Abstract
Background. Metabolic acidosis, usually manifested by low serum bicarbonate level, is common in chronic kidney disease (CKD) and appears to be associated with higher mortality in dialysis patients. It is not known whether a similar association is present in patients with non-dialysis-dependent CKD (NDD-CKD).
Methods. We used multivariable-adjusted Cox models to examine the association between baseline and time-variable serum bicarbonate (measured as total CO2) with the outcomes of all-cause mortality and the composite of pre-dialysis mortality or end-stage renal disease in 1240 male patients with moderate and advanced NDD-CKD.
Results. Serum bicarbonate showed a significant U-shaped association with all-cause mortality, with the highest mortality rate observed in patients with baseline serum bicarbonate levels <22 mmol/L [multivariable-adjusted hazard ratio (95% confidence interval) for patients with serum bicarbonate <22 mmol/L versus ≥22 mmol/L: 1.33 (1.05–1.69), P = 0.02] and the lowest mortality observed in patients with baseline serum bicarbonate of 26–29 mmol/L. The associations between lower serum bicarbonate level and mortality were more accentuated in subgroups of patients with better nutritional status and lower inflammation.
Conclusions. Both lower and higher serum bicarbonates are associated with increased all-cause mortality in patients with moderate and advanced NDD-CKD. Clinical trials are needed to determine if therapeutic interventions aimed at optimizing serum bicarbonate can result in improved outcomes in this population.
Keywords: bicarbonate, chronic kidney disease, mortality
Introduction
Metabolic acidosis is a common complication of chronic kidney disease (CKD) that can have multiple deleterious metabolic consequences such as uraemic bone disease, protein-energy wasting, impaired myocardial function and glucose homeostasis, accumulation of beta-2 microglobulin, chronic inflammation and disturbances in growth hormone and thyroid function [1–3]. While many of these complications of metabolic acidosis have been linked to adverse clinical outcomes in CKD, it remains unclear if metabolic acidosis itself has a direct effect on such outcomes. Abnormal serum bicarbonate levels have been associated with increased mortality in patients receiving maintenance dialysis [4–6], with associations found for both metabolic acidosis and alkalosis in the Dialysis Outcomes and Practice Patterns Study (DOPPS) [4] and a Fresenius database-based study [5]. The impact of metabolic acidosis and metabolic alkalosis has, however, not been studied extensively in patients with non-dialysis-dependent CKD (NDD-CKD). We examined all-cause mortality and the composite of pre-dialysis mortality or end-stage renal disease (ESRD) associated with serum bicarbonate levels in a cohort of patients with moderate and advanced NDD-CKD.
Subjects and methods
Study population and data collection
The study population consisted of 1259 patients evaluated and treated for NDD-CKD at Salem Veteran Affairs Medical Center (VAMC) between 1 January 1990 and 30 June 2007 and followed up until 1 April 2008. Thirteen female patients and 6 patients whose race was other than White or Black were excluded; the final study population consisted of 1240 patients.
Baseline characteristics of the population were recorded at the time of the patients’ initial evaluation in the nephrology clinic, and included demographic and anthropometric characteristics, co-morbid conditions and laboratory results, as detailed before [7,8]. Use of certain medications [angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARB), statins, phosphate binders and activated vitamin D products] was assessed over the entire follow-up period and used as a surrogate marker for quality of clinical care. Glomerular filtration rate (GFR) was estimated using the abbreviated equation developed for the Modification of Diet in Renal Disease Study [9] and categorized according to the staging system introduced by the Kidney/Dialysis Outcome Quality Initiative (K/DOQI) Clinical Practice Guidelines for CKD: Evaluation, Classification, and Stratification [10]. Laboratory parameters including serum total CO2, which is generally used as an indirect measure of serum bicarbonate concentration [11], were recorded longitudinally throughout the follow-up period and were averaged over 6-month time periods for time-dependent analyses. Serum total CO2 was measured by a pH rate of change method (reference range: 20–34 mmol/L, coefficient of variation: 4.5%) using a Beckman Synchron LXi analyser (Beckman, Fullerton, CA, USA). All the biochemical measurements were performed in a single laboratory at the Salem VAMC.
