Abstract
AIM: To determine the immunomodulatory effect of Shiitake (a mushroom extract), we tested its effect on liver-mediated immune regulation in a model of immune-mediated colitis.
METHODS: Four groups of mice were studied. Colitis was induced by intracolonic instillation of TNBS in groups A and B. Groups A and C were treated daily with Shiitake extract, while groups B and D received bovine serum albumin. Mice were evaluated for development of macroscopic and microscopic. The immune effects of Shiitake were determined by FACS analysis of intra-hepatic and intrasplenic lymphocytes and IFN-γ ELISPOT assay.
RESULTS: Administration of Shiitake resulted in marked alleviation of colitis, manifested by significant improvement in the macroscopic and microscopic scores, and by reduction in IFN-γ-producing colonies in group A, compared to group B mice (1.5 pfu/mL vs 3.7 pfu/mL, respectively). This beneficial effect was associated with a significant increase in the intra-hepatic CD8+ lymphocyte trapping, demonstrated by an increased intrasplenic/intrahepatic CD4/CD8 lymphocyte ratio. These effects were accompanied by a 17% increase in the number of intrahepatic natural killer T (NKT) cells. A similar effect was observed when Shiitake was administered to animals without disease induction.
CONCLUSION: Shiitake extract affected liver-mediated immune regulation by altering the NKT lymphocyte distribution and increasing intrahepatic CD8+ T lymphocyte trapping, thereby leading to alleviation of immune-mediated colitis.
Keywords: Mushrooms, Colitis, Immune modulation, Shiitake, Natural killer T cell
INTRODUCTION
Mushrooms have been valued by humans throughout history as a food product and for medical purposes[1]. Mushrooms have been used as a medicine in the Far East since ancient times. Extracts and isolated metabolites from mushrooms are known to modulate immune responses[2], resulting in enhanced innate and acquired disease resistance. The major immunomodulatory effects of the active substances derived from mushrooms include mitogenicity and activation of immune effector cells, such as lymphocytes, macrophages, and natural killer cells. Activation of these cells can result in the production of cytokines, including interleukins (ILs), tumor necrosis factor alpha (TNF-α), and interferon gamma (INF-γ)[2]. The ability of select mushroom extracts to modulate the differentiation capacity of CD4+ T cells into mature Th1 and/or Th2 subsets has been documented recently[3].
Other recent studies have suggested that these extracts may have a profound effect on Th1- or Th2-immune mediated disorders[4]. A number of bioactive molecules, mostly polysaccharides with anti-tumor properties have been identified in mushroom-derived formulations[5].
Lentinan, a (1-3)-β-glucan from Lentinus edodes (Shiitake), is licensed as an immunostimulatory drug[6]. Pre-treatment of mice with lentinan results in increased concentrations of TNF-α, IL-12, and IFN-γ, as well as an increase in the number of Listeria monocytogenes-specific CD8 T cells in the spleen. The bacterial burden in the spleen and liver of mice was reduced significantly during primary and secondary listeria infection after lentinan pre-treatment of mice. In addition, Lentinus edodes and its active component, the polysaccharide lentinan, have been found to be effective against several tumors, including prostate and gastric tumors, and leukemia[7,8]. However, the mechanism of action of lentinan is not clearly understood and there is currently no data on its effect on immune-mediated diseases.
The role of liver in the pathogenesis of various immune-mediated disorders is well known[9]. Liver contains a mixture of lymphocytes including both conventional T and B cells, as well as a distinct population of resident liver lymphocytes. Furthermore, liver is involved in the trapping and destruction of activated T cells[10]. As of this writing, the precise role of the liver in immune cell trapping and destruction is not fully understood, although one theory suggests that cells already in the process of apoptosis are sequestrated in the liver[11,12]. A second theory suggests that the liver plays an active role by destroying activated T cells through a local tolerance mechanism that causes clonal deletion[13,14].
The goal of the present study was to determine the effect of Shiitake on colonic inflammation in a murine model of immune-mediated colitis and to examine the role of liver in systemic tolerance induction in this setting.
MATERIALS AND METHODS
Animals
Normal inbred 2-4 mo old C57BL male mice were obtained from Harlan, Israel, and maintained in the Animal Core of the Hadassah-Hebrew University Medical School. Mice were maintained on standard laboratory feed and kept in 12-h light/dark cycles. All of the experiments were performed in accordance with the institute’s ethical committee for animal handling.
Experimental design
Four groups of mice consisting of 10 animals each were studied. Mice in experimental group A were fed Shiitake extract (50 g/mouse), starting 2 d before (day 2), until 9 d after induction of colitis by intra-rectal administration of trinitrobenzene sulfonic acid (TNBS, day 9) as described previously[15]. Mice in group B were fed bovine serum albumin (BSA, 50 g/mouse). Group C was fed with Shiitake extract (50 g/mouse) from day 0 of experiment until day 9. Mice in control group D were fed with BSA, 50 g/mouse from day 0 to day 9. Mice were sacrificed on day 10.
