Abstract
AIM: To determine intestinal permeability, the serum tumor necrosis factor (TNF)-α level and urine nitric oxide (NO) metabolites are altered in liver cirrhosis (LC) with or without ascites.
METHODS: Fifty-three patients with LC and 26 healthy control subjects were enrolled in the study. The intestinal permeability value is expressed as the percentage of polyethylene glycol (PEG) 400 and 3350 retrieval in 8-h urine samples as determined by high performance liquid chromatography. Serum TNF-α concentrations and urine NO metabolites were determined using an enzyme-linked immunosorbent assay (ELISA) and Greiss reaction method, respectively.
RESULTS: The intestinal permeability index was significantly higher in patients with LC with ascites than in healthy control subjects or patients with LC without ascites (0.88 ± 0.12 vs 0.52 ± 0.05 or 0.53 ± 0.03, P < 0.05) and correlated with urine nitrite excretion (r = 0.98). Interestingly, the serum TNF-α concentra-tion was significantly higher in LC without ascites than in control subjects or in LC with ascites (198.9 ± 55.8 pg/mL vs 40.9 ± 12.3 pg/mL or 32.1 ± 13.3 pg/mL, P < 0.05). Urine nitrite excretion was significantly higher in LC with ascites than in the control subjects or in LC without ascites (1170.9 ± 28.7 μmol/L vs 903.1 ± 55.1 μmol/L or 956.7 ± 47.7 μmol/L, P < 0.05).
CONCLUSION: Increased intestinal macromolecular permeability and NO is probably of importance in the pathophysiology and progression of LC with ascites, but the serum TNF-α concentration was not related to LC with ascites.
Keywords: Intestinal permeability, Tumor necrosis factor-α, Nitric oxide, Liver cirrhosis, Ascites
INTRODUCTION
It has been shown that the gut, as a reservoir of enteric bacteria in the body, plays a protective role as mucosal barrier function, immunoglobulin secretion, and local and systemic macrophage system, but under liver cirrhosis (LC) with portal hypertension a correlative connection between liver damage and the functional activity of the intestine with mucosal abnormalities exist[1–3]. Increased intestinal permeability (IPI) with bacterial translocation and endotoxemia have been implicated in the pathogenesis of chronic liver injury and as contributory factors in the development of dangerous complications, such as encephalopathy and bacterial infections in LC[4]. However, other investigators have suggested that intestinal permeability is probably of limited importance in the pathophysiology of bacterial infections in patients with LC[5]. Intestinal permeability in LC has been reported as being increased or normal[6–10]. The development of systemic endotoxemia may in turn act through the release of cytokines, to further increase intestinal permeability, impair host immunity and promote bacterial translocation from the gut, thus resulting in a vicious circle[11,12]. It has been proposed that some of these cytokines play a role in several known cirrhosis-related complications, such as hyperdynamic circulation, susceptibility to infection, and hepatic encephalopathy[13]. Tumor necrosis factor (TNF)-α is a 17 kDa cytotoxic protein produced by mononuclear cells on activation by bacterial endotoxin and tissue injury[13]. However, the TNF-α level in LC has been reported with controversial findings, as it may or may not correlate with an advanced stage of disease and a worse outcome[14–18].
Nitric oxide (NO) has a role in cirrhosis. Endotoxemia, possibly from gut-derived bacterial translocation, causes induction of NO synthase leading to increase vascular NO production, which is the primary stimulus for the development of vasodilatation in cirrhosis and its accompanying clinical manifestations[19]. While NO is an unstable molecule, one means of investigating NO formation is to measure nitrite (NO2-), which is one of two primary stable non-volatile breakdown products of NO. A dose dependent increase in nitrite has been demonstrated to occur when macrophages are activated with lipopolysaccharide (LPS) both in vitro and in vivo[20]. Therefore, we speculated that endotoxin mediated increases in the NO metabolite nitrite in urine are related to the magnitude of intestinal macromolecular permeability and hence to LC related complications.
Limited data exists on the state of intestinal macromolecular permeability using polyethylene glycol (PEG) (400 and 3350) in cirrhotic patients with or without ascites. To clarify the role of intestinal macromolecular permeability, the serum TNF-α level and nitrite level in urine to the development of LC with ascites, we investigated whether intestinal macromolecular permeability is altered in patients LC with or without ascites, and its relationship with the serum TNF-α level and NO metabolite level in urine.