Statistical analyses
After descriptive analyses, skewed variables were log-transformed. Missing data points for comorbidity index (1% missing), body mass index (BMI, 18% missing), serum phosphorus (7% missing), serum albumin (4% missing), blood cholesterol (8% missing), haemoglobin (2% missing), white blood cell count (WBC, 5% missing), percent lymphocytes in WBC (6% missing) and 24-h urine protein (8% missing) were imputed using linear regression with all other patient characteristics serving as independent variables. Smoking (5% missing) was analysed by creating a dummy variable corresponding to missing smoking status. Serum bicarbonate was analysed both as a continuous variable and as a categorical measure after dividing it into categories of <22 (corresponding to the 10th percentile in our sample and to the lower limit of desirable bicarbonate level in CKD according to K-DOQI guidelines [12]), 22–25 (10th–50th percentile), 26–29 (51st–90th percentile) and >29 mmol/L (>90th percentile). Serum bicarbonate was also dichotomized into levels of <22 mmol/L and ≥22 mmol/L.
Outcomes analysis: the starting time for survival analysis was the date of the first encounter in the Nephrology Clinic at Salem VAMC. Outcome measures were overall (pre- and post-dialysis) all-cause mortality (ascertained from VA electronic records) and the composite of pre-dialysis mortality or ESRD (defined as initiation of maintenance dialysis therapy and ascertained from local hospital records including Medicare Form 2728). Patients were considered lost to follow-up if no contact was documented with them for more than 6 months, and they were censored in survival analyses at the date of the last documented contact. Associations between baseline serum bicarbonate levels and the above outcomes were evaluated in fixed-covariate Cox models, with adjustment for potential confounders. Time-dependent Cox models were used to capture the impact of temporal changes in serum bicarbonate and other potential confounders. Variables to be included in multivariable models included those that showed a significant association with baseline serum bicarbonate and surrogate markers of malnutrition and inflammation (body mass index, serum cholesterol, albumin, white blood cell count and percentage of lymphocytes). Selection of variables to be included in the final multivariable models was performed by a multivariable regression spline function using backward elimination of weak predictors using a closed test approach [13]; age, race and surrogate markers of malnutrition and inflammation were forced in the models. Nonlinear associations were examined by including polynomial terms and by using restricted cubic splines; analyses were restricted to values above the 5th and below the 95th percentile of the predictor variable in order to maintain the stability of the spline models. The joint significance of polynomial terms was assessed by using the Wald test.
Subgroup analyses were performed in patients divided by demographic and comorbid conditions and by levels of kidney function and nutritional markers. Sensitivity analyses were performed by using only non-imputed values of independent variables and by restricting analyses to a more contemporary cohort of patients enrolled after 1 January 2001. P values of <0.05 were considered significant. Statistical analyses were performed using STATA statistical software version 10 (STATA Corporation, College Station, TX, USA). The study protocol was approved by the Research and Development Committee at the Salem VAMC.
Results
The mean (±SD) age of the cohort was 68 ± 11 years, 24% were Black and the mean estimated GFR was 37 ± 17 mL/min/1.73 m2. Most patients had CKD stages 3 (57%) and 4 (30%), with fewer patients having CKD stages 1 (2%), 2 (8%) and 5 (4%). Seven hundred and fifty-one patients (61%) were enrolled after 1 January 2001. A total of 622 patients died [mortality rate: 124/1000 patient-years, 95% confidence interval (CI): 115–134] and 267 reached ESRD (ESRD rate: 62/1000 patient-years, 95% CI: 55–70) during a median follow-up of 3.3 years. Thirty-five patients (2.8%) were lost to follow-up, and their characteristics were not significantly different (data not shown).
