Abstract
Background. Cardiovascular disease is a leading cause of death among renal transplant recipients. Aortic calcification is associated with increased mortality in dialysis subjects. The significance of aortic calcification among renal transplant recipients is unknown. Our objective was to prospectively examine the association of aortic calcification with cardiovascular events and all-cause mortality among asymptomatic incident renal transplant recipients.
Methods. One hundred and twelve renal transplant recipients underwent electron beam computed tomography. Aortic calcification was scored by the Agatston method. The mean follow-up time was 5.1 years. Cardiovascular events (heart failure, coronary artery disease, peripheral arterial disease and stroke) and all-cause mortality were recorded.
Results. The cohort consisted of 62% Caucasians, 38% African Americans and 62% male gender. The mean age was 49.0 ± 12.5 years. Thirty-four percent had aortic calcification. During follow-up, 12 cardiovascular events and 10 deaths were recorded. Subjects with aortic calcification had more cardiovascular events compared to those without aortic calcification (23.7 versus 4.1%, P = 0.001). Recipients with aortic calcification had higher mortality compared to those without aortic calcification but it did not reach statistical significance (15.8 versus 5.4%, P = 0.07). The univariate hazard ratio of aortic calcification score in a proportional hazard Cox model to assess event-free survival was 1.15 (1.04–1.27, P = 0.01). Diabetes and aortic calcification score were independently associated with survival. In addition to the predictors above, dialysis vintage was an independent predictor for combined future cardiovascular event and mortality.
Conclusions. In conclusion, aortic calcification is prevalent among renal transplant recipients and is predictive of future cardiovascular events. Aortic calcification is easily identified by non-invasive testing, and should be considered when assessing cardiovascular risk in asymptomatic renal transplant recipients.
Keywords: cardiovascular events, renal transplantation, vascular calcification
Introduction
Chronic kidney disease (CKD) is associated with increasing risk for cardiovascular disease and cardiovascular events. Individuals with only moderately impaired renal function (e.g. CKD stages 2 and 3) are at an increased risk for cardiovascular disease as manifested by congestive heart failure and coronary artery disease [1]. End-stage renal disease (ESRD) patients are at the highest risk for cardiovascular disease. Renal transplantation is the best therapy for ESRD. However, despite an improvement in survival [2], renal transplant recipients remain at an increased risk for cardiovascular disease compared to the general population.
Aortic calcification is a risk marker for cardiovascular disease, as it has been associated with coronary artery disease and stroke in the general population [3]. Aortic calcification has also been associated with heart failure [4]. Aortic stiffness, which leads to left ventricular hypertrophy, myocardial fibrosis and subsequent congestive heart failure and sudden death, is linked to aortic calcification. Aortic calcification is associated with age, hypertension and history of cardiovascular disease [5]. Among those without hypertension, aortic calcification was reported to be more prevalent in women [5]. Risk factors for aortic calcification in the dialysis population include calcium–phosphorus product, age, dialysis vintage, blood pressure, smoking and diabetes mellitus [6]. In the dialysis population, aortic calcification is associated with vascular stiffness [7] and peripheral arterial disease [8].
Aortic calcification can be easily quantified using several non-invasive measures. However, there is a limited understanding of the predictive role of aortic calcification in this high-risk population. Our objective was to prospectively determine in a cohort of asymptomatic incident renal transplant recipients if aortic calcification predicts cardiovascular events and all-cause mortality.
Materials and methods
Subjects
A total of 112 consecutive incident renal transplant recipients from a single institution were enrolled in the study. A baseline questionnaire and medical record review provided biochemical, clinical and demographic data. Subjects were eligible for the study if they had no prior history of coronary artery revascularization, myocardial infarction or anginal symptoms as elicited by the Rose questionnaire [9]. Patients with a history of peripheral arterial disease or cerebrovascular accidents were not excluded from the study. This cohort has been previously described in studies related to coronary artery calcification [10,11].
Institutional Review Board approved this study and we have obtained written informed consent from all subjects.
