Abstract
Transforming growth factor beta (TGFβ) plays an important role in cancer, but accurate measurement of circulating TGFβ is complicated by the high TGFβ content of platelets which can release TGFβ ex vivo. We evaluated the use of citrate-theophylline-adenosine-dipyridamole (CTAD) tubes to reduce preanalytical variation in TGFβ measurements caused by ex vivo platelet activation. CTAD substantially reduced ex vivo platelet activation relative to traditional plasma collections in normal donors, which correlated with a decrease in measured TGFβ levels. We show that TGFβ levels are elevated in the majority of cancer patients with skeletal metastases, and that within-patient variability of these levels is relatively low over several weeks. Patients with elevated TGFβ could be subdivided into groups with or without evidence of platelet contribution to measured TGFβ levels. The use of CTAD tubes allows a better determination of a patient’s TGFβ status, and may improve classification of patients with oncologic disease.
Keywords: TGFβ, cancer, biomarker, CTAD
Primary objective
Transforming growth factor β (TGFβ) is a dimeric 25 kD cytokine synthesized by multiple cell types. Three isoforms exist (TGFβ1, TGFβ2, and TGFβ3), of which TGFβ1 is the dominant form in humans. (For the remainder of this report, TGFβ will refer to TGFβ1 unless otherwise specified). Although the majority of tissues in the human body are capable of synthesizing TGFβ under appropriate conditions, two of the tissues with the highest TGFβ content in normal donors are platelets (20 mg/kg) (Assoian and Sporn 1986) and bone (200 μg/kg) (Seyedin et al 1985). The role of TGFβ in normal biology is intricate and depends largely on the environment in which TGFβ is secreted. These actions can include suppression of cell proliferation, enhanced extracellular matrix deposition, and immunomodulatory effects (Blobe et al 2000). The role of TGFβ in cancer is even more complex, where TGFβ has been proposed to have roles as both a tumor suppressor in the premalignant phase of disease and as a pro-oncogenic factor in later stage metastatic disease (Roberts and Wakefield 2003; Siegel and Massague 2003). Because of this, TGFβ may play an important role in the pathological processes of cancer.
The use of TGFβ as a biomarker in cancer has been explored for years with varying success. Immunohistochemical analysis of biopsy material has shown increased TGFβ staining in many different tumor types (Gordinier et al 1999; Nio et al 2005), which correlated to clinical parameters such as metastasis and disease progression in some studies (Hazelbag et al 2004; Yamamoto et al 2004; Culhaci et al 2005). Because TGFβ is readily secreted by most cell types, a number of groups have evaluated the use of circulating TGFβ levels as a potential cancer biomarker. Results from such approaches have been variable, with some reports suggesting that circulating TGFβ levels have clinical utility in predicting disease progression (Shariat et al 2001), while others show no relationship between circulating TGFβ and outcome (Wakefield et al 1995).
One factor that complicates the use of circulating TGFβ levels as a biomarker is the potential for ex vivo release of TGFβ from platelet stores due to platelet activation. It has long been appreciated that platelets are a rich source of TGFβ (Assoian and Sporn 1986), and that as a result serum measurements of TGFβ are largely driven by platelet count and do not accurately reflect true circulating levels of TGFβ (Kropf et al 1997). As a result, plasma is the preferred matrix for TGFβ assessment. However, some level of platelet activation occurs even with routine plasma collection, and the amount of ex vivo activation can vary significantly depending upon the type of anticoagulant tube used (Golanski et al 1996; Neufeld et al 1999; Philippe et al 2004). For this reason, methods to reduce the level of platelet activation in plasma samples would be useful to help limit overestimation of in vivo TGFβ levels due to ex vivo activation of platelets.
In this study, we have evaluated the use of citrate-theophylline-adenosine-dipyridamole (CTAD) tubes as a means to provide more accurate TGFβ measurements in platelet-poor plasma from cancer patients. CTAD tubes contain citrate anticoagulant and a mixture of theophylline, adenosine, and dipyramidole, which serve to reduce ex vivo platelet activation by increasing cAMP and inhibiting calcium-mediated platelet activation (Contant et al 1983; Macey et al 2002). We have characterized the effects of this matrix on a commonly used TGFβ ELISA, and applied this approach to the measurement of TGFβ in healthy volunteers and in oncology patients with skeletal metastases. We demonstrate that CTAD tubes are an acceptable matrix for TGFβ analysis, and that the reduction of ex vivo platelet activation using these tubes leads to a concomitant reduction in measured TGFβ levels in healthy volunteers. Using this technique we demonstrate that the majority of oncology patients with skeletal metastases have elevations in circulating TGFβ levels, and that the within-patient variability of these measurements are relatively low over a two-week period. In addition, the use of CTAD plasma coupled with measurement of platelet activation markers allows differentiation of patients with or without substantial platelet contributions to measured TGFβ levels. This approach is an extension of the previous works showing that control of platelet activation is critical to the assessment of circulating TGFb levels (Reinhold et al 1997).