Preparation and administration of the mushrooms
Source: Mushroom spawn (Lentinus edodes 4087) used in this study was purchased from Sylvan (France).
Mushroom culture: Mushrooms were grown on a 1:1 mixture of cotton and wheat straws. The straws were oven dried at 60°C for 24 h and milled to 1-3 cm particle size. The straw mixture was wetted to 70% water content and packed into 4 kg polypropylene bags containing a microporous filter. The bags were steam sterilized at 100°C for 2 h, then cooled to 25°C for inoculation with 2% spawn w/w. The culture was incubated at 25°C for 30 d. For fruiting, the temperature was reduced to 16°C with a relative humidity of 90%, 12 h daily light and CO2 concentration of 600-800 ppm. The fruiting bodies were then oven dried at 60°C for 24 h and milled to 1 mm particle size.
Experimental colitis: Colitis was induced by a single enema of TNBS (Sigma, USA)[16]. Fifty milligrams of TNBS was dissolved in 20% ethanol (total volume 1 mL) and instilled through a rubber catheter 10 cm into the colon via the anus, without any bowel preparation. The animals were kept fasting for 24 h prior to the procedure and were anesthetized with Ketalar. After instillation, the mice were maintained in the supine position until recovery from the anesthesia to prevent immediate leakage of the instillate.
Assessment of colonic damage: Assessment of colonic damage was performed 10 d following induction of colitis[15]. Ten centimeters of the distal colonic tissue was removed and opened by a longitudinal incision and gently washed with saline. The freshly opened colonic segments were examined by an independent observer blinded to the treatment. The extent of the mucosal damage was assessed. Four macroscopic parameters were determined: degree of colonic ulceration, intestinal and peritoneal adhesions, wall thickness, and degree of mucosal edema. Each parameter was graded on a scale from 0 (completely normal) to 4 (most severe).
Colonic histology: For each animal, 6 specimens of colonic tissue from the distal 10 cm were removed for histological analysis. The tissues were fixed in formaldehyde, then sliced into 4 to 6 mm pieces, dehydrated in ethanol, embedded in paraffin wax, sectioned, and stained with hematoxylin and eosin. The degree of inflammation on microscopic sections of the colon was graded semi-quantitatively from 0 to 4. Grade 0: Normal with no signs of inflammation; Grade 1: very low level of leukocyte infiltration; Grade 2: Low level of leukocyte infiltration; and Grade 3: High level of infiltration with high vascular density and bowel wall thickening; Grade 4: Transmural infiltration, with loss of goblet cells, high vascular density, wall thickening, and disruption of normal bowel architecture. Two experienced examiners who were blinded to the study group performed the grading.
Evaluation of the role of natural killer T (NKT) lymphocytes and the liver in CD4 trapping
Liver and spleen lymphocyte isolation: Splenocytes and liver lymphocytes were isolated as described previously with the following modifications[17]. The inferior vena cava was cut above the diaphragm and the liver was flushed with 5 mL cold PBS until it became pale in color. The connective tissue and the gallbladder were removed, and the liver was placed in a 10 mL dish containing cold sterile PBS. Splenocytes and liver lymphocytes were isolated by crushing the spleen and liver through a stainless mesh (size 60, Sigma Chemical Co., St. Louis MO)[16]. The resulting cell suspension was placed in a 50 mL tube for 3 min, washed twice with cold PBS (1250 r/min for 10 min), and all insoluble debris was removed. Cells were re-suspended in PBS, and passed through a nylon mesh pre-soaked in PBS. Unbound cells were collected and washed twice in 45 mL PBS. For liver and spleen lymphocyte isolation, the cells were suspended in a 50 mL tube containing 7 mL PBS, and underlayed with 20 mL Histopaque 1077 (Sigma Diagnostics, St. Louis, MO). The tube was centrifuged at 1640 r/min for 15 min at room temperature. Cells at the density interface were collected, diluted in a 50 mL tube, and washed twice with ice-cold PBS (pelleting at 1250 r/min for 10 min). Approximately 1 × 106 cells per mouse liver were recovered. The cell viability, assessed by Trypan blue staining was > 95%. Both splenocytes and liver-associated lymphocytes were isolated from every animal in all the experimental groups.
