Abstract
Background. Aortic valve sclerosis (AVS) and mitral annulus calcification (MAC) are highly prevalent in patients with end-stage renal disease. It is less well established whether milder kidney disease is associated with cardiac calcifications. We evaluated the relationships between renal function and MAC, aortic annular calcification (AAC) and AVS in the elderly.
Methods. From the Cardiovascular Health Study, a community-based cohort of ambulatory adults ≥ age 65, a total of 3929 individuals (mean ± SD age 74 ± 5 years, 60% women) were evaluated with two-dimensional echocardiography. Renal function was assessed by means of creatinine-based estimated glomerular filtration rate (eGFR) and cystatin C.
Results. The prevalences of MAC and AAC were significantly higher in individuals with an eGFR < 45 mL/ min/1.73 m2 (P < 0.01 for each), and cystatin C levels were significantly higher in individuals with MAC or AAC compared to individuals without these cardiac calcifications (P < 0.001 for each). After multivariate-adjustment, an eGFR <45 mL/min/1.73 m2 was significantly associated with MAC [odds ratio 1.54 (95% CI 1.16–2.06), P = 0.003] and not associated with AAC [1.30 (0.97–1.74), P = 0.085] and AVS [1.15 (0.86–1.53), P = 0.355]. In addition, cystatin C levels were independently associated with MAC [odds ratio per SD 1.12 (1.05–1.21), P = 0.001] and not associated with AAC [1.07 (1.00–1.15), P = 0.054] and AVS [0.99 (0.93–1.06), P = 0.82]. Furthermore, the prevalence of multiple cardiac calcifications was higher in subjects with an eGFR < 45 mL/ min/1.73 m2 and increased per quartile of cystatin C (P-values < 0.001). In addition, a significant trend was observed between an eGFR < 45 mL/min/1.73 m2, increasing levels of cystatin C and the number of cardiac calcifications (P < 0.05).
Conclusions. In a community-based cohort of the elderly, moderate kidney disease as defined by an eGFR <45 mL/min/1.73m2 and elevated levels of cystatin C was associated with prevalent MAC. In addition, a significant trend was observed between an eGFR <45 mL/min/1.73m2, increasing levels of cystatin C and the number of cardiac calcifications. No associations were found between renal function and AAC or AVS.
Keywords: chronic kidney disease, cohort, creatinine, cystatin C, elderly
Introduction
Cardiovascular disease is the most common cause of morbidity and mortality in patients with chronic kidney disease [1]. This increased risk is even present in individuals with mild to moderate reductions in renal function [2]. Several mechanisms might be responsible for this increased risk. The association between chronic kidney disease and mortality may be explained by an increased prevalence and severity of cardiovascular risk factors, or be an outcome of an underlying pathological process that affects both renal function and risk of cardiovascular mortality (for example, atherosclerosis). The association between renal function and cardiovascular disease may also reflect a risk factor induced by impaired renal function, which then causes cardiovascular disease, such as hypertension, an elevated inflammatory state or altered calcium and phosphorus metabolism leading to cardiac calcifications.
Cardiac calcifications such as mitral annular calcification (MAC), aortic annular calcification (AAC) or aortic valve sclerosis (AVS) are common in individuals with ESRD [3] and cardiac and vascular calcifications are important predictors of all-cause and cardiovascular mortality in ESRD patients [4–6]. However, the association of less severe renal dysfunction with cardiac calcifications is less well established, particularly among older adults in whom cardiac calcifications are most common. In the Jackson Cohort of the Atherosclerotic Risk in Communities Study [7], the Framingham Offspring Study [8] and the Multi-Ethnic Study of Atherosclerosis (MESA) [9], cross-sectional associations were seen between chronic kidney disease and MAC. In addition, the Cardiovascular Health Study (CHS) showed a higher prevalence of chronic kidney disease in older adults with combined MAC, AAC and AVS (P = 0.04) [10]. However, in the Framingham Offspring study, chronic kidney disease was not associated with AVS or AAC [8] and a borderline association was detected between AVS and kidney function in MESA [9]. Except for MESA, all studies used creatinine to define chronic kidney disease. The Jackson cohort defined chronic kidney disease as serum creatinine >1.6 mg/dL for men and >1.4 mg/dL for women; the Framingham Offspring study defined chronic kidney disease as a creatinine-based estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2, and CHS as serum creatinine level ≥1.5 mg/dL.