Methods and procedures
Patient population
After obtaining Institution Review Board approval of the clinical protocol and informed consent from patients, samples were collected from oncology patients from three different institutions as part of an ongoing clinical trial designed to examine TGFβ-related biomarkers (Baselga et al 2008). Patients were enrolled if they had previously-documented cancer involving skeletal metastases. 49 patients participated in this study, and CTAD tubes were collected 4 times over a 2–4 week period with no limitations on state at collection (diurnal, fasted.fed, etc). Patients received no oncolytic therapies during this period. Tumor types in this study included breast cancer (23 patients), prostate cancer (15) multiple myeloma (7), renal cell carcinoma (3), and lymphoma (1).
Sample collection and TGFβ1 analysis
Samples were collected into either tripotassium ethylene-diamine tetra-acetate (K3EDTA) or CTAD tubes (Becton, Dickinson and Co, Franklin Lakes, NJ). A modified protocol was used for these collections. Briefly, a tourniquet was initially used to locate the vein, then removed before collection. After removal of the tourniquet, a single discard tube was drawn to allow washout of any activation products caused by initiation of phlebotomy, followed by the CTAD tube. Tubes were then spun for 15 min at 2500 g, and the central portion of the plasma layer transferred to a second tube. This tube was then spun again for 15 min at 2500 g, and the supernatant transferred to a final tube for storage at −70 °C until analysis.
TGFβ levels were analyzed using a commercially available ELISA (R&D Systems, Minneapolis, MN, catalog # DB100) per the manufacturer’s protocol. Briefly, plasma samples underwent initial acidification/neutralization steps immediately prior to analysis to activate latent TGFβ to an immunoreactive form, under conditions which have been shown to minimize re-association with TGFβ binding proteins (Kropf et al 1997). The ELISA assay utilized soluble TGFβ type II receptor coated on the ELISA plate for antigen capture, with detection via a HRP-linked polyclonal specific for TGFβ1. Recombinant human TGFβ1 (rhTGFβ) included in the kit was used as a standard and for the spiking experiments that follow below.
Analytical performance of the TGFβ1 assay in CTAD matrix was evaluated as follows: Assay precision was determined using 4 samples ranging from 0.05 ng/ml to 15.8 ng/ml, which were run in triplicate in 5 runs over a 3 day period. Within- and between-day variance were expressed as the coefficient of variation (standard deviation/mean) for these samples. Matrix effects were assessed by spiking known concentrations of the rhTGFβ standard into CTAD samples, and evaluating recovery relative to buffer controls. Dilutional linearity was determined by performing a dilution series ranging from 1:2 to 1:20. Dilutions were run in triplicate, and the percent variation in observed values compared to expected results was calculated for each dilution. Freeze-thaw stability was assessed using 4 aliquots of samples stored at −70 °C which were subjected to between 1 and 4 freeze-thaw cycles. All 4 aliquots were then assayed in triplicate in a single run.
Clinical trial samples were stored at −70 °C until the conclusion of the study, then batch analyzed for TGFβ levels. In addition to TGFβ, separate aliquots from these same CTAD plasma samples were also batch analyzed for two markers of platelet activation, platelet factor 4 (PF4) and beta-thromboglobulin (BTG) using commercial enzyme immunoassays (Asserachrom® PF4 and Asserachrom® BTG, both from Diagnostica Stago, Asnieres-Sur-Siene, France).
Main outcomes and results
Evaluation of CTAD matrix for TGFβ measurements
We initially examined the analytical performance of the TGFβ1 ELISA assay in CTAD plasma to ensure that this matrix did not adversely affect assay performance. As shown in Table 1, analytical parameters for the TGFβ1 assay in CTAD plasma were comparable to that reported for EDTA plasma by the manufacturer (Table 1). Within- and between-day coefficient of variation (CV) ranged from 5.9%–13.4% and 9.1%–29%, respectively, with the highest variability seen at 0.05 ng/ml. The 0.05 ng/ml concentration was below manufacturer’s recommendations, but was initially evaluated in an attempt to extend the reportable range of the assay in the expectation that TGFβ levels in CTAD plasma would be lower than reported for EDTA plasma due to decreased platelet activation. No evidence of a matrix effect was seen in either the recovery or dilutional linearity experiments, with less than 10% variation from expected results at most concentrations. Freeze-thaw stability of TGFβ was observed for two freeze-thaw cycles in CTAD with <10% loss of signal, but further freeze-thaw cycles led to a progressive loss of signal (>15% change from original values).
