Abstract
Psoriasis is a common chronic and disabling inflammatory disease that has an enormous physical, functional and psychosocial impact on patients’ quality of life. To date several conventional therapies are available for the treatment of this condition (eg, cyclosporine, methotrexate, retinoids, and psoralen plus ultraviolet A) which, although providing clinical response, do not maintain long-lasting disease remission and at times show poor tolerability with potential toxicity thus limiting their use. A challenge in psoriasis management is to utilize precociously an adequate therapy and to achieve effective and safe maintenance of its clearance by improving both skin and joint manifestations as well as to prevent joint destruction and disability. Recent improvement in the knowledge of the pathogenesis of this disease was fundamental for the development of novel targeted treatment options that may be effective, safer and well tolerated on long-term administration periods, thus improving patient’s quality of life. These novel agents, which are called “biologics”, target specifically tumor necrosis factor-α (infliximab, etanercept and adalimumab) or T cells (alefacept and efalizumab).
Keywords: psoriasis, biologics, anti-TNF-α, anti-T cells
Introduction
Psoriasis is a common, chronic, relapsing inflammatory skin disease that can be associated with significant morbidity. Patients affected by severe psoriasis constitute approximately 20%–30% of all patients with this disease and often require systemic treatment which has a major economic impact on the Health Service (Sampogna et al 2004; Stern et al 2004; Smith et al 2005).
The aim of a chronic treatment is a balance between preventing disease-associated morbidity and disability, and minimizing side effects and organ toxicity consequent to prolonged use of a single agent. Standard systemic therapies available for the treatment of moderate-to-severe plaque psoriasis include photochemotherapy, retinoids, cyclosporine, methotrexate, and fumarates. Although many patients benefit from many of these therapies and are able to achieve disease control, few are ever completely disease free and some are ineligible because of side effects or comorbidities. In addition, the unrestricted long-term administration is not recommended due to the potential cumulative toxicity and the possibility of treatment-resistance (Griffiths et al 2000; de Rie et al 2004; Rapp and Feldman 2004; Stern et al 2004; Saraceno and Griffiths 2006).
Over the last 5–7 years, there has been a significant advance in devising new drugs, the so-called biologics, which emerged as potentially alternative valuable therapeutic options for severe psoriasis and may be used safely over the long term with less toxicity than other traditional systemic treatments. The recent advances in the understanding of the immunopathogenesis of psoriasis and the identification of key cytokines and immune cells, eg, tumor necrosis factor (TNF)-α and T-cells, revolutionized the management of this chronic disease. Biologics describe agents designed to block specific molecular steps important in the pathogenesis of psoriasis, and include two main groups: (i) agents targeting the cytokine TNF-α (eg, etanercept, infliximab, adalimumab) and (ii) agents targeting T cells or antigen-presenting cells (eg, efalizumab, alefacept) (Rapp and Feldman 2004; Papp 2005a).
Fundamental principles of the immunopathogenesis of psoriasis
Psoriasis is a complex disease that is recently considered an immune-mediated, organ-specific (skin, or skin and joints) inflammatory condition, in which intralesional T lymphocytes trigger primed basal stem keratinocytes to proliferate and perpetuate the disease process. Moreover, the complex interactions between susceptibility genes, immunologic effector mechanisms and environmental trigger factors (eg, infections, antigens, drugs, physical and/or emotional stress) elicit the disease process in the skin. Although epidermal hyperproliferation and terminal differentiation are the fundamental abnormalities in psoriatic skin, there is an immune-mediated inflammatory process involving cytokines, chemokines, antigen-presenting cells (APCs), eg, Langherans cells, neutrophils and natural killer T cells, and mature skin-homing peripheral CD4+ and CD8+ T lymphocytes (Krueger et al 1984; Bhalearao and Bowcock 1998; Gaspari 2006).