The associations of other more sensitive markers of chronic kidney disease, such as cystatin C, and cardiac calcifications have not been extensively evaluated. An exception is MESA that demonstrated that cystatin C is associated with MAC predominantly in individuals with diabetes [9]. However, MESA used computed tomography to assess MAC and AVS instead of echocardiography [9]. Cystatin C is a 122-amino acid, 13 kD protein produced by nearly all human cells and belongs to the competitive inhibitors of cysteine proteinases. The protein is freely filtered by the renal glomerulus with no reabsorption into the bloodstream and approximates direct measures of GFR more precisely than creatinine [11]. In the present study, we evaluated the association between renal function, as assessed by eGFR and cystatin C, and MAC, AAC and AVS in 3929 older adults from CHS.
Patients and methods
The CHS is a prospective observational cohort designed to assess risk factors and cardiovascular outcomes in older adults. Participants aged ≥65 years were sampled from Medicare enrollment lists in four US communities. Persons were excluded from the CHS if they were wheel-chair bound or institutionalized, were receiving active treatment for cancer or were unable to participate in the examination. The study was approved by the institutional review board at each participating centre, and informed consent was obtained. The design, rationale and examination details of the CHS have been published elsewhere [12]. Between 1989 and 1990, an initial CHS cohort of 5201 participants was enrolled [13], and a second cohort of African American individuals (n = 687) was enrolled between 1992 and 1993. Serum cystatin C and serum creatinine levels were measured in participants who attended the annual study visit in 1992 or 1993 and from whom serum was available (n = 4637) [14]. A total of 4029 participants had an echocardiogram performed in the 1994–95 examination cycle [10]. We excluded 42 participants with significant aortic stenosis and 58 participants with inadequate images for evaluation of aortic and mitral calcification. In total, 3929 individuals were eligible for analyses [10].
Echocardiography
Two-dimensional echocardiographic studies were recorded on videotape with the use of a cardiac ultrasound machine (model SSH-160A, Toshiba, Tustin, CA, USA) as previously described [15].
MAC was defined by an intense echocardiograph-producing structure located at the junction of the atrioventricular groove and posterior mitral leaflet on the parasternal long-axis, apical four-chamber or parasternal short-axis view. Severity was qualitatively determined in parasternal short-axis view at the level of the mitral annulus as mild (focal, limited increased echodensity of the mitral annulus), moderate (marked echodensity involving more than one-third but less than half of the ring circumference) or severe (marked echodensity involving half or more of the circumference of the ring or with intrusion of the calcification into the left ventricular inflow tract). Mildly calcified mitral annulus was found in 1495 participants, moderate in 133 and severe in 12 individuals [10]. The three classes of calcification severity were combined to create a binary outcome.
AAC was defined as increased echodensity of the aortic root at the insertion of the aortic cusps. The severity was qualitatively determined as mild (limited increased echodensity of aortic annulus), moderate (extensive echodensity involving more than half of ring circumference, but with preservation of leaflet mobility) and severe (extensive echodensity involving entire circumference of aortic ring, and with limitation of leaflet excursion). There were 1698 participants with mild, 49 with moderate and 1 with severe AAC [10]. The three classes of calcification severity were combined to create a binary outcome. In total, in 163 patients out of the 4029 with available echocardiograms, AAC could not be assessed due to technical reasons.
AVS was identified by aortic cusp thickening, normal aortic cusp excursion and peak trans-aortic valve flow velocity <2.0 m/s. AVS was found in 2114 participants [10].