Table 1.
Analytical performance of TGFβ ELISA in CTAD matrix
| CTAD | EDTA | |||
|---|---|---|---|---|
| Precision | Within-day | Concentrations tested (ng/ml) | 0.05, 0.2, 8.0,15.8 | 0.88, 5.14, 11.13 |
| CV | 13.4%, 8.3%, 5.8%, 5.9% | 7.3%, 4.9%, 3.7% | ||
| Between-day | Concentrations tested (ng/ml) | 0.05, 0.2, 8.0, 15.8 | 1.18, 4.23, 7.21 | |
| CV | 29%, 10.8%, 11.5%, 9.1% | 12.7%, 10.3%, 9.8% | ||
| Reportable range | 0.75–240 ng/ml | 0.36–240 ng/ml | ||
| Dilutional linearity | Dilutions tested | 1:2, 1:5, 1:10, 1:20 | 1:2, 1:4 | |
| % of expected value | 100%, 98%, 97%, 120% | 103%, 103% | ||
| Freeze-thaw stablility | Stable to 2 freeze/thaw cycles | NR |
Notes: Experiments were performed as described in methods. CVi and % values are listed in order corresponding to tested concentrations for all experiments. Performance parameters in EDTA were taken from the manufacturer’s package insert for the TGFβ ELISA. The analytical performance of the assay in CTAD plasma was comparable with that reported for EDTA plasma.
Abbreviations: CTAD, citrate-theophylline-adenosine-dipyridamole; CVi, within-patient variability; EDTA, ethylenediamine tetra-acetate; NR, not reported; TGFβ, transforming growth factor beta.
Impact of CTAD tubes on ex vivo platelet activation and TGFβ measurement
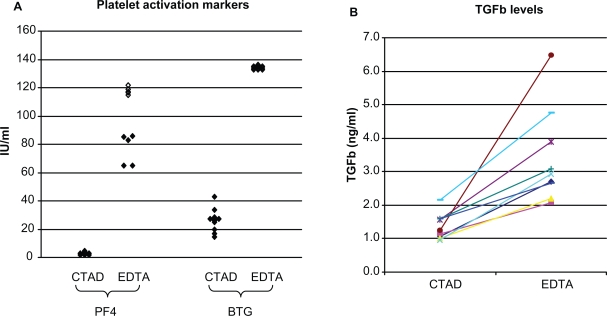
Normal donor samples were next used to evaluate the differences between EDTA and CTAD tubes on ex vivo TGFβ release, and to generate an estimate of the reference range for TGFβ in the CTAD matrix. Paired EDTA and CTAD tubes were drawn from 10 normal donors and evaluated for TGFβ as well as two markers of platelet activation, platelet factor 4 and beta-thromboglobulin. PF4 and BTG are contained in platelet alpha granules in approximately equimolar amounts (Kaplan and Owen 1981), and are released following platelet activation or platelet lysis. In our study, BTG and PF4 levels were both elevated in EDTA tubes relative to CTAD for all donors, confirming that CTAD confers an advantage in limiting ex vivo platelet activation relative to EDTA (Figure 1A). A concurrent increase in TGFβ levels was also seen in the EDTA tubes from all donors, resulting in a 67%–430% bias in TGFβ levels in the EDTA plasma relative to the CTAD plasma (Figure 1B). An additional 15 CTAD samples were then drawn from normal donors to estimate the reference range for TGFβ in this matrix. The median TGFβ level from all 25 samples was 1.3 ng/ml (range 0.8–2.2 ng/ml), with a 95th percentile of 1.7 ng/ml, which was established as the upper limit of normal for the following oncology study.
Figure 1.
Effect of CTAD matrix on platelet activation and TGFβ measurements. A) Markers of platelet activation. Both PF4 and BTG were substantially elevated in samples collected in EDTA tubes from normal donors, confirming that some level of ex vivo platelet activation occurs in EDTA plasma draws. (Open symbols represent values outside the range of the assay). B) Within-donor TGFβ levels in CTAD and EDTA tubes. EDTA TGFβ levels were higher than CTAD tube levels for all donors, ranging from 1.7- to 5.3-fold higher.
