Abstract
The immune response to T helper (Th) cell determinants of a variety of antigens is often poor and limits severely the potential efficacy of current therapeutic measures through vaccination. Here, we report that an immunologically silent tumor determinant can be rendered immunogenic if linked with a dominant determinant of a parasite antigen, suggesting the existence of functional Th–Th cooperation in vivo. This phenomenon could be mimicked in part by signaling either through CD40 to the antigen-presenting cells or through OX40 to the tumor-determinant reactive T cells, with maximal effects obtained by combined anti-CD40 and anti-OX40 treatment in vivo. The data suggest that CD4 T cells reactive with a dominant determinant provide help to other CD4 T cells through up-regulating the costimulatory ability of antigen-presenting cells, in much the same way as help for CD8 cells. CD4 help for CD4 T cells represents a new immunological principle and offers new practical solutions for vaccine therapy against cancer and other diseases in which antigenic help is limiting.
Associative recognition is a basic regulatory mechanism of the immune response and refers to the phenomenon in which immunity to one determinant of a multideterminant antigen enhances the response to other determinants. The cooperative effect is mediated by a determinant recognized by T helper cells (Th) and has been demonstrated for B cell responses (T–B cooperation) (1) and cytotoxic T lymphocytes responses (Th–cytotoxic T lymphocyte cooperation) (2). A similar form of associative recognition between two Th cell determinants (Th–Th cooperation) has not been described yet but could prove valuable to responses against poorly immunogenic antigens such as tumor antigens. The immune response against tumor antigens is hindered, among other factors, by down-regulation of MHC molecules, self-tolerance, and functional hierarchy in the immunogenicity of T cell determinants. It is now clear that T cell determinants of protein antigens can be categorized into dominant, subdominant, and cryptic to reflect a different degree of immunogenicity in vivo (3). Consequently, as T cells reactive with dominant determinants of tumor antigens are eliminated in the thymus during negative selection, the adult immune repertoire is composed mainly of precursor cells with moderate avidity for subdominant and cryptic determinants. Thus, methods to heighten the response of CD4 and CD8 T lymphocytes of the residual repertoire against poorly immunogenic determinants are needed to develop better immunogens against cancer.
MUC-1, a glycosylated molecule of high molecular weight expressed in malignant tumors of epithelial origin (4, 5), induces both CD8 (6–8) and CD4 T lymphocytes (9, 10) against an antigenic core consisting of a tandem repeat of 20 amino acid residues. For these reasons, MUC-1 is a useful model antigen for targeted manipulations of the immune response. Specific T cell responses against MUC-1 were induced in C57BL/6 mice using a model of epitope-based genetic vaccination, somatic transgene immunization (11). This approach is based on the inoculation into the spleen of adult mice of plasmid DNA comprising an Ig heavy (H) chain gene controlled by a B cell-specific promoter to target resident B cells (12). The use of Ig genes modified in the complementarity-determining regions (CDR) to code for heterologous epitopes (13) allows one to direct the in vivo synthesis and secretion by B cells of transgenic Ig, which immunize the host against the B and T cell epitopes expressed in their V region (14, 15). Unlike conventional DNA vaccination (16), which induces Th1 responses (17), somatic transgene immunization activates Th0 cells that produce IL-2, IFN-γ, and IL-4 (15). One advantage of somatic transgene immunization is that heterologous epitopes can be assembled on the Ig V region in various assortment and combination to maximize immunogenicity but also to provide easy tools to study the reciprocal regulation between distinct antigenic determinants during the immune response in vivo.
Here, we report that an immunologically silent Th cell determinant of a tumor antigen can be rendered immunogenic if presented in linked association with a dominant Th cell determinant from the circumsporozoite antigen of the human malaria parasite Plasmodium falciparum. This phenomenon, help for helpers, seems to be mediated in part by signaling through CD40 to the antigen-presenting cells (APC), suggesting a similar mechanism to that of CD4 help for CD8 T cells, and can be replaced by OX40 signaling to the T cells. The results suggest the existence of functional Th–Th cooperation in vivo.
Materials and Methods
Mice.
Eight to ten-week-old C57BL/6 mice were purchased from The Jackson Laboratory and were kept in the Bonner Hall animal facility of the University of California, San Diego.
Plasmid DNAs.
Plasmids γ1NV2VTSA3, γ1VTSA3, γ1NV2DTRP3, γ1DTRP3, and γ1NV2 were engineered according to previously published methods (18). For γ1NV2VTSA3, a pair of complementary oligonucleotides 5′GTACCCGTCACCTCGGCCCCGGACACCAGGCCGGCCCCG3′ (sense) and 5′GTACCGGGGCCGGCCTGGTGTCCGGGGCCGAGGTGACGG3′ (antisense) coding for VTSAPDTRPAP was synthesized and annealed, and the product was cloned in the Asp-718 site in CDR3 (18). The pair of complementary oligonucleotides, 5′CATGGTAATGCAAACCCAAATGTAGATCCCAATGCCAACCCA3′ (sense) and 5′CATGTGGGTTGGCATTGGGATCTACATTTGGGTTTGCATTAC3′ (antisense) coding for NANP–NVDP–NANP, was cloned in the NcoI site in CDR2. Similarly, for γ1NV2DTRP3, a pair of complementary oligonucleotides, 5′GTACCCGACACCAGGCCGGACACCAGGCCGGA3′ (sense) and 5′GTACCGGCCTGGTGTCCGGCCTGGTGTCCGGC3′ (antisense) coding for [DTRP]3, was synthesized and annealed, and the product was cloned in the Asp-718 site in CDR3. γ1VTSA3, γ1DTRP3, and γ1NV2 were engineered accordingly using the above primers. Plasmids were purified using Qiagen Megaprep kit (Qiagen, Chatsworth, CA) and stored at −20°C until use.