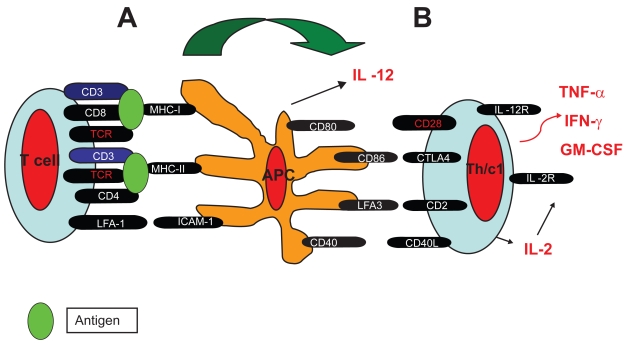
In fully developed psoriatic skin lesions, innate immune cells (eg, neutrophils, dendritic APCs and natural killer T cells), adaptive immune T cells, and an inflammatory infiltrate are found (Gaspari 2006). Both CD4+ and CD8+ T lymphocytes are present, with the CD4+ T lymphocytes being present mostly in the dermis. There are two subsets of CD8+ T lymphocytes: an epidermal homing subset expressing CD103 (integrin α E) and a subset that remains in the dermis, which may be in transit to or from the epidermis. These mature peripheral T lymphocytes in psoriatic lesions are skin-homing activated memory cells CLA (cutaneous leukocyte antigen+), HLA antigen-DR+, which express α/β TCR. The epidermal CD8+ T lymphocytes also express CD103 that allows them to interact with E-cadherin, facilitating their migration into the epidermis and their ability to bind to epidermal cells. Therefore, both CD4+ and CD8+ T cells respond to processed polypeptides presented by mature APCs in the skin. The possibilities for antigen recognition by these cells include self polypeptides (epidermal or keratinocyte-derived), those derived from microbial agents, or microbial superantigens. Psoriatic plaque development and maintenance is dependent on the pathologic collaboration of T lymphocytes (CD4+ cells, CD8+ cells and natural killer T cells) and dendritic APCs. The APCs are responsible for activation of infiltrating T cells, by cell-cell interactions of which immunologic synapse is central for antigen recognition. Antigen recognition by T lymphocytes requires that mature APCs process complex polypeptides, load them onto self-major histocompatibility complex class I or II molecules with a variety of costimulatory signals (eg, CD86, CD80, CD40, lymphocyte function-associated antigen-3, CD54) for which there are receptors on the surface of T lymphocytes, present the processed peptides to the T cells, and finally growth factor production by the activated T lymphocytes (Figures 1–2) (Bhalerao and Bowcock 1998; Austin et al 1999; Prinz 2003; Griffiths 2003).
Figure 1.
Immunologic synapse. A: Innate T lymphocyte (T-cell)-antigen presenting cell (APC) interaction and antigen recognition. B: Activation of innate T cells into adaptive, Th1 (T-helper) and Tc1 (T-cytotoxic), and immunologic cascade.
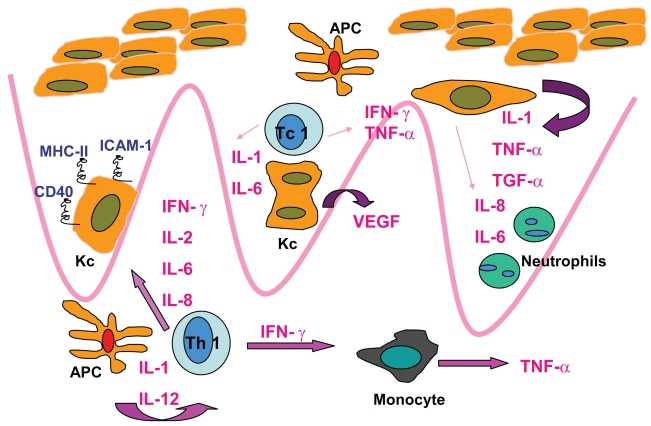
Figure 2.
Immunopathogensis of psoriasis.
Abbreviations: Kc, keratinocyte; APC, antigen-presenting cell; Tc1, T-cytotoxic lymphocyte; Th1, T-helper lymphocyte.
Moreover, in skin lesions and in blood there is a deficiency of T-regulatory cell suppressor activity that may lead to unrestrained T cell proliferation and chronic overproduction of chemokines and Th1-derived cytokines, eg, interferon-γ, interleukin-2, TNF-α, etc. These proinflammatory cytokines and growth factors target epidermal keratinocytes to hyperproliferate, develop abnormal differentiation and become resistant to apoptotic signals. Furthermore, epidermal hyperproliferation is supported by neoangiogenesis which is also driven by T cell-derived growth factors and inflammatory cytokines (Austin et al 1999; Prinz 2003; Banchereau et al 2004; Gaspari 2006).