Flow cytometry analysis for determination of CD4+, CD8+ and NKT lymphocyte subsets: Following lymphocyte isolation, triplicate samples of 2 × 105 to 5 × 105 cells/500 μL PBS were placed in Falcon 2052 tubes, incubated with 4 mL 1% BSA for 10 min, and centrifuged at 1400 r/min for 5 min. The cells were resuspended in 10 μL FCS with 1:20 FITC-anti mouse CD3 antibody, 1:20 PE-anti mouse CD4 antibody, 1:20 APC-anti mouse CD8 antibody, or 1:20 FITC-anti mouse NK1.1 antibody (NKR-P1C, Pharmingen, USA), and mixed every 10 min for 30 min. The cells were washed twice in 1% BSA, and kept at 4°C until reading. In the control group, only 5 μL of 1% BSA was added. Analytical cell sorting was performed on 1 × 104 cells from each group with a fluorescence-activated cell sorter (FACSTAR plus, Becton Dickinson). Only live cells were counted, and background fluorescence from non-antibody-treated lymphocytes was subtracted. Gates were set by forward- and side-scatter to exclude dead cells and red blood cells. Data was analyzed by the Consort 30 two-color contour plot (Becton Dickinson, Oxnard, CA) or Cellquest program.
Antigen specific IFN ELISPOT assays: IFN spot forming cells (SFC), were identified using a modified subject-specific, antigen-directed ELISPOT assay (Mabtech, Nacka, Sweden)[18]. Filtration plates (96 well), coated with high protein binding hydrophobic PVDF membrane were used (Millipore Corp., Bedford, MA, USA). The plates were coated with 1-D1K anti-IFN coating antibody (15 mg/mL, Mabtech, Nacka, Sweden) for 24 h at 4°C. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient separation of 2 mL whole blood samples, collected in acid citrate dextrose tubes, and processed within 1 h. The PBMC were washed twice in RPMI 1640 with 10% fetal bovine serum. The cells were cultured in 96 well plates (1 × 105 cells/well) with RPMI 1640 and 10% FBS. Triplicate samples were prepared with 2 doses of the study drug from each subject (5 and 10 g/mL) or phytohemagglutinin (PHA, 2.5 g/mL) without antigen. Plates were incubated for 48 h at 37°C under 5% CO2. The plates were then washed and 100 μl biotinylated antibody (7-B6-1-biotin, Mabtech, Nacka, Sweden) at a concentration of 1 μg/mL in filtered PBS with 0.5% FBS. Plates were incubated for 3 h at room temperature. Following washing, 100 μL of streptavidin-alkaline phosphatase was added, and the plates were incubated for 90 min at room temperature. The plates were washed and substrate (BioRad, Richmond, CA) was added for 30 min, until reddish-purple spots appeared. Using a dissection microscope, dark spots, reflecting IFN-γ-secreting clones, were counted. The results were expressed as mean IFN-γ-secreting cells per 105 PBMC (in triplicate), after subtraction of the mean spots from wells without the study drug.
Statistical analysis
Statistical analysis was performed using the student’s t test. P < 0.05 was considered significant.
RESULTS
Effect of Shiitake on survival
Shiitake administration was associated with significant improvement in the survival of animals in group A, compared with the control group B (BSA control). Of the mice in group A, 100% survived vs 44% in group B. All mice in control groups C and D survived.
Effect of Shiitake on severity of experimental colitis
Macroscopic scoring of colitis: The severity of colitis in mice treated with Shiitake mushroom showed marked improvement compared with mice in group B, that were fed BSA (mean macroscopic score 0.75 ± 0.2 and 3.2 ± 0.1 for groups A and B, respectively, P < 0.005, Figure 1A). No significant signs of colitis were observed in mice in groups C and D.
Figure 1.
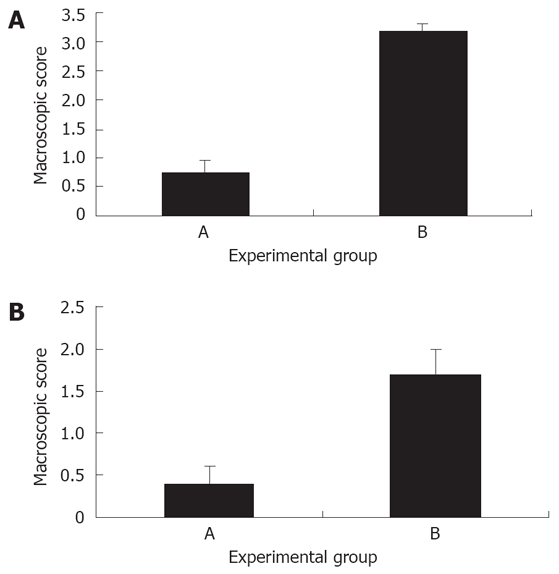
A: Effect of Shiitake administration on the macroscopic score of experimental colitis: Shiitake administration significantly alleviated the severity of colitis in group A mice compared with mice in control group B; B: Effect of Shiitake administration on the microscopic score of colitis: Shiitake administration alleviated the severity of colitis in group A mice compared with mice in control group B.