Synthetic Peptides.
MUC-1-derived synthetic peptides VTSAPDTRPAP, TSAPDTRPA, and DTRP–DTRP–DTRP were synthesized at the Peptide Facility of the California Institute of Technology (Pasadena, CA). Synthetic peptide NANP–NVDP–NANP was synthesized at the Peptide Facility of the La Jolla Institute for Allergy and Immunology (La Jolla, CA).
DNA Immunizations.
Mice were inoculated intraspleen with 100 μg of plasmid DNA in 50 μl of sterile saline solution (19). In the experiment in which plasmids were coinjected (mixing experiment), γ1VTSA3 and γ1NV2 were mixed in equal amounts (50 μg) in the same syringe and injected as a single (50 μl) inoculum. In the experiment in which these plasmids were administered as two injections in two opposite sites along the longitudinal axis of the spleen, each plasmid was injected at 100 μg in 50 μl. Control mice receiving γ1NV2 were also injected with 50 μl of sterile saline.
In Vivo Antibody Treatment.
Ligation of CD40 in vivo was performed as follows. Mice immunized with γ1VTSA3 were treated with 100 μg of the CD40-activating monoclonal antibody FGK45 (20) in sterile saline (0.2 ml) intravenously on days 1 and 5 for a total of two injections (200 μg). Control mice were similarly injected with a rat IgG2a antibody of unrelated specificity. Ligation of OX40 in vivo was performed as follows. Mice immunized with γ1VTSA3 were treated with 100 μg of the OX40-activating monoclonal antibody OX86 (21) in sterile saline (0.2 ml) intraperitoneally on day 2 (100 μg). In combination treatment, mice were injected with 100 μg of antibody FGK45 intravenously on days 1 and 5, and 100 μg of antibody OX86 intraperitoneally on day 2 for a total of three injections and 300 μg of antibodies. Control mice were similarly injected with rat IgG. To block CD40L, mice immunized with γ1NV2VTSA3 were treated with 250 μg of CD40L blocking monoclonal antibody MR1 (20) or hamster IgG control antibody in sterile saline (0.2 ml) intraperitoneally every other day starting on day −1 for a total of four injections (1 mg).
T Cell Assays.
Spleen cell suspensions were cultured (2 × 106 cells/ml) in RPMI 1640 medium (Irvine Scientific) supplemented with Hepes buffer, glutamine, 7.5% FCS, and 50 μM 2-mercaptoethanol, with or without synthetic peptide (50 μg/ml) unless otherwise specified in triplicate. The cells were incubated at 37°C in 10% CO2 for 3 days. [3H]Thymidine was added at 1 μCi per well, and the cells were incubated for 16–18 h at 37°C. Cells were harvested onto glass fiber filter mats using a Tomtec cell harvester, and the radioactivity was measured in a liquid scintillation counter (Betaplate, Wallac, Tuku, Finland). Results are expressed as stimulation index calculated as the ratio of (cpm of cells cultured in the presence of synthetic peptide)/(cpm of cells cultured in the absence of peptide). ConA stimulation was used as positive control. Culture supernatants harvested 40 h after initial seeding were tested for IL-2 activity in a bioassay and amounts of IL-4 and IFN-γ by ELISA as described (15). Tests were done in duplicate.
Separation of CD4+ and CD8+ T Cells.
CD4+ and CD8+ T cells were isolated by antibody plus complement-mediated depletion from splenocytes harvested 14 days (see Fig. 3) after DNA inoculation as previously described (15).
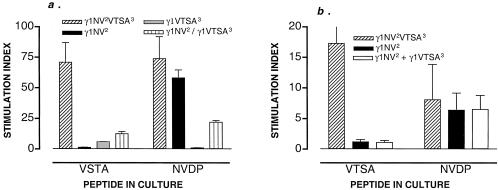
Figure 3.