Laboratory methods
Serum creatinine was measured using the Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, NY, USA), a colorimetric method. The mean coefficient of variation for monthly controls was 1.94% (range 1.16–3.90). Creatinine-based eGFR was calculated by the simplified modification of diet in renal disease (MDRD) equation, defined as GFR = 186.3 × (serum creatinine)−1.154 × age−0.203 × (0.742 for women) × (1.212 if black) [16,17]. eGFR was categorized into three groups: >60 mL/min per 1.73 m2, 45–59 mL/min per 1.73 m2 and <45 mL/min per 1.73 m2. Stages 1 and 2 of the K/DOQI guidelines [18] were combined in the current study due to the increasing uncertainty at values >60 mL/min/1.73 m2 as stated by the UK consensus conference on early chronic kidney disease [19], and the lack of albuminuria and haematuria measurements in the current study. The use of stages 3A and 3B has been advocated previously in this Journal [20] and we combined stages 3B, 4 and 5 due to the small number of subjects in stages 4 and 5 (n = 25). Serum creatinine in the MDRD study and subsequently in a subset of individuals included in the Third National Health and Nutrition Examination Survey were both analysed at the Cleveland Clinic Laboratory. Because creatinine values vary across clinical laboratories, we calibrated the creatinine concentration in our current study to the Cleveland Clinic Laboratory indirectly as previously described [21]. Cystatin-C was measured using a BNII nephelometer (Dade Behring Inc., Deerfield, IL, USA) and a particle-enhanced immunonephelometric assay (N Latex Cystatin-C, Dade Behring) [22]. The assay range is 0.195–7.330 mg/L, with the reference range for young, healthy individuals reported as 0.53–0.95 mg/L. Intra-assay coefficients of variation (CVs) range from 2.0 to 2.8% and inter-assay CVs range from 2.3 to 3.1%.
Statistical analysis
Continuous data were reported as mean ± standard deviation or median (interquartile range) if the data were skewed. Categorical data were presented as percentages. Differences between subgroups were evaluated by Student's t-test for the normally distributed continuous variables, or with the Mann–Whitney test if data were skewed. Differences in categorical data were compared with the χ2 test. Logistic regression analysis was used to calculate the odds ratio (OR) with 95% CI for having MAC, AAC or AVS. The OR of cardiac calcifications was estimated for individuals with a mild decrease in eGFR (45–59 mL/min per 1.73 m2) and for those with a moderate decrease in eGFR (<45 mL/min per 1.73 m2) using individuals with normal eGFR (>60 mL/min per 1.73 m2) as the referent group. Cystatin C was included into the model both continuously (per SD increase) as in quartiles. P-values were calculated for ordinal trend across the different categories of the kidney function measurements. Except for age, gender and race which were forced into all models, covariates were identified with use of a model that included cystatin C as a predictor of each outcome; each candidate variable was entered separately, and variables that changed the parameter estimate (beta coefficient) of cystatin C by 5% or more were retained in the final model [14]. For each outcome, the same covariates were entered into the models for eGFR, and cystatin C. Reference groups without the condition of interest were allowed to contain either one or both of the other two forms of cardiac calcification as done previously in CHS [10]. A Poisson regression analysis was used to analyse the association between kidney function measurements and number of cardiac calcifications (one, two or three). Statistical analyses were performed with SPSS 14.0 software for Windows (SPSS Inc., Chicago, IL, USA) and STATA 9.0 (College Station, TX, USA). A P-value < 0.05 was considered statistically significant.
Results
Cardiac calcifications were relatively common in these 3929 older adults (age 74 ± 5 years, 60% women): MAC was present in 42%, AAC in 44% and AVS in 54% of the individuals. The characteristics of individuals with and without the specific cardiac calcifications are shown in Table 1. Individuals with MAC, AAC and AVS were significantly older and more likely to use anti-hypertensive medication and have prevalent cardiovascular disease (P < 0.05). In addition, participants with MAC were more likely to be white and had higher blood pressure levels, LDL-cholesterol levels, waist to hip ratio, fibrinogen levels and prevalence of diabetes (P < 0.05). Participants with AVS were more likely to be male and had higher systolic blood pressure, lower HDL-cholesterol and higher waist to hip ratio (P < 0.05). The small differences in participant characteristics (e.g. blood pressure, age) were statistically significant, but may not be clinically relevant.
Table 1.