Study end points
The primary end points for the study were cardiovascular events, defined as coronary artery disease (coronary artery bypass surgery, percutaneous intervention or myocardial infarction), heart failure, cerebrovascular accident or peripheral arterial disease (revascularization or amputation) and all-cause mortality. Heart failure was defined as hospital admission with an acute exacerbation. Patients were followed prospectively from the time of transplant to the occurrence of the first cardiovascular event and/or death. Follow-up was until 30 September 2008.
Electron beam computed tomography
We used electron beam computed tomography to evaluate thoracic aortic calcification in all subjects. Subjects were eligible if baseline electron beam computed tomography was performed within 6 months of transplant. Our methods have been described previously [10]. In summary, subjects underwent a single scan performed on a C-150 Imatron scanner (GE, San Francisco, CA, USA) within 6 months post-transplant. The same scanner was used for all subjects. Electrocardiographic triggering of scans was used to ensure that all images were obtained at the same phase in the cardiac cycle. Serial, contiguous, 3-mm-thick transverse images were obtained commencing at the root of the aorta cephalad to the coronary sinuses and proceeding caudad to the entire coronary tree. No contrast-enhancing agent was used. Quantity of aortic and coronary artery calcification was based on the calcific area, average density and the number of plaques in the coronary arteries and thoracic aorta [12]. Specifically, a total calcium score was calculated by multiplying the area of the calcified plaque by a coefficient based on the peak computed tomography number as originally described by Agatston [13]. The aortic calcification score was measured in three segments: root, ascending and descending aorta. We divided the standard deviation of each segment by the total score and the sum of all segments created a single total aortic score. The acquired images were reviewed on a TeraRecon workstation using the Aquarius program, version 2.1 (TeraRecon, San Mateo, CA, USA). During the study period, there were two readers for the electron beam computed tomography results who were blinded to the clinical course of the subjects.
Immunosuppression
Our standard immunosuppressive regimen comprised induction with rabbit antithymocyte globulin, followed by maintenance therapy with a calcineurin inhibitor (tacrolimus in the majority of patients) mycophenolate mofetil and low-dose prednisone (5 mg/day).
Data collected
The following information was collected for all subjects: demographics, comorbidities including hypertension, hypercholesterolaemia, diabetes, dialysis vintage, therapy for renal osteodystrophy including vitamin D, and phosphorus binders, statin use and smoking status. Physiologic parameters routinely performed for clinical care including haemoglobin, creatinine, calcium and phosphorus were abstracted. Estimated glomerular filtration rate was calculated at 3 months post-transplant using the Modification of Diet in Renal Disease formula [14]. A pre-transplant sample was analysed for a complete lipid profile (total cholesterol, triglycerides, apolipoprotein A1, apolipoprotein B and high-density lipoprotein). All plasma lipid assays were analysed using commercially available reagents from Sigma Diagnostics on an autoanalyser. High-sensitivity C-reactive protein was measured using commercially available reagents from Wako Diagnostics (Richmond, VA, USA). We used a sandwich enzyme-linked immunosorbent assay commercially available from R&D Systems (Minneapolis, MN, USA) to measure soluble intracellular adhesion molecule.
Statistical methods
Demographic and laboratory parameters were summarized by means ± standard deviations and medians for continuous measures and as percentages for categorical variables. Categorical values were analysed by chi-squared tests. Normally distributed variables were analysed by a t-test. A Kaplan–Meier analysis was used to compare event-free survival in patients with and without aortic calcification. A Cox proportional hazard analysis was used to evaluate the independent effect of aortic calcification on event-free survival and all-cause mortality. Results were considered statistically significant if the corresponding P-value ≤ 0.05. All analyses were done using Stata 10 (StataCorp, College Station, TX, USA).
Results
The cohort was reflective of our centre's ethnic diversity with 62% Caucasians and 38% African Americans. Males comprised 62% of the cohort. The mean age was 49.0 ± 12.5 years (range 18.4–72.7). The most common cause of renal disease listed was hypertension (54%) followed by diabetes (31%). These aetiologies were not mutually exclusive. Diabetes was present in 39 subjects (35%).