(a) Coinjection of plasmids yields a diminished response to the cryptic determinant induced by Th–Th associative recognition (mixing experiment). Mice were immunized with the plasmids indicated. The γ1NV2/γ1VTSA3 group received the two plasmids mixed in the same syringe as a single inoculum. T cell proliferation was measured on cells harvested on day 14 and cultured for 3 days on synthetic peptide (50 μg/ml) VTSAPDTRPAP (VTSA) or NANP–NVDP–NANP (NVDP). Values represent the mean stimulation index ± SD of 4 mice per group. Data representative of two independent experiments. Values (cpm) in mice immunized with γ1NV2VTSA3 are as follows: medium alone (1,205 ± 308), plus VTSA (85,555 ± 7,500); plus NVDP (89,170 ± 4,530). Values (cpm) in mice immunized with γ1NV2 are as follows: medium alone (1,205 ± 308), plus VTSA (1,566 ± 74); plus NVDP (70,250 ± 3,543). Values (cpm) in mice immunized with γ1VTSA3 are as follows: medium alone (1,605 ± 203), plus VTSA (1,444 ± 107); plus NVDP (1,205 ± 75). Values (cpm) in mice immunized with γ1NV2/γ1VTSA3 are as follows: medium alone (1,405 ± 75), plus VTSA (17,562 ± 1,251); plus NVDP (30,910 ± 78). (b) Injection of plasmids in two different sites abrogates the response to the cryptic determinant induced by Th–Th associative recognition (two sites experiment). Mice were immunized with the plasmids indicated. In the γ1NV2 + γ1VTSA3 group, mice were injected with the individual plasmids in two opposite sites of the spleen. Mice receiving γ1NV2 were also injected with 50 μl of sterile saline solution on the opposite site to account for hemodynamic effects. T cell proliferation was measured on cells harvested on day 14 that were cultured for 3 days on synthetic peptide (50 μg/ml) VTSAPDTRPAP (VTSA) or NANP–NVDP–NANP (NVDP). Values represent the mean stimulation index ± SD of 5 mice per group. Values (cpm) in mice immunized with γ1NV2VTSA3 are as follows: medium alone (2,716 ± 804), plus VTSA (47,051 ± 1,687); plus NVDP (22,516 ± 4,806). Values (cpm) in mice immunized with γ1NV2 are as follows: medium alone (3,302 ± 105), plus VTSA (3,963 ± 105); plus NVDP (21,137 ± 2,150). Values (cpm) in mice immunized with γ1NV2 + γ1VTSA3 (injection into two sites) are as follows: medium alone (2,545 ± 107), plus VTSA (2,799 ± 409); plus NVDP (16,445 ± 4,094). Experiment representative of three independent experiments.
Results
The Response to Subimmunogenic Th Determinants via Th–Th Cooperation.
In the course of pilot studies, we observed that anti-MUC-1 responses using transgenes coding for single MUC-1 determinants could not be induced. Experiments were then set to investigate the possibility of enhancing these responses through the positive effect imparted by a strong Th cell determinant in a way reminiscent of the enhancing effect exerted by CD4 T cells on CD8 T cell responses (22). To this end, we engineered transgenes coding for the sequence VTSAPDTRPAP of the tandem repeat domain VTSAPDTRPAPGSTAP of MUC-1 sequence along with the 12-mer sequence NANP–NVDP–NANP, referred to as NVDP or NV (ref. 2) of the circumsporozoite antigen of P. falciparum malaria parasite (15), a dominant Th cell determinant in H-2b mice (23). The resulting plasmid, γ1NV2VTSA3 (Fig. 1), induced a strong response against both determinants. We reasoned that because somatic transgene immunization is 100% effective in inducing primary effector CD4+ T cells (15), the anti-MUC-1 response triggered by “dual determinant” transgenes could reflect a form of Th–Th associative recognition.
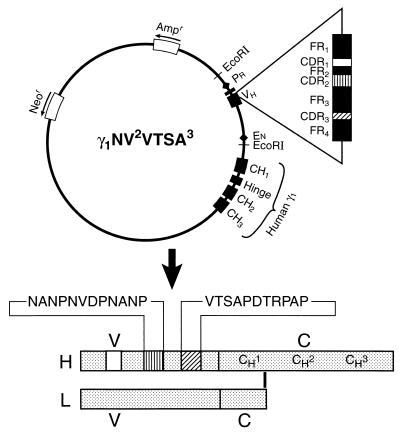
Figure 1.
Schematic representation of plasmid vector γ1NV 2VTSA3. This vector codes for a chimeric (mouse/human) H chain gene where The VH region is the 2.3-kb EcoRI fragment containing the rearranged VDJ of a murine VH7183 gene (14). The human γ1 constant region (CH1, CH2, and CH3) gene is in genomic configuration. Promoter (Pr) and enhancer (En) elements for tissue-specific expression and the neomycin (Neor) and ampicillin (Ampr) resistance genes are indicated. The VH region is mutagenized to code for two heterologous determinants, as indicated in the structure of the translated transgenic H chain product. The light chain is provided by the host cell. The amino acids NANP–NVDP–NANP (NVDP) in CDR2 and VTSAPDTRPAP (VTSA) in CDR3 are from the circumsporozoite protein of the malaria P. falciparum and MUC-1 antigens, respectively. CDR, complementarity-determining region; FR, framework region; H, heavy (chain); L, light (chain); V, variable region; C, constant region. Not to scale.
Lymphocytes of mice immunized with γ1VTSA3 did not proliferate against VTSAPDTRPAP and did not produce IL-2, IFN-γ, and IL-4, cytokines typically produced by primary effector CD4 T cells during somatic transgene immunization (15). In contrast, all mice vaccinated with the dual determinant transgene developed a strong and specific T cell response to VTSAPDTRPAP (MUC-1) (Fig. 2a). Lymphocytes restimulated in vitro with a different MUC-1 peptide did not proliferate. Because immunization with plasmid γ1NV2 coding for NANP–NVDP–NANP (malaria) in CDR2 failed to induce any reactivity against VTSAPDTRPAP (MUC-1) (not shown), cross-reactivity between VTSAPDTRPAP and NANP–NVDP–NANP could be ruled out. The effect was limited to CD4+ T cells as determined using separated CD4 and CD8 T cell populations (Fig. 2a Inset). As previously noted for the response against NANP–NVDP–NANP (15), anti-VTSAPDTRPAP CD4 T cells secreted IL-2, IFN-γ, and IL-4 proportionally to cell proliferation (not shown). CD4 effectors were not cytotoxic for peptide-pulsed RMAS (H-2b) (not shown). Provisionally, we concluded that associative recognition of the two Th cell determinants mediated the activation of precursor CD4 T cells specific for the immunologically silent VTSAPDTRPAP determinant.