Characteristics of participants with and without mitral annular calcifications (MAC), aortic annular calcifications (AAC) and aortic valve sclerosis (AVS)
| MAC | AAC | AVS | ||||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | |
| N = 2289 | N = 1640 | N = 2101 | N = 1710 | N = 1815 | N = 2114 | |
| Age (years) | 73.7 ± 4.6 | 75.3 ± 5.3* | 73.6 ± 4.6 | 75.2 ± 5.2* | 74.1 ± 5.0 | 74.6 ± 5.0* |
| Male | 41% | 39% | 39% | 41% | 37% | 43%* |
| White | 57% | 43%* | 54% | 46%* | 47% | 53% |
| Black | 65% | 35% | 59% | 41% | 43% | 57% |
| Systolic blood pressure (mmHg) | 135 ± 21 | 137 ± 21* | 135 ± 21 | 137 ± 21 | 135 ± 21 | 137 ± 22* |
| Diastolic blood pressure (mmHg) | 72 ± 11 | 71 ± 12* | 72 ± 11 | 71 ± 11 | 71 ± 11 | 72 ± 11 |
| Anti-hypertensive medication | 46% | 53%* | 46% | 51%* | 47% | 51%* |
| Cholesterol (mg/dL) | 208 ± 37 | 212 ± 40* | 209 ± 36 | 210 ± 40 | 209 ± 37 | 210 ± 39 |
| LDL-cholesterol (mg/dL) | 126 ± 33 | 130 ± 33* | 127 ± 33 | 129 ± 32 | 127 ± 33 | 129 ± 33 |
| HDL-cholesterol (mg/dL) | 54 ± 15 | 53 ± 14 | 54 ± 15 | 53 ± 14 | 54 ± 15 | 53 ± 14* |
| Triglycerides (mg/dL) | 143 ± 83 | 147 ± 90 | 144 ± 82 | 145 ± 91 | 144 ± 83 | 145 ± 89 |
| Lipid-lowering medication | 7.3% | 8.9% | 7.2% | 9.1% | 7.4% | 8.4% |
| Body mass index | 26.8 ± 4.5 | 27.1 ± 4.7 | 27.0 ± 4.6 | 26.9 ± 4.5 | 27.0 ± 4.8 | 26.9 ± 4.5 |
| Waist to hip ratio | 0.94 ± 0.08 | 0.95 ± 0.07* | 0.94 ± 0.08 | 0.95 ± 0.08 | 0.94 ± 0.08 | 0.95 ± 0.08* |
| Ever smoker | 53% | 53% | 54% | 51% | 52% | 54% |
| History of diabetes | 13% | 16%* | 14% | 15% | 14% | 15% |
| C-reactive protein (mg/L) | 2.5 (1.2–5.7) | 2.6 (1.2–5.7) | 2.5 (1.2–5.7) | 2.6 (1.2–5.6) | 2.7 (1.3–5.9) | 2.5 (1.1–5.6) |
| Haemoglobin | 13.7 ± 1.3 | 13.7 ± 1.3 | 13.8 ± 1.3 | 13.7 ± 1.3 | 13.7 ± 1.3 | 13.7 ± 1.3 |
| Fibrinogen | 323 ± 64 | 329 ± 67* | 324 ± 65 | 328 ± 66 | 325 ± 65 | 326 ± 65 |
| Prevalent cardiovascular disease | 25% | 33%* | 25% | 31%* | 26% | 30%* |
MAC, AAC and AVS were defined as present or absent, i.e. summing mild, moderate or severe versus none.
*P < 0.05.
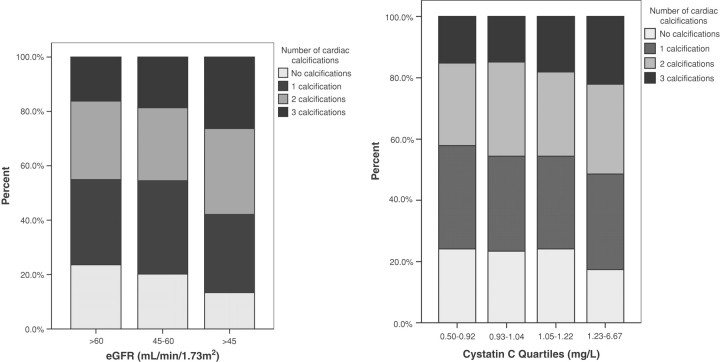
In unadjusted analyses (Table 2), the prevalences of MAC and AAC were significantly higher in individuals with moderate kidney disease as defined by an eGFR < 45 mL/ min/1.73 m2 (P < 0.001 and P = 0.008, respectively) and levels of cystatin C were significantly higher in individuals with MAC and AAC in comparison to individuals without (P < 0.001 for each). In addition, as shown in Figure 1, the prevalence of multiple cardiac calcifications was higher in subjects with an eGFR < 45 mL/ min/1.73 m2 and increased per quartile of cystatin C (P-value <0.001). Significant differences were not seen for eGFR or cystatin C and AVS. After multivariable adjustment, an eGFR < 45 mL/min/1.73 m2 was significantly associated with prevalent MAC [multivariable OR = 1.54 (95% CI = 1.16–2.06), P = 0.003] and not associated with AAC [1.30 (0.97–1.74), P = 0.085] and AVS [1.15 (0.86–1.53), P = 0.355] (Table 3). In addition, a significant trend was observed between an eGFR <45 mL/min/1.73 m2 and the number of cardiac calcifications as shown in Table 5. If eGFR was evaluated in quartiles, no significant associations were seen between quartiles of eGFR and prevalent MAC, AAC or AVS (data not shown).