Baseline characteristics in patients divided by categories of baseline serum bicarbonate are shown in Table 1. Patients with lower serum bicarbonate were more likely to be Black and active smokers and less likely to have prevalent diabetes mellitus and cardiovascular disease; they had higher comorbidity index, systolic blood pressure, serum phosphorus and proteinuria and lower eGFR, serum calcium and blood haemoglobin levels. The use of ACEI/ARB and statins was less frequent, but the use of phosphate binding medications was more frequent in patients with lower serum bicarbonate; the use of calcitriol did not differ by categories of serum bicarbonate level (Table 1).
Table 1.
Baseline characteristics of individuals stratified by categories of baseline serum bicarbonate concentration
| Baseline serum bicarbonate (mmol/L) | P-value | ||||
|---|---|---|---|---|---|
| <22 (N = 134) | 22–25.9 (N = 442) | 26–29 (N = 516) | >29 (N = 148) | ||
| Age (years) | 68.2 ± 11.7 | 68.6 ± 10.5 | 67.9 ± 10.9 | 68.9 ± 10.7 | 0.6 |
| Race (Black) | 41 (31) | 120 (27) | 114 (22) | 23 (16) | 0.006 |
| DM | 58 (43) | 243 (55) | 301 (58) | 79 (54) | 0.019 |
| ASCVD | 70 (52) | 241 (54) | 297 (57) | 98 (66) | 0.057 |
| Smoking | 43 (34) | 102 (24) | 128 (26) | 28 (20) | 0.059 |
| Comorbidity index | 3.1 ± 1.8 | 2.4 ± 1.6 | 2.4 ± 1.7 | 2.4 ± 1.6 | 0.001 |
| Calcitriol use | 43 (32) | 147 (33) | 154 (30) | 39 (26) | 0.4 |
| Calcium containing medication use | 60 (45) | 134 (30) | 122 (24) | 21 (15) | <0.001 |
| Sevelamer HCl use | 20 (15) | 54 (12) | 51 (10) | 9 (6) | 0.06 |
| ACEI/ARB use | 87 (65) | 331 (75) | 406 (79) | 116 (78) | 0.008 |
| Statin use | 57 (43) | 287 (65) | 360 (70) | 105 (71) | <0.001 |
| BMI (kg/m2) | 27.5 ± 5.5 | 29.0 ± 5.6 | 29.8 ± 6.0 | 29.9 ± 6.4 | 0.002 |
| SBP (mmHg) | 151 ± 28 | 152 ± 26 | 149 ± 26 | 144 ± 24 | 0.007 |
| DBP (mmHg) | 74 ± 15 | 75 ± 16 | 74 ± 15 | 73 ± 16 | 0.4 |
| eGFR (mL/min/1.73 m2) | 27.2 ± 15.8 | 34.8 ± 15.7 | 40.0 ± 16.2 | 45.9 ± 19.1 | <0.0001 |
| Serum albumin (g/dL) | 3.6 ± 0.6 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.6 ±0.5 | 0.6 |
| Serum cholesterol (mg/dL) | 184 ± 52 | 191 ± 55 | 191 ± 54 | 188 ± 70 | 0.5 |
| Serum calcium (mg/dL) | 8.9 ± 0.6 | 9.2 ± 0.6 | 9.2 ± 0.5 | 9.3 ± 0.5 | <0.0001 |
| Serum phosphorus (mg/dL) | 4.2 ± 1.1 | 3.8 ± 0.8 | 3.7 ± 0.7 | 3.8 ± 0.7 | <0.0001 |
| Blood Hgb (g/dL) | 11.4 ± 1.8 | 12.5 ± 1.8 | 13.0 ± 1.8 | 13.4 ± 1.8 | <0.0001 |
| Blood WBC (1000/mm3) | 7.1 (6.7–7.6) | 7.4 (7.2–7.6) | 7.2 (7.1–7.4) | 7.3 (6.9–7.7) | 0.7 |
| Blood lymphocytes (%WBC) | 22.8 ± 8.5 | 23.2 ± 8.3 | 23.5 ± 8.6 | 22.7 ± 9.2 | 0.7 |
| Proteinuria (mg/24 h) | 1150 (900–1470) | 776 (673–896) | 687 (603–784) | 564 (426–745) | 0.0006 |
Data are presented as means ± SD, number (% of total) or geometric means (95% confidence interval).