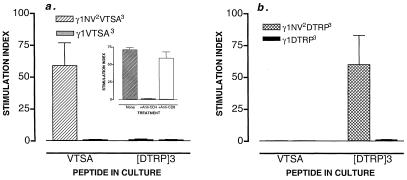
Figure 2.
Th–Th associative recognition triggers a response to the MUC-1 determinant VTSAPDTRPAP. (a) The proliferative response of spleen cells from C57BL/6 mice immunized with the plasmids indicated is shown. Spleen cells harvested on day 14 were cultured in RPMI with 7.5% FCS with synthetic peptide (50 μg/ml) VTSAPDTRPAP (VTSA) for 3 days at 37°C. Synthetic peptide [DTRP]3 served as a control. Values represent the mean stimulation index ± SD (above controls with no peptide) of 10 mice per group corresponding to two independent experiments. Values (cpm) in mice immunized with γ1NV2VTSA3 are as follows: medium alone (1,605 ± 203), plus VTSA (94,695 ± 3,450); plus [DTRP]3 (1,926 ± 105). Values (cpm) in mice immunized with γ1VTSA (3) are as follows: medium alone (1,605 ± 203), plus VTSA (1,444 ± 107); plus [DTRP]3 (1,427 ± 75). (Inset) T cell proliferation against the cryptic determinant in mice immunized with γ1NV2VTSA3 is restricted to CD4+ T cells. Fourteen days after DNA inoculation, spleen cell populations were prepared and depleted of CD8+ or CD4+ T cells by antibody plus complement. Unfractionated cells are shown as reference. Values represent the mean stimulation index ± SD of four mice per group. Values of cytokines in CD4+ and CD8+ populations were, respectively, IL-2 (1400 ± 70 pg/ml vs. <70), IFN-γ (438 ± 16 pg/ml vs. <50), and IL-4 (397 ± 78 pg/ml vs. <40). (b) Th–Th associative recognition triggers a response to the MUC-1 (DTRP)3 determinant. The proliferative response of spleen cells from C57BL/6 mice immunized with the plasmids indicated is shown. Spleen cells harvested on day 14 were cultured in RPMI with 7.5% FCS with synthetic peptide (50 μg/ml) (DTRP)3 for 3 days at 37°C. Synthetic peptide VTSAPDTRPAP (VTSA) served as a control. Values represent the mean stimulation index ± SD (above controls with no peptide) of 10 mice per group corresponding to two independent experiments. Values (cpm) in mice immunized with γ1NV2DTRP3 are as follows: medium alone (1,605 ± 203), plus VTSA (1,350 ± 126); plus [DTRP]3 (81,000 ± 2,747). Values (cpm) in mice immunized with γ1DTRP3 are as follows: medium alone (1,222 ± 107), plus VTSA (1,127 ± 98); plus [DTRP]3 (1,074 ± 132).
Serum levels of transgenic Ig were measured to determine whether the effect observed was due to different expression of the two transgenes in vivo. On day 15, the serum concentration was remarkably similar (28 ± 2 and 30 ± 1.6 ng/ml) for mice immunized with γ1VTSA3 (no. 4) and mice immunized with γ1NV2VTSA3 (no. 4). Thus, in vivo the synthetic machinery was similar for both transgenes. To rule out a bystander inhibitory effect by the VTSAPDTRPAP sequence, experiments were repeated using a different MUC-1 peptide sequence, the dodecapeptide DTRP–DTRP–DTRP ([DTRP]3) also from the tandem repeat domain VTSAPDTRPAPGSTAP. Whereas spleen lymphocytes of mice immunized with γ1DTRP3 did not proliferate against ([DTRP]3), mice immunized with the dual determinant transgene γ1NV2DTRP3 mounted a strong and specific T cell response against both dodecapeptides (Fig. 2b). It should be noted that the response against the subimmunogenic determinants cannot be explained on the basis of a differential effect by bacterial CpG in the plasmids because these are all identical except for the CDR2 and CDR3 coding regions.
Cellular Requirements in Th–Th Cooperation.
To ascertain the cellular requirements for Th–Th cooperation, we performed two additional experiments. In the first experiment, mice were injected with a mixture of plasmids γ1VTSA3 (coding for VTSAPDTRPAP in CDR3) and γ1NV2 (coding for NANP–NVDP–NANP in CDR2) as a single inoculum. The resulting anti-VTSAPDTRPAP (MUC-1) response was diminished by about 85% compared with that in mice immunized with the dual determinant transgene (Fig. 3a). The response to NANP–NVDP–NANP (malaria) was also decreased, albeit only 2- to 3-fold. In the second experiment, plasmids γ1VTSA3 and γ1NV2 were injected separately in two opposite sites along the longitudinal axis of the spleen. In this case, the response to VTSAPDTRPAP (MUC-1) was entirely abrogated, whereas the response to NANP–NVDP–NANP (malaria) was unmodified over controls (Fig. 3b). The production of cytokines was either decreased (coinjection) or abrogated (separate injections) to reflect the proliferative response (Table 1). Collectively, it seems that maximal response against the immunologically silent determinant occurs when the two Th determinants are in linked association, arguing in favor of processing and presentation of the two Th cell determinants by the same APC.
Table 1.