Table 2.
Renal function parameters of participants with and without mitral annular calcifications (MAC), aortic annular calcifications (AAC) and aortic valve sclerosis (AVS)
| MAC | AAC | AVS | ||||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | |
| N = 2289 | N = 1640 | N = 2101 | N = 1710 | N = 1815 | N = 2114 | |
| eGFR | ||||||
| >60 mL/min/1.73 m2 | 59.5% | 40.5% | 56.3% | 43.7% | 46.6% | 53.4% |
| 45–60 mL/min/1.73 m2 | 57.9% | 42.1% | 54.0% | 46.0% | 45.1% | 54.9% |
| <45 mL/min/1.73 m2 | 43.9% | 56.1%* | 45.4% | 54.6%* | 40.7% | 59.3% |
| eGFR (mL/min/1.72 m2) | 70.2 ± 16.1 | 69.4 ± 17.5 | 70.2 ± 16.1 | 69.6 ± 17.3 | 70.0 ± 16.2 | 69.8 ± 17.1 |
| Cystatin C (mg/L) | 1.07 ± 0.25 | 1.12 ± 0.33* | 1.07 ± 0.25 | 1.11 ± 0.33* | 1.08 ± 0.26 | 1.09 ± 0.31 |
MAC, AAC and AVS were defined as present or absent, i.e. summing mild, moderate or severe versus none.
*P < 0.05.
Fig. 1.
Percentage of subjects with none, one, two or three calcifications divided by categories of estimated glomerular filtration rate (eGFR) and quartiles of cystatin C. The prevalence of multiple cardiac calcifications was significant higher in subjects with an eGFR < 45 mL/min/1.73 m2 and increased per quartile of cystatin C (both P-values < 0.001).
Table 3.
Association between creatinine-based estimated glomerular filtration rate (eGFR) and mitral annular calcifications (MAC), aortic annular calcifications (AAC) and aortic valve sclerosis (AVS)
| eGFR | ||||
|---|---|---|---|---|
| >60 mL/min/1.73 m2 | 45–60 mL/min/1.73 m2 | <45 mL/min/1.73 m2 | P for trend | |
| N = 2777 | N = 710 | N = 214 | ||
| MAC | ||||
| Model 1a | Reference | 0.97 (0.82–1.15) | 1.65 (1.24–2.20) | 0.019 |
| Model 2b | Reference | 0.94 (0.79–1.11) | 1.54 (1.16–2.06) | 0.082 |
| AAC | ||||
| Model 1a | Reference | 0.98 (0.82–1.17) | 1.36 (1.02–1.82) | 0.169 |
| Model 2b | Reference | 0.95 (0.80–1.14) | 1.30 (0.97–1.74) | 0.340 |
| AVS | ||||
| Model 1a | Reference | 1.01 (0.85–1.20) | 1.22 (0.91–1.62) | 0.299 |
| Model 2c | Reference | 0.98 (0.82–1.16) | 1.15 (0.86–1.53) | 0.599 |
MAC, AAC and AVS were defined as present or absent, i.e. summing mild, moderate or severe versus none. Data presented as OR (95% confidence intervals).
aAdjusted for age, gender and race.
bAdjusted for age, gender, race, prevalent cardiovascular disease and HDL-cholesterol.
cAdjusted for age, gender, race, prevalent cardiovascular disease, hypertension, HDL-cholesterol and waist-hip-ratio.