Histological assessment of colitis: Similar results were obtained in the total microscopic score of colitis. The mean microscopic score was 0.4 ± 0.2 in group A vs 1.7 ± 0.3 in group B (Figure 1B). Histological evaluation of colonic tissues showed a marked reduction in the inflammatory response in Shiitake fed mice (Figure 2A). By contrast, mice in group B showed severe colitis, manifested by inflammatory infiltration of the mucosa, and patchy necrosis of the mucosa and submucosa with purulent and fibrinoid material extending up to the muscle layer. The muscle and serosal layers showed infiltration by acute and chronic inflammatory cells (Figure 2B).
Figure 2.
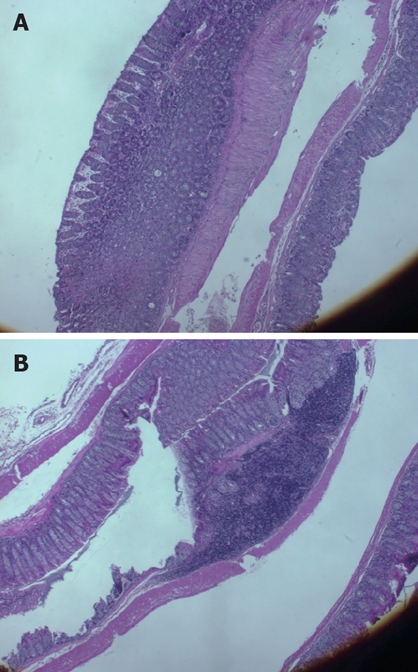
Histological evaluation of colonic tissues showed a marked reduction in the inflammatory response in Shiitake fed mice (A). By contrast, severe colitis was observed in mice in group B, manifested by inflammatory infiltration of the mucosa, and patchy necrosis of the mucosa and submucosa, with purulent and fibrinoid material extending to the muscle layer (B).
Effect of Shiitake on NKT lymphocyte distribution
The number of intra-hepatic NKT cells was determined in all experimental groups. The number of liver NKT cells was increased in group A, compared to group B (29.1% vs 18.6%, Figure 3A). Liver NKT cells were also increased in group C compared with group D (32.1% vs 24.1%). The intrasplenic NKT cell counts, reflecting the total systemic pools of NKT cells, were also determined. A decrease in NKT cell number was observed in group A compared with group B, and in group C compared with group D (1.1 vs 2.4 and 1.6 vs 3.3, respectively). To determine whether the intra-hepatic NKT increase was part of a systemic increase in NKT cells, the intrasplenic/intrahepatic NKT cell ratio was calculated. This ratio was significantly reduced in the Shiitake fed mice (groups A and C vs groups B and D, Figure 3B).
Figure 3.
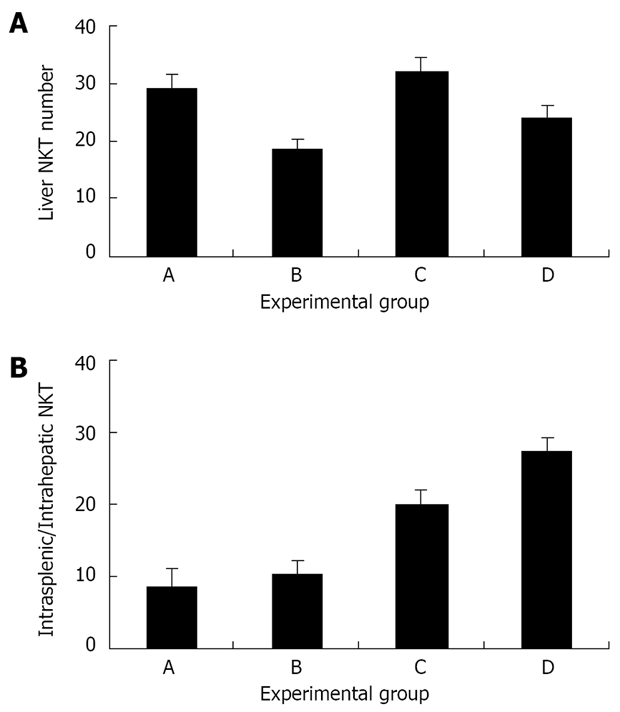
A: The effect of Shiitake administration on intrahepatic NKT cells. Liver NKT cell numbers were increased in groups A and C (which received Shiitake), compared with mice in group B and in control group D; B: The effect of Shiitake administration on the intrasplenic/ intrahepatic NKT cell ratio.