The baseline electron beam computed tomography was performed on average 2.6 ± 1.9 months after transplantation. The mean baseline aortic calcification score for the complete cohort was 208.1 ± 783.4 with a median of 0. Within the cohort, 38 subjects (34%) had aortic calcification. Demographics of the cohort according to the presence or absence of aortic calcification are presented in Table 1. The group with no aortic calcification was younger, less likely to be African American, with shorter dialysis vintage or preemptive transplant, lower coronary artery calcification scores and had higher albumin values compared to subjects with aortic calcification. Sixty-two percent of the cohort had coronary artery calcification. While 31.3% had both coronary and aortic calcification, 35% had no calcification at either site. Patients with aortic calcification also had higher mean and median coronary artery calcification scores.
Table 1.
Baseline characteristics of the cohort according to the presence or absence of aortic calcification
| Characteristic | Calcification (n = 38) | No calcification (n = 74) | P-value |
|---|---|---|---|
| Male (%) | 68.4 | 58.1 | 0.29 |
| Age (year) | 56 ± 8 | 45 ± 12 | <0.001 |
| Black race (%) | 52.6 | 29.7 | 0.02 |
| Dialysis vintage (year) | 3.3 ± 3.0 | 1.8 ± 2.2 | 0.003 |
| Preemptive transplant (%) | 13.2 | 29.7 | 0.05 |
| Living donor transplant (%) | 28.9 | 44.6 | 0.11 |
| BMI (kg/m2) | 26.7 ± 5.0 | 27.5 ± 5.2 | 0.48 |
| Diabetes (%) | 44.7 | 29.7 | 0.11 |
| Hypertension (%) | 92.1 | 93.2 | 0.83 |
| Active smoker (%) | 10.8 | 9.5 | 0.82 |
| ACE inhibitor or ARB use (%) | 13.2 | 21.6 | 0.32 |
| CVD (%) | 7.9 | 9.5 | 0.78 |
| CRP (mg/L) | 5.2 ± 7.4 | 3.5 ± 5.1 | 0.09 |
| Total cholesterol (mg/dL) | 170 ± 37 | 174 ± 47 | 0.66 |
| Triglycerides (mg/dL) | 188 ±150 | 180 ± 170 | 0.82 |
| LDL (mg/dL) | 91 ± 30 | 95 ± 38 | 0.61 |
| HDL (mg/dL) | 43 ± 13 | 46 ± 19 | 0.40 |
| sICAM (ng/mL) | 261 ± 78 | 293 ± 80 | 0.06 |
| Systolic blood pressure (mmHg) | 138 ± 27 | 134 ± 20 | 0.38 |
| Diastolic blood pressure (mmHg) | 73 ± 12 | 74 ± 12 | 0.64 |
| ApoA1 (mg/dL) | 120 ± 26 | 125 ± 27 | 0.34 |
| Lipoprotein A (mg/dL) | 36 ± 32 | 47 ± 48 | 0.15 |
| Calcium (mg/dL) | 9.3 ± 1.4 | 9.2 ± 0.86 | 0.68 |
| Phosphorus (mg/dL) | 5.0 ± 1.3 | 5.1 ± 1.3 | 0.68 |
| Albumin (g/dL) | 3.59 ± 0.5 | 3.91 ± 0.44 | 0.001 |
| CAC mean | 709.9 (842.3) | 192.0 (506.1) | <0.001 |
| CAC median | 436.4 | 3.9 | <0.001 |
ACE, angiotensin-converting enzyme; ApoA1, apolipoprotein A; ARB, angiotensin receptor blocker; BMI, body mass index; CAC, coronary artery calcium score; CRP, C-reactive protein; CVD, cardiovascular disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; sICAM, soluble intracellular adhesion molecule.