MATERIALS AND METHODS
Patients and healthy control subjects
Participating patients and healthy control subjects were comprised of 26 patients with LC with ascites, 27 patients with LC without ascites and 26 age and sex-matched healthy individuals with a normal medical history, physical examination and blood chemistry. Subjects with known infection, gastrointestinal or renal disease or diabetes mellitus were excluded from the study. Also excluded were patients that received substances known to affect intestinal permeability test results such as lactulose, non-steroidal anti-inflammatory drugs, or alcohol, in the previous 2 wk. The Institutional Review Board of the Seonam University Health Sciences Center (Namwon, Korea) approved the study. All subjects in this study gave informed consent. The diagnosis of LC was based on the typical findings of hepatic cirrhotic appearance, splenomegaly, esophageal varices, and ascites by ultrasonography and an upper gastrointestinal endoscopy, and laboratory results (prolonged prothrombin time, hypoalbuminemia with or without elevated liver enzymes). The severity of liver disease was determined according to Child-Pugh criteria.
Measurement of intestinal macromolecular permeability
Urine samples used in this study were collected during 8 h from subjects that fasted overnight (last meal before 8 PM the day before). Subjects ingested a 100 mL test solution containing 1 g of PEG 400 and 10 g of PEG 3350 in water. Each subject ingested the PEG solution 1 h before a breakfast meal. Urine samples were been kept frozen (-20°C) until processing for analysis. In this study, we attempted to detect PEG 400 as a low molecular weight (MW) marker and 3350 as a higher MW marker simultaneously in urine samples by high performance liquid chromatography (HPLC) using evaporative light scattering detection. About 2 mL of urine was filtered through a 0.45 μm syringe filter (Nylon membrane) and stored at 4°C until analysis. All of the 1 mL-vial urine samples for analysis were directly placed into a Waters 717+ autosampler with refrigerator (10°C). The HPLC column was a 5 μm PLRP-S 100 A column (150 mm × 4.6 mm, Polymer Laboratories, Amherst, MA USA) packed with PS/DVB polymeric beads. To remove particles in the urine sample, a disposable Security Guard kit (Phenomenex, Torrance, CA USA) was used with the HPLC column. A gradient mobile phase (acetonitrile/H2O) for an elution of 40-60 min was used to separate efficiently all hydrophilic and hydrophobic compounds. As the HPLC eluents were controlled by a gradient controller program, we tried to set the program to allow the impurities elute first while the marker compounds (PEG 400 and PEG 3350) eluted later without peak overlap. The eluted components were analyzed by an evaporative light scattering detector (PL-ELSD 2100 under conditions of evaporation -85°C, nebulizer 85°C and gas flow 1.0; Polymer Laboratories). Calibration curves were obtained in the range of 200-1500 mg/L for PEG 400 and 10-200 mg/L for PEG 3350, respectively. The intestinal permeability was calculated by the concentration of the PEG marker compound and total urine volume. The calculated intestinal permeability index (IPI, in %), PEG retrieval ratio, is an expression of the PEG 3350 intestinal permeability, relative to PEG 400.
Measurement of serum TNF-α
With in a 12 h period after oral administration of the PEG solution, 10 milliliters of a blood sample was taken from a forearm vein of each individual. All blood samples were anticoagulated with EDTA and then plasma was separated by centrifugation at 1600 g for 15 min. Plasma samples were stored at -70°C until analysis. The serum TNF-α concentration was determined by the enzyme-linked immunosorbent assay (ELISA) technique (Quantikine® human TNF-α, R & D Systems, Minneapolis, MN USA), according to the manufacturer instructions.
Measurement of urinary nitrite excretion
About 2 mL of urine was filtered through a 10 000 MW filter (Millipore Microcon YM-10) and was assayed for the NO metabolite nitrite by the Greiss reaction using Parameter TM Total NO/Nitrite/Nitrate kit (R &D Systems). The total concentration of nitrite was determined by absorbance at 540 nm after urine nitrate (NO3-) was converted to nitrite (NO2-) by the NADPH-dependent nitrate reductase.
Statistical analysis
Data are reported as mean values and standard errors (mean ± SE) or percentage according to variables. Differences among the three groups were analyzed by ANOVA. When a significant effect occurred, Scheffe post hoc comparisons were used to test differences among the means. An independent samples t-test was used to compare test results between two groups. A nonparametric test, the Mann-Whitney test, was used to compare independently two groups that had fewer than 10 samples. We calculated Pearson’s correlation coefficient for associations between two variables. SPSS statistical software (version 11.0) was used for the statistical analysis. A two-tailed significant level of 5% was chosen as a type I error.
RESULTS
Characteristics of the participating patients
There were no significant differences regarding age and gender between the cirrhotic patients with or without ascites and healthy control subjects. The distribution of causes of LC were alcohol (n = 31), viral infection (HBV 14, HCV 7; n = 21) and alcohol combined with HBV infection (n = 1). Renal function as assessed based on blood urea and creatinine levels was normal in all patients and control subjects. Details of the demographics, etiology, severity, complications of the LC and concurrent infections are outlined in Table 1.
Table 1.