Effect of Shiitake on the intrahepatic and systemic CD4/CD8 ratios
The intra-hepatic CD4/CD8 ratio was reduced signifi-cantly in mice that were fed Shiitake compared with those fed BSA (1.5 for group A vs 2.4 in group B, P < 0.005, Figure 4). A marked decrease in the intra-hepatic CD4/CD8 ratio was also noted in group C compared with the naïve group D (1.5 vs 2, P < 0.005, Figure 4). Interestingly, an opposite effect was noted when the intrasplenic CD4/CD8 ratios were calculated. Intra-hepatic CD8 trapping in Shiitake fed mice was associated with an increase in the peripheral (intrasplenic) CD4/CD8 ratio compared with BSA fed mice (4 and 2.1 for groups A and B, respectively; P < 0.005; Figure 4). However, even in mice without colitis, feeding Shiitake caused the peripheral CD4/CD8 ratio to decrease compared with naïve mice (2.3 in group C vs 3.9 in group D, P < 0.005, Figure 4). The intrasplenic/intrahepatic CD4/CD8 ratio was also calculated. This ratio was increased in groups A, C, D, while it was reduced significantly in group B (2.6, 1.5, 1.80 vs 0.9, respectively; P < 0.005 for all; Figure 5). The increased peripheral/liver CD4/CD8 ratio suggests CD8 lymphocyte trapping in the liver during systemic tolerance induction.
Figure 4.
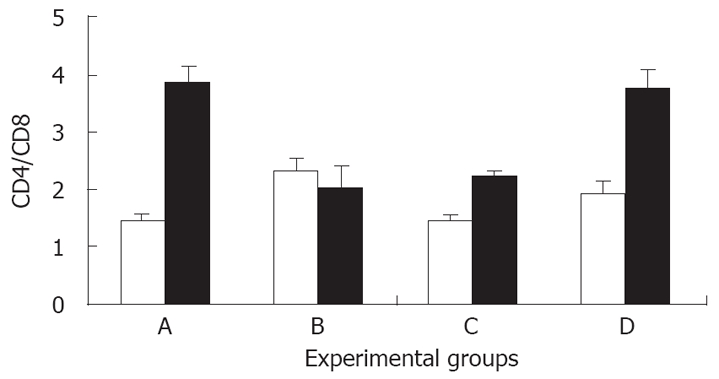
Effect of Shiitake administration on the intra-hepatic (open bars) and intrasplenic (black bars) CD4/CD8 ratio.
Figure 5.
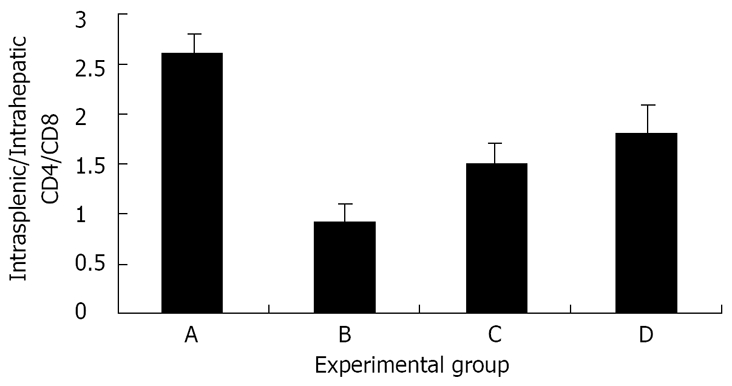
The effect of Shiitake administration on the intrasplenic/intrahepatic CD4/CD8 ratio: The peripheral (spleen)/intrahepatic (liver) CD4/CD8 ratio was markedly increased in groups A, C, and D. By contrast, the ratio was markedly reduced in group B.
Effect of Shiitake on antigen-directed IFN ELISPOT
The number of antigen specific T-cell colonies secreting IFN-γ was reduced significantly in group A compared with group B (1.5 pfu/mL vs 3.7 pfu/mL, P < 0.005), suggesting a specific effect of Shiitake on T cells involved in Th1-mediated immune colitis.
DISCUSSION
Previous studies have shown that Lentinan, a (1-3)-β-glucan obtained from Lentinus edodes, has anti-tumor activity, but the purported immunomodulatory effect has not yet been tested in immune-mediated disorders[19–21]. The present study indicates that a Shiitake-derived formulation has a beneficial effect in an animal model of TNBS-induced colitis. Administration of this formulation significantly improved the survival rate, and alleviated the macroscopic and microscopic evidence (scores) of colitis. These results were associated with a dramatic reduction in the number of antigen-specific IFN-γ-producing colonies. The present study has shown that the liver has a role in mediating systemic induction of tolerance in the setting of our experimental model. This role comprises of sequestering and possibly destroying potentially harmful effector cells, resulting in disease amelioration. The systemic effect of Shiitake in reducing TNBS-induced colitis, was mediated by the trapping of CD8 T cells in the liver. Altered NKT lymphocyte distribution was also associated with the protective effect of Shiitake.
Shiitake and its active component, the polysac-charide lentinan, have previously been shown to be effective against different tumors[20,21]. Several studies have shown that these compounds are effective against gastrointestinal tumors, gynecologic tumors, as well as against leukemia and lymphoma[22,23]. In most of these studies Shiitake was used to augment the effect of other drugs[24]. It has been suggested that mushroom-derived factors do not attack the cancer cells directly, but rather produce their anti-tumor effects by activating different immune responses in the host[7,25].