Table 5.
Association between creatinine-based estimated glomerular filtration rate (eGFR), cystatin C and number of cardiac calcificationsa
| Odds ratio (95% CI) | P-valueb | |
|---|---|---|
| eGFR | ||
| >60 mL/min/1.73 m2 | Reference | |
| 45–60 mL/min/1.73 m2 | 0.98 (0.91–1.05) | 0.566 |
| <45 mL/min/1.73 m2 | 1.15 (1.03–1.29) | 0.015 |
| Cystatin C (mg/L) | 1.12 (1.03–1.23) | 0.013 |
Data presented as OR (95% confidence intervals).
aThe total number of cardiac calcifications, summing the presence or absence of mitral annular calcifications (MAC), aortic annular calcifications (AAC) and aortic valve sclerosis (AVS). Each defined as the sum of mild, moderate or severe versus none, with the reference group being individuals with no calcifications in any of these structures.
bAdjusted for age, gender and race.
After multivariable adjustment, cystatin C levels were significantly associated with the presence of MAC (OR per SD = 1.12, P = 0.001) and not associated with the presence of AAC (OR per SD = 1.07, P = 0.054) and AVS (OR per SD = 0.99, P = 0.82) (Table 4). After adjustment for age and gender, the proportion of participants with MAC and AAC, but not AVS, increased significantly across quartiles of cystatin C. However, the association between quartiles of cystatin C and AAC became non-significant after further adjustment for other risk factors (Table 4). In addition, a clear linear trend was found between increasing cystatin C levels and the number of cardiac calcifications as shown in Table 5.
Table 4.
Association between cystatin C levels, presented as quartiles and continuously (per standard deviation), and mitral annular calcifications (MAC), aortic annular calcifications (AAC) and aortic valve sclerosis (AVS)
| Cystatin C quartiles | Cystatin C (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| 0.50–0.92 mg/L | 0.93–1.04 mg/L | 1.05–1.22 mg/L | 1.23–6.67 mg/L | P for trend | Per standard deviation | P-value | |
| MAC | |||||||
| Model 1a | Reference | 1.08 (0.90–1.30) | 1.17 (0.97–1.41) | 1.34 (1.10–1.63) | 0.003 | 1.16 (1.08–1.24) | <0.001 |
| Model 2b | Reference | 1.07 (0.89–1.29) | 1.12 (0.93–1.35) | 1.23 (1.00–1.50) | 0.048 | 1.12 (1.05–1.21) | 0.001 |
| AAC | |||||||
| Model 1a | Reference | 1.16 (0.96–1.39) | 1.03 (0.85–1.24) | 1.27 (1.04–1.56) | 0.076 | 1.10 (1.02–1.18) | 0.009 |
| Model 2b | Reference | 1.14 (0.95–1.38) | 0.99 (0.82–1.19) | 1.19 (0.97–1.46) | 0.29 | 1.07 (1.00–1.15) | 0.054 |
| AVS | |||||||
| Model 1a | Reference | 0.90 (0.76–1.08) | 0.89 (0.74–1.07) | 0.96 (0.79–1.17) | 0.59 | 1.01 (0.95–1.09) | 0.68 |
| Model 2c | Reference | 0.89 (0.74–1.07) | 0.86 (0.71–1.03) | 0.90 (0.74–1.10) | 0.23 | 0.99 (0.93–1.06) | 0.82 |
MAC, AAC and AVS were defined as present or absent, i.e. summing mild, moderate or severe versus none. Data presented as OR (95% confidence intervals).
aAdjusted for age, gender and race.
bAdjusted for age, gender, race, prevalent cardiovascular disease and HDL-cholesterol.
cAdjusted for age, gender, race, prevalent cardiovascular disease, hypertension, HDL-cholesterol and waist-hip-ratio.
