gasco A. Bioorg Med Chem. 2005;13:4750. doi: 10.1016/j.bmc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Ponzio G. Gazz Chim Ital. 1931;61:943. [Google Scholar]
- 24.Nirode WF, Luis JM, Wicker JF, Wachter NM. Bioorg Med Chem. 2006;16:2299. doi: 10.1016/j.bmcl.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Kitahara K, Toma T, Shimokawa J, Fukuyama T. Org Lett. 2008;10:2259. doi: 10.1021/ol800677p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and NMR data for all final compounds can be found in the Supporting Information.