Abbreviations: BTG, beta-thromboglobulin; CTAD, citrate-theophylline-adenosine-dipyridamole; EDTA, ethylenediamine tetra-acetate; PF4, platelet factor 4; TGFβ, transforming growth factor beta.
Levels and variability of TGFβ measurements in oncology patients with advanced disease
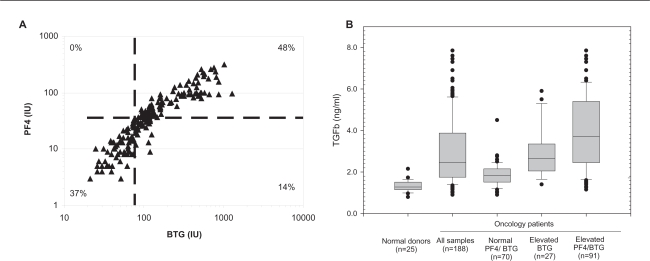
A total of 188 samples were collected in CTAD tubes from 49 oncology patients, and TGFβ, PF4 and BTG levels were measured as described. A surprising observation in this study was that 118 samples (63%) showed evidence of platelet activation, despite the use of CTAD tubes. There was good correlation between the two platelet activation markers in individual samples, with the majority of samples showing agreement between PF4 and BTG (Figure 2A). TGFβ levels were significantly increased in the samples from oncology patients relative to normal donors (p < 0.05, t-test), with 77% of the samples collected falling above the upper limit of normal established in the healthy donor population. As one would expect, samples with evidence of platelet activation showed the highest levels of TGFβ (Figure 2B). However, TGFβ levels were also elevated in approximately half of the samples with normal PF4 and BTG levels, suggesting that in those patients TGFβ elevation may be driven by a mechanism independent of platelet release (such as elevated in vivo production of TGFβ from another source).
Figure 2.
CTAD plasma TGFβ levels in normal donors and oncology patients. A) Relationship of platelet-activation markers in clinical samples. Dashed lines represent the upper limit of normal for both markers, %’s indicated fraction of all samples falling in each quadrant. The overall correlation between platelet activation markers was good across all samples (r2 = 0.71, Pearson correlation coefficient), with agreement between PF4 and BTG in 85% of the samples. B) TGFβ levels in oncology patients related to platelet activation. Boxes represent the median and central 50th percentile of observations, whiskers represent the 10th and 90th percentiles with individual outliers noted, dashed line represents upper limit established in normal donors (1.7 ng/ml). A single sample with an extremely elevated TGFβ level (13.2 pg/ml, BTG/PF4 +ve) is not shown to improve scaling. Samples collected from oncology patients had significantly higher circulating TGFβ levels than those from normal donors (p < 0.05 students t-test for all groups when compared to normal donors). This was true regardless of platelet activation status, although TGFβ levels were higher in samples with evidence of platelet activation.
Abbreviations: BTG, beta-thromboglobulin; CTAD, citrate-theophylline-adenosine-dipyridamole; PF4, platelet factor 4; TGFβ, transforming growth factor beta.
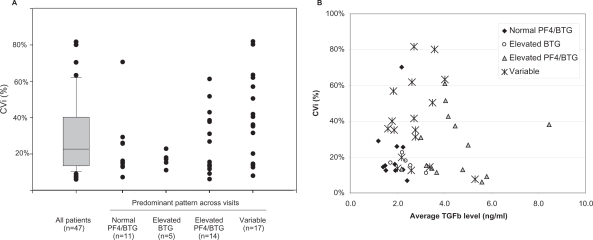
We next considered the within-patient variability (CVi) of TGFβ levels in the 47 patients who had at least 3 samples collected. The median CVi was relatively low for these patients (22.7%), with the majority of patients showing <40% variation in TGFβ levels over the two week period tested (Figure 3A). In addition to this consistency in TGFβ levels, an interesting finding was that many patients also showed consistent patterns of platelet activation markers over repeated visits. The variability of TGFβ measurements was lowest in the patients who had consistently normal PF4 and BTG levels, while high CVi values were more common in the patients showing evidence of platelet activation. This increased variability in the platelet-activated subset was not simply attributable to an increase in measured TGFβ levels, as many patients with high TGFβ levels showed relatively low CVi (Figure 3B).
Figure 3.