Mushroom derivates can affect different parts of the immune system, including macrophage activation by induction of TNF-α, IL-6, and IL-1, dendritic cell activation, and various effects on T cells[5]. Lentinus edodes has been described as a T-cell adjuvant, skewing the Th1/Th2 balance towards Th1 through the specific induction of IL-12 from activated macrophages[26–28].
In addition to the protective effects against cancer, Shiitake has also been shown to be hepatoprotective as well as an anti-fibrotic agent. In dimethylnitrosamine-induced hepatitis, Shiitake decreased the serum aminotransferase levels by inhibiting the over-accumula-tion of collagen fibrils and suppressing the over-expression of genes for smooth muscle α-actin and heat shock protein-47[29]. Shiitake also inhibited, in a dose-dependent manner and without cytotoxicity, the morphologic changes and proliferation of isolated rat hepatic stellate cells (HSCs), which play a central role in liver fibrosis[29]. Interestingly, the hepatoprotective effects of Shiitake were also observed after oral administration[30]. In another model, the D-galactosamine (GalN)-induced liver injury in mice, oral administration of Shiitake decreased the release of aminotransferases and reduced the degree of histological injury[30].
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting condition of unknown etiology that exhibits a variety of autoimmune features[31,32]. Studies in animal models and in humans implicate an abnormal intestinal epithelial cell barrier function, excessive production of Th1 or Th2 cytokines, and the unrestrained activation of CD4+ TCRαβ+ T cells in the pathogenesis of this disorder[32]. Induction of systemic tolerance towards disease-associated antigens has been validated as a method to alter the immune response and alleviate colitis in both animals and humans[17,18].
Rectal administration of TNBS in mice induces chronic intestinal inflammation similar to that seen in Crohn’s disease in humans. Stimulated cells in the inflamed mucosa produce increased amounts of IFN-γ. IL-2, and IL-12, along with reduced IL-4 expression[33]. Administration of low dose colitis-extracted protein has been shown to inhibit the host colonic inflammatory response and to alleviate colitis in this model[17]. Tolerance induction led to an immunological shift from a pro-inflammatory Th1 type response to an anti-inflammatory Th2 type.
NKT regulatory lymphocytes differentiate through thymic and extrathymic pathways[34]. These cells are characterized as CD4+ or CD4-CD8- and CD16-, express αβ TCRint, and share the surface molecules with NK cells, including NK1.1 and CD122[35]. NKT cells exist in low numbers in the peripheral blood as well as in most other tissues, but are abundant in the liver[35]. The expression of NK1.1-CD1 ligand in the liver is likely responsible for this phenomenon. Upon stimulation, these cells produce significant quantities of IL-4 and IFN-γ, and exhibit enhanced cytolytic activity[36]. Our group and others have recently shown that this subset of lymphocytes may play an important role in the induction of peripheral tolerance[33,37]. Induction of peripheral tolerance via oral administration of an antigen or FK506 treatment, have both been associated with a significant increase in NKT LAL production and cytotoxic activity[15]. Relevant to this study, NKT lymphocytes have also been shown to play an active role in the immune modulation of experimental colitis. Adoptive transfer of tolerized NKT cells mediate the transfer of tolerance to recipient mice and prevent the induction of disease[15].
In the present study, the beneficial effect of Shiitake was associated with increased number of liver NKT cells irrespective of the induction of colitis. This change was accompanied by a systemic decrease in NKT cell number in treated animals. To determine whether the intra-hepatic NKT increase was part of a systemic increase in NKT cells or retention of these cells in the liver, the intrahepatic/intrasplenic NKT cell ratio was calculated. While no overall increase in the number of systemic NKT cells was found, this ratio was increased significantly in Shiitake treated mice. Thus, NKT residency in the liver was increased. Taken together, these data suggest that the Shiitake-derived formulation used in the present study has a substantial effect on NKT regulatory lymphocytes. As such, such formulations may carry the potential to alleviate similar NKT-dependent disorders.
In addition to the observed changes in NKT cells, the beneficial effect of Shiitake was associated with increased intrasplenic/intrahepatic ratio of CD4/CD8, suggesting a preferential trapping of CD8 lymphocytes in the liver during systemic tolerance induction. This effect was similar to that observed in other previously described methods of tolerance induction, where the liver was shown to play an active role[9,10]. Similar to its effect on NKT regulatory cell distribution, the effect of Shiitake on intrahepatic lymphocyte sequestration was observed in treated animals whether or not colitis was induced. This finding suggests that Shiitake acts as a genuine immune modulator in both healthy and disease conditions, although a more pronounced effect was noted in disease states.
In summary, the Shiitake-derived formulation had a favorable effect on immune mediated colitis. This effect was associated with alterations in the NKT lymphocyte distribution, and on intra-hepatic CD8 lymphocyte trapping during tolerance induction. Further identification of the specific effects caused by several mushroom derived compounds may facilitate the development of oral administration of these products for the treatment of immune-mediated disorders, including IBD.