TNF-α appears to be a critical cytokine in the pathogenesis of T cell-mediated autoimmune diseases, including psoriasis, where it is demonstrated that it is crucial for keratinocyte hyperproliferation, endothelial cell regulation, and recruitment/effector function of memory T cells (Vassalli 1992; Ettehadi et al 1994; Kimber et al 2000). Moreover, TNF-α levels are increased in psoriatic lesions, compared with those in uninvolved skin in psoriatic patients and in the healthy skin of nonpsoriatic individuals. Serum and lesional TNF levels decrease after effective psoriasis therapy correlating with clinical improvement in the disease. These observations suggested the use of anti-TNF-α agents with the intent to interfere with the proinflammatory effects of this cytokine. Therefore, several TNF-α antagonists have been successfully utilized for the treatment of severe psoriasis (Gordon and Ruderman 2006).
Targeted treatment options
Advances in the knowledge of the major role of T cells in the pathogenesis of psoriasis and the understanding of chronic inflammatory pathways has led to the development of several biologic agents that may target specific inflammatory steps. Many accessory molecules involved in the so-called immunologic synapse, have been targeted by biologic therapies (alefacept and efalizumab) which showed a significant efficacy in psoriasis by interfering with the effector CD4+ and CD8+ T lymphocyte activation and secretion of the cytokines (eg, IFN-γ and TNF-α) found in psoriatic lesions. The first agent approved to treat adult patients affected by moderate-to-severe plaque psoriasis was alefacept (Amevive®, Biogen Idec Inc.) and it is currently registered for use in several countries, including United States (US) but not the European Union (EU). Efalizumab (Raptiva®, Genetech, Inc.; Serono International S.A.) is also registered for the same indication in many countries, including US and EU (Ellis and Krueger 2001; Krueger et al 2002; Lebwhol et al 2003; Jullien et al 2004; Rapp and Feldman 2004; Leonardi et al 2005).
Therapeutic targeting of TNF-α with biologic agents such as etanercept, infliximab, and adalimumab has proven the important role of this inflammation mediator in psoriasis. Etanercept (Enbrel®, Amgen, Inc.; Wyeth Pharmaceuticals) is registered for the treatment of psoriasis and psoriatic arthritis (PsA) in EU and US among other countries. Another TNF-α antagonist, infliximab (Remicade®, Centocor, Inc.; Schering-Plough Corp.), has presented Phase III clinical trial data for the treatment of psoriasis and recently was registered for use in EU. Currently evaluated in Phase III clinical trials, adalimumab (Humira®, Abbott Laboratories) is at an earlier stage of development for psoriasis (Leonardi et al 2003; Rapp and Feldman 2004; Papp et al 2005a; Reich et al 2005a). (Table 1)
Table 1.
Biologics in psoriasis
| Class | Structure | Origin | Indications | Dosage | |
|---|---|---|---|---|---|
| Alefacept | T cell inhibitor | Soluble recombinant fusion protein against CD2 (LFA-3 protein subunit + human IgG1 fragment domain) | Humanized | Moderate-to-severe plaque psoriasis | 15 mg/week SC injections |
| Efalizumab | T cell inhibitor | Monoclonal antibody against the CD11a | Humanized | Moderate-to- severe plaque psoriasis | 1 mg/kg/week SC injections |
| Infliximab | TNF-α inhibitor | Monoclonal antibody against TNF-α | Chimeric (human/murine) | Rheumatoid arthritis, Crohn’s disease, moderate-to- severe plaque psoriasis and psoriatic arthritis | 3–5 mg/kg EV infusion at time 0, 2 and 6-weeks (induction) and every 8-weeks (maintenance) |
| Etanercept | TNF-α inhibitor | Soluble TNF-α receptor (recombinant fusion protein of human TNF receptor + human IgG1) | Human | Rheumatoid arthritis, moderate-to- severe plaque psoriasis and psoriatic arthritis | 25–50 mg twice a week SC injections |
| Adalimumab | TNF-α inhibitor | Monoclonal antibody against TNF-α | Human | Rheumatoid arthritis, psoriatic arthritis | 40 mg once a week or once every 2-weeks SC injections |
T cell inhibitors
Alefacept
Alefacept is a recombinant protein that binds to CD2 on memory-effector T lymphocytes, inhibiting their activation and reducing the number of these cells. It is a fusion protein composed of an LFA-3 protein and human IgG1 fragment crystallizable (Fc) domain. The drug is administered by intramuscular (IM) injection or intravenous (IV) infusion. Patients treated with alefacept have experienced a reduction in CD45RO+ memory T cells, which correlates with the clinical improvement. To date, no clinically significant signs of immunosuppression, opportunistic infections, or increase in malignancy have been observed (Ellis and Krueger 2001; Lebwhol et al 2003).
