Abstract
AIM: To compare a lansoprazole-based triple versus quadruple therapy for Helicobacter pylori (H pylori) eradication with emphasis on side effect profile, patient compliance and eradication rate at a rural district general hospital in Wales, United Kingdom.
METHODS: One hundred one patients with H pylori infection were included in the study. Patients were randomised to receive triple therapy comprising of lansoprazole 30 mg, amoxycillin 1 g, clarithromycin 500 mg, all b.d. (LAC), or quadruple therapy comprising of lansoprazole 30 mg b.d., metronidazole 500 mg t.d.s., bismuth subcitrate 240 mg b.d., and tetracycline chloride 500 mg q.d.s. (LMBT). Cure was defined as a negative 13C urea breath test 2 mo after treatment.
RESULTS: Seven patients were withdrawn after randomisation. Fifty patients were assigned to LAC group and 44 to LMBT group. The intention-to-treat cure rates were 92% and 91%, whereas the per-protocol cure rates were 92% and 97%, respectively. Side effects were common, with 56% experiencing moderate to severe symptoms in the LAC group and 59% in the LMBT group. Symptoms of vomiting, diarrhoea and black stools were significantly more common in the LMBT group. Patient compliance was 100% for triple therapy and 86% for quadruple therapy (P < 0.01). One-third of patients in both groups were still taking acid-reducing medications at six-month follow-up.
CONCLUSION: One-week triple and quadruple therapies have similar intention-to-treat eradication rates. Certain side effects are more common with quadruple therapy, which can compromise patient compliance. Patient education or modifications to the regimen are alternative options to improve compliance of the quadruple regimen.
Keywords: Helicobacter pylori, Triple therapy, Quadruple therapy, Side effects, Treatment compliance, Eradication rate
INTRODUCTION
European studies have shown that quadruple therapy, even though more effective with a cure rate of over 95% by per protocol analysis[1–3], is less popular compared to a standard triple therapy for eradication of Helicobacter pylori (H pylori) infection. The reasons for this are the complexity of the regimen and also its side effects. Scheduling drugs four or more times a day reduces compliance[4,5]. However, some studies have suggested that quadruple therapy has a similar magnitude of adherence and adverse effects compared to triple the-rapies[6,7].
Triple therapies are the mainstay of current treat-ment but resistance to clarithromycin is reducing its effectiveness. In the presence of resistance to clarithro-mycin, some studies have shown eradication rate below 80% and even as low as 25%-61% with standard triple therapy containing clarithromycin, amoxycillin and a proton-pump inhibitor[7–11]. Clarithromycin resistance is also increasing in our region[12,13].
Quadruple therapy is used mainly as a second-line therapy after failed eradication with triple therapy[14–18]. Earlier consensus meeting reports including the Maastricht II Consensus Report on the management of H pylori infection have recommended the use of quadruple therapy for 1 wk as second-line therapy for H pylori infection[19–21]. However, updated reports have now recommended quadruple therapy as an alternative first-line eradication therapy[22–24].
The objective of the study was to compare a standard lansoprazole-based triple therapy (HeliClear®) to a lansoprazole-based quadruple therapy as first-line therapy in a surgical practice in a predominantly Caucasian population in North Wales.
MATERIALS AND METHODS
We conducted a prospective randomised trial of patients under the care of an upper gastrointestinal surgeon at Ysbyty Gwynedd, a rural District General Hospital in North Wales. The population served by Ysbyty Gwynedd is predominantly (98.8%) white and there are about 120 new cases of H pylori each year from a population of around 180 000. Twenty-four percent of strains were resistant to metronidazole, 7% to clarithromycin and 4% to both. There was resistance to tetracycline in 1 out of 363 isolates and none to amoxycillin[12].
The Local Ethics Committee of the participating hospitals approved the study. From June 2001 to November 2005, 101 patients with diagnosis of infection proven by gastric histology or urease test or culture were included in the study. Two positive tests were required for inclusion. The inclusion and exclusion criteria are shown in Table 1.
Table 1.