The association between renal function and cardiac calcifications was not significantly modified by the history of cardiovascular disease or traditional cardiovascular risk factors (P for interactions >0.1). In addition, no significant interaction was present between classes of eGFR and cystatin C levels on cardiac calcifications (P = 0.649). In the current study, cystatin C levels were significantly associated with the different stages of eGFR. The mean cystatin C level was 1.00 ± 0.17 mg/L in subjects with an eGFR >60 mL/min/1.73 m2, 1.24 ± 0.22 mg/L in subjects with an eGFR between 45 and 60 mL/min/1.73 m2 and 1.72 ± 0.56 mg/L in subjects with an eGFR <45 mL/min/1.73 m2 (P < 0.001).
Discussion
In the present large cohort of elderly adults, moderate kidney disease as defined by an eGFR <45 mL/min/1.73 m2 and elevated levels of cystatin C was associated with prevalent MAC. In addition, a significant trend was observed between an eGFR <45 mL/min/1.73 m2, increasing levels of cystatin C and the number of cardiac calcifications. Chronic kidney disease defined by eGFR or cystatin C was not associated with AAC or AVS after adjustment for cardiovascular risk factors.
The observed association between chronic kidney disease as assessed by elevated levels of cystatin C and an eGFR <45 mL/min/1.73 m2, and MAC may be explained by several mechanisms. The increased risk of annular calcification may be due to the cumulative effect of unmeasured or incompletely measured cardiovascular risk factors that cluster with renal function impairment or may reflect a common underlying pathological process such as atherosclerosis. Alternatively, the observed association between renal function and perivalvular calcification might be due to a more causal intermediary factor that results from renal dysfunction, such as an increased inflammatory state, hypertension or a deregulation of mineral metabolism [23,24]. Thus, renal dysfunction may also be a more direct cause of annular calcification.
In the present study, eGFR was less strongly associated with MAC than was cystatin C. Cystatin C may be a more specific marker of kidney function than eGFR, particularly among older adults, as cystatin C is independent of muscle mass and less affected by age and sex than serum creatinine [11]. Conversely, creatinine-based prediction equations may be less precise in more moderate ranges of eGFR, as suggested by prior studies [25,26]. Among older adults without chronic kidney disease (eGFR >60 mL/ min/1.73 m2), cystatin C was predictive of cardiovascular and kidney disease, whereas creatinine levels were only marginally associated with these outcomes [27]. In the present study, no association could be found between calcifications and eGFR divided into quartiles, but an eGFR <45 mL/min/1.73 m2 was associated with the presence of MAC. This observation supports the conclusion that eGFR may be less precise in higher ranges and becomes more predictive in patients with moderate kidney disease. In the present study, only 5.8% of individuals had an eGFR <45 mL/min/1.73 m2, suggesting that cystatin C may be a marker of preclinical kidney disease not yet detected by eGFR levels. In the current study, cystatin C levels were significantly associated with the different stages of eGFR.
Moderate renal dysfunction, as assessed by an eGFR < 45 mL/min/1.73 m2 and elevated levels of cystatin C, was not associated with AAC and AVS. Whether the relation between renal function and MAC is truly biologically different from AAC or AVS, or whether other factors may have influenced the results, cannot be concluded from the present study. Interestingly, in the Framingham Offspring Study, a significant association was also only seen between chronic kidney disease and MAC, not AAC or AVS [8]. In addition, Ribeiro et al. found that calcium–phosphate product levels in dialysis patients were significantly associated with MAC, but not with AAC [28]. These findings suggest that derangement of the calcium–phosphate metabolism may play an important role in the development of MAC but may be less important in the development of AAC or AVS. AAC may be more a degenerative disease due to increasing age, while MAC might be caused by a metabolic derangement related to chronic kidney disease and therefore more associated with eGFR and cystatin C levels. However, some common pathological pathway might also be present, considering the overlap in participants with both MAC and AAC (26% of the total population).
Our study has potential limitations. The cross-sectional design precludes conclusions of the temporal relation between renal function and cardiac calcifications. In addition, odds ratios may overestimate the relative risk because cardiac calcifications are common in the current study. A poor calcium and phosphate balance might (partly) explain the observed relationships, but calcium, phosphorus and parathyroid hormone were not measured in the present study. Residual confounding due to unmeasured or incompletely measured factors cannot be excluded. In addition, the majority of the outcome was mild calcification and including more severe abnormalities may have strengthened the results. The findings were observed in older adults and may not be generalizable to younger populations; on the other hand, due to increasing risk with age, the relationships between renal dysfunction and cardiac calcifications might be best evaluated in older populations.