Alefacept has been evaluated as a weekly 7.5 mg IV administration and as a weekly IM 15 mg injection however, only the IM dose is currently available. The efficacy and safety of alefacept 15 mg, weekly administered for 12-weeks, were evaluated in a randomized, placebo-controlled, Phase III trial of adult patients with plaque psoriasis (body surface area >10%). Two weeks after the treatment phase was completed (study week-14), the Psoriasis Area and Severity Index (PASI) improved by at least 75% from baseline (PASI-75) in 21% of the 166 patients who received alefacept 15 mg per week, with 42% achieving at least a 50% improvement from baseline (PASI-50). This compares with rates of 5 and 18%, respectively, for patients randomized to placebo (n = 168; P < 0.001 for both comparisons). Alefacept therapy appeared to be well tolerated, even with long-term use (Lebwhol et al 2003).
A multicentre (63 sites in Europe, US, and Canada), randomized, double-blind, placebo-controlled, parallel-group study, comparing 10 mg and 15 mg of alefacept and placebo administered IM once-weekly for 12-weeks, demonstrated that treatment with 15 mg of alefacept provided a higher PASI reduction from baseline with adverse events similar to that of placebo (Ortonne 2003).
The primary concern with alefacept is T lymphocyte depletion. Patients with CD4+ T lymphocyte counts below normal should not initiate therapy with alefacept. It also recommended to monitor twice-weekly the T cells. Weekly monitoring of CD4+ T cells is also recommended in patients treated in the United States (Papp 2005a).
Efalizumab
Efalizumab is a humanized monoclonal antibody against the CD11a molecule. CD11a and CD18 are subunits of leukocyte function-associated antigen 1 (LFA-1), a T cell surface molecule important in T cell activation, T cell migration into skin, and cytotoxic T cell function. This drug binds to CD11a on T cells blocking the interaction between LFA-1 and intercellular adhesion molecule 1 (ICAM-1), its partner molecule for adhesion. The blockade is reversible and does not deplete T cells. The currently available formulation of efalizumab is delivered as a once-weekly subcutaneous (SC) injection (Jullien et al 2004).
Multiple Phase III clinical trials have demonstrated the efficacy, safety, and health-related quality-of-life (HRQOL) benefits of 12-weeks of SC efalizumab therapy (1 mg/kg/week) in patients with moderate-to-severe chronic plaque psoriasis. A total of 556 adult psoriatic patients were randomized to receive efalizumab (n = 369) or placebo (n = 187) double-blind for 12-weeks; all patients were then eligible to receive extended efalizumab open-label treatment for an additional 12-weeks. Efalizumab treatment showed a significant effect relative to placebo after 12-weeks, and the extended treatment conferred additional clinical benefit. Efalizumab has shown good safety profile and no opportunistic infections, no clinical signs of immunosuppression, hepatotoxicity or nephrotoxicity associated to its use. The Phase III trials showed no evidence of T cell depletion or increased risk of end-organ toxicity, malignancy, or infection. The most common adverse events associated with efalizumab administration are acute flu-like symptoms (eg, headache, chills, fever, myalgia, vomiting, and nausea) observed primarily after the first two doses. The incidence of acute adverse events in patients treated with efalizumab at the following doses is comparable to that observed in the placebo group. Worsening of psoriasis and psoriasis variants has been observed in 3% of efalizumab patients during therapy and in 14% of patients following abrupt discontinuation of efalizumab, respectively a generalized inflammatory reaction and a rebound. Furthermore, new-onset or worsening arthritis has been infrequently reported during clinical trials (Leonardi et al 2003; Sterry et al 2004; Menter et al 2005a).