Inclusion criteria and exclusion criteria
| Criteria | |
| Inclusion criteria | Dyspeptic symptoms |
| Has recent OGD (duodenal ulcer; gastric ulcer; gastritis or non-ulcer dyspepsia) | |
| Positive for H pylori on histology and culture or CLO test or 13C-urea breath test | |
| Exclusion criteria | Age less than 18 or above 75 yr |
| Symptomatic gallstones | |
| Treated with antibiotic or bismuth-containing drugs during the month prior to inclusion | |
| Treated with proton pump inhibitor during the week prior to inclusion | |
| Disturbed gastrointestinal physiology (gastric surgery; vagotomy; Zollinger-Ellison syndrome; chronic ingestion of NSAIDs) | |
| Concomitant serious disease | |
| Concomitant medications that may adversely interact with the study drugs (e.g. warfarin, anti-epileptics) | |
| Pregnancy and breast-feeding | |
| Childbearing age without adequate contraception | |
| Allergy to drugs used in the study | |
| Mental illness | |
| Heavy drinking or abuse of drugs | |
Patients were recruited into the trial once they had met the criteria and given fully informed written consent. Patients were recruited from the outpatient departments at one district general hospital and a satellite hospital served by the same team of doctors. The patients received a 7-d course of either a triple regimen (LAC) or a quadruple regimen (LMBT) (Table 2).
Table 2.
Regimens used in the trial
| Triple therapy regimen (LAC) | Quadruple therapy regimen (LMBT) |
| Lansoprazole (30 mg b.d.) | Lansoprazole (30 mg b.d.) |
| Amoxycillin (1 g b.d.) | Metronidazole (400 mg t.d.s.) |
| Clarithromycin (500 mg b.d.) | Bismuth subcitrate (240 mg b.d.) |
| Tetracycline chloride (500 mg q.d.s.) |
Randomisation took place at the hospital pharmacies when the patients collected their medications with a note from the recruiting doctor. The pharmacists dispensed the medications adhering to the order on a random list of therapy regimens.
A printed chart showing the names of the drugs, the number of pills to take and the time schedule was given to all participants to improve understanding and compliance with treatment.
Compliance was evaluated by patient’s record of each dosage taken onto the chart during the week of therapy. Any tablet that was not consumed needed to be brought back to the clinic for pill count. The patients were asked to record the reasons for missed dosages. They were also asked to record any side effects and their severity during the therapy. Proton pump inhibitors and other acid-reducing medications were not allowed after treatment. The patients returned for interview at 6 wk after therapy. The efficacy of treatment was evaluated by means of the 13C-urea breath test performed following the standard European protocol at 8 wk following the start of therapy[12]. Patients were reviewed again at 6 mo after therapy to assess symptoms and use of any medications after determining their post therapy H pylori status. Patients who tested positive were offered the alternate regimen and retested after a gap of 2 mo.
Statistical analysis
Proportions were compared using Fisher’s Exact Test. Quantitative variables were compared using t-test and non-parametric variables were compared using Mann–Whitney U test. Non-categorical values are given as the mean ± SD. Calculations were performed using the SPSS for Windows statistical package.
RESULTS
One hundred one patients were randomized into the trial but seven patients were withdrawn from the study after randomization (one because of diagnosis of bronchial carcinoma, one because of diagnosis of gallstones, two withdrew from the study and three were non-compliant to study protocol) (Figure 1).
Figure 1.
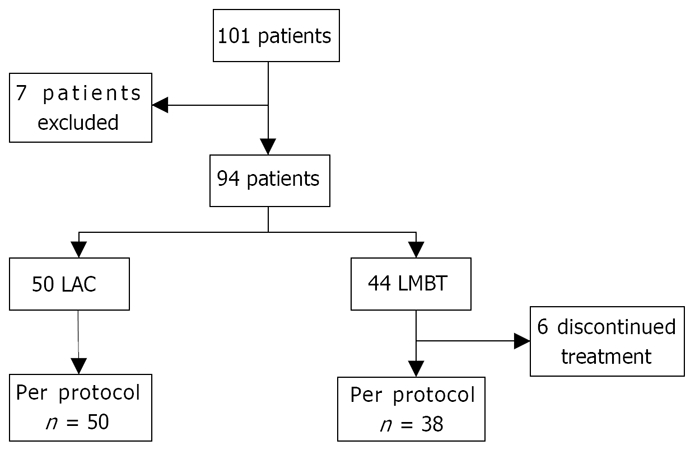
CONSORT flow diagram showing entries and withdrawals from the study.