The mean follow-up was 5.1 ± 1.6 years. During the study period, 12 cardiovascular events and 10 deaths were recorded. Three recipients with cardiovascular events later expired. The cardiovascular events included heart failure (n = 5), coronary artery disease (n = 5) and peripheral arterial disease (n = 2). Only one subject with a history of peripheral arterial disease or cerebrovascular accident had a new cardiovascular event during the follow-up period. The causes of death were infection (n = 2), gastrointestinal bleed (n = 1), unknown (n = 4) and cardiac-related death (n = 3).
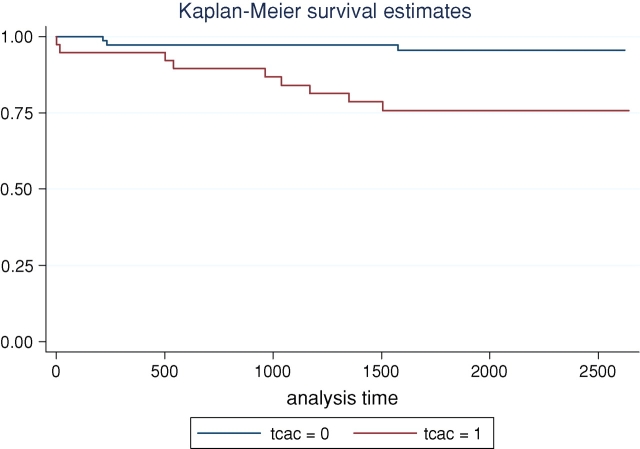
Recipients with aortic calcification had more cardiovascular events compared to those without aortic calcification (23.7% versus 4.1%, P = 0.001). Figure 1 depicts the unadjusted cardiovascular event-free survival curves for recipients with and without aortic calcification (P = 0.001). Recipients with aortic calcification had higher mortality compared to those without aortic calcification but it did not reach a statistical significance (15.8 versus 5.4%, P = 0.07).
Fig. 1.
Cardiovascular event-free survival by Kaplan–Meier analysis, according to the presence or absence of aortic calcification (tcac) among renal transplant recipients.
Variables in Table 1 with a P-value <0.1 were used in a proportional hazard Cox model to assess event-free survival. The univariate hazard ratio of aortic calcification score was 1.15 (1.04–1.27, P = 0.01). Diabetes and aortic calcification score were independently associated with survival (Table 2). Soluble intracellular adhesion molecule levels had a small but significant protective effect. In addition to the predictors above, dialysis vintage was an independent predictor for future cardiovascular event and mortality.
Table 2.
Risk factors associated with cardiovascular events and mortality
| Hazard | 95% Confidence | ||
|---|---|---|---|
| Variable | ratio | interval | P-value |
| Risk factors associated with cardiovascular events | |||
| Diabetes | 8.01 | 2.35–27.6 | 0.001 |
| AC score | 1.27 | 1.09–1.49 | 0.003 |
| sICAM | 0.99 | 0.98–1.0 | 0.004 |
| Risk factors associated with cardiovascular events and mortality | |||
| Diabetes | 8.57 | 2.70–27.3 | <0.001 |
| AC score | 1.26 | 1.09–1.46 | 0.002 |
| sICAM | 0.99 | 0.99–0.99 | 0.026 |
| Dialysis vintage | 1.21 | 1.02–1.44 | 0.034 |
AC, aortic calcification; sICAM, soluble intracellular adhesion molecule.
Discussion
This is the first study to demonstrate that aortic calcification is predictive of cardiovascular events in a group of incident renal transplant recipients without prior history of coronary revascularization. In addition, we found that aortic calcification was an independent predictor of event-free survival in renal transplant recipients.
The prevalence of aortic calcification in our cohort is much lower than the 85% reported in a prevalent transplant cohort, which had received a transplant on average 8 years before the scan [15]. It was also lower than that reported in a recent study of incident dialysis patients [7]. Although dialysis vintage was similar, subjects in our cohort were younger and comprised asymptomatic incident renal transplant recipients without a history of previous coronary revascularization or myocardial infarction; other studies included subjects with known coronary artery disease.
Several reports have documented the clinical importance of vascular calcification among dialysis patients; however, there is a paucity of studies in the renal transplant population. Vascular calcification among dialysis patients is thought to be a result of derangements in bone mineral metabolism and the use of therapies to correct those abnormalities. Calcification of peripheral arteries as detected by ultrasound and plain radiographs has been associated with all-cause and cardiovascular mortality in dialysis patients [16,17]. Aortic calcification is also associated with all-cause and cardiovascular mortality among dialysis patients [18,19]. Cardiovascular mortality among dialysis patients is frequently associated with congestive heart failure and sudden death. Ventricular hypertrophy and myocardial fibrosis, which lead to heart failure and sudden death from fatal arrhythmias, are thought to result from increased aortic stiffness, which is associated with aortic and coronary calcification [7,20].
Age and dialysis vintage are associated with presence of aortic calcification at the time of transplant in our study and this finding has been confirmed by others previously [15,19,21,22]. Dialysis vintage has been associated with risk for cardiovascular events post-transplant [23]. Pre-transplant vascular calcification burden, transplant-related factors, hyperparathyroidism, hypertension and new-onset diabetes mellitus after transplant, all may be contributing factors to this phenomenon. In our study, aortic calcification was not associated with history of smoking or systolic blood pressure as reported by others [22].
There has been a strong association between nutrition, inflammation and atherosclerosis in chronic kidney disease [24]. Our study confirms this association in renal transplant recipients. Atherosclerosis is an inflammatory process. C-reactive protein is a marker of inflammation. We found that C-reactive protein was associated with aortic calcification presence at the time of transplant and aortic calcification progression post-transplant (data not shown). A recent cross-sectional study found an association of aortic calcification with C-reactive protein in dialysis subjects [25]. C-reactive protein has been found to be associated with presence of aortic calcification in some [26], but not all studies [27]. C-reactive protein did not predict aortic calcification progression in the general population [26]. We did not find that C-reactive protein predicted event-free survival independently.
One of the earliest events in the formation of atherosclerotic plaque is the binding of leukocytes to intracellular adhesion molecule-1. In a small study of dialysis patients, soluble intracellular adhesion molecule was shown to be an independent risk factor for mortality independent of age, nutritional status, history of cardiovascular disease and high-sensitivity C-reactive protein [28]. The concentration of soluble intracellular adhesion molecule is modified by the use of blood pressure medications such as angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists [29]. Although we believe that medication use may explain the difference in soluble intracellular adhesion molecule levels in our study (as patients with greater risk factors for atherosclerosis and heart failure may be preferentially placed on angiotensin-converting enzyme inhibitors and angiotensin receptor blockers), we were unable to find differential use of these medications between calcification groups in our study.
Diabetes has been associated with aortic calcification presence in prevalent dialysis subjects [19]. In our cohort, diabetes was associated with worse event-free survival and mortality. It is known that patients with diabetes are more likely to have heart failure [30] and have prolonged hospital length of stay when admitted for acute heart failure exacerbation [31].
Given the high burden of cardiovascular disease in renal transplant recipients, developing means to better assess cardiovascular disease risk is important, especially due to the high prevalence of asymptomatic disease. Identification of aortic calcification among asymptomatic renal transplant recipients will identify a high-risk group for cardiovascular events and all-cause mortality. Vascular calcification is a marker of cardiovascular disease that can easily be assessed by several non-invasive means including electron beam computed tomography, plain radiographs and ultrasound. Although we used electron beam computed tomography in this study to identify aortic calcification, lesions can also be identified using plain soft-tissue X-ray. In the general population, aortic arch calcification identified by chest radiograph is associated with coronary artery disease and stroke, as well as increased risk for cardiovascular death in those younger than 65 years [32–35]. Given the ease of identifying aortic calcification, this may be a simple means by which to further risk stratify renal transplant recipients.
We recruited a cohort without a history of myocardial infarction or coronary revascularization. Forty-two percent of cardiovascular events were related to heart failure, which is associated aortic calcification. Our results indicate that aortic calcification is a risk marker for all cardiovascular events including heart failure.