COMMENTS
Background
Natural killer T (NKT) lymphocytes play a regulatory role in various immune-mediated disorders. To determine the immunomodulatory impact of Shiitake we tested its effect on liver-mediated immune regulation in a model of immune-mediated colitis. Shiitake extract affected liver-mediated immune regulation by altering NKT lymphocyte distribution and increasing intra-hepatic CD8+ T lymphocyte trapping, thereby leading to alleviation of immune-mediated colitis.
Research frontiers
The liver is a site for lymphocyte clearance, and plays an important role in determining the CD4+/CD8+ balance during tolerance induction.
Innovations and breakthroughs
Shiitake extract altered NKT lymphocyte distribution and increased intra-hepatic CD8+ T lymphocyte trapping.
Applications
Shiitake extracts can serve as immune modulatory tools in various immune mediated disorders.
Peer review
The present study looked at the role of mushroom extract on tolerance induction, using an established model of TNBS colitis. It was observed that there was alleviation of microscopic and macroscopic colitis and better survival with Shiitake treatment, accompanied with changes in intra-hepatic NKT, CD4, CD8 populations with reduced IFN secreting colonies in the peripheral blood. The authors concluded that Shiitake modulates the immune response in the liver to a more tolerogenic state, and results in improvement of the colitis.
Supported by (in part) The Roman-Epstein Liver Research Foundation (to Y.I.)
Peer reviewers: Dr. Wing-Kin Syn, Department of Medicine, Division of Gastroenterology, Duke University MC, GSRB-1, Suite 1073, DUMC 3256, 595 LaSalle Street, Durham 27710, United States; Mario U Mondelli, Prof, Department of Infectious Diseases, Fondazione IRCCS Policlinico San Matteo and University of Pavia, Laboratori Area Infettivologica, Dipartimento di Malattie Infettive, Fondazione IRCCS Policlinico San Matteo, via Taramelli 5, Pavia 27100, Italy
S- Editor Zhong XY L- Editor Anand BS E- Editor Lin YP
References
- 1.Yin Y, Fu W, Fu M, He G, Traore L. The immune effects of edible fungus polysaccharides compounds in mice. Asia Pac J Clin Nutr. 2007;16 Suppl 1:258–260. [PubMed] [Google Scholar]
- 2.Lull C, Wichers HJ, Savelkoul HF. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm. 2005;2005:63–80. doi: 10.1155/MI.2005.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodama N, Murata Y, Nanba H. Administration of a polysaccharide from Grifola frondosa stimulates immune function of normal mice. J Med Food. 2004;7:141–145. doi: 10.1089/1096620041224012. [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Kodama N, Nanba H. Effect of maitake (Grifola frondosa) D-fraction on the control of the T lymph node Th-1/Th-2 proportion. Biol Pharm Bull. 2002;25:536–540. doi: 10.1248/bpb.25.536. [DOI] [PubMed] [Google Scholar]
- 5.Moradali MF, Mostafavi H, Ghods S, Hedjaroude GA. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi) Int Immunopharmacol. 2007;7:701–724. doi: 10.1016/j.intimp.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Kupfahl C, Geginat G, Hof H. Lentinan has a stimulatory effect on innate and adaptive immunity against murine Listeria monocytogenes infection. Int Immunopharmacol. 2006;6:686–696. doi: 10.1016/j.intimp.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes) J Altern Complement Med. 2002;8:581–589. doi: 10.1089/107555302320825093. [DOI] [PubMed] [Google Scholar]
- 8.Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med. 1999;221:281–293. doi: 10.1046/j.1525-1373.1999.d01-86.x. [DOI] [PubMed] [Google Scholar]
- 9.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 10.Shibolet O, Alper R, Zolotarov L, Trop S, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. The role of intrahepatic CD8+ T cell trapping and NK1.1+ cells in liver-mediated immune regulation. Clin Immunol. 2004;111:82–92. doi: 10.1016/j.clim.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Polakos NK, Klein I, Richter MV, Zaiss DM, Giannandrea M, Crispe IN, Topham DJ. Early intrahepatic accumulation of CD8+ T cells provides a source of effectors for nonhepatic immune responses. J Immunol. 2007;179:201–210. doi: 10.4049/jimmunol.179.1.201. [DOI] [PubMed] [Google Scholar]
- 12.Wuensch SA, Pierce RH, Crispe IN. Local intrahepatic CD8+ T cell activation by a non-self-antigen results in full functional differentiation. J Immunol. 2006;177:1689–1697. doi: 10.4049/jimmunol.177.3.1689. [DOI] [PubMed] [Google Scholar]
- 13.John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol. 2004;172:5222–5229. doi: 10.4049/jimmunol.172.9.5222. [DOI] [PubMed] [Google Scholar]