Fifty patients were assigned to the LAC group and 44 to the LMBT group. The demographic and clinical characteristics of the groups were comparable (Table 3).
Table 3.
Patient characteristics
|
Therapy |
||
| LAC (n = 50) | LMBT (n = 44) | |
| Age | 55.2 ± 10.9 | 53.7 ± 11.4 |
| Gender (male: female) | 26:24 | 27:17 |
| Active smoking | 10 (20%) | 16 (36%) |
| NSAID use | 4 (8%) | 3 (7%) |
| Ethanol abuse (> 14 U/wk) | 4 (8%) | 3 (7%) |
| Previous therapy with antacids | 4 (8%) | 8 (18%) |
| Time between treat-ment and UBT (mo) | 2.2 ± 0.7 | 2.1 ± 0.5 |
| Gastric ulcer | 1 | 1 |
| Duodenal ulcer | 3 | 1 |
| Gastritis | 36 | 33 |
| Duodenitis | 6 | 8 |
| Diagnosis of H pylori infection (Urease: Culture: Biopsy) | 42:29:45 | 37:27:44 |
NSAID: Non-steroidal anti-inflammatory drug; UBT: Urea breath test.
Compliance and side-effects
Compliance was excellent in the LAC group with all the patients completing the 7-d therapy. In contrast, 6 patients (14%) in the LMBT group failed to complete the treatment (P < 0.01). In spite of this three had a negative breath test.
Four out of the six patients had attributed moderate/severe nausea as the reason for discontinuing treatment. One had severe diarrhoea and another had nausea, vomiting and diarrhoea.
Side effects were reported by vast majority of patients in both groups, 45 patients (90%) in the LAC group and 42 patients (95%) in the LMBT group.
The most frequent symptoms in the LAC group were dry mouth (54%) and taste disturbance (46%). Patients in the LMBT group experienced significantly more nausea (45%), vomiting (20%), diarrhoea (57%) and black stool (80%) (Table 4).
Table 4.
Incidence of side effects n (%)
|
Therapy |
P-value | ||
| LAC (n = 50) | LMBT (n = 44) | ||
| Nausea | 11 (22) | 20 (45) | < 0.05 |
| Vomiting | 0 (0) | 9 (20) | < 0.01 |
| Diarrhoea | 14 (28) | 25 (57) | < 0.01 |
| Headache | 12 (24) | 19 (44) | |
| Dizziness | 9 (18) | 11 (25) | |
| Blurred vision | 5 (10) | 6 (14) | |
| Itching | 5 (10) | 5 (11) | |
| Rash | 1 (2) | 2 (5) | |
| Dry mouth | 27 (54) | 19 (43) | |
| Sore mouth | 4 (8) | 0 (0) | |
| Glossitis | 2 (4) | 1 (2) | |
| Black tongue | 6 (12) | 6 (14) | |
| Black stool | 5 (10) | 35 (80) | < 0.01 |
| Taste disturbance | 23 (46) | 14 (32) | |
| Arthralgia | 3 (6) | 1 (2) | |
Each symptom was graded as mild, moderate or severe. In the LAC group, mild symptoms were observed in 17 patients (34%), moderate symptoms observed in 25 patients (50%) and severe symptoms observed in 3 patients (6%). In the LMBT group, mild symptoms were observed in 19 patients (43%), moderate symptoms observed in 13 patients (30%) and severe symptoms observed in 10 patients (23%) (Table 5, P < 0.05). Despite most of the patients experiencing some side effects, none were severe enough to require hospitalization.
Table 5.
Severity of side-effects (n)
| Therapy | ||
| Severity | LAC | LMBT |
| None | 5 | 2 |
| Mild | 17 | 19 |
| Moderate | 25 | 13 |
| Severe | 3 | 10 |
| Total | 50 | 44 |
Severity score: 1 = mild, does not cause any concern; 2 = moderate, but not bad enough to discontinue treatment; 3 = severe or incapacitating, forced to discontinue treatment.