Cytokines in cultures from mice immunized with transgenes carrying individual Th cell determinants
| Exp.* | DNA | Peptide in culture
|
|||||
|---|---|---|---|---|---|---|---|
| VTSA
|
NVDP
|
||||||
| IL-2 | IFN-γ | IL-4 | IL-2 | IFN-γ | IL-4 | ||
| 1 | γ1NV2VTSA3 | 1612 ± 472 | 290 ± 52 | 290 ± 118 | 1825 ± 654 | 334 ± 68 | 368 ± 126 |
| γ1VTSA3 | <70 | <50 | <40 | <70 | <50 | <40 | |
| γ1NV2 | <70 | <50 | <40 | 1950 ± 430 | 233 ± 30 | 186 ± 83 | |
| γ1NV2/γ1VTSA3 | 862 ± 225 | 155 ± 53 | <40 | 1537 ± 166 | 209 ± 56 | <40 | |
| 2 | γ1NV2VTSA3 | 1182 ± 290 | 243 ± 48 | 235 ± 35 | 848 ± 219 | 243 ± 38 | 105 ± 26 |
| γ1NV2 | <70 | <50 | <40 | 1171 ± 508 | 815 ± 444 | 58 ± 16 | |
| γ1NV2 + γ1VTSA3 | <70 | <50 | <40 | 450 ± 125 | 120 ± 7 | <40 | |
Spleen cells were harvested on day 14 after DNA immunization. Cells were cultured for 3 days in the presence of synthetic peptide. Supernatants were tested for cytokine content as detailed in Materials and Methods. Cytokines are expressed in pg/ml. Symbols: /, coinjected (mixing experiment); +, separately injected.
Temporal Dynamics of the Response.
Analysis of the temporal dynamics of the responses against NANP–NVDP–NANP and VTSAPDTRPAP revealed a clear-cut difference. Whereas 3 days after DNA inoculation there was neither cell proliferation nor production of cytokines, by day 5 there was a marked Th cell response against NANP–NVDP–NANP (malaria) but not against VTSAPDTRPAP (MUC-1). By day 7, all mice responded to both determinants, albeit the response against the dominant determinant was of greater magnitude (Fig. 4). The production of cytokines reflected the magnitude and the specificity of the proliferative responses (Table 2). The data indicate, therefore, that during Th–Th cooperation, the reactivity against the subimmunogenic determinant is delayed of approximately 48 h over that against the dominant determinant. The fact that the two responses are specific and refracted in time argues against the possibility that responsiveness against the subimmunogenic determinant results from a bystander effect due to the cytokines produced in response to the dominant determinant.
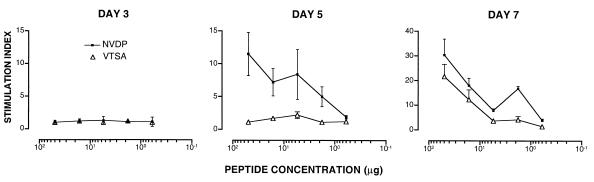
Figure 4.
The temporal kinetics of Th–Th associative recognition. C57BL/6 mice were immunized with plasmid γ1NV2VTSA3, and the proliferative response of spleen cells was measured at the time of harvest indicated. The concentration (μg/ml) of synthetic peptide VTSA or NVDP in culture is indicated. Values represent the mean stimulation index ± SD of 4 mice per group.
Table 2.
Temporal dynamics of cytokine production
| Day | μg/ml | Peptide in culture
|
|||||
|---|---|---|---|---|---|---|---|
| VTSA
|
NVDP
|
||||||
| IL-2 | IFN-γ | IL-4 | IL-2 | IFN-γ | IL-4 | ||
| 3 | 50 | <70 | <50 | <40 | <70 | <50 | <40 |
| 16.5 | <70 | <50 | <40 | <70 | <50 | <40 | |
| 5.5 | <70 | <50 | <40 | <70 | <50 | <40 | |
| 1.8 | <70 | <50 | <40 | <70 | <50 | <40 | |
| 5 | 50 | <70 | <50 | <40 | 298 ± 65 | 1835 ± 224 | 80 ± 67 |
| 16.5 | <70 | <50 | <40 | 160 ± 55 | 2170 ± 340 | 116 ± 67 | |
| 5.5 | <70 | <50 | <40 | 125 ± 32 | 568 ± 1076 | <40 | |
| 1.8 | <70 | <50 | <40 | 54 ± 13 | 225 ± 391 | <40 | |
| 7 | 50 | 1197 ± 689 | 466 ± 137 | <40 | 1112 ± 484 | 1438 ± 247 | <40 |
| 16.5 | 455 ± 204 | 159 ± 119 | <40 | 1082 ± 383 | 607 ± 256 | <40 | |
| 5.5 | 349 ± 236 | <50 | <40 | 535 ± 168 | 84 ± 84 | <40 | |
| 1.8 | 57 ± 12 | <50 | <40 | 250 ± 74 | <50 | <40 | |
Mice were immunized with plasmid γ1NV2VTSA3. Spleen cells were harvested on day 14 after DNA immunization. Cells were cultured for 3 days in the presence of synthetic peptide. Supernatants were tested for cytokine content as detailed in Materials and Methods. Values correspond to experiment shown in Fig. 4. Cytokine levels are expressed in pg/ml.
A Role for CD40 and OX40 in Th–Th Cooperation.