Demographics and characteristics of the subjects (mean ± SE )
| Cirrhotics with ascites (n = 26) | Cirrhotics without ascites (n = 27) | Healthy controls (n = 26) | |
| Age (yr) | 54.7 ± 9.6 | 53.9 ± 9.7 | 50.3 ± 9.2 |
| Sex (M/F) | 23/3 | 21/6 | 17/9 |
| Etiology | |||
| Alcohol | 16 | 15 | |
| Viral1 | 9 | 12 | |
| Alcohol/viral2 | 1 | 0 | |
| Child class (A/B/C) | 1/16/9 | 22/5/0 | |
| Child-Pugh score | 8.8 ± 0.44b | 6.3 ± 0.34 | |
| Serum albumin (g/dL) | 2.8 ± 0.11b | 3.5 ± 0.12 | |
| Serum bilirubin (mg/dL) | 5.1 ± 0.92b | 2.0 ± 0.43 | |
| Prothrombin time (s) | 15.7 ± 0.50a | 14.3 ± 0.40 | |
| AST (IU/L) | 78.3 ± 9.45 | 74.8 ± 11.4 | |
| ALT (IU/L) | 34.1 ± 4.8 | 51.5 ±8.9 | |
| Encephalopathy | 9a | 2 | |
| Esophageal varix | 11 | 9 |
Viral etiology-cirrhosis with ascites (HBV-6, HCV-3) and cirrhosis without ascites (HBV-8, HCV-4);
Viral etiology-HBV-1.
P < 0.05,
P < 0.01 vs cirrhosis without ascites.
Intestinal macromolecular permeability
Mean values for PEG 400 and 3350 retrieval were 46.5 ± 3.22 and 0.24 ± 0.03 in control subjects, 44.1 ± 5.17 and 0.21 ± 0.02 in patients with LC without ascites and 37.4 ± 3.55 and 0.31 ± 0.04 in patients with LC with ascites, respectively. The mean values for the IPI were different in patients from the healthy control subjects and patients with LC without ascites reflected the expected low diffusion of PEG 3350, being significantly higher in patients with LC with ascites (0.52 ± 0.05 and 0.53 ± 0.03 vs 0.88 ± 0.12, P < 0.05) (Figure 1). However, there was no significant difference between the healthy control subjects and patients with LC without ascites (Table 2).
Figure 1.
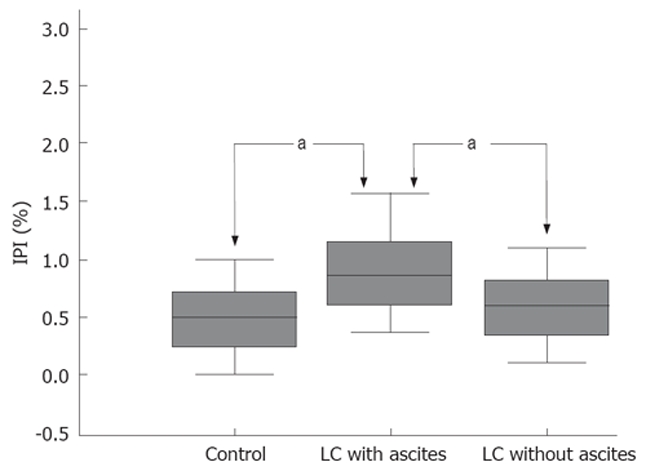
Polyethylene glycol retrieval ratio (PEG 3350/400) in the healthy control subjects and the cirrhotic patients. aP < 0.05.
Table 2.
Intestinal permeability, serum TNF-α and urine nitrite levels in the healthy control subjects and cirrhotic patients (mean ± SE )
| Cirrhotics with ascites (n = 26) | Cirrhotics without ascites (n = 27) | Healthy controls (n = 26) | |
| PEG400 | 37.4 ± 3.55 | 44.1 ± 5.17 | 46.5 ± 3.22 |
| PEG3350 | 0.31 ± 0.04 | 0.21 ± 0.02 | 0.24 ± 0.03 |
| IPI | 0.88 ± 0.12a | 0.53 ± 0.03 | 0.52 ± 0.05 |
| Nitrite | 1170.9 ± 28.7a | 956.7 ± 47.7 | 903.1 ± 55.1 |
| TNF-α | 32.1 ± 13.3 | 198.9 ± 55.8a | 40.9 ± 12.3 |
PEG: Polyethylene glycol; IPI: Intestinal permeability index.
P < 0.05.