13C-urea breath test
All 94 patients returned for a 13C-urea breath test 2 mo after eradication therapy. Four patients (8%) from the LAC group and four patients from LMBT (9%) had positive results indicating failure of H pylori eradication. Three of the four patients had an incomplete quadruple therapy (Table 6).
Table 6.
13C-urea breath test results n (%)
|
Therapy |
P-value | ||
| LAC (n = 50) | LMBT (n = 44) | ||
| Returned for UBT | 50 (100) | 44 (100) | - |
| Completed therapy | 50 (100) | 38 (86) | < 0.01 |
| UBT result | 46 negative, 4 positive | 37 negative, 1 positive | |
| Not completed therapy | 0 (0%) | 6 (14) | < 0.01 |
| UBT result | - | 3 positive, 3 negative | - |
| Intention-to-treat cure rate | 92% (46/50) | 91% (40/44) | |
| Per-protocol cure rate | 92% (46/50) | 97%(37/38) | |
UBT: Urea breath test.
All the eight patients who tested positive with 13C-urea breath test had the alternate regimen. Three of four patients, who had initially LAC and then LMBT therapy, were negative on the second breath test.
Six-month follow-up
Eighty-six patients (91.5%) returned for a 6-mo follow-up. Over one-third of patients had recurrent or persistent symptoms and remained on long-term acid-reduction therapy (with proton-pump inhibitors, H2-antagonist or other antacids) even after successful eradication (Table 7).
Table 7.
Symptomatic outcome at 6-mo follow-up n (%)
|
Therapy |
||
| LAC (n = 50) | LBMT (n = 44) | |
| Follow-up at 6 mo | 46 (92) | 40 (91) |
| Persistent symptoms | 4 (8) | 7 (16) |
| Recurrent symptoms | 17 (34) | 9 (20) |
| Repeat eradication therapy | 1 (2) | 1 (2) |
| Long-term acid-reduction therapy | 17 (34) | 14 (32) |
DISCUSSION
This study has shown that a lansoprazole–based quadruple therapy is as effective as triple therapy in a predominantly white population in the UK (intention-to-treat rate: 91% vs 92% respectively). The resistance to clarithromycin (7%) is beginning to diminish the effectiveness of the triple therapy (92% per protocol eradication) whereas metronidazole resistance (24%) did not affect quadruple therapy (97% per protocol eradication)[25].
Side effects are common in both regimens occurring in around 90% of patients. However, severe side effects occurred more frequently with quadruple therapy (23% vs 6%) and this reduced compliance.
Four out of the six patients taking quadruple therapy stopped because of nausea and vomiting, which was probably due to metronidazole. Replacing metronidazole with amoxycillin should reduce these side effects and increase compliance[26]. Interestingly, dry mouth was noticed more in the triple therapy group even though lansoprazole was the most likely cause.
The intention-to-treat cure rate of quadruple therapy (LMBT) was comparable to triple therapy (LAC) in spite of lower compliance. Educating patients about the possible common side effects and the importance of complete eradication should provide a very high cure rate as the per protocol cure rate was 97% for quadruple therapy.
Quadruple therapy is very cost effective and should be considered as a first-line therapy especially when there are economic constraints. Lansoprazole-based quadruple therapy costs £17 as against £38 for the triple therapy for a one-week course[27]. The difference of £21 per treatment can be relieved from economic burden for the health service to treat this common condition.
Patients have to be warned that about one sixth of them will have persistent symptoms and about third of them will develop recurrent symptoms with a similar proportion needing long-term treatment with a proton-pump inhibitor, H2-antagonist or other antacids.