Optimal priming of CD4 T cells requires contact-dependent interactions with APC. After ligation of the T cell receptor by the MHC–peptide complex, antigen-independent signals are delivered through binary molecular interactions between the membrane of the APC and the T cell (24). In somatic transgene immunization, the transgene is productively harbored in splenic B cells (12), making it plausible that B cells are likely to serve as the initial APC to present peptides of the endogenously synthesized Ig heavy (H) chain transgene (25). This position is strengthened by the recent report that resting small B cells are able to present self-idiotype peptides to CD4 T cells in vivo (26). During a T–B interaction, up-regulation of CD40L on T cells begins 3–5 h after recognition of antigen (27). This promotes ligation of CD40 on the B cell, which results within 24 h in up-regulation of costimulatory molecules such as B7.2 and OX-40L on the same cell (28). The net result is a rapid heightening of the costimulatory potential between interacting cells. Therefore, in a model where the effects of Th–Th cooperation are mediated by augmented costimulation, signaling through the CD40–CD40L and OX40–OX40L receptor–ligand pairs could be relevant.
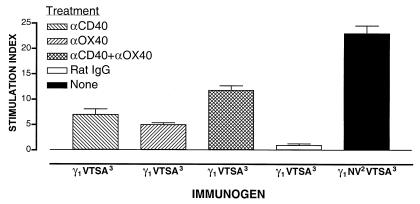
To probe the involvement of CD40 and OX40 and to see whether CD4 help could be replaced by signaling through these molecules, mice immunized with plasmid γ1VTSA3 were treated with monoclonal antibody FGK45, a CD40 agonist, monoclonal antibody OX86, an OX40 agonist, or a combination of both. Ligation of CD40 resulted in a clear T cell response against VTSAPDTRPAP (MUC-1) (Fig. 5) with production of IL-2 (266 ± 81 pg/ml) and IFN-γ and IL-4 below detectable levels. Thus, ligation of CD40 at the time of priming mimicked in part (≈30%) the effect of Th–Th cooperation on the response against the immunologically silent determinant. The reaction was specific for CD4 T cells in that proliferation occurred only in the separated CD4 cell population (not shown). Not surprisingly, blockade of CD40L with the anti-CD40L monoclonal antibody MR1 in mice immunized with the dual determinant transgene abrogated both the response to the cryptic as well as to dominant determinant by 98% and 97% (not shown). Consequently, it became impossible to discriminate between priming of the dominant T cell population and help for the cryptic determinant. The inhibitory effect was not related to blockade of the secretion of transgenic Ig, as anti-CD40L-treated and control groups had comparable serum values (42 ± 10 vs. 36.6 ± 4.7 ng/ml). Treatment with an isotype-matched monoclonal antibody induced no effect.
Figure 5.
Ligation of CD40 or OX40 mediates a T cell response against a cryptic MUC-1 determinant. C57BL/6 mice were immunized with plasmid γ1VTSA3 and treated with either 100 μg of the CD40-activating monoclonal antibody FGK45 (20) intravenously on days 1 and 5, or 100 μg of the OX40-activating monoclonal antibody OX86 (21) intraperitoneally on day 2. In the combination treatment, mice were injected with 100 μg of antibody FGK45 intravenously on days 1 and 5, and 100 μg of antibody OX86 intraperitoneally on day 2 for a total of three injections. Control mice were similarly injected with rat IgG. Mice immunized with plasmid γ1NV2VTSA3 did not receive any treatment and served as a positive control for the response against the cryptic determinant. Each group consisted of four mice. Values represent the stimulation index ± SD of the proliferative response of spleen cells harvested on day 7. Mice were immunized at the same time to avoid intertest variations. Values (cpm) in mice immunized with γ1VTSA3 are as follows: treated with anti-CD40: medium alone (931 ± 37), plus VTSA (6,537 ± 951); treated with anti-OX40: medium alone (1,318 ± 233), plus VTSA (6,562 ± 987); treated with anti-CD40 plus anti-OX40: medium alone (834 ± 89), plus VTSA (9,835 ± 538); and treated with rat IgG: medium alone (538 ± 63), plus VTSA (541 ± 77). Values (cpm) in mice immunized with γ1 NV2VTSA3 are as follows: medium alone (1,953 ± 21), plus VTSA (45,292 ± 3,524).
The results with anti-CD40 suggested that the likely mechanism of action of the dominant epitope was to increase the costimulatory ability of the APC by providing CD40 signals from CD40L. Because recent results show that OX40 represents an important costimulatory signal for a CD4 response (29, 30), we hypothesized that targeting OX40 on the T cell would bypass the requirement for CD40 signals and directly induce a response against the cryptic determinant. Ligation of OX40 by treatment with the anti-OX40 monoclonal antibody OX86 also resulted in a clear T cell response against VTSAPDTRPAP (MUC-1) (Fig. 5) with production of IL-2 (509 ± 122 pg/ml) and IFN-γ and IL-4 below detectable levels. Thus, ligation of OX40 at the time of priming mimicked in part (≈22%) the effect of Th–Th cooperation on the response against the immunologically silent determinant. As shown, neither ligation of CD40 nor of OX40 succeeded in completely reproducing the effect of the dominant determinant in driving the response against the cryptic determinant. We then tested the possibility that the simultaneous ligation of CD40 and OX40 could yield a response against the cryptic determinant comparable to that generated through the effect of Th–Th cooperation. A combination treatment produced a greater effect than a single treatment, but this was only about 50% of the response against the same determinant obtained through Th–Th cooperation (Fig. 5). Cells restimulated in culture with VTSAPDTRPAP (MUC-1) peptide produced IL-2 (482 ± 163 pg/ml) but not IFN-γ or IL-4. By comparison, the cultures from mice immunized with the dual determinant transgene produced IL-2 (646 ± 21 pg/ml) and IFN-γ (455 ± 22 pg/ml) but not IL-4. Taken together, the results indicate that Th–Th cooperation is dependent, at least in part, on a threshold costimulatory potential created by T cells activated by the dominant determinant and that CD40 and OX40 may both be involved.
