Abstract
Background. Prostate-specific antigen (PSA) occurs in different molecular forms in serum: free PSA (fPSA) and complexed PSA (cPSA), the sum of which corresponds to total PSA (tPSA). In addition to tPSA, percent fPSA is widely used in the detection of prostate cancer. Free PSA, ∼28 kDa, is eliminated by glomerular filtration. Previous data showed that men with end-stage renal dysfunction requiring chronic dialysis have increased percent fPSA. In this study, we evaluated whether moderate-to-severe chronic renal dysfunction, but with no need for dialysis, also importantly affects percent fPSA.
Methods. The study group consisted of 101 men (median age 57 years, interquartile range 46–68) with chronic kidney disease and no diagnosis of prostate cancer. Their median glomerular filtration rate (GFR) was 23 mL/min/1.73 m2 (interquartile range 16–33; range 8–83), determined by iohexol clearance. Controls included 5264 men (median age 57 years, interquartile range 54–62) attending a prostate cancer screening program with no diagnosis of prostate cancer during 8 years of follow-up.
Results. With adjustment for age, median fPSA levels and percent fPSA were significantly higher (P < 0.001) in patients with renal dysfunction, 0.45 μg/L and 47.2%, respectively, compared to controls, 0.29 μg/L and 29.9%, respectively. Regression analysis in the study group showed a significant association between GFR and percent fPSA (P = 0.036).
Conclusions. The percent fPSA is importantly influenced by moderately impaired renal function in men with chronic kidney disease. For such men, use of the current clinical decision limits for percent fPSA could cause some men with prostate cancer to be misdiagnosed as having benign disease, and therefore fPSA should not be used to diagnose prostate cancer in these patients.
Keywords: diagnosis, glomerular filtration rate, chronic kidney disease, prostate cancer, prostate-specific antigen
Introduction
The measurement of serum prostate-specific antigen (PSA) is an important clinical tool for the detection and monitoring of prostate cancer. PSA occurs in several molecular forms in the blood, with two predominant forms: free, non-complexed PSA (fPSA) with a molecular mass of ∼28 kDa and complexed PSA (cPSA), a stable ∼90 kDa complex with alpha-1-antichymotrypsin. PSA also occurs in complex with alpha-1-antitrypsin and alpha-2-macroglobulin but at much lower levels and of uncertain clinical value [1,2]. The sum of fPSA and cPSA roughly corresponds to the conventional immunodetected total PSA (tPSA) [3]. Increased release of tPSA in the blood is caused by prostate cancer, but also by other prostate disorders such as benign enlargement and prostatitis. However, fPSA as a percentage of tPSA (percent fPSA) is lower in men with prostate cancer than in men with benign disorders. Therefore, percent fPSA enhances discrimination of prostate cancer [4], and it is frequently used to enhance the diagnostic efficacy of PSA for early detection of prostate cancer in men with moderately elevated tPSA [5].
Half-life for fPSA in serum is short (4–33 h) [6–10]. This, combined with its low molecular mass, suggests elimination by glomerular filtration. In contrast, cPSA has slow elimination kinetics and 3-fold larger molecular mass, which prevents glomerular filtration; these observations suggest other routes of elimination, most likely by liver metabolism [11,12]. After kidney transplantation and graft reperfusion, serum concentrations of fPSA decline rapidly, which supports the hypothesis that the free, non-complexed PSA is eliminated from blood circulation by glomerular filtration [13–15]. Renal dysfunction may therefore alter the relative proportions of the two PSA forms by decreasing the elimination of fPSA, thereby increasing percent fPSA. This has been confirmed by findings in men on haemodialysis [16–19] and in men with peritoneal dialysis treatment [19] who had significantly higher percent fPSA. This raises the possibility that renal dysfunction may compromise the diagnostic accuracy of percent fPSA and suggests that a high percent fPSA should not be considered as a sign of benign prostatic disease in men with strongly reduced glomerular filtration rate (GFR) requiring chronic dialysis.
To our knowledge, no reports have described the effect of less severe renal dysfunction on fPSA. In this study, we evaluate the effect of different degrees of renal dysfunction on serum PSA and percent fPSA, in men without dialysis treatment.