A sub-analysis relating intestinal permeability to the severity of LC for all patients as indicated by the Child-Pugh class showed significant differences between class A , B and C for PEG 3350 (0.20 ± 0.02, 0.25 ± 0.03 vs 0.42 ± 0.08, P < 0.05) and IPI (0.52 ± 0.04, 0.72 ± 0.07 vs 1.12 ± 0.27, P < 0.05). According to sub-analysis relating IPI to the presence of complications of LC for patients as indicated by encephalopathy and hypoalbuminemia, there were significant differences (P < 0.05), but not for patients as indicated by a prolonged prothrombin time, esophageal varix or hyperbilirubinemia.
Serum TNF-α level
The concentration of serum TNF-α was 198.9 ± 55.8 pg/mL in patients with LC without ascites, 32.1 ± 13.3 pg/mL in LC with ascites and 40.9 ± 12.3 pg/mL in the control subjects (Figure 2). A group comparison by the Mann-Whitney test showed that the serum TNF-α level was significantly higher in patients with LC without ascites than in the control subjects and in patients with LC with ascites (P < 0.05) (Table 2). According to the sub-analysis relating the TNF-α level to the severity of LC for all patients as indicated by the Child-Pugh class, class A was higher than class B and C (218.8 ± 43.4 vs 78.9 ± 26.3 and 17.7 ± 3.1, respectively) and class B was higher than class C, and there were significant differences between them (P < 0.05). According to the sub-analysis relating the TNF-α level to the presence of complications of LC for patients as indicated by the presence of hypoalbuminemia, there were significant differences (P < 0.05), but not for patients as indicated by encephalopathy, prolonged prothrombin time, esophageal varix and hyperbilirubinemia (Table 3).
Figure 2.
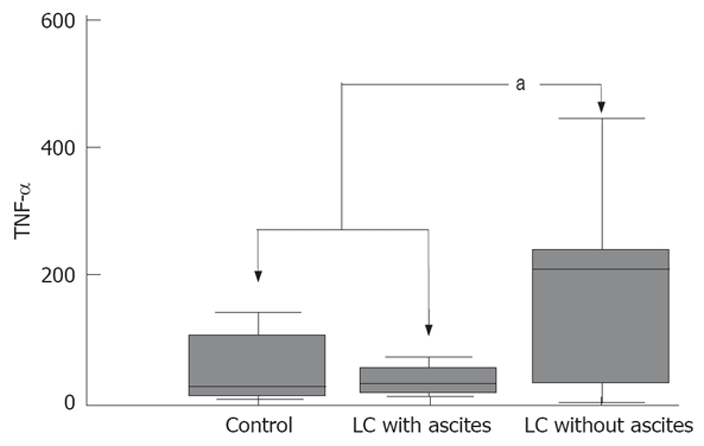
Tumor necrosis factor-α (TNF-α) levels in the healthy control subjects and the cirrhotic patients. aP < 0.05.
Table 3.
Analysis relating intestinal permeability, nitrite level and TNF-α level to the clinical and laboratory findings in the cirrhotic patients (mean ± SE )
| n | PEG400 | PEG3350 | IPI (%) | Nitrite (μmol/L) | TNF-α (pg/mL) | |
| Child-Pugh classification | ||||||
| A | 23 | 42.6 ± 5.7 | 0.20 ± 0.02 | 0.52 ± 0.04 | 1137.3 ± 45.4 | 218.8 ± 43.4a |
| B | 21 | 38.6 ± 4.4 | 0.25 ± 0.03 | 0.72 ± 0.07 | 967.7 ± 67.5 | 78.9 ± 26.3 |
| C | 9 | 41.5 ± 6.0 | 0.42 ± 0.08a | 1.12 ± 0.27a | 1086.3 ± 36.4 | 17.7 ± 3.1 |
| Serum albumin (g/dL) | ||||||
| > 3.4 | 16 | 41.4 ± 4.9 | 0.23 ± 0.03 | 0.52 ± 0.05 | 1013.2 ± 65.7 | 183.6 ± 43.5 |
| < 3.4 | 37 | 40.6 ± 4.0 | 0.27 ± 0.02a | 0.78 ± 0.08a | 1114.7 ± 43.6 | 105.8 ± 28.4a |
| Serum bilirubin (mg/dL) | ||||||
| > 1.2 | 33 | 44.7 ± 4.5 | 0.29 ± 0.03 | 0.76 ± 0.09 | 1078.1 ± 45.7 | 76.6 ± 18.4 |
| < 1.2 | 20 | 34.4 ± 3.7 | 0.20 ± 0.03 | 0.60 ± 0.07 | 1049.6 ± 61.2 | 216.3 ± 51.2 |
| Prothrombin time (s) | ||||||
| > 13 | 43 | 40.66 ± 3.6 | 0.27 ± 0.03 | 0.75 ± 0.07 | 1052.6 ± 40.4 | 121.2 ± 26.8 |
| < 13 | 10 | 41.56 ± 7.0 | 0.21 ± 0.03 | 0.50 ± 0.07 | 1074.9 ± 88.8 | 163.8 ± 55.4 |
| Encephalopathy | ||||||
| No | 42 | 41.58 ± 3.79 | 0.24 ± 0.02 | 0.67 ± 0.07 | 1097.6 ± 41.48 | 139.1 ± 27.52 |
| Yes | 11 | 37.98 ± 5.00 | 0.33 ± 0.04 | 0.82 ± 0.13a | 1030.0 ± 77.84 | 91.9 ± 49.89 |
| Esophageal varix | ||||||
| No | 33 | 42.6 ± 4.54 | 0.24 ± 0.03 | 0.72 ± 0.08 | 1119.9 ± 39.7 | 145.6 ± 33.74 |
| Yes | 20 | 37.86 ± 3.16 | 0.29 ± 0.03 | 0.67 ± 0.10 | 1007.7 ± 70.1 | 102.4 ± 31.30 |
PEG: Polyethylene glycol; IPI: Intestinal permeability index.