DM, diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; Hgb, haemoglobin; WBC, white blood cell count.
Comparisons are made by ANOVA or the chi-square test.
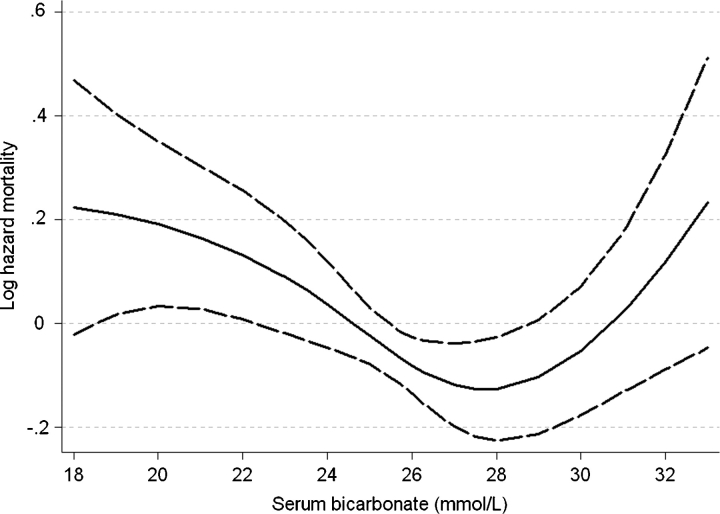
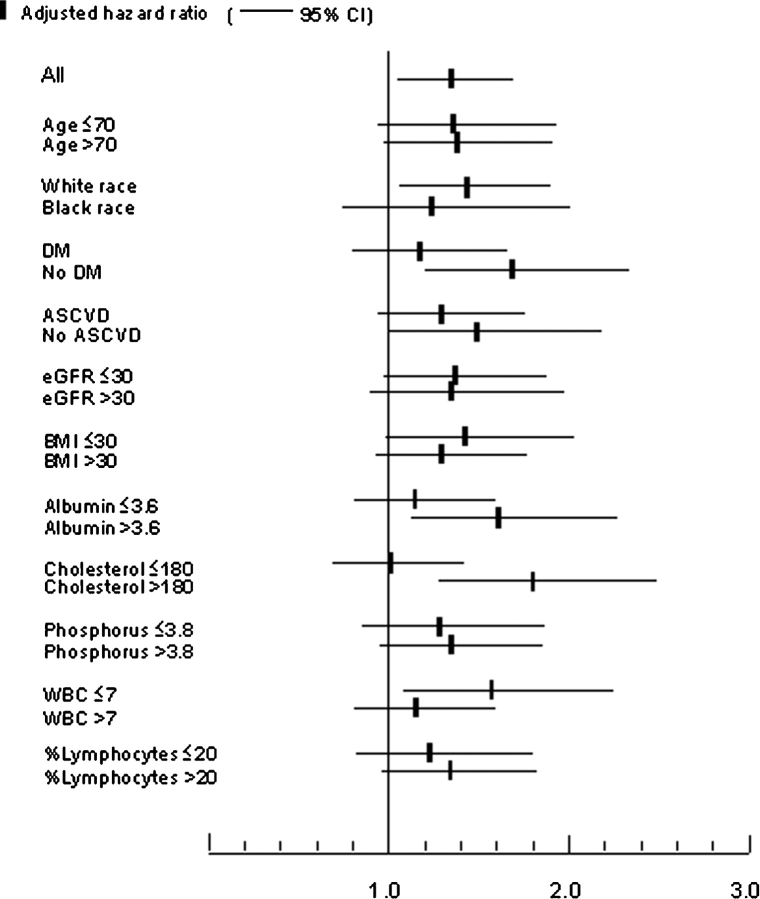
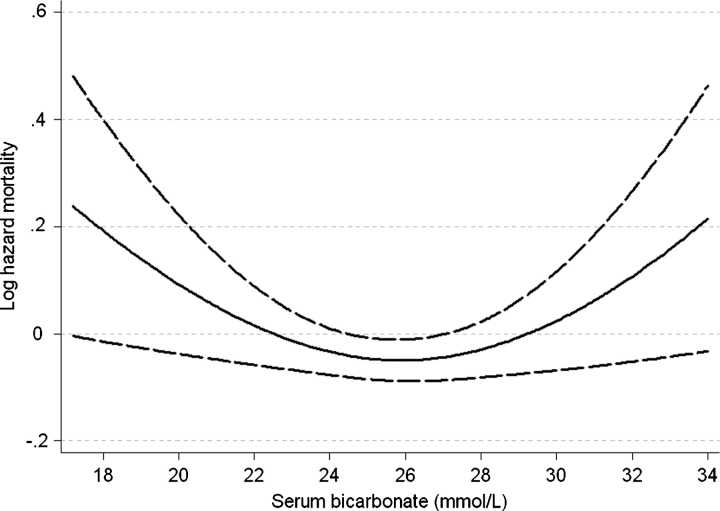
Patients with lower serum bicarbonate displayed a higher mortality rate in fixed-covariate Cox models after adjustment for age, race, BMI, comorbidity index, diabetes mellitus, cardiovascular disease, smoking, systolic blood pressure, eGFR, serum phosphorus and albumin, white blood cell count (WBC), percentage of lymphocytes in WBC count and use of ACEI/ARB, statins and phosphate binders, but the association between serum bicarbonate and all-cause mortality was non-linear, with a relative increase in mortality also seen in patients with serum bicarbonate of >29 mmol/L (Figure 1, P = 0.008 for the joint significance of the linear, quadratic and cubic terms). Patients with the lowest serum bicarbonate experienced the highest mortality rates: compared to patients with serum bicarbonate levels of 26–29 mmol/L, those with serum bicarbonate of <22, 22–25 and >29 mmol/L experienced a multivariable-adjusted hazard ratio (95% CI) of 1.43 (1.10–1.87), 1.11 (0.92–1.35) and 1.24 (0.94–1.64). Compared to patients with serum bicarbonate of ≥22 mmol/L, those with levels <22 mmol/L had a multivariable-adjusted hazard ratio (95% CI) of all cause mortality of 1.33 (1.05–1.69), P = 0.02. This association was more pronounced in subgroups