Modified seven-day quadruple therapy, by reducing the frequency of tetracycline chloride and bismuth subcitrate from four times to three times daily, has also been tried successfully as a first-line treatment with cure rate and compliance rate of over 90%[2]. Bateson has shown that a twice-daily quadruple therapy using lansoprazole, tetracycline, clarithromycin and metronidazole is effective (95.5% eradication rate) in UK patients with duodenal ulcer but this pre-dated resistance to clarithromycin and metronidazole[28]. Amoxycillin has been shown to improve eradication in resistant patients and perhaps a trial of a twice-daily quadruple therapy substituting amoxycillin for metronidazole should be considered[26]. Other approaches to the problem of antibiotic resistance include a sequential therapy that substituted amoxycillin with tinidazole during the first 5 d of a 10-d triple therapy with pantoprazole, amoxycillin and clarithromycin, which has been shown to achieve a significantly higher eradication rate[29]. Pretreatment sensitivity testing has been confirmed to be cost effective by significantly improved eradication in a study that used omeprazole and two antibiotics chosen based on susceptibility testing, compared to omeprazole, clarithromycin and metronidazole standard triple therapy[30].
Recent randomised studies that compared triple therapy with quadruple therapy as a first-line treatment option for H pylori and some reports showed superior eradication rates with the quadruple therapy[6,31,32] whereas others have shown no difference[33,34]. Quadruple therapy is becoming the standard treatment as resistance to clarithromycin, and to a lesser extent metronidazole, is reducing the efficacy of triple therapies. The side effects may be reduced by replacing metronidazole with amoxycillin but patients should be better educated about the side effects in order to improve compliance and cure rates.
COMMENTS
Background
The treatment for Helicobacter pylori (H pylori) is becoming less effective as the organism is becoming resistant to the commonly used antibiotics in triple therapies. Quadruple therapies were less popular because of their side effects but still have good eradication rates.
Research frontiers
This study compares lansoprazole-based triple and quadruple therapy for H pylori infection in white Caucasians in rural Wales, an area with low resistance to Clarithromycin and moderate resistance to metronidazole.
Innovations and breakthroughs
Both regimens had high eradications rates (> 90%) showing that resistance has not yet significantly affected this UK population. Even better rates (97%) can be achieved with quadruple therapy if patients are able to complete the full course. Patients need to be educated about the side effects and importance of completing the course to achieve the higher eradication rates.
Applications
Quadruple therapies provide a cost effective and highly successful treatment for H pylori. The side effects and compliance may be improved by substituting amoxycillin for metronidazole-an area for future research.
Terminology
Triple therapy is a regimen of a proton pump inhibitor and two antibiotics. Quadruple therapy is a regimen of a proton pump inhibitor, a bismuth compound and two antibiotics.
Peer review
The authors compared lansoprazole-based triple and quadruple therapy in the eradication of H pylori. They found that both regimens were equally effective and that quadruple therapy was less costly even though 6 patients had to discontinue treatment because of side effects. This is an important study.
Acknowledgments
We are indebted to Ms Meinir Williams for her dedicated secretarial assistance.
Supported by Wyeth, United Kingdom and North West Wales NHS Trust
Peer reviewer: Marco Romano, MD, Professor, Dipartimento di Internistica Clinica e Sperimentale-Gastroenterologia, II Policlinico, Edificio 3, II piano, Via Pansini 5, Napoli 80131, Italy
S- Editor Li DL L- Editor Li M E- Editor Lin YP
References
- 1.de Boer WA, Driessen WM, Potters VP, Tytgat GN. Randomized study comparing 1 with 2 weeks of quadruple therapy for eradicating Helicobacter pylori. Am J Gastroenterol. 1994;89:1993–1997. [PubMed] [Google Scholar]
- 2.Calvet X, Garcia N, Gene E, Campo R, Brullet E, Sanfeliu I. Modified seven-day, quadruple therapy as a first line Helicobacter pylori treatment. Aliment Pharmacol Ther. 2001;15:1061–1065. doi: 10.1046/j.1365-2036.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 3.de Boer SY, v d Meeberg PC, Siem H, de Boer WA. Comparison of four-day and seven-day pantoprazole-based quadruple therapy as a routine treatment for Helicobacter pylori infection. Neth J Med. 2003;61:218–222. [PubMed] [Google Scholar]
- 4.Buring SM, Winner LH, Hatton RC, Doering PL. Discontinuation rates of Helicobacter pylori treatment regimens: a meta-analysis. Pharmacotherapy. 1999;19:324–332. doi: 10.1592/phco.19.4.324.30939. [DOI] [PubMed] [Google Scholar]
- 5.Fennerty MB, Lieberman DA, Vakil N, Magaret N, Faigel DO, Helfand M. Effectiveness of Helicobacter pylori therapies in a clinical practice setting. Arch Intern Med. 1999;159:1562–1566. doi: 10.1001/archinte.159.14.1562. [DOI] [PubMed] [Google Scholar]
- 6.Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spenard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 7.Fischbach LA, van Zanten S, Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004;20:1071–1082. doi: 10.1111/j.1365-2036.2004.02248.x. [DOI] [PubMed] [Google Scholar]
- 8.Laine L, Frantz JE, Baker A, Neil GA. A United States multicentre trial of dual and proton pump inhibitor-based triple therapies for Helicobacter pylori. Aliment Pharmacol Ther. 1997;11:913–917. doi: 10.1046/j.1365-2036.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- 9.Comet R, Calvet X, Navarro M, Garcia N, Sanfeliu I. [Seven-day omeprazole, clarithromycin, and amoxicillin for the therapy of Helicobacter pylori infection] Gastroenterol Hepatol. 1998;21:81–83. [PubMed] [Google Scholar]
- 10.Pipkin GA, Williamson R, Wood JR. Review article: one-week clarithromycin triple therapy regimens for eradication of Helicobacter pylori. Aliment Pharmacol Ther. 1998;12:823–837. doi: 10.1046/j.1365-2036.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- 11.Calvet X, Lopez-Lorente M, Cubells M, Bare M, Golvez E, Molina E. Two-week dual vs. one-week triple therapy for cure of Helicobacter pylori infection in primary care: a multicentre, randomized trial. Aliment Pharmacol Ther. 1999;13:781–786. doi: 10.1046/j.1365-2036.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- 12.Elviss NC, Owen RJ, Xerry J, Walker AM, Davies K. Helicobacter pylori antibiotic resistance patterns and genotypes in adult dyspeptic patients from a regional population in North Wales. J Antimicrob Chemother. 2004;54:435–440. doi: 10.1093/jac/dkh343. [DOI] [PubMed] [Google Scholar]
- 13.Chisholm SA, Teare EL, Davies K, Owen RJ. Surveillance of primary antibiotic resistance of Helicobacter pylori at centres in England and Wales over a six-year period (2000-2005) Euro Surveill. 2007;12:E3–E4. doi: 10.2807/esm.12.07.00721-en. [DOI] [PubMed] [Google Scholar]
- 14.Gisbert JP, Gisbert JL, Marcos S, Gravalos RG, Carpio D, Pajares JM. Seven-day 'rescue' therapy after Helicobacter pylori treatment failure: omeprazole, bismuth, tetracycline and metronidazole vs. ranitidine bismuth citrate, tetra-cycline and metronidazole. Aliment Pharmacol Ther. 1999;13:1311–1316. doi: 10.1046/j.1365-2036.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee JM, Breslin NP, Hyde DK, Buckley MJ, O'Morain CA. Treatment options for Helicobacter pylori infection when proton pump inhibitor-based triple therapy fails in clinical practice. Aliment Pharmacol Ther. 1999;13:489–496. doi: 10.1046/j.1365-2036.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 16.Gomollon F, Ducons JA, Ferrero M, Garcia Cabezudo J, Guirao R, Simon MA, Montoro M. Quadruple therapy is effective for eradicating Helicobacter pylori after failure of triple proton-pump inhibitor-based therapy: a detailed, prospective analysis of 21 consecutive cases. Helicobacter. 1999;4:222–225. doi: 10.1046/j.1523-5378.1999.99307.x. [DOI] [PubMed] [Google Scholar]