P < 0.05.
Urinary nitrite excretion
Urinary nitrite excretion was significantly increased in patients with LC with ascites as compared to patients with LC without ascites or the healthy control subjects (1170.9 ± 28.7 μmol/L vs 956.7 ± 47.7 μmol/L or 903.1 ± 55.1 μmol/L, P < 0.05) (Figure 3 and Table 2). According to a sub-analysis relating urinary nitrite excretion to the severity of LC as measured by the Child-Pugh class and to the presence of complications of LC for patients as indicated by the presence of encephalopathy, hypoalbuminemia, prolonged prothrombin time, esophageal varix and hyperbilirubinemia, there were no significant differences (Table 3).
Figure 3.
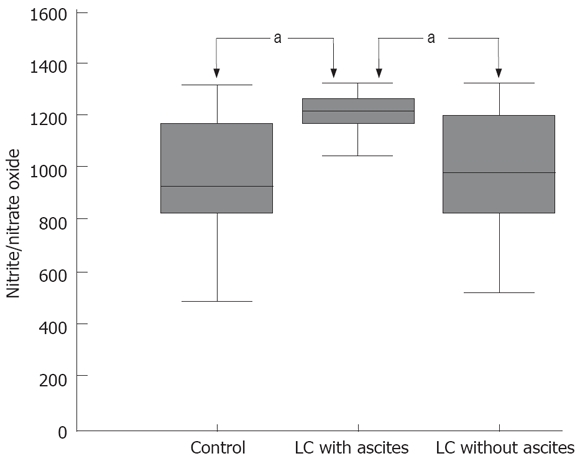
Urinary nitrite excretion in the healthy control subjects and the cirrhotic patients. aP < 0.05.
Correlation and statistical analysis
Patients with alcoholic versus non-alcoholic cirrhosis did not differ significantly (P > 0.05) in the intestinal macromolecular permeability (PEG 400, 3350 retrieval and IPI), serum TNF-α level and urine nitrite level. There was a positive correlation between the Child-Pugh score and increasing intestinal macromolecular permeability (r = 0.494 for PEG 3350 and r = 0.447 for IPI, P < 0.01), but no significant correlation between the Child-Pugh score and the TNF-α level or urinary nitrite level among patients with LC with or without ascites. No significant correlation was observed between PEG 400, 3350 percentage retrieval, IPI and the serum TNF-α level, between the TNF-α level, and urine nitrite level but there was a significant correlation between IPI and urine nitrite excretion (r = 0.98, P < 0.05) among patients with LC with or without ascites.
DISCUSSION
The concept of altered intestinal permeability is important and has been implicated in a number of pathological situations, including celiac disease associated with antigen permeability, allergic intestinal diseases such as digestive hypersensitivity, inflammatory diseases such as Crohn’s disease, ulcerative colitis, acute pancreatitis, alcoholic liver disease and LC associated with substance permeability during inflammation[5,21,22].
The pathogenic mechanisms implicated in the failure of intestinal barrier in cirrhosis have not been fully elucidated as yet and remains to be investigated. Toxic metabolites of alcohol are known to induce alterations of enterocyte tight junctions, which may increase paracellular permeability[23]. However, other inflammatory conditions may alter barrier integrity, as measured by increased gut permeation. An alternative mechanism may be the proinflammatory cytokines, which can be produced locally by epithelial cells or may reach the intestinal mucosa from an inflammatory focus distant from the bowel[24]. Interestingly, in vitro studies in cell monolayers suggest that cytokines may mediate these permeation effects by changes in the production of NO[25]. The mechanism for this effect is not known, but may involve relaxation of the cytoskeleton or oxidation/nitration of cytoskeleton proteins[26].