In conclusion, in this community-based cohort of older adults, moderate kidney disease as defined by an eGFR <45 mL/min/1.73 m2 and elevated levels of cystatin C was associated with prevalent MAC. In addition, a significant trend was observed between an eGFR <45 mL/min/ 1.73 m2, increasing levels of cystatin C and the number of cardiac calcifications. Moderate kidney disease defined by eGFR or cystatin C was not associated with AVS or AAC. Additional studies are needed in the future to investigate the underlying mechanisms responsible for the association between moderate kidney dysfunction and cardiac calcifications and cardiovascular disease in general.
Acknowledgments
Dr F.W. Asselbergs is a research fellow of the Netherlands Heart Foundation (2003T010) and the Dutch Inter University Cardiology Institute Netherlands. Drs Shlipak, Fried and Katz are funded by R01 HL073208-01. Dr Shlipak is also supported by the American Federation for Aging Research and National Institute on Aging (Paul Beeson Scholars Program), by the Robert Wood Johnson Foundation (Generalist Faculty Scholars Program) and by R01 DK066488. Drs Fried, Shlipak and Siscovick are also supported by R01 AG027002. The Cardiovascular Health Study (CHS) is supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129 and N01-HC-15103 from the National Heart, Lung and Blood Institute (NHLBI). A full list of participating CHS investigators and institutions can be found at www.chs-nhlbi.org.
Conflict of interest statement. None declared.
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Henry RM, Kostense PJ, Bos G, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn study. Kidney Int. 2002;62:1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x. [DOI] [PubMed] [Google Scholar]
- 3.Maher ER, Young G, Smyth-Walsh B, et al. Aortic and mitral valve calcification in patients with end-stage renal disease. Lancet. 1987;2:875–877. doi: 10.1016/s0140-6736(87)91370-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang AY, Wang M, Woo J, et al. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: a prospective study. J Am Soc Nephrol. 2003;14:159–168. doi: 10.1097/01.asn.0000038685.95946.83. [DOI] [PubMed] [Google Scholar]
- 5.London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 6.Sharma R, Pellerin D, Gaze DC, et al. Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis. 2007;191:348–354. doi: 10.1016/j.atherosclerosis.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Fox E, Harkins D, Taylor H, et al. Epidemiology of mitral annular calcification and its predictive value for coronary events in African Americans: the Jackson Cohort of the Atherosclerotic Risk in Communities Study. Am Heart J. 2004;148:979–984. doi: 10.1016/j.ahj.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Larson MG, Vasan RS, et al. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham Heart Study. J Am Soc Nephrol. 2006;17:521–527. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
- 9.Ix JH, Shlipak MG, Katz R, et al. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2007;50:412–420. doi: 10.1053/j.ajkd.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Barasch E, Gottdiener JS, Larsen EK, et al. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS) Am Heart J. 2006;151:39–47. doi: 10.1016/j.ahj.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 11.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin c is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 13.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 14.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 15.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, et al. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Verhave JC, Fesler P, Ribstein J, et al. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005;46:233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 18.K/DOQI. Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 19. UK Consensus Conference on Early Chronic Kidney Disease 2007; http://www.renal.org/CKDguide/consensus.html .
- 20.Abutaleb N. Why we should sub-divide CKD stage 3 into early (3a) and late (3b) components. Nephrol Dial Transplant. 2007;22:2728–2729. doi: 10.1093/ndt/gfm349. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 22.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring nephelometer II system. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 23.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang AY, Woo J, Wang M, et al. Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol. 2001;12:1927–1936. doi: 10.1681/ASN.V1291927. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Wasen E, Isoaho R, Mattila K, et al. Estimation of glomerular filtration rate in the elderly: a comparison of creatinine-based formulae with serum cystatin C. J Intern Med. 2004;256:70–78. doi: 10.1111/j.1365-2796.2004.01340.x. [DOI] [PubMed] [Google Scholar]
- 27.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro S, Ramos A, Brandao A, et al. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol Dial Transplant. 1998;13:2037–2040. doi: 10.1093/ndt/13.8.2037. [DOI] [PubMed] [Google Scholar]