Although, the study represents the largest series of transplant patients with aortic calcification, the sample size is relatively small. The proportion of patients with aortic calcification may also be less in this study than the general transplant population as 24% of our cohort had preemptive transplants. Few cardiovascular events occurred as expected due to the inclusion criteria, so future studies in larger cohorts followed for a longer period of time post-transplant are needed.
In conclusion, aortic calcification is prevalent among renal transplant recipients without a prior history of coronary revascularization. Aortic calcification is predictive of cardiovascular events and is easily identified by non-invasive testing. Identifying aortic calcification should be considered when assessing cardiovascular risk in renal transplant recipients without a history of coronary artery disease, particularly in those with a history of diabetes, older or with elevated C-reactive protein.
Acknowledgments
This study was supported by National Institutes of Health (research supplement R01 DK 67390 to S.S.D., R03 DK 60709 and K08 DK 02626 to S.E.R.). S.S.D. was also supported by the Josiah Macy, Jr Foundation, and S.E.R. by AHA (GIA 0655479U). S.G. was supported by Genzyme, Bristol-Meyers Squibb and Parexel.
Conflict of interest statement. None declared.
References
- 1.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System USRDS 2005 Annual Data Report. Bethesda, MD: United States Renal Data System, 2006.
- 3.Schousboe JT, Taylor BC, Kiel DP, et al. Abdominal aortic calcification detected on lateral spine images from a bone densitometer predicts incident myocardial infarction or stroke in older women. J Bone Miner Res. 2008;23(3):409–416. doi: 10.1359/jbmr.071024. [DOI] [PubMed] [Google Scholar]
- 4.Walsh CR, Cupples LA, Levy D, et al. Abdominal aortic calcific deposits are associated with increased risk for congestive heart failure: the Framingham Heart Study. Am Heart J. 2002;144:733–739. doi: 10.1067/mhj.2002.124404. [DOI] [PubMed] [Google Scholar]
- 5.Oyama N, Gona P, Salton CJ, et al. The differential impact of age, sex, and hypertension on aortic atherosclerosis. The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28(1):155–159. doi: 10.1161/ATVBAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 6.Shigematsu T, Kono T, Satoh K, et al. Phosphate overload accelerates vascular calcium deposition in end-stage renal disease patients. Nephrol Dial Transplant. 2003;18:iii86–iii89. doi: 10.1093/ndt/gfg1022. [DOI] [PubMed] [Google Scholar]
- 7.Raggi P, Bellasi A, Ferramosca E, et al. Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int. 2007;71:802–807. doi: 10.1038/sj.ki.5002164. [DOI] [PubMed] [Google Scholar]
- 8.Fabbian F, Catalano C, Orlandi V, et al. Evaluation of aortic arch calcification in hemodialysis patients. J Nephrol. 2005;18:289–293. [PubMed] [Google Scholar]
- 9.Heyden S, Bartel AG, Tabesh E, et al. Angina pectoris and the Rose questionnaire. Arch Intern Med. 1971;128:961–964. [PubMed] [Google Scholar]
- 10.Rosas SE, Mensah K, Weinstein RB, et al. Coronary artery calcification in renal transplant recipients. Am J Transplant. 2005;5:1942–1947. doi: 10.1111/j.1600-6143.2005.00955.x. [DOI] [PubMed] [Google Scholar]
- 11.Schankel K, Robinson J, Bloom R, et al. Determinants of coronary artery calcification progression in renal transplant recipients. Am J Transplant. 2007;7(9):2158–2164. doi: 10.1111/j.1600-6143.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- 12.Rumberger JA, Brundage BH, Rader DJ, et al. Electron bean computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons [erratum appears in Mayo Clin Proc 1999 May; 74(5): 538] Mayo Clin Proc. 1999;74:243–252. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Levy AS, Bosch JP, Lewis JB, et al. (Modification of Diet in Renal Disease Study Group) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen PTH, Coche E, Goffin E, et al. Prevalence and determinants of Corcnary artery calcification assessed by chest CT in renal transplant recipients. Am J Nephrol. 2007;27:329–335. doi: 10.1159/000102978. [DOI] [PubMed] [Google Scholar]