While the 12-week, double-blind, placebo-controlled, first-treatment (FT) CLEAR trial period demonstrated the efficacy/safety of efalizumab in moderate-to-severe plaque psoriasis including refractory or contraindicated patients for other systemic treatments, a further study of Sterry and colleagues (2006) assessed the efficacy/safety during an open-label extended 24-week continuous treatment. Among efalizumab-treated patients who had <75% PASI improvement at week-12 FT, extended treatment led to PASI75 in 26.6%; patients who had PASI ≥50 and ≤75 improvement at week-12 FT, extended treatment led to PASI75 in 47.5%; among patients achieving PASI75 at week-12 FT, median time of relapse was 58 days; and, re-treatment after relapse led to an improvement of mean PASI of 62.3% from study baseline. Safety results were consistent of other previous studies and efficacy results demonstrate additional benefit of continuing efalizumab (Sterry et al 2006).
TNF-α inhibitors
Infliximab
Infliximab is a chimeric monoclonal antibody that binds membrane-bound and soluble TNF-α. It was the first blocker studied for the treatment of psoriasis, approved by the FDA to treat moderate-to-highly active rheumatoid arthritis in combination with methotrexate, moderate-to-highly active Crohn’s disease, and both rheumatoid arthritis and Crohn’s disease who have failed to respond to conventional therapies. This drug is administrated as IV infusion (Rapp and Feldman 2004; Papp 2005a).
Efficacy of infliximab has been demonstrated in psoriasis patients in randomized, placebo-controlled Phase II trials. Results of an international multicenter, randomized, placebo-controlled Phase III trial of adult patients with plaque psoriasis treated with infliximab 5 mg/kg were recently reported. At week-10 of treatment, the PASI-75 response rate was 80.4% in the infliximab-treated group and 2.6% in the placebo-treated group. Response was sustained through week-24. However, excluding patients who missed 2 infusions, the PASI-75 rate at week-50 was 70.5%. The moderate reduction in efficacy may be due to the development of inhibitory antibodies in a percentage of the patients (Reich et al 2005a). The safety profile for infliximab during this extended treatment Phase III trial appears to be comparable to those observed during earlier studies. The incidence of adverse events and serious adverse events in infliximab-treated patients was slightly elevated through the first 24-weeks of treatment, and one patient who received infliximab died of sepsis. Elevations in aminotransferases were also observed in some infliximab-treated patients. In this trial, infection rates were comparable between the treated and placebo groups (Reich et al 2005a).
The results of a Phase III, multicentre, double-blind clinical trial which included 378 psoriatic patients demonstrated the effectiveness of infliximab 5 mg/kg in both induction and maintenance regimen. At week-10, 80% of patients treated with infliximab achieved PASI75 and 57% PASI90, compared with 3% and 1% in the placebo group (p < 0.0001). At week-24, PASI75 and PASI90 were maintained (p < 0.0001). At week-50, 61% achieved PASI75 and 45% achieved PASI90 in the infliximab group (Reich et al 2005b).
In a randomized comparison of continuous (every-8-week) vs. intermittent (as-needed) infliximab maintenance regimens for the treatment of moderate-to-severe psoriasis demonstrated that, through week-50, response was best in the continuous infliximab therapy group (Menter et al 2007).
It is also demonstrated that therapy with infliximab at the dose of 5 mg/kg significantly improves the signs and symptoms of arthritis, dactylitis and enthesitis in patients with active PsA that had been resistan to disease-modifying antirheumatic drugs (DMARDs). Results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) showed that a continuous infliximab treatment led to sustained benefit through 50-weeks with a favorable benefit-to-risk ratio (Antoni et al 2005).