- 17.Sicilia B, Sierra E, Lago A, Villar M, Garcia S, Gomollon F. [High eradication rates in Helicobacter pylori infection in patients with duodenal ulcer who failed previous eradication therapy] Med Clin (Barc) 2000;115:641–643. doi: 10.1016/s0025-7753(00)71649-6. [DOI] [PubMed] [Google Scholar]
- 18.Boixeda D, Bermejo F, Martin-De-Argila C, Lopez-Sanroman A, Defarges V, Hernandez-Ranz F, Milicua JM, Garcia-Plaza A. Efficacy of quadruple therapy with pantoprazole, bismuth, tetracycline and metronidazole as rescue treatment for Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16:1457–1460. doi: 10.1046/j.1365-2036.2002.01304.x. [DOI] [PubMed] [Google Scholar]
- 19.Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:1–12. doi: 10.1111/j.1440-1746.1998.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 20.Gisbert JP, Calvet X, Gomollon F, Sainz R. [Treatment for the eradication of Helicobacter pylori. Recommendations of the Spanish Consensus Conference] Med Clin (Barc) 2000;114:185–195. doi: 10.1016/s0025-7753(00)71237-1. [DOI] [PubMed] [Google Scholar]
- 21.Malfertheiner P, Megraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 22.Hunt R, Fallone C, Veldhuyzan van Zanten S, Sherman P, Smaill F, Flook N, Thomson A. Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori--an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004;18:547–554. doi: 10.1155/2004/326767. [DOI] [PubMed] [Google Scholar]
- 23.Vilaichone RK, Mahachai V, Graham DY. Helicobacter pylori diagnosis and management. Gastroenterol Clin North Am. 2006;35:229–247. doi: 10.1016/j.gtc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Wouden EJ, Thijs JC, van Zwet AA, Sluiter WJ, Kleibeuker JH. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am J Gastroenterol. 1999;94:1751–1759. doi: 10.1111/j.1572-0241.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 26.Chi CH, Lin CY, Sheu BS, Yang HB, Huang AH, Wu JJ. Quadruple therapy containing amoxicillin and tetracycline is an effective regimen to rescue failed triple therapy by overcoming the antimicrobial resistance of Helicobacter pylori. Aliment Pharmacol Ther. 2003;18:347–353. doi: 10.1046/j.1365-2036.2003.01653.x. [DOI] [PubMed] [Google Scholar]
- 27.British Medical Association, Royal Pharmaceutical Society of Great Britain. British National Formulary. 42nd ed. Wallingford: Pharmaceutical Press. 2001:41, 43, 169, 286. [Google Scholar]
- 28.Bateson MC. Quadruple therapy for symptomatic spontaneous duodenal ulcer disease. Postgrad Med J. 2001;77:447–450. doi: 10.1136/pmj.77.909.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, Hassan C, Bernabucci V, Tampieri A, Morini S. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556–563. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 30.Romano M, Marmo R, Cuomo A, De Simone T, Mucherino C, Iovene MR, Montella F, Tufano MA, Del Vecchio Blanco C, Nardone G. Pretreatment antimicrobial susceptibility testing is cost saving in the eradication of Helicobacter pylori. Clin Gastroenterol Hepatol. 2003;1:273–278. [PubMed] [Google Scholar]
- 31.Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002;123:1763–1769. doi: 10.1053/gast.2002.37051. [DOI] [PubMed] [Google Scholar]
- 32.Uygun A, Kadayifci A, Safali M, Ilgan S, Bagci S. The efficacy of bismuth containing quadruple therapy as a first-line treatment option for Helicobacter pylori. J Dig Dis. 2007;8:211–215. doi: 10.1111/j.1751-2980.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 33.Calvet X, Ducons J, Guardiola J, Tito L, Andreu V, Bory F, Guirao R. One-week triple vs. quadruple therapy for Helicobacter pylori infection - a randomized trial. Aliment Pharmacol Ther. 2002;16:1261–1267. doi: 10.1046/j.1365-2036.2002.01278.x. [DOI] [PubMed] [Google Scholar]
- 34.Jang HJ, Choi MH, Kim YS, Seo YA, Baik KH, Baik IH, Eun CS, Kim JB, Kae SH, Kim DJ, et al. [Effectiveness of triple therapy and quadruple therapy for Helicobacter pylori eradication] Korean J Gastroenterol. 2005;46:368–372. [PubMed] [Google Scholar]