- 14.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 15.Trop S, Ilan Y. NK 1.1+ T cell: a two-faced lymphocyte in immune modulation of the IL-4/IFN-gamma paradigm. J Clin Immunol. 2002;22:270–280. doi: 10.1023/a:1019974005134. [DOI] [PubMed] [Google Scholar]
- 16.Shibolet O, Alper R, Avraham Y, Berry EM, Ilan Y. Immunomodulation of experimental colitis via caloric restriction: role of Nk1.1+ T cells. Clin Immunol. 2002;105:48–56. doi: 10.1006/clim.2002.5260. [DOI] [PubMed] [Google Scholar]
- 17.Ilan Y, Weksler-Zangen S, Ben-Horin S, Diment J, Sauter B, Rabbani E, Engelhardt D, Chowdhury NR, Chowdhury JR, Goldin E. Treatment of experimental colitis by oral tolerance induction: a central role for suppressor lymphocytes. Am J Gastroenterol. 2000;95:966–973. doi: 10.1111/j.1572-0241.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- 18.Margalit M, Israeli E, Shibolet O, Zigmond E, Klein A, Hemed N, Donegan JJ, Rabbani E, Goldin E, Ilan Y. A double-blind clinical trial for treatment of Crohn's disease by oral administration of Alequel, a mixture of autologous colon-extracted proteins: a patient-tailored approach. Am J Gastroenterol. 2006;101:561–568. doi: 10.1111/j.1572-0241.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 19.Vetvicka V, Vetvickova J, Frank J, Yvin JC. Enhancing effects of new biological response modifier beta-1,3 glucan sulfate PS3 on immune reactions. Biomed Pharmacother. 2008;62:283–288. doi: 10.1016/j.biopha.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Mizuno M. Anti-tumor polysaccharides from mushrooms during storage. Biofactors. 2000;12:275–281. doi: 10.1002/biof.5520120141. [DOI] [PubMed] [Google Scholar]
- 21.Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000;5:4–27. [PubMed] [Google Scholar]
- 22.Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. Perspect Biol Med. 2006;49:159–170. doi: 10.1353/pbm.2006.0034. [DOI] [PubMed] [Google Scholar]
- 23.Aoyagi K, Koufuji K, Yano S, Murakami N, Miyagi M, Takeda J, Shirouzu K. Long-term survival after gastric cancer with liver metastasis: a report of two cases. Kurume Med J. 2001;48:335–338. doi: 10.2739/kurumemedj.48.335. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Li X, Xu X, Zeng F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr Res. 2005;340:1515–1521. doi: 10.1016/j.carres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 25.Vetvicka V, Yvin JC. Effects of marine beta-1,3 glucan on immune reactions. Int Immunopharmacol. 2004;4:721–730. doi: 10.1016/j.intimp.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 27.Murata Y, Shimamura T, Tagami T, Takatsuki F, Hamuro J. The skewing to Th1 induced by lentinan is directed through the distinctive cytokine production by macrophages with elevated intracellular glutathione content. Int Immunopharmacol. 2002;2:673–689. doi: 10.1016/s1567-5769(01)00212-0. [DOI] [PubMed] [Google Scholar]
- 28.Ooi VE, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem. 2000;7:715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- 29.Akamatsu S, Watanabe A, Tamesada M, Nakamura R, Hayashi S, Kodama D, Kawase M, Yagi K. Hepatoprotective effect of extracts from Lentinus edodes mycelia on dimethylnitrosamine-induced liver injury. Biol Pharm Bull. 2004;27:1957–1960. doi: 10.1248/bpb.27.1957. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe A, Kobayashi M, Hayashi S, Kodama D, Isoda K, Kondoh M, Kawase M, Tamesada M, Yagi K. Protection against D-galactosamine-induced acute liver injury by oral administration of extracts from Lentinus edodes mycelia. Biol Pharm Bull. 2006;29:1651–1654. doi: 10.1248/bpb.29.1651. [DOI] [PubMed] [Google Scholar]
- 31.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 32.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menachem Y, Trop S, Kolker O, Shibolet O, Alper R, Nagler A, Ilan Y. Adoptive transfer of NK 1.1+ lymphocytes in immune-mediated colitis: a pro-inflammatory or a tolerizing subgroup of cells? Microbes Infect. 2005;7:825–835. doi: 10.1016/j.micinf.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Nowak M, Stein-Streilein J. Invariant NKT cells and tolerance. Int Rev Immunol. 2007;26:95–119. doi: 10.1080/08830180601070195. [DOI] [PubMed] [Google Scholar]
- 35.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 36.Godfrey DI, McConville MJ, Pellicci DG. Chewing the fat on natural killer T cell development. J Exp Med. 2006;203:2229–2232. doi: 10.1084/jem.20061787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu KO, Porcelli SA. The diverse functions of CD1d estricted NKT cells and their potential for immunotherapy. Immunol Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]