Infliximab has been associated with several adverse events. Infusion reactions, reported in 19% of patients in clinical trials, usually consist of fever or chills, chest pain, hypotension, hypertension, and dyspnea. Neutralizing antibodies are formed, and patients can develop a serum-sickness reaction days after administration of the drug. Multiple reports have indicated mild and serious infections during treatment with infliximab, in particular reactivation of latent tuberculosis (TB). The available in vitro and epidemiologic evidence for the TNF inhibitors (infliximab and etanercept) shows that the risk of development of active TB is higher with infliximab. Furthermore, a decrease of TNF-α activity is demonstrated to be correlated with an increase of susceptibility to TB. The reason that only some patients succumb to rapidly disseminated infection is unknown, but may be related to the extent of TNF blockade in different individuals. This difference in inhibition may also explain why the incidence of TB seems to be increased with infliximab in comparison with the other TNF inhibitors. However, it has not been observed an increased risk of serious infection in infliximab-treated patients. One of the major concerns with the use of TNF inhibitors is the potential development of lymphomas. The majority of cases (81%) were non-Hodgkin’s lymphomas. Induction of antinuclear antibodies and anti-DNA antibodies is observed in some patients treated with infliximab and etanercept. Recently, the induction of true lupus erythematosus by TNF inhibitors has been observed (Reich et al 2005a).
Etanercept
Etanercept is a TNF-α receptor recombinant fusion protein, comprising domains of the 75-kDa human TNF receptor and human IgG1, which competitively inhibits the interaction of this cytokine with cell-surface receptors and binds soluble TNF, preventing its mediated cellular responses and modulating the activity of other proinflammatory cytokines regulated by TNF. Etanercept is self-administered as SC injections once or twice-weekly (Rapp and Feldman 2004; Papp 2005a).
The efficacy and safety benefits of etanercept after 12 and 24-weeks of therapy in patients with moderate-to-severe chronic plaque psoriasis have been demonstrated in Phase III clinical trials.
In the EU the recommended dosage is 25 mg administered SC twice-weekly for up to 24-weeks, although dosing at 50 mg twice-weekly is also possible for the first 12-weeks followed by a dose reduction to 25 mg twice-weekly. Moreover, on the basis of published clinical data, European guidelines indicate that nonresponders should discontinue etanercept after 12-weeks and that re-treatment is possible after discontinuation. In the US, the recommended dosing of etanercept is 50 mg twice-weekly for the first 12-weeks of treatment followed by 50 mg etanercept once-weekly.
A double-blind, randomized, placebo-controlled Phase III clinical study demonstrated the safety and efficacy of 12 and 24-weeks of etanercept treatment. Results showed that etanercept was well tolerated and the adverse events observed during the first 12-week placebo-controlled period were mild-to-moderate and the etanercept and placebo groups showed no significant differences (Leonardi et al 2003; Papp et al 2005b).
Etanercept has been used safely over the past few years. Injection-site reactions are the main adverse events noted. There have been observations of demyelinating disorders such as multiple sclerosis, allergic reactions, and aplastic anemia. It possesses a good safety profile in regard to the risk of malignancy and infection. There was no apparent temporal association between the onset of clinical TB and the introduction of etanercept therapy. TNF antagonists might induce new-onset heart failure or exacerbate existing disease (Rapp and Feldman 2004; Papp 2005a).
The safety data from an integrated database of 1347 patients from 3 randomized, double-blind, placebo-controlled clinical trials were analyzed by Gottlieb and colleagues (2006). Rates of adverse events in the first 12-weeks of etanercept monotherapy in the 3 trials were similar among all active groups as well as each active group compared with the placebo group, and no dose-related toxicities were reported.
A recent randomized, open-label study evaluated the effectiveness and safety of continuous versus interrupted etanercept therapy. All patients received uninterrupted etanercept 50 mg twice weekly during the first 12-weeks, followed by either continuous or interrupted 50 mg once weekly in the next 12-weeks. At week-12, comparable high proportions of responders were in the continuous (71.3%) and interrupted (72%) groups. At week-24, the proportion of responders was greater in the continuous group (71% vs 59.5% of the interrupted group; p < 0.0001). Both treatment groups were generally well tolerated (Moore et al 2007).
Adalimumab
Adalimumab is a fully human anti-TNF-α monoclonal antibody administered as 40 mg subcutaneously once every other week. It has been approved by the FDA for the treatment of rheumatoid arthritis and currently being evaluated in Phase II and III clinical studies for the treatment of moderate-to-severe plaque psoriasis and PsA. These trials showed impressive PASI responses, with 53% of patients on 40 mg every other week and 80% of patients on 40 mg weekly achieving a 75% reduction in the PASI. Adverse events reported were similar to placebo, with headache, injection site pain, nausea, elevated tryglicerides, cough, sinus congestion, and fatigue. Adalimumab was found to be effective for psoriasis refractory to other treatments including infliximab and etanercept (Backer 2004; Chen et al 2004; Rapp and Feldman 2004; Menter et al 2005b; Papp 2005a; Winterfield et al 2005).