Within-patient variability (CVi) of TGFβ measurements in oncology patients. Boxes represent the median and central 50th percentile of observations, whiskers represent the 10th and 90th percentiles with individual outliers noted. A) CVi of repeated measurement calculated for all patients with at least 3 visits. Subgroups are defined as patients who had the specified pattern in ≥3 of 4 visits. Variability was lowest in the patients with consistent patterns of platelet activation markers. B) Relationship between within-patient variability and TGFβ levels. X axis represents average TGFβ level across all visits for each patient. There was no correlation between CVi and TGFβ levels for any of the groups.
Abbreviations: BTG, beta-thromboglobulin; CTAD, citrate-theophylline-adenosine-dipyridamole; EDTA, ethylenediamine tetra-acetate; PF4, platelet factor 4; TGFβ, transforming growth factor beta.
Conclusions
In this study we have shown that CTAD tubes are a suitable anticoagulant for TGFβ analysis, and that a commercially available TGFβ1 ELISA assay shows acceptable performance characteristics in this matrix. As predicted, the use of CTAD tubes led to a decreased level of ex vivo platelet activation when compared to traditional plasma collections in K3EDTA, which corresponded to a decrease in measured TGFβ levels in normal donors. The contribution of ex vivo platelet activation on TGFβ levels in normal donors was substantial in EDTA, resulting in as much as a 5-fold increase in measured TGFβ in these samples. We have seen similar results with VEGF analysis in CTAD vs EDTA tubes (data not shown), and a recent publication has shown similar results for a number of platelet-derived growth factors including TGFβ (Zimmermann et al 2005), suggesting that this approach may be relevant to the measurement of many circulating cytokines.
TGFβ levels were elevated in the majority of the oncology patients enrolled in this trial. This was not entirely unexpected, as enrollment was limited to patients with skeletal metastases which is a population where TGFβ has been shown to play a significant role (Kang et al 2005). There were substantial differences in TGFβ levels between patients, with average levels over the 4 visits ranging from 1.2–8.5 ng/ml across the groups of patients enrolled. However, even with this wide range of TGFβ values across the population, within-patient variability was relatively low in most patients. This is encouraging for potential use of TGFβ as a biomarker, because it implies that limited sampling would be required to accurately assess a patient’s TGFβ status. We believe that the use of CTAD tubes contributed to this consistency, by reducing the variability that would be introduced by sporadic ex vivo platelet activation during the collection process. This is supported by the observation that the subset of patients where PF4 and BTG were sporadically elevated showed the highest levels of within-patient variability.
A surprising observation in this study was that a large number of samples showed elevated levels of BTG and PF4 despite the use of CTAD tubes to limit ex vivo platelet activation. The cause of this is not clear. One possibility is that this represents ex vivo platelet activation, either due to poor sample processing or an inherent increase in platelet reactivity in some oncology patients. Alternatively, the elevations in PF4 and BTG may indicate an increased in vivo platelet activation in a subset of these oncology patients. This would explain the within-patient consistency of activation marker patterns across multiple visits, and could reflect the hypercoagulable state commonly found in patients with advanced cancer (Caine et al 2002). It is interesting to note that patients with elevated TGFβ levels in this study can be largely divided into two groups using these platelet markers. Patients with repeated evidence of platelet activation showed the highest levels of TGFβ, consistent with the observation that platelets contain large amounts of TGFβ. However, an additional group of patients had elevations of TGFβ in the absence of any evidence of platelet activation. It is possible that these patients represent a subset where TGFβ secretion from the tumor milieu is the primary driver of elevated circulating levels. Further work will be required to define if there is any biological relevance to differentiating between these subgroups in terms of predicting either the disease prognosis or response to TGFβ-targeted therapies.