of patients with higher albumin and cholesterol and lower WBC, but only the interaction with blood cholesterol level reached statistical significance (P = 0.047 for the interaction term) (Figure 2). Time-dependent analyses revealed a similar U-shaped association between serum bicarbonate and all-cause mortality, but the lowest mortality rate was observed in patients with serum bicarbonate levels of ∼26 mmol/L (Figure 3).
Fig. 1.
Multivariable-adjusted log hazards ratios (95% confidence intervals) of all-cause mortality associated with baseline levels of serum bicarbonate in a fixed-covariate Cox model adjusted for age, race, body mass index, comorbidity index, diabetes mellitus, cardiovascular disease, smoking, systolic blood pressure, estimated glomerular filtration rate, serum phosphorus and albumin, white blood cell count, percentage of lymphocytes and use of medications.
Fig. 2.
Multivariable-adjusted hazards ratios (95% confidence intervals) of all-cause mortality associated with a baseline serum bicarbonate of <22 mmol/L (versus ≥22 mmol/L) in select subgroups of patients.
Fig. 3.
Multivariable-adjusted log hazards ratios (95% confidence intervals) of all-cause mortality associated with levels of serum bicarbonate in a time-dependent Cox model adjusted for age, race, diabetes mellitus, cardiovascular disease, body mass index, systolic blood pressure, estimated glomerular filtration rate, serum phosphorus and albumin, white blood cell count, percentage of lymphocytes and use of medications.
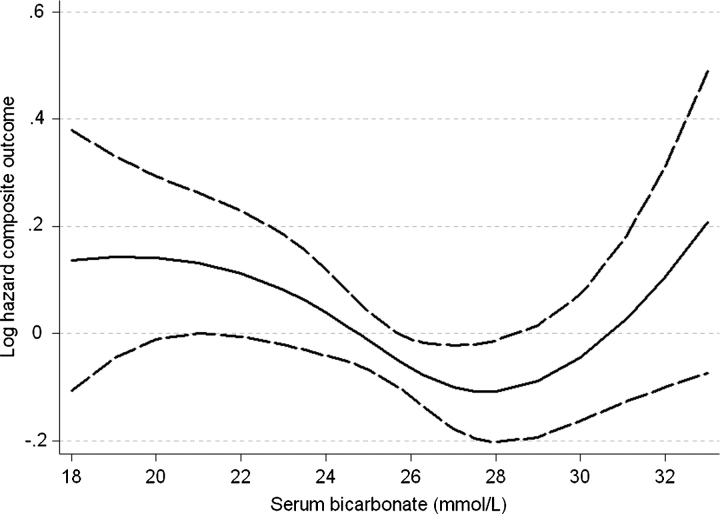
The association between serum bicarbonate and the composite outcome of pre-dialysis mortality or ESRD was similar to its association with mortality in fixed-covariate (Figure 4) and in time-dependent Cox models (data not shown). The association of serum bicarbonate with the studied outcomes remained similar when including non-imputed independent variables in multivariable models and when restricting analyses to the more contemporary cohort of patients enrolled after 1 January 2001 (data not shown).
Fig. 4.
Multivariable-adjusted log hazards ratios (95% confidence intervals) of the composite outcome of pre-dialysis mortality or end-stage renal disease associated with baseline levels of serum bicarbonate in a fixed-covariate Cox model adjusted for age, race, body mass index, comorbidity index, diabetes mellitus, cardiovascular disease, systolic blood pressure, proteinuria, estimated glomerular filtration rate, serum phosphorus and albumin, white blood cell count, percentage of lymphocytes and use of medications.
Discussion
We describe the association between serum bicarbonate level and all-cause mortality in a large group of patients with moderate and advanced NDD-CKD. Serum bicarbonate displayed a non-linear relationship with this outcome, with both lower and higher bicarbonate concentrations showing an association with higher mortality. The results were similar in fixed-covariate and time-dependent survival analyses, although the ideal serum bicarbonate concentration appeared to be slightly lower in the time-dependent models.
The mechanism of action whereby higher serum bicarbonate could have a positive impact on survival is not clear. Metabolic acidosis is common in CKD patients receiving chronic dialysis therapy, due to the impaired ability of the kidneys to account for the ∼1 mEq/kg body weight per day of hydrogen ions generated metabolically in adult patients [11], with lesser severity in patients with NDD-CKD, depending on the degree of their kidney dysfunction. Effects of metabolic acidosis in CKD include the exacerbation of uraemic bone disorders, worsened protein-energy wasting (including muscle wasting and decreased albumin synthesis), impaired myocardial function and glucose homeostasis, accumulation of beta-2 microglobulin, chronic inflammation and disturbances in growth hormone and thyroid function [1–3]. Several of the consequences of metabolic acidosis have been associated with adverse outcomes such as increased mortality in CKD, but the mechanism(s) underlying such associations often remain obscure, and it is unclear to what extent metabolic acidosis itself can be directly linked to adverse clinical outcomes. In a study of 56 385 maintenance haemodialysis patients, a serum bicarbonate concentration <22 mEq/L was associated with lower mortality in unadjusted analyses, but with higher mortality after extensive adjustments for markers of malnutrition and inflammation [6]. Lower serum bicarbonate was associated with both better nutritional status and worse outcomes in two other large observational studies of dialysis patients [4,5]. In the DOPPS study by Bommer et al. [4], the midweek pre-dialysis serum bicarbonate level was correlated inversely with protein catabolic rate, serum albumin and serum phosphorus levels, and the lowest mortality was seen in patients who had a serum bicarbonate of 20.1–21 mEq/L. Similar to our study, the association with serum bicarbonate in the DOPPS study and in the study by Lowrie et al. [5] from the Fresenius database was U-shaped, with higher serum bicarbonate level also being associated with worse mortality. In spite of the similarity of the associations, there are important differences between the acid–base status of dialysis-dependent and non-dialysis-dependent patients with CKD, as dialysis-dependent patients are exposed to large loads of exogenous bicarbonate in a short period of time during haemodialysis, which can cause significant over-correction of metabolic acidosis with resultant negative effects related to alkalaemia. Since similar events are unlikely in NDD-CKD, the mechanisms underlying the associations seen in these two groups may be different and require separate studying. Negative effects of higher serum bicarbonate could in general be related to residual confounding by unaccounted morbidity (severe lung disease or congestive heart failure), medication use (diuretics) or nutritional factors, or could be the results of a truly deleterious mechanism related to severe alkalaemia, such as hypokalaemia, hypocalcaemia or hypomagnesaemia, with resultant cardiac arrhythmias [5,14].