Results of a double-blind, randomized, placebo-controlled study showed that patients with moderate-to-severe active PsA and chronic plaque psoriasis treated with adalimumab significantly improved joint and skin manifestations, inhibited structural changes on radiographs, lessened disability due to joint damage, and improved quality of life. At week-12, 58% of the adalimumab-treated patients achieved an ACR20 response, compared with 14% of the placebo-treated patients (P < 0.001). At week-24, similar ACR20 response rates were maintained. Among the 69 adalimumab-treated patients evaluated with the Psoriasis Area and Severity Index (PASI), 59% achieved a 75% PASI improvement response at 24-weeks, compared with 1% of the 69 placebo-treated patients evaluated (P < 0.001) (Mease et al 2005).
Summary
Biologic agents appear to offer an effective and safe alternative to conventional systemic therapies and phototherapy for the treatment of moderate-to-severe psoriasis. To date, patients treated with infliximab, etanercept, adalimumab, efalizumab, alefacept have achieved successful therapy of psoriasis without the organ toxicity seen with the long-term standard systemic treatments. However, these agents have potential limitations which include the expected high costs of treatment, lack of long-term follow-up, and the selected nature of the patient populations treated.
References
- Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52(4):1227–36. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–9. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- Backer DE. Adalimumab: human recombinant immunoglobulin G1 anti-tumor necrosis factor monoclonal antibody. Rev Gastroenterol Disord. 2004;4:196–210. [PubMed] [Google Scholar]
- Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–50. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- Bhalerao J, Bowcock AM. The genetics of psoriasis: a complex disorder of the skin and immune system. Hum Mol Genet. 1998;7:1537–45. doi: 10.1093/hmg/7.10.1537. [DOI] [PubMed] [Google Scholar]
- Chen DM, Gordon K, Leonardi MD, et al. Adalimumab efficacy and safety in patients with moderate to severe chronic plaque psoriasis: Preliminary findings from a 12-week dose-ranging trial. J Am Acad Dermatol. 2004;50(3 Pt II) and PS 491; Ps. [Google Scholar]
- de Rie MA, Goedkoop AY, Bos JD. Overview of psoriasis. Dermatol Ther. 2004;17:341–9. doi: 10.1111/j.1396-0296.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–55. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- Ettehadi P, Greaves MW, Wallach D, et al. Elevated tumor necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994;96:146–51. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54(3):S67–80. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Gordon KB, Ruderman EM. The treatment of psoriasis and psoriatic arthritis: An interdisciplinary approach. J Am Acad Dermatol. 2006;54(3):S85–91. doi: 10.1016/j.jaad.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Leonardi CL, Goffe BS, et al. Etanercept monotherapy in patients with psoriasis: a summary of safety, based on an integrated multistudy database. J Am Acad Dermatol. 2006;54:S92–100. doi: 10.1016/j.jaad.2005.10.053. [DOI] [PubMed] [Google Scholar]
- Griffiths CEM, Clark CM, Chalmers RJG, et al. A systematic review of treatments for severe psoriasis. Health Technol Assess. 2000;4:1–125. doi: 10.3310/hta4400. [DOI] [PubMed] [Google Scholar]
- Griffiths CE. The immunological basis of psoriasis. J Eur Acad Dermatol Venereol. 2003;17:1–5. doi: 10.1046/j.1468-3083.17.s2.1.x. [DOI] [PubMed] [Google Scholar]
- Jullien D, Prinz JC, Langley RGB, et al. T-cell modulation for the treatment of chronic plaque psoriasis with efalizumab (Raptiva): mechanisms of action. Dermatology. 2004;208:297–306. doi: 10.1159/000077660. [DOI] [PubMed] [Google Scholar]
- Kimber I, Cumberbatch M, Dearman RJ, et al. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br J Dermatol. 2000;142:401–12. doi: 10.1046/j.1365-2133.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- Krueger Gg, Bergstresser PR, Lowe NJ, et al. Psoriasis. J Am Acad Dermatol. 1984;11:937–47. doi: 10.1016/s0190-9622(84)80018-3. [DOI] [PubMed] [Google Scholar]
- Krueger GG, Papp KA, Stough DB, et al. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2002;47:821–33. doi: 10.1067/mjd.2002.127247. [DOI] [PubMed] [Google Scholar]