In the current study, PEG with different molecular masses was used to assess gut permeability, as it combines unique attributes in its chemical structure. It is non-toxic, water-soluble, as is endotoxin, and not metabolized either by the host or by intestinal bacteria[27]. After transmigration into the blood, the polar PEG-molecule is excreted with the urine. Because of its homogeneous chemical properties, its appropriately adaptable molecular mass and its linear, chain-like shape (mimicking the comparable structure of endotoxin)[28], PEG seems to be an appropriate probe for the assess-ment of LPS translocation through the intestine. All of these demands cannot be met by other commonly used permeability marker compounds such as mono- or disaccharides, sugar alcohols, complexes with radioactive nuclides (51Cr-EDTA, 99mTc-DTPA), proteins, or even combinations of these compounds[29].
The simultaneous use of two test marker compounds allows the expression of global intestinal permeability as an index reflecting the transfer value of the less permeable test marker (PEG 3350) relative to the most diffusible probe (PEG 400). Since pre-absorption factors such as gastric emptying, dilution by digestive secretions and post-absorption factors such as systemic distribution and renal clearance are assumed to affect both molecules equally, the value of this index should then be directly comparable from one individual to another[30].
It has been suggested that intestinal permeability markers pass through either a transcellular or a paracellular pathway. However, it is difficult to determine that endotoxins or other bacterial toxins from the gut lumen into the portal system pass paracellularly or transcellularly in cirrhotic patients, as there was no significant difference of PEG 400 and 3350 retrieval between the control subjects and cirrhotic patients with or without ascites in this study. To address this issue, further studies are needed for morphological or molecular changes of intestinal mucosa in LC during the PEG test. Distribution of PEG in ascites might have caused a lower urinary excretion rate and thus underestimated possible permeability changes in patients with ascites. However, Kalaitzakis et al assessing intestinal permeability with 51Cr-EDTA concluded that a loss of 51Cr-EDTA into the ascitic compartment was unlikely and paracentesis had no significant effect on the urinary 51Cr-EDTA excretion, which suggests that ascites in itself does not unduly affect the test results[5]. In the present study, ascitic fluid from three cirrhotic patients was tested for PEG and it was not detected; therefore, the possibility of lower urinary excretion rates due to distribution of PEG in ascites can be ruled out.
Previous studies have shown an association between IPI and severity of LC assessed according to the Child-Pugh classification[8,9], but other studies have failed to reproduce these results[6]. In the present study, we observed significantly higher PEG 3350 retrieval and IPI in Child-Pugh class C patients as compared with that in class A and B patients. Methodological and/or patient selection differences should be taken into account when interpreting the results of this study.
In this study, there were no concomitant infections and a significantly higher TNF-α level in cirrhotic patients without ascites than healthy control subjects or patients with LC with ascites was seen; thus, there was a tendency for a negative correlation between the TNF-α level and Child-Pugh class in the advanced stage of LC. In advanced cirrhosis, hepatic damage and inflammation are reduced due to a decreased liver reserve and marked fibrosis, and consequently, ALT levels decrease. Additionally, diminished amounts of cytokine-producing cells such as hepatocytes and Kupffer cells may lead to a decrease of TNF-α production[15]. In LC, several inflammatory states and commonly occurring infections may be another source of TNF-α production and could explain contradicting results. Increased production of TNF-α associated with inflammation and tissue necrosis is seen not only in hepatitis, but also in other inflammatory conditions. In the present study, IPI and urinary nitrite excretion were significantly higher in patients with LC with ascites as compared to patients with LC without ascites or healthy control subjects, with a significant correlation. Since NO is thought to have a wide range of biological functions other than vasodilatation, it is likely to affect both the progress and the clinical features of LC as well as the hemodynamics in cirrhotic patients. For example, NO is a potent inducer of increased membrane permeability in the vascular endothelium and intestinal mucosa, possibly contributing to the accumulation of ascites and to bacterial translocation[31]. In the current study, although TNF-α was thought to induce NO synthesis (NOS) through the inducible NOS and endothelial NOS[32,33], there was no significant correlation between TNF-α level and NO level in LC. This is the same to some studies, where such a relation could not be observed[34,35]. It has been suggested that some other factors including TNF-α contribute to elevation of NO in LC.
A simple comparison of the published data with the findings of the present study is not easy to make. There were differences between the reported results of intestinal permeability, which means differences in the methods of assessment, including the composition of the probe solution and analytic techniques employed, as well as differences in the patient populations and in the causes and severity of LC. In conclusion, our results suggest that increased intestinal macromolecular permeability and NO are probably of importance in the pathophysiology and progression of LC with ascites, and furthermore, IPI may be a contributory factor in the development of encephalopathy in LC.