Subjects and methods
Patients
We investigated men with chronic kidney disease undergoing regular medical examination at the Department of Nephrology and Transplantation, University Hospital (UMAS), Malmö, Sweden. During a routine visit for GFR determination with iohexol clearance, men without history of prostate cancer or urinary tract symptoms were invited to participate in the study, and all 105 consecutive men accepted the invitation. Using the local cancer registry updated to 31 December 2006, we investigated if any of the participating men had a prior diagnosis of prostate cancer. Two study participants had been diagnosed with prostate cancer and, hence, were excluded. Further, two men were excluded as their PSA levels were below analytical detection limits. Therefore, our study cohort included 101 men (median age 57 years; range 22–85) with a median iohexol clearance of 23 mL/min/1.73 m2 (range, 8–83). The included men were diagnosed with diabetic nephropathy (n = 20), glomerulonephritis (n = 21), non-specified renal disease (n = 13), nephrosclerosis (n = 15), a group of miscellaneous renal diseases (n = 10) and 22 renal transplants with various degrees of renal function (Table 1). Informed consent was obtained from all patients. The Committee of Ethics at Lund University approved the study design.
Table 1.
Characteristics of the study participants
| Median (interquartile range) | |||
|---|---|---|---|
| Characteristic | Study patients (n = 101) | Controls (n = 5264) | P-value |
| Age (years) | 57 (46, 68) | 57 (54, 62) | |
| GFRa (mL/min/1.73 m2) | 23 (16, 33) | – | |
| Creatinine (μmol/L) | 226 (161, 297) | – | |
| Total PSA (μg/L) | 1.08 (0.59, 1.87) | 0.95 (0.61, 1.55) | 0.15 |
| Free PSA (μg/L) | 0.45 (0.28, 0.76) | 0.29 (0.20, 0.43) | <0.001 |
| Percent free PSA | 47.2 (37.5, 55.1) | 29.9 (22.4, 38.8) | <0.001 |
| Renal diseases, no. of patients (%) | |||
| Diabetic nephropathy | 20 (20%) | – | |
| Renal transplants | 22 (22%) | – | |
| Glomerulonephritis | 21 (21%) | – | |
| Renal disease (non-specified) | 13 (13%) | – | |
| Nephrosclerosis | 15 (15%) | – | |
| Miscellaneous renal diseases | 10 (10%) | – | |
aGFR measured by iohexol clearance. P-values were obtained by linear regression, with adjustment for age.
Blood collection
Blood samples for PSA analysis were collected at the same time as the routine determinations of glomerular filtration rates. The blood samples were allowed to clot for 15 min at room temperature and subsequently centrifuged at 3500 g for 10 min and then immediately stored at −70°C pending analysis. Measurements of creatinine, tPSA and fPSA were made in all blood samples.
Controls
We used data from a biennial prostate cancer screening program that was part of the European Randomized Screening for Prostate Cancer (ERSPC) study. The program was conducted in Göteborg, Sweden, from 1995 to 2002 [20]. Informed consent was obtained from all participants. The screening group consisted of 10 000 randomly selected men aged 51–66 years who were invited for a first round of PSA testing between 1995 and 1996, and then re-invited every second year for 8 years. A total of 5855 men participated in the first round of screening; 559 cases of prostate cancer were detected during the follow-up (up to 31 December 2004) and excluded from the cohort (n = 5296). From this cohort, we included 5264 men (median age 57 years) as controls in our study. Thirty-two men were not included due to missing values (n = 6), PSA levels below the detection limit (n = 18) or PSA data suggestive of laboratory interference (n = 8).
Laboratory methods
The time-resolved ProStatus PSA free/total fluoroimmunoassay was used to measure tPSA and fPSA, from which percent fPSA was calculated, in serum from both study patients and controls. The characteristics of the Prostatus® assay was previously comprehensively documented [21] and measures fPSA and cPSA, in an equimolar fashion and the cross-reaction for PSA-ACT in the fPSA assay is <0.2%, with a lower limit of detection of 0.05 μg/L for fPSA and 0.04 μg/L for tPSA [22,23]. The Prostatus® assay was calibrated according to a WHO standardization in 2003, and therefore all control samples were increased with a factor of 1.10 for fPSA and 1.13 for tPSA. Study patients were all analysed with the calibrated assay.
Serum creatinine was determined by the Kodak Ektachem 700 XR-C enzymatic method (Eastman Kodak, Rochester, New York, USA) using the enzymes creatinine amidohydrolase and creatinine amidinohydrolase. The reference range was 55–116 μmol/L.