Discussion
The results presented are consistent with the view that cooperation between two Th cells can occur in a way analogous to cooperation between CD4 and CD8 T cells. From a functional standpoint Th–Th cooperation allows one to turn an immunologically silent Th determinant into an immunogenic one. T help for helpers, as the present experiments suggest, is an important new principle to further our understanding on reciprocal regulation among hierarchically distinct Th cell determinants (3) in the adaptive immune response.
The reasons for differential immunogenicity of Th cell determinants, separate activation of CD4 T cells, and the role of APC in this phenomenon can only be speculated. Our data suggest that Th–Th cooperation requires that the two Th determinants be presented by the same APC. As documented, coinjection of two “single determinant” transgenes yielded a poor response against the subimmunogenic determinant, implying that the modest effect was probably sustained by a small number of doubly transfected B cells. Whereas a direct role by the dominant determinant in driving the response to the subimmunogenic determinant is not disputed, the difference in the kinetics of the response (Fig. 4) could reflect a difference in (i) precursor frequency for each of the two determinants, (ii) affinity of the two peptides for MHC class II molecules, or (iii) ability to activate APC. Whereas precursor frequency in both instances may be low, peptide affinity and differential activation of APC may both be involved. In fact, activation of naïve CD4 T cells is determined by the balance between the costimulatory potential and the affinity of peptide–MHC complex for the T cell receptor (31). Whatever the case may be, our data imply that the same APC can process and present two Ig V region determinants without negative effects by the dominant peptide on processing (e.g., truncation) or binding to the MHC molecule of the subimmunogenic peptide.
Our results suggest that Th–Th cooperation may require APC activation through the CD40–CD40L receptor–ligand pair. This is reminiscent of the demonstration that a CD40–CD40L interaction is involved in Th–cytotoxic T lymphocyte cooperation and that CD40 ligation can substitute for T cell help in the activation of CD8 T lymphocytes (32). In addition, recent data have shown that OX40 is also an important costimulatory molecule in the CD4 response (29, 30, 33, 34) and that CD40 ligation increases OX40L expression (35). Our data show that in vivo ligation of OX40 on T cells also mediates a response to the cryptic determinant. Therefore, we propose that the mechanism of help induced by the dominant Th cell determinant is to promote APC activation through CD40, which in turn up-regulates molecules such as B7–1 and B7–2 and also OX40. The combined action of these molecules then allows the subimmunogenic determinant to be presented in such a manner that it is now immunogenic. Taken together, our experiments and interpretation suggest that Th–Th cooperation is consistent with a three-cell model in which the T cell response to the dominant determinant results in APC activation via up-regulation of CD40L on the T cell and ligation of CD40 on the APC. This is followed, among other changes, by up-regulation of OX40L on the APC and heightens the threshold of costimulation to a point that presentation of, and response to, the cryptic determinant by a second CD4 T cell becomes possible. Although we know of no precedent for antigen-specific Th–Th cooperation in vivo, a form of bystander Th–Th cooperation via dendritic cell activation can be inferred from earlier in vitro studies (36). It is possible, however, that a form of Th–Th cooperation as the one described here may readily occur in response to large multivalent antigen without having been previously identified.
The present results offer a new way of looking at the behavior of weak Th cell determinants and strategies to induce effector CD4 T cells and overcome tolerance. Recently, it was shown that CD4 T cell tolerance can be overcome by CD40 (32), and similarly OX40 ligation can also prevent and reverse tolerance (M.C., unpublished results), suggesting that a defect in costimulation and APC function is associated with the poor immunogenicity of antigens. Th–Th cooperation applies, therefore, to the design of new immunotherapeutic vaccines that intend to induce CD4 T cell immunity because, as shown here, an increase in the costimulatory potential via CD40 or OX40 is an effective way to overcome the refractoriness of the immune system vis-à-vis a cryptic determinant. It is interesting to note that the combined anti-CD40 and anti-OX40 treatment yielded a better response against the cryptic determinant than single ligation treatments, suggesting a synergistic effect in vivo. However, not even the combined ligation of CD40 and OX40 was as effective in providing help as the dominant determinant in the transgene. This shows that immunization with the dual determinant transgene via Th–Th cooperation is a better way to promote immunity against poorly immunogenic determinants and implies that in all likelihood there is no better help for a helper cell than the one provided through a dominant Th cell determinant.
Exploitation of Th–Th cooperation may be applicable to cancer and other situations in which CD4 immunity is deficient, such as in the course of HIV infection and in neonatal immunity. As a new immunological principle, it also provides new theoretical ground to understand termination of tolerance against self-antigens and the initiation of CD4-mediated autoreactivity, not only through cross-reactivity with exogenous antigens but also from the random insertion and assortment of self- and viral sequences in the genome (37). It also corroborates the view that T cell autoreactivity against cryptic determinants can originate from determinant spreading, a form of intramolecular amplification cascade in which determinants that are cryptic after primary immunization become immunogenic as the disease progresses (38).