COMMENTS
Background
Increased intestinal permeability (IPI) with bacterial translocation and endotoxemia have been implicated in the pathogenesis of chronic liver injury and as contributory factors in the development of dangerous complications, such as encephalopathy and bacterial infections in liver cirrhosis (LC). However, limited data exists on the state of intestinal macromolecular permeability using PEG (400 and 3350) in cirrhotic patients with or without ascites. To clarify the role of intestinal macromolecular permeability, the serum tumor necrosis factor (TNF)-α level and nitrite level in urine to the development of LC with ascites, the authors investigated whether intestinal macromolecular permeability is altered in patients LC with or without ascites, and its relationship with the serum TNF-α level and NO metabolite level in urine.
Innovations and breakthroughs
The authors investigated the relation between intestinal permeability in compensated and decompensated cirrhosis in relation to TNF-α and urine nitrite oxide levels. Their results suggest that increased intestinal macromolecular permeability and NO are probably of importance in the pathophysiology and progression of LC with ascites, and furthermore, IPI may be a contributory factor in the development of encephalopathy in LC.
Applications
All the findings of the current study will provide useful information for the understanding and the treatment of LC.
Peer review
This is an interesting study on a relevant topic. The main results of the study are that the increased permeability and nitrite oxide is of importance in the pathophysiology of decompensated cirrhosis.
Acknowledgments
We express our gratitude to Ho Young Na and Gun Young Hong for assisting with the sample collection and Cheol Hyun Kim for assistance with the statistical analysis.
Supported by A grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea, No.0520190-1
Peer reviewer: Dr. Soren Moller, Department of Clinical Physiology 239, Hvidovre Hospital, Kettegaard alle 30, DK-2650 Hvidovre DK-2650, Denmark
S- Editor Li DL L- Editor Rippe RA E- Editor Zhang WB
References
- 1.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 2.DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Budillon G, Parrilli G, Pacella M, Cuomo R, Menzies IS. Investigation of intestine and liver function in cirrhosis using combined sugar oral loads. J Hepatol. 1985;1:513–524. doi: 10.1016/s0168-8278(85)80749-2. [DOI] [PubMed] [Google Scholar]
- 4.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalaitzakis E, Johansson JE, Bjarnason I, Bjornsson E. Intestinal permeability in cirrhotic patients with and without ascites. Scand J Gastroenterol. 2006;41:326–330. doi: 10.1080/00365520510024278. [DOI] [PubMed] [Google Scholar]
- 6.Fujii T, Seki T, Maruoka M, Tanaka J, Kawashima Y, Watanabe T, Sawamura T, Inoue K. Lactulose-L-rhamnose intestinal permeability test in patients with liver cirrhosis. Hepatol Res. 2001;19:158–169. doi: 10.1016/s1386-6346(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 7.Huglo D, De Botton S, Canva-Delcambre V, Colombel JF, Wallaert B, Steinling M, Marchandise X. Simultaneous determination of pulmonary and intestinal permeability in patients with alcoholic liver cirrhosis. Eur J Nucl Med. 2001;28:1505–1511. doi: 10.1007/s002590100589. [DOI] [PubMed] [Google Scholar]
- 8.Campillo B, Pernet P, Bories PN, Richardet JP, Devanlay M, Aussel C. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol. 1999;11:755–759. doi: 10.1097/00042737-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, Gutierrez A, Carnices F, Palazon JM, et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482–1486. [PubMed] [Google Scholar]
- 10.Zuckerman MJ, Menzies IS, Ho H, Gregory GG, Casner NA, Crane RS, Hernandez JA. Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci. 2004;49:621–626. doi: 10.1023/b:ddas.0000026307.56909.21. [DOI] [PubMed] [Google Scholar]
- 11.Michie HR, Manogue KR, Spriggs DR, Revhaug A, O'Dwyer S, Dinarello CA, Cerami A, Wolff SM, Wilmore DW. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- 12.O'Dwyer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459–1464. doi: 10.1001/archsurg.1988.01400360029003. [DOI] [PubMed] [Google Scholar]
- 13.Odeh M, Sabo E, Srugo I, Oliven A. Serum levels of tumor necrosis factor-alpha correlate with severity of hepatic encephalopathy due to chronic liver failure. Liver Int. 2004;24:110–116. doi: 10.1111/j.1478-3231.2004.0894.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiki I, Yilmaz O, Erdem F, Gundogdu M, Demircan B, Bilici M. Tumour necrosis factor-alpha levels in hepatitis B virus-related chronic active hepatitis and liver cirrhosis and its relationship to Knodell and Child-Pugh scores. Int J Clin Pract. 2006;60:1075–1079. doi: 10.1111/j.1742-1241.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Yue B, Wang GQ, Lu SL. Serum and ascites levels of macrophage migration inhibitory factor, TNF-alpha and IL-6 in patients with chronic virus hepatitis B and hepatitis cirrhosis. Hepatobiliary Pancreat Dis Int. 2002;1:577–580. [PubMed] [Google Scholar]
- 16.Giron-Gonzalez JA, Martinez-Sierra C, Rodriguez-Ramos C, Macias MA, Rendon P, Diaz F, Fernandez-Gutierrez C, Martin-Herrera L. Implication of inflammation-related cytokines in the natural history of liver cirrhosis. Liver Int. 2004;24:437–445. doi: 10.1111/j.1478-3231.2004.0951.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee FY, Lu RH, Tsai YT, Lin HC, Hou MC, Li CP, Liao TM, Lin LF, Wang SS, Lee SD. Plasma interleukin-6 levels in patients with cirrhosis. Relationship to endotoxemia, tumor necrosis factor-alpha, and hyperdynamic circulation. Scand J Gastroenterol. 1996;31:500–505. doi: 10.3109/00365529609006772. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson AS, Gretzer C, Wallerstedt S. Elevation of cytokines in peritoneal fluid and blood in patients with liver cirrhosis. Hepatogastroenterology. 2004;51:505–509. [PubMed] [Google Scholar]
- 19.Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337:776–778. doi: 10.1016/0140-6736(91)91384-7. [DOI] [PubMed] [Google Scholar]
- 20.Oudenhoven IM, Klaasen HL, Lapre JA, Weerkamp AH, Van der Meer R. Nitric oxide-derived urinary nitrate as a marker of intestinal bacterial translocation in rats. Gastroenterology. 1994;107:47–53. doi: 10.1016/0016-5085(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 21.DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rahman SH, Ammori BJ, Larvin M, McMahon MJ. Increased nitric oxide excretion in patients with severe acute pancreatitis: evidence of an endotoxin mediated inflammatory response? Gut. 2003;52:270–274. doi: 10.1136/gut.52.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 24.McKay DM, Baird AW. Cytokine regulation of epithelial permeability and ion transport. Gut. 1999;44:283–289. doi: 10.1136/gut.44.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 26.Banan A, Fields JZ, Zhang Y, Keshavarzian A. iNOS upregulation mediates oxidant-induced disruption of F-actin and barrier of intestinal monolayers. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1234–G1246. doi: 10.1152/ajpgi.2001.280.6.G1234. [DOI] [PubMed] [Google Scholar]
- 27.Philipsen EK, Batsberg W, Christensen AB. Gastrointestinal permeability to polyethylene glycol: an evaluation of urinary recovery of an oral load of polyethylene glycol as a parameter of intestinal permeability in man. Eur J Clin Invest. 1988;18:139–145. doi: 10.1111/j.1365-2362.1988.tb02404.x. [DOI] [PubMed] [Google Scholar]
- 28.Parlesak A, Bode JC, Bode C. Parallel determination of gut permeability in man with M(r) 400, M(r) 1500, M(r) 4000 and M(r) 10,000 polyethylene glycol. Eur J Clin Chem Clin Biochem. 1994;32:813–820. doi: 10.1515/cclm.1994.32.11.813. [DOI] [PubMed] [Google Scholar]
- 29.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 30.Loret S, Nollevaux G, Remacle R, Klimek M, Barakat I, Deloyer P, Grandfils C, Dandrifosse G. Analysis of PEG 400 and 4000 in urine for gut permeability assessment using solid phase extraction and gel permeation chromatography with refractometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:195–202. doi: 10.1016/j.jchromb.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J, Vilardell F, Mourelle M, Moncada S. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139–1143. [PubMed] [Google Scholar]
- 32.Elsing C, Harenberg S, Stremmel W, Herrmann T. Serum levels of soluble Fas, nitric oxide and cytokines in acute decompensated cirrhotic patients. World J Gastroenterol. 2007;13:421–425. doi: 10.3748/wjg.v13.i3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genesca J, Gonzalez A, Segura R, Catalan R, Marti R, Varela E, Cadelina G, Martinez M, Lopez-Talavera JC, Esteban R, et al. Interleukin-6, nitric oxide, and the clinical and hemodynamic alterations of patients with liver cirrhosis. Am J Gastroenterol. 1999;94:169–177. doi: 10.1111/j.1572-0241.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- 34.Barsacchi R, Perrotta C, Bulotta S, Moncada S, Borgese N, Clementi E. Activation of endothelial nitric-oxide synthase by tumor necrosis factor-alpha: a novel pathway involving sequential activation of neutral sphingomyelinase, phosphatidylinositol-3' kinase, and Akt. Mol Pharmacol. 2003;63:886–895. doi: 10.1124/mol.63.4.886. [DOI] [PubMed] [Google Scholar]
- 35.Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104:1223–1233. doi: 10.1172/JCI7458. [DOI] [PMC free article] [PubMed] [Google Scholar]