We found that the association of mortality with serum bicarbonate levels in our study was more pronounced in subgroups with better nutritional status; this underscores the importance of nutrition and inflammation as confounders of serum bicarbonate, in that they can act both as independent predictors of outcomes and as determinants of serum bicarbonate levels. Higher protein intake can be one of the causes of a lower serum bicarbonate level in patients with CKD [15,16], and the deleterious impact of lower serum bicarbonate could to some extent be masked by the benefits associated with better nutrition. Conversely, metabolic acidosis can in itself have a negative impact on nutritional status [1,11,17], with its proteolytic effect possibly mediated by inhibition of insulin signalling through phosphoinositide 3-kinase [18]. Further strengthening the link between metabolic acidosis and hypercatabolism in uraemia are studies showing that interventions aimed at correcting metabolic acidosis in dialysis patients can improve various aspects of malnutrition [19–23]. Clinical trials will need to explore if interventions targeting metabolic acidosis could improve hard clinical outcomes such as mortality, and to clarify if such an improvement is mediated through effects on nutrition and inflammation.
Several limitations of our study need to be mentioned. The historical and observational nature of the study only allows us to establish associations, but not causal relationships between serum bicarbonate level and mortality. Our study sample was limited to male patients from a single medical centre; hence, our results may not apply to the larger population with NDD-CKD. The enrolment of patients over an extended period of time in our study makes it possible that secular trends in medical practices could have affected patient outcomes differently based on the time of enrolment. To address this issue, we examined more contemporary patients separately and found similar in outcomes. Non-random distribution of patients in different bicarbonate categories makes it possible that uneven medical care in the different groups could affect outcomes. We addressed this concern by assessing the use of several medical interventions that are commonly applied in patients with CKD (such as ACEI/ARB, statins, phosphate binders and activated vitamin D products) and found that some of them were used less often, but others more often in patients with lower serum bicarbonate levels, making it unlikely that overall poorer medical care would have been present in these patients. We did not have data on potentially relevant variables such as diuretic use and serum potassium levels; thus, residual confounding from these factors could have influenced our results.
Lower serum bicarbonate levels are associated with higher all-cause mortality in patients with moderate and advanced NDD-CKD, whereas serum bicarbonate between ∼24 to 29 mmol/L appears associated with the greatest survival, even after controlling for the confounding effect of nutritional status and inflammation. Clinical trials are needed to determine if therapies aimed at achieving an optimal serum bicarbonate concentration could improve such outcomes. Bicarbonate replacement therapy is affordable and easy to apply, making it thus a potentially attractive therapeutic strategy.
Acknowledgments
Parts of this material were submitted for presentation at the American Society of Nephrology Renal Week 2008, 4–9 November, Philadelphia, PA, USA. This study was supported by an investigator-initiated grant from Genzyme to CPK and by grant 1R01DK078106-01 from NIDDK to CPK and KKZ.