The GFR was determined by measuring the plasma clearance of iohexol according to a one-compartment model, where samples were taken after the distribution phase, in the assumed monoexponential part of the plasma decay curve. Iohexol was analysed by the HPLC technique [24,25]. The reference range was 80–125 mL/min/1.73 m2 up to the age of 50 years, 60–110 mL/min/1.73 m2 between 50 and 65 years and 50–90 mL/min/1.73 m2 above 65 years.
Statistical methods
Statistical comparisons of biomarker levels between the control and study groups were performed using linear regression with adjustment for age. The association between percent fPSA and GFR was evaluated using linear regression analysis. P-values of <0.05 were considered to be statistically significant, and statistical analyses were conducted using Stata 9.0 (Stata Corp., College Station, TX, USA).
Results
The characteristics of study patients and controls are presented in Table 1. In the current study, we evaluated PSA levels and percent fPSA in 101 men with chronic kidney disease but without need for dialysis. According to the criteria of the Kidney Disease Quality Outcome Initiative (K/DOQI) [26], the majority of our study patients had moderate-to-severe renal insufficiency (defined as GFR between 15 and 59 mL/min/1.73 m2). Among our study patients, 96% had GFR <59 mL/min/1.73 m2, but patients with other degrees of chronic kidney diseases were also included. The median GFR was 23 mL/min/1.73 m2.
There was no significant difference in tPSA in the study patients compared with controls (median 1.08 μg/L versus 0.95 μg/L; P = 0.15). Both median fPSA and percent fPSA, however, were significantly higher (P < 0.001) in the study patients (0.45 μg/L and 47.2%, respectively) than in the controls (0.29 μg/L and 29.9%, respectively) (Table 1). Among the study patients, 69 (68%) had percent fPSA >40% and 39 (39%) had percent fPSA >50%.
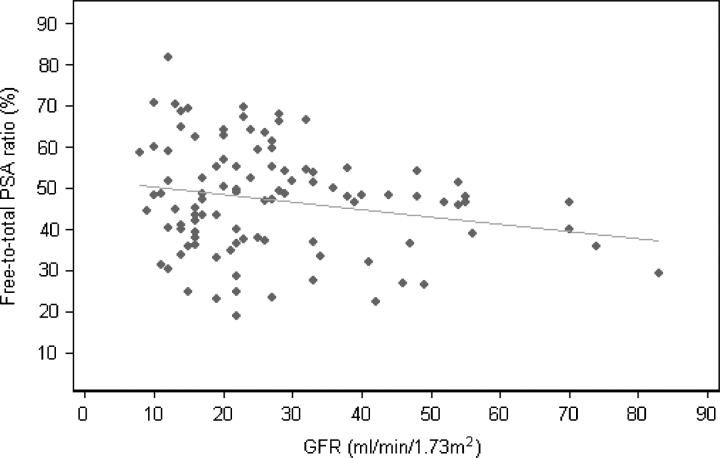
We also investigated the association between GFR and percent fPSA in the study group (Figure 1). Regression analysis showed a significant association of lower GFR with higher percent fPSA (P = 0.036). We considered whether the regression function might be used to adjust a given percent fPSA in light of GFR. However, Figure 1 clearly shows that the relationship between percent fPSA and GFR is subject to heteroscedacity: men with lower GFR values had greater variability in percent fPSA than men with higher GFR values. This makes regressions imprecise: for example, the percent fPSA predicted from the linear regression model for a man with GFR <20 mL/min/1.73 m2 ranged from 48% to 50%, while the observed levels for these patients ranged from 23% to 81%. We attempted to stabilize the variance through Box Cox transformation of GFR and percent fPSA; however, no transformation provided an adequate regression model.
Fig. 1.
Scatter plot showing the relationship between percent fPSA and glomerular filtration rate (GFR) in 101 men with chronic kidney disease. The light grey line is from a linear regression model to predict percent fPSA based on GFR.
Discussion
In addition to tPSA, percent fPSA is frequently measured to enhance the discrimination of prostate cancer from benign prostate disorders. It has been confirmed extensively that a high percent fPSA indicates a lesser risk of cancer. By using a cut-off of <25%, Catalona et al. have reported that the number of unnecessary biopsies could be reduced by 20% while maintaining a 95% sensitivity for prostate cancer detection in men with tPSA in the range 4–10 μg/L [27]. Diminished renal elimination of fPSA may, therefore, affect percent fPSA and its accuracy as a diagnostic tool for prostate cancer. Several reports confirm that men with terminal renal failure requiring chronic dialysis have significantly higher percent fPSA compared to men without known renal dysfunction [16–19].