In summary, we believe that the use of CTAD tubes coupled with PF4 and BTG measurements will improve the ability to accurately assess a patient’s true TGFβ status by reducing the background noise introduced by ex vivo platelet activation. Removing a potential source of preanalytical error should reduce the inherent variability of TGFβ measurements in patients, and allow for a better evaluation of the true utility of circulating TGFβ levels as a cancer biomarker. Further work applying this technique to well-annotated clinical specimens will be required to determine the value of circulating TGFβ levels as a potential prognostic tool.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986;102:1217–23. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Rothenberg ML, Tabernero J, et al. TGF-β signaling related markers in cancer patients with bone metastasis. Biomarkers. 13:217–36. doi: 10.1080/13547500701676019. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Caine GJ, Stonelake PS, Lip GY, et al. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia (New York) 2002;4:465–73. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contant G, Gouault-Heilmann M, Martinoli JL. Heparin inactivation during blood storage: Its prevention by blood collection in citric acid, theophylline, adenosine, dipyridamole-C.T.A.D. mixture. Thromb Res. 1983;31:365–74. doi: 10.1016/0049-3848(83)90337-7. [DOI] [PubMed] [Google Scholar]
- Culhaci N, Sagol O, Karademir S, et al. Expression of transforming growth factor-beta-1 and p27Kip1 in pancreatic adenocarcinomas: relation with cell-cycle-associated proteins and clinicopathologic characteristics. BMC Cancer. 2005;5:98. doi: 10.1186/1471-2407-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golanski J, Pietrucha T, Baj Z, et al. Molecular insights into the anticoagulant-induced spontaneous activation of platelets in whole blood – various anticoagulants are not equal. Thromb Res. 1996;83:199–216. doi: 10.1016/0049-3848(96)00129-6. [DOI] [PubMed] [Google Scholar]
- Gordinier ME, Zhang HZ, Patenia R, et al. Quantitative analysis of transforming growth factor beta 1 and 2 in ovarian carcinoma. Clin Cancer Res. 1999;5:2498–505. [PubMed] [Google Scholar]
- Hazelbag S, Kenter GG, Gorter A, et al. Prognostic relevance of TGF-beta1 and PAI-1 in cervical cancer. Int J Cancer. 2004;112:1020–8. doi: 10.1002/ijc.20512. [DOI] [PubMed] [Google Scholar]
- Kang Y, He W, Tulley S, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA. 2005;102:13909–14. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KL, Owen J. Plasma levels of beta-thromboglobulin and platelet factor 4 as indices of platelet activation in vivo. Blood. 1981;57:199–202. [PubMed] [Google Scholar]
- Kropf J, Schurek JO, Wollner A, et al. Immunological measurement of transforming growth factor-beta 1 (TGF-β1) in blood; assay development and comparison. Clin Chem. 1997;43:1965–74. [PubMed] [Google Scholar]
- Macey M, Azam U, McCarthy D, et al. Evaluation of the anticoagulants EDTA and citrate, theophylline, adenosine, and dipyridamole (CTAD) for assessing platelet activation on the ADVIA 120 hematology system. Clin Chem. 2002;48:891–9. [PubMed] [Google Scholar]
- Neufeld M, Nowak-Gottl U, Junker R. Citrate-theophylline-adenine-dipyridamol buffer is preferable to citrate buffer as an anticoagulant for flow cytometric measurement of platelet activation. Clin Chem. 1999;45:2030–3. [PubMed] [Google Scholar]
- Nio Y, Omori H, Hashimoto K, et al. Immunohistochemical expression of receptor-tyrosine kinase c-kit protein and TGF-beta1 in invasive ductal carcinoma of the pancreas. Anticancer Res. 2005;25:3523–9. [PubMed] [Google Scholar]
- Philippe J, De Logi E, Baele G. Comparison of five different citrated tubes and their in vitro effects on platelet activation. Clin Chem. 2004;50:656–8. doi: 10.1373/clinchem.2003.028704. [DOI] [PubMed] [Google Scholar]
- Reinhold D, Bank U, Buhling F, et al. A detailed protocol for the measurement of TGFb1 in human blood samples. J Immun Methods. 1997;209:203–6. doi: 10.1016/s0022-1759(97)00160-9. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis [comment] Proc Natl Acad Sci USA. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedin SM, Thomas TC, Thompson AY, et al. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc Natl Acad Sci USA. 1985;82:2267–71. doi: 10.1073/pnas.82.8.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariat SF, Shalev M, Menesses-Diaz A, et al. Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19:2856–64. doi: 10.1200/JCO.2001.19.11.2856. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nature Rev Cancer. 2003;3:807–21. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Letterio JJ, Chen T, et al. Transforming growth factor-beta1 circulates in normal human plasma and is unchanged in advanced metastatic breast cancer. Clin Cancer Res. 1995;1:129–36. [PubMed] [Google Scholar]
- Yamamoto T, Akisue T, Marui T, et al. Expression of transforming growth factor beta isoforms and their receptors in malignant fibrous histiocytoma of soft tissues. Clin Cancer Res. 2004;10:5804–7. doi: 10.1158/1078-0432.CCR-0770-03. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Koenig J, Zingsem J, et al. Effect of specimen anticoagulation on the measurement of circulating platelet-derived growth factors. Clin Chem. 2005;51:2365–8. doi: 10.1373/clinchem.2005.055558. [DOI] [PubMed] [Google Scholar]