- 16.Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 17.London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel DM, Raggi P, Smits G, et al. Factors associated with mortality in patients new to haemodialysis. Nephrol Dial Transplant. 2007;22(12):3568–3572. doi: 10.1093/ndt/gfm424. [DOI] [PubMed] [Google Scholar]
- 19.Okuno S, Ishimura E, Kitakani K, et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417–425. doi: 10.1053/j.ajkd.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 20.van Popele NM, Mattace-Raso FU, Vliegenthart R, et al. Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: the Rotterdam Study. J Hypertens. 2006;24:2371–2376. doi: 10.1097/01.hjh.0000251896.62873.c4. [DOI] [PubMed] [Google Scholar]
- 21.Moe SM, O’Neill KD, Fineberg N, et al. Assessment of vascular calcification in ESRD patients using spiral CT. Nephrol Dial Transplant. 2003;18:1152–1158. doi: 10.1093/ndt/gfg093. [DOI] [PubMed] [Google Scholar]
- 22.Taniwaki H, Ishimura E, Tabata T, et al. Aortic calcifications in haemodialysis patients with diabetes mellitus. Nephrol Dial Transplant. 2005;20:2472–2478. doi: 10.1093/ndt/gfi039. [DOI] [PubMed] [Google Scholar]
- 23.Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int. 1998;53:767–772. doi: 10.1046/j.1523-1755.1998.00787.x. [DOI] [PubMed] [Google Scholar]
- 24.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamada K, Fujimoto S, Nishiura R, et al. Risk factors of the progression of abdominal aortic in patients on chronic haemodialysis. Nephrol Dial Transplant. 2007;22:2032–2037. doi: 10.1093/ndt/gfm031. [DOI] [PubMed] [Google Scholar]
- 26.Elias-Smale SE, Kardys I, Oudkerk M, et al. C-reactive protein is related to extent and progression of coronary and extra-coronary atherosclerosis; results from the Rotterdam study. Atherosclerosis. 2007;195:e195–e202. doi: 10.1016/j.atherosclerosis.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Khera A, de Lemos JA, Peshock RM, et al. Relationship between C-reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation. 2006;113:38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 28.Stenvinkel P, Lindholm B, Heimburger M, et al. Elevated serum levels of soluble adhesion molecules predict death in pre-dialysis patients: association with malnutrition, inflammation, and cardiovascular disease. Nephrol Dial Transplant. 2000;15:1624–1630. doi: 10.1093/ndt/15.10.1624. [DOI] [PubMed] [Google Scholar]
- 29.Suliman ME, Qureshi AR, Heimburger O, et al. Soluble adhesion molecules in end-stage renal disease: a predictor of outcome. Nephrol Dial Transplant. 2006;21:1603–1610. doi: 10.1093/ndt/gfl005. [DOI] [PubMed] [Google Scholar]
- 30.Nichols GA, Gullion CM, Koro CE, et al. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 31.Gebreegziabher Y, McCullough PA, Bubb C, et al. Admission hyperglycemia and length of hospital stay in patients with diabetes and heart failure: a prospective cohort study. Congest Heart Fail. 2008;14:117–120. doi: 10.1111/j.1751-7133.2008.07569.x. [DOI] [PubMed] [Google Scholar]
- 32.Iribarren C, Sidney S, Sternfeld B, et al. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–2815. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 33.Witteman JC, Kannel WB, Wolf PA, et al. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 34.Yun KH, Jeong MH, Oh SK, et al. Clinical significance of aortic knob width and calcification in unstable angina. Circ J. 2006;70:1280–1283. doi: 10.1253/circj.70.1280. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Galvin HK, Johnson SC, et al. Aortic calcification on plain chest radiography increases risk for coronary artery disease. Chest. 2002;121:1468–1471. doi: 10.1378/chest.121.5.1468. [DOI] [PubMed] [Google Scholar]