This study shows that men with chronic kidney disease and impaired renal function have significantly higher serum levels of fPSA (median 0.45 μg/L versus 0.29 μg/L) and significantly higher percent fPSA (median 47% versus 30%) compared to controls. As many as 68% of the study patients presented with a percent fPSA >40%. We have earlier reported that patients on haemodialysis demonstrated fPSA levels of 0.42 μg/L and percent fPSA ∼40% [19]. Thus, the present study demonstrates that increased percent fPSA is not only restricted to men with terminal renal insufficiency but also occurs in men with moderate-to-severe renal dysfunction. For such men, the detection and monitoring of prostate cancer using the current clinical decision limits for percent fPSA could cause some men to be misdiagnosed as having benign disease. Since a cut-point of the degree of renal dysfunction at which the percent fPSA first becomes affected could not be determined, should refrain these patients from using percent fPSA for the detection or monitoring of prostate cancer. Total PSA levels did not differ significantly between our study patients and the controls, although there was a trend towards higher tPSA levels among the study patients.
The inclusion of a very large number of control patients was intended to ensure that the vast majority of control participants would have normal renal function, although renal function was not evaluated in controls. A large survey in the USA demonstrated that only 0.3% of ‘normal’ persons have GFR <30 mL/min/1.73 m2, and that no more than ∼4% have GFR 30–60 mL/min/1.73 m2 [26].
The data in this study do not allow us to determine the degree of renal dysfunction at which the percent fPSA first becomes affected. Determining such a cut-point would require a greater number of study patients. We have, however, shown that patients with renal dysfunction as a group are clearly distinguished from those with presumed normal renal function with respect to fPSA and percent fPSA.
Our results show that the currently established percent fPSA cut-off points are inappropriate not only for men on dialysis but also for patients with moderate-to-severe renal dysfunction. Impaired renal function is thus an important factor to consider in the evaluation of percent fPSA for detection of prostate cancer.
Acknowledgments
We thank Jan-Åke Nilsson for help with statistics, and Gun-Britt Eriksson and Kerstin Håkansson for laboratory work. This work was supported by the National Cancer Institute contract P50-CA92629-SPORE PILOT Project 7, the Swedish Cancer Society project no. 3555, European Union 6th Framework contract LSHC-CT-2004-503011 (P-Mark) Fundatión Federico SA, the Cancer Research Fund of University Hospital, Malmö, Region Skåne Research Fund and the Faculty of Medicine, Lund University.
Conflict of interest statement. Dr Hans Lilja is a patent holder of free PSA assays. The authors declare no conflict of interest.
References
- 1.Stenman UH, Leinonen J, Alfthan H, et al. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–226. [PubMed] [Google Scholar]
- 2.Christensson A, Laurell CB, Lilja H. Enzymatic activity of Prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990;194:755–763. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- 3.Lilja H, Christensson A, Dahlen U, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37:1618–1625. [PubMed] [Google Scholar]
- 4.Christensson A, Bjork T, Nilsson O, et al. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100–105. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 5.Roddam AW, Duffy MJ, Hamdy FC, et al. Use of prostate-specific antigen (PSA) isoforms for the detection of prostate cancer in men with a PSA level of 2–10 ng/ml: systematic review and meta-analysis. Eur Urol. 2005;48:386–399. doi: 10.1016/j.eururo.2005.04.015. discussion 398–389. [DOI] [PubMed] [Google Scholar]
- 6.Partin AW, Piantadosi S, Subong EN, et al. Clearance rate of serum-free and total PSA following radical retropubic prostatectomy. Prostate Suppl. 1996;7:35–39. [PubMed] [Google Scholar]
- 7.Bjork T, Ljungberg B, Piironen T, et al. Rapid exponential elimination of free prostate-specific antigen contrasts the slow, capacity-limited elimination of PSA complexed to alpha 1-antichymotrypsin from serum. Urology. 1998;51:57–62. doi: 10.1016/s0090-4295(97)00572-4. [DOI] [PubMed] [Google Scholar]