Conflict of interest statement. CPK and KKZ have received grant support and/or honoraria from Genzyme, Shire and Novo Nordisk.
References
- 1.Kopple JD, Kalantar-Zadeh K, Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl. 2005:S21–S27. doi: 10.1111/j.1523-1755.2005.09503.x. [DOI] [PubMed] [Google Scholar]
- 2.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45:978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Franch HA, Mitch WE. Catabolism in uremia: the impact of metabolic acidosis. J Am Soc Nephrol. 1998;9:S78–S81. [PubMed] [Google Scholar]
- 4.Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:661–671. [PubMed] [Google Scholar]
- 5.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 6.Wu DY, McAllister CJ, Kilpatrick RD, et al. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1:70–78. doi: 10.2215/CJN.00010505. [DOI] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Trivedi BK, Anderson JE. Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: a historical prospective cohort study. Adv Chronic Kidney Dis. 2006;13:183–188. doi: 10.1053/j.ackd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, et al. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant. 2006;21:1257–1262. doi: 10.1093/ndt/gfk057. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 10.National Kidney Foundation (NKF). K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Mehrotra R, Fouque D, et al. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial. 2004;17:455–465. doi: 10.1111/j.0894-0959.2004.17606.x. [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2000;35:S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 13.Royston P, Sauerbrei W. Multivariable modeling using cubic regression splines: a principled approach. Stata J. 2007;7:45–70. [Google Scholar]
- 14.Kovesdy CP, Regidor DL, Mehrotra R, et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz I, Maher T, Hulter HN, et al. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983;24:670–680. doi: 10.1038/ki.1983.210. [DOI] [PubMed] [Google Scholar]
- 16.Uribarri J, Levin NW, Delmez J, et al. Association of acidosis and nutritional parameters in hemodialysis patients. Am J Kidney Dis. 1999;34:493–499. doi: 10.1016/s0272-6386(99)70077-6. [DOI] [PubMed] [Google Scholar]
- 17.Ballmer PE, McNurlan MA, Hulter HN, et al. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest. 1995;95:39–45. doi: 10.1172/JCI117668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franch HA, Raissi S, Wang X, et al. Acidosis impairs insulin receptor substrate-1-associated phosphoinositide 3-kinase signaling in muscle cells: consequences on proteolysis. Am J Physiol Renal Physiol. 2004;287:F700–F706. doi: 10.1152/ajprenal.00440.2003. [DOI] [PubMed] [Google Scholar]
- 19.Graham KA, Reaich D, Channon SM, et al. Correction of acidosis in CAPD decreases whole body protein degradation. Kidney Int. 1996;49:1396–1400. doi: 10.1038/ki.1996.196. [DOI] [PubMed] [Google Scholar]
- 20.Graham KA, Reaich D, Channon SM, et al. Correction of acidosis in hemodialysis decreases whole-body protein degradation. J Am Soc Nephrol. 1997;8:632–637. doi: 10.1681/ASN.V84632. [DOI] [PubMed] [Google Scholar]
- 21.Kooman JP, Deutz NE, Zijlmans P, et al. The influence of bicarbonate supplementation on plasma levels of branched-chain amino acids in haemodialysis patients with metabolic acidosis. Nephrol Dial Transplant. 1997;12:2397–2401. doi: 10.1093/ndt/12.11.2397. [DOI] [PubMed] [Google Scholar]
- 22.Lofberg E, Wernerman J, Anderstam B, et al. Correction of acidosis in dialysis patients increases branched-chain and total essential amino acid levels in muscle. Clin Nephrol. 1997;48:230–237. [PubMed] [Google Scholar]
- 23.Verove C, Maisonneuve N, El AA, et al. Effect of the correction of metabolic acidosis on nutritional status in elderly patients with chronic renal failure. J Ren Nutr. 2002;12:224–228. doi: 10.1053/jren.2002.35298. [DOI] [PubMed] [Google Scholar]