- Lebwhol M, Christophers E, Langley R, et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139:719–27. doi: 10.1001/archderm.139.6.719. [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–22. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Papp KA, Gordon KB, et al. Extended efazilumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. J Am Acad Dermatol. 2005;52:425–33. doi: 10.1016/j.jaad.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52(10):3279–89. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- Menter A, Gordon K, Carey W, et al. Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis. Arch Dermatol. 2005a;141:31–8. doi: 10.1001/archderm.141.1.31. [DOI] [PubMed] [Google Scholar]
- Menter M, Gordon K, Leonardi C, et al. Adalimumab efficacy and safety results in patients with moderate-to-severe chronic plaque psoriasis with and without psoriatic arthritis. Poster 2713 presented at the American academy of Dermatology Annual Meeting.2005b. [Google Scholar]
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe psoriasis. J Am Acad Dermatol. 2007;56:31. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Moore A, Gordon KB, Gottlieb A, et al. A randomized, open-label trial of continuous versus interrupted etanercept therapy in the treatment of psoriasis. J Am Acad Dermatol. 2007;56:598–603. doi: 10.1016/j.jaad.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Ortonne JP. clinical response to alefacept: results of a phase 3 study of intramuscular administration of alefacept in patients with chronic plaque psoriasis. J Acad Dermatol Venereol. 2003;2:12–16. doi: 10.1046/j.1468-3083.17.s2.3.x. [DOI] [PubMed] [Google Scholar]
- Papp KA. The long-term efficacy and safety of new biological therapies for psoriasis. Arch Dermatol Res. 2005a;298:7–15. doi: 10.1007/s00403-006-0660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. B J Dermatol. 2005b;152:1304–12. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- Prinz JC. The role of T cells in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:257–70. doi: 10.1046/j.1468-3083.2003.00720.x. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Feldman SR. The promise and challenge of new biological treatments for psoriasis: how do they impact quality of life. Dermatol Ther. 2004;17:376–82. doi: 10.1111/j.1396-0296.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005b;366:1367–74. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- Reich K, Nestle FO, Papp K, et al. express study Investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: A Phase III, multicentre, double-blind trial. Lancet. 2005a;366:1367–74. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- Sampogna F, Sera F, Abeni D, et al. Measures of clinical severity, quality of life, and psycological distress in patients with psoriasis: A cluster analysis. J Invest Dermatol. 2004;122:602–7. doi: 10.1046/j.0022-202X.2003.09101.x. [DOI] [PubMed] [Google Scholar]
- Saraceno R, Griffiths CEM. A European perspective on the challenges of managing psoriasis. J Am Acad Dermatol. 2006;54(3):S81–3. doi: 10.1016/j.jaad.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Smith CH, Anstey AV, Barker AD, et al. British Association of Dermatologists guidelines for use of biological interventions in psoriasis. B J Dermatol. 2005;153:486–97. doi: 10.1111/j.1365-2133.2005.06893.x. [DOI] [PubMed] [Google Scholar]
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Invest Dermatol symp Proc. 2004;9:136–9. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- Sterry W, Dubertret L, Papp K, et al. Efalizumab for patients with moderate to severe chronic plaque psoriasis: results of the international, randomized, controlled phase III clinical experience Raptiva (CLEAR) trial. J Invest Dermatol. 2004;123(2):A64. doi: 10.1111/j.1365-2133.2006.07344.x. [DOI] [PubMed] [Google Scholar]
- Sterry W, Stingl G, Langley RG, et al. Clinical Experience Acquired with Raptiva (CLEAR) trial in patients woth moderate-to-severe plaque psoriasis: results from extended treatment in an international, Phase III, placebo-controlled trial. J Dtsch Dermatol Ges. 2006;4:947–56. doi: 10.1111/j.1610-0387.2006.06111.x. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Winterfield LS, Menter A, Gordon K, et al. Psoriasis treatment: current and emerging directed therapies. Ann Rheum Dis. 2005;64(2):87–92. doi: 10.1136/ard.2004.032276. [DOI] [PMC free article] [PubMed] [Google Scholar]