- 8.Lilja H, Haese A, Bjork T, et al. Significance and metabolism of complexed and noncomplexed prostate specific antigen forms, and human glandular kallikrein 2 in clinically localized prostate cancer before and after radical prostatectomy. J Urol. 1999;162:2029–2034. doi: 10.1016/S0022-5347(05)68093-7. discussion 2034–2025. [DOI] [PubMed] [Google Scholar]
- 9.Martin BJ, Cheli C, Davis R, et al. cPSA and fPSA elimination in African-American men. Prostate Cancer Prostatic Dis. 2003;6:163–168. doi: 10.1038/sj.pcan.4500649. [DOI] [PubMed] [Google Scholar]
- 10.Gregorakis AK, Malovrouvas D, Stefanakis S, et al. Free/Total PSA (F/T ratio) kinetics in patients with clinically localized prostate cancer undergoing radical prostatectomy. Clin Chim Acta. 2005;357:196–201. doi: 10.1016/j.cccn.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Kilic S, Yalcinkaya S, Guntekin E, et al. Determination of the site of metabolism of total, free, and complexed prostate-specific antigen. Urology. 1998;52:470–473. doi: 10.1016/s0090-4295(98)00208-8. [DOI] [PubMed] [Google Scholar]
- 12.Birkenmeier G, Struck F, Gebhardt R. Clearance mechanism of prostate specific antigen and its complexes with alpha2-macroglobulin and alpha1-antichymotrypsin. J Urol. 1999;162:897–901. doi: 10.1097/00005392-199909010-00086. [DOI] [PubMed] [Google Scholar]
- 13.Bruun L, Ekberg H, Bjork T, et al. Rapid elimination by glomerular filtration of free prostate specific antigen and human kallikrein 2 after renal transplantation. J Urol. 2004;171:1432–1435. doi: 10.1097/01.ju.0000118580.19344.68. [DOI] [PubMed] [Google Scholar]
- 14.Fischer K, Hamza A, Wicht A, et al. Shift of the f/t-PSA quotient in relation to renal insufficiency: consequences for the early detection of prostate carcinoma in patients with terminal renal failure. Anticancer Res. 2007;27:1945–1948. [PubMed] [Google Scholar]
- 15.Kamali K, Zargar MA. Effects of renal transplantation on serum-free and total PSA levels. Transplant Proc. 2007;39:1027–1028. doi: 10.1016/j.transproceed.2007.03.089. [DOI] [PubMed] [Google Scholar]
- 16.Sasagawa I, Kubota Y, Hayami S, et al. Serum levels of total and free prostate specific antigen in men on hemodialysis. J Urol. 1998;160:83–85. [PubMed] [Google Scholar]
- 17.Douville P, Tiberi M. Effect of terminal renal failure on the ratio of free to total prostate-specific antigen. Tumour Biol. 1998;19:113–117. doi: 10.1159/000029981. [DOI] [PubMed] [Google Scholar]
- 18.Djavan B, Shariat S, Ghawidel K, et al. Impact of chronic dialysis on serum PSA, free PSA, and free/total PSA ratio: is prostate cancer detection compromised in patients receiving long-term dialysis? Urology. 1999;53:1169–1174. doi: 10.1016/s0090-4295(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 19.Bruun L, Bjork T, Lilja H, et al. Percent-free prostate specific antigen is elevated in men on haemodialysis or peritoneal dialysis treatment. Nephrol Dial Transplant. 2003;18:598–603. doi: 10.1093/ndt/18.3.598. [DOI] [PubMed] [Google Scholar]
- 20.Hugosson J, Aus G, Lilja H, et al. Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer. 2004;100:1397–1405. doi: 10.1002/cncr.20126. [DOI] [PubMed] [Google Scholar]
- 21.Ulmert D, Becker C, Nilsson JA, et al. Reproducibility and accuracy of measurements of free and total prostate-specific antigen in serum vs. plasma after long-term storage at -20 degrees C. Clin Chem. 2006;52:235–239. doi: 10.1373/clinchem.2005.050641. [DOI] [PubMed] [Google Scholar]
- 22.Mitrunen K, Pettersson K, Piironen T, et al. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–1120. [PubMed] [Google Scholar]
- 23.Pettersson K, Piironen T, Seppala M, et al. Free and complexed prostate-specific antigen (PSA): in vitro stability, epitope map, and development of immunofluorometric assays for specific and sensitive detection of free PSA and PSA-alpha 1-antichymotrypsin complex. Clin Chem. 1995;41:1480–1488. [PubMed] [Google Scholar]
- 24.Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972;30:271–274. doi: 10.3109/00365517209084290. [DOI] [PubMed] [Google Scholar]
- 25.Krutzen E, Back SE, Nilsson-Ehle I, et al. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984;104:955–961. [PubMed] [Google Scholar]
- 26.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 27.Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542–1547. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]