Acknowledgments
We thank Kent T. Miner for the valuable help provided. This work was supported by National Institutes of Health Grant R01 CA77427 and a grant from the Elsa U. Pardee Foundation (to M.Z.), National Institutes of Health Grant AI 36259 (to M.C.), and American Cancer Society Grant CIM-97369 (to S.P.S.).
Abbreviations
- APC
antigen-presenting cells
- CDR
complementarity-determining region
- Th
T helper
Footnotes
See commentary on page 12950.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230429197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230429197
References
- 1.Mitchison N A. Eur J Immunol. 1971;1:18–27. doi: 10.1002/eji.1830010104. [DOI] [PubMed] [Google Scholar]
- 2.Cassell D, Forman J. Ann NY Acad Sci. 1988;532:51–60. doi: 10.1111/j.1749-6632.1988.tb36325.x. [DOI] [PubMed] [Google Scholar]
- 3.Sercarz E E, Lehmann P V, Ametani A, Benichou G, Miller A, Moudgil K. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 4.Gendler S J, Spicer A P. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 5.Graham R A, Taylor-Papadimitriou J. In: Tumor Immunology: Immunotherapy and Cancer Vaccines. Dalgleish A G, Browning M J, Sikova K, editors. New York: Cambridge Univ. Press; 1996. pp. 269–286. [Google Scholar]
- 6.Jerome K R, Domenech N, Finn O J. J Immunol. 1993;151:1654–1662. [PubMed] [Google Scholar]
- 7.Ioannides C G, Fisk B, Jerome K R, Irimura T, Wharton J T, Finn O J. J Immunol. 1993;151:3693–3703. [PubMed] [Google Scholar]
- 8.Finn O J, Jerome K R, Henderson R A, Pecher G, Domenech N, Maagarian-Blander J, Barratt-Boyes S M. Immunol Rev. 1995;145:61–89. doi: 10.1111/j.1600-065x.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 9.Tempero R M, VanLith M L, Morikane K, Rowse G J, Gendler S J, Hollingsworth M A. J Immunol. 1998;161:5500–5506. [PubMed] [Google Scholar]
- 10.Pecher G, Finn O J. Proc Natl Acad Sci USA. 1996;93:1699–1704. doi: 10.1073/pnas.93.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanetti M, Gerloni M, Xiong S. The Immunologist. 1999;7:79–84. [Google Scholar]
- 12.Xiong S, Gerloni M, Zanetti M. Proc Natl Acad Sci USA. 1997;94:6352–6357. doi: 10.1073/pnas.94.12.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanetti M. Nature (London) 1992;355:466–477. [Google Scholar]
- 14.Gerloni M, Ballou W R, Billetta R, Zanetti M. Nature Biotech. 1997;15:876–881. doi: 10.1038/nbt0997-876. [DOI] [PubMed] [Google Scholar]
- 15.Gerloni M, Miner K T, Xiong S, Croft M, Zanetti M. J Immunol. 1999;162:3782–3789. [PubMed] [Google Scholar]
- 16.Ulmer J B, Sadoff J C, Liu M A. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 17.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S L, Spiegelberg H L, Carson D A. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong S, Gerloni M, Zanetti M. Nat Biotechnol. 1997;15:882–886. doi: 10.1038/nbt0997-882. [DOI] [PubMed] [Google Scholar]
- 19.Gerloni M, Billetta R, Xiong S, Zanetti M. DNA Cell Biol. 1997;16:611–625. doi: 10.1089/dna.1997.16.611. [DOI] [PubMed] [Google Scholar]
- 20.Noelle R J. Proc Natl Acad Sci USA. 1992;89:6550–6554. [Google Scholar]
- 21.al-Shamkhani A, Birkeland M L, Puklavec M, Brown M H, James W, Barclay A N. Eur J Immunol. 1996;26:1695–1699. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- 22.Keene J A, Forman J. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardin E H, Nussenzweig R S. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 24.Grewal I S, Flavell R A. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 25.Weiss S, Bogen B. Cell. 1991;64:767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- 26.Munthe L A, Kyte J A, Bogen B. Eur J Immunol. 1999;29:4043–4052. doi: 10.1002/(SICI)1521-4141(199912)29:12<4043::AID-IMMU4043>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Jaiswal A I, Croft M. J Immunol. 1997;159:2282–2291. [PubMed] [Google Scholar]
- 28.Croft M, Dubey C. Crit Rev Immunol. 1997;17:89–118. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- 29.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann M F. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 30.Murata K, Ishii N, Takano H, Miura S, Ndhlovu L C, Nose M, Noda T, Sugamura K. J Exp Med. 2000;191:365–374. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers P R, Grey H M, Croft M. J Immunol. 1998;160:3698–3704. [PubMed] [Google Scholar]
- 32.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. Nature (London) 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 33.Gramaglia I, Weinberg A D, Lemon M, Croft M. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 34.Gramaglia I, Jember A, Pippig S D, Weinberg A D, Killeen N, Croft M. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 35.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- 36.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klenerman P, Hengartner H, Zinkernagel R M. Nature (London) 1997;390:298–301. doi: 10.1038/36876. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann P V, Forsthuber T, Miller A, Sercarz E E. Nature (London) 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]