Abstract
OBJECTIVES
To preliminarily examine the association between cardiac output, a measure of systemic blood flow, and structural brain magnetic resonance imaging indices of white matter hyperintensities (WMHs).
DESIGN
Cross-sectional.
SETTING
University medical setting.
PARTICIPANTS
Thirty-six older adults without dementia with prevalent cardiovascular disease (aged 56–85).
MEASUREMENTS
Cardiac output, WMHs.
RESULTS
Partial correlations, adjusting for age and history of hypertension, yielded an inverse relationship between WMHs adjacent to subcortical nuclei and cardiac output (correlation coefficient = −0.48, P = .03); as cardiac output decreased, WMHs increased significantly. No significant associations were found between cardiac output and total WMHs or periventricular WMHs.
CONCLUSION
These preliminary data suggest that systemic blood flow, measured according to cardiac output, is inversely associated with WMHs adjacent to the subcortical nuclei. Cerebrovascular degeneration and the chronicity of hypoperfusion may exacerbate the susceptibility of white matter integrity to alterations in blood flow in older adults.
Keywords: systemic perfusion, aging, cardiovascular disease, MRI
White matter hyperintensities (WMHs) are a common finding on brain magnetic resonance imaging (MRI) that become increasingly prevalent with age.1 These imaging markers are often summarized according to neuroanatomical region, and include periventricular WMHs (PWMHs) and deep WMHs (DWMHs). PWMHs are contiguous to the margins of the lateral ventricles, and DWMHs include the deep white matter of the centrum semiovale extending into subcortical regions.2
In addition to age, several vascular risk factors have been implicated in the presence and progression of WMHs in geriatric cohorts3,4 with the most consistent being hypertension.5–7 For instance, elevations in midlife blood pressure are associated with greater numbers of WMHs in later life, suggesting a causal relation between hypertension and WMH development.8
Neuropathological studies have documented that the etiology of WMHs may vary as a function of regional location. PWMHs are reportedly related to subependymal gliosis or disruption of the ependymal lining,9 whereas DWMHs reflect rarefaction of myelin, gliosis, and fiber loss that is ischemic in nature.10 Extensive DWMHs are associated with additional axonal loss, demyelination, glial loss, and spongiosis.11
Although some evidence suggests common pathologies across areas of WMHs (e.g., gliosis), distinct etiological mechanisms of injury may exist for various clinical populations with qualitatively different vascular risk profiles. Acknowledging that brain MRI cannot elucidate the exact pathology of WMHs, it may aid in the identification of novel risk factors that etiologically contribute to the presence and progression of WMHs.
Cardiac output, a measure of systemic perfusion, is one cardiovascular variable that has received less attention but may be an important risk factor for cognitive aging and WMHs. Cardiac output is a measure of forward stroke volume that reflects the amount of blood exiting the heart measured in liters per minute (L/min). There are autoregulatory mechanisms that augment blood flow to the brain at the expense of muscle tissue and other organs during critical moments of sudden reductions in systemic blood flow (e.g., cardiac arrest),12 although such autoregulatory mechanisms may be less effective when reductions in systemic blood flow are chronic. Furthermore, these mechanisms may change as a function of age-associated vasculature breakdown. Examination of the relationships between reduced cardiac output and regional distributions of WMHs may yield important information regarding systemic blood flow as a possible risk factor for WMHs.
The primary purpose of the present study was to preliminarily assess relations between systemic perfusion, measured according to cardiac output, and WMHs adjacent to the lateral ventricles (PWMHs) and subcortical nuclei (subcortical WMHs). Previous work demonstrated a significant relationship between executive dysfunction and systemic hypoperfusion measured according to cardiac output.13 It was hypothesized that low cardiac output would be associated with WMHs adjacent to the subcortical nuclei, because subcortical structures implicated in the frontal-subcortical circuitry mediate some elements of executive functioning,14 and ischemia and neurocognitive dysfunction associated with frontal-subcortical system compromise has been reported15 even in a healthy aging cohort.16 Recent research suggests that total WMHs may more accurately capture relationships between causal factors and regional WMHs,17 so cardiac output was also analyzed in relation to total WMH burden. The second purpose of the present study was to assess the interaction between age and cardiac output as it relates to subcortical WMHs.
METHODS
Participants
Participants were 36 community-dwelling individuals participating in a prospective study examining the effects of cardiac disease on cognitive function in older people. Participants were recruited from local hospitals, rehabilitation programs, private practices, and general advertisements. Inclusion criteria required a documented history of cardiovascular disease, defined as prior myocardial infarction, heart failure, coronary artery disease, cardiac surgery, or hypertension. Participants spoke English and had normal or corrected hearing and vision. Exclusion criteria were end-stage heart disease; history of traumatic brain injury with loss of consciousness for longer than 10 minutes; neurological disease (e.g., dementia, Parkinson’s disease), major psychiatric illness (e.g., schizophrenia); current depressed mood assessed according to the Beck Depression Inventory;18 previous drug or alcohol abuse requiring hospitalization; and MRI contraindication, including ferrous metal implants or pacemakers. The local institutional review board approved this study, and written informed consent was obtained from all participants before assessments. Participants consisted of 24 men and 12 women. Mean global cognitive functioning, assessed according to the Mini-Mental State Examination19 and the Dementia Rating Scale,20 was in the normal range for all participants. Clinical characteristics and neuropsychological variables are displayed in Table 1.
Table 1.
Clinical Characteristics
| Variable | Value |
|---|---|
| Age, mean ± SD | 71.5 ± 7.5 |
| Education level, years, mean ± SD | 13.9 ± 2.2 |
| Beck Depression Inventory, mean ± SD | 4.4 ± 2.9 |
| Mini-Mental State Examination score, mean ± SD | 28.9 ± 1.2 |
| Dementia Rating Scale score, mean ± SD | 138.1 ± 4.6 |
| Cardiac output, L/min, mean ± SD | 4.6 ± 1.5 |
| Female,% | 33 |
| Hypertension,% | 81 |
| Prior myocardial infarction,% | 44 |
| Prior coronary artery bypass,% | 42 |
| History of hypercholesterolemia,% | 42 |
| Family history of heart disease,% | 36 |
| History of atrial fibrillation,% | 11 |
| Current diabetes mellitus,% | 11 |
| History of heart failure,% | 8 |
SD = standard deviation.
Echocardiogram
A complete transthoracic echocardiogram was obtained from each participant according to standards from the American Society of Echocardiography. Cardiac output is the amount of blood (L/min) that is pumped from the heart and is a function of stroke volume and heart rate. Forward stroke volume can be calculated as the mean velocity of blood flow leaving the left ventricle, recorded using Doppler echocardiography, multiplied by the area of left ventricular outflow tract, measured from the two-dimensional echo image [cardiac output = (time velocity integral × cross-sectional area) × heart rate]. This noninvasive method is strongly correlated with more-invasive measures of cardiac output.21
Brain MRI and WMH Quantification
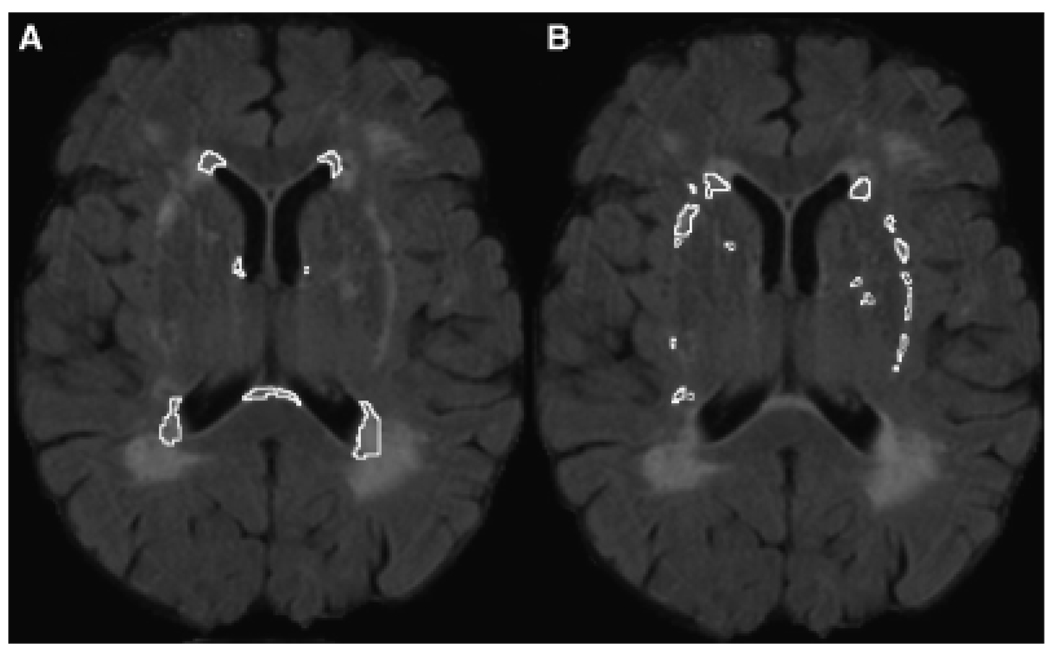
The brain MRI protocol used has been described in detail elsewhere.22 Briefly, brain MRI scans were obtained using a Siemens Symphony 1.5 Tesla unit. A standard imaging protocol was used that consisted of sagittal T1- (repetition time (TR)/echo time (TE) = 500/30) and T2-weighted (TR/TE = 2,500/80) conventional spin-echo localizer images, as well as axial T1, T2, and fluid attenuated inversion recovery (FLAIR; TR/TE = 6,000/105, slice thickness = 5 mm/2 mm gap) images. The FLAIR sequence was used for this study, because its suppression of cerebrospinal fluid signal increases the sensitivity to detecting WMHs. Postprocessing was conducted using ANALYZE (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN). The skull was stripped, and the brain stem and cerebellum were removed manually, leaving only the cerebral brain parenchyma. A semiautomated thresholding method was applied to the FLAIR images to isolate the hyperintensities in the two regions of interest: periventricular (WMHs confluent with the lateral ventricles) and subcortical (WMHs adjacent to subcortical nuclei, including the basal ganglia and thalamus). Figure 1 illustrates the neuronanatomy captured by these two regions. Total WMHs equaled the sum of the periventricular and subcortical areas and WMHs associated with the corona radiata. Intra- and interrater reliability for regional quantification was consistently greater than 0.90.
Figure 1.
White matter hyperintensity (WMH) regions of interest. (A) Periventricular WMHs (WMHs confluent with the lateral ventricles); (B) = subcortical WMHs (WMHs adjacent to subcortical gray matter, including the basal ganglia and thalamus).
Total brain volume was calculated using threshold histogram values that were consistent with brain parenchyma. Brain volume was applied as a correction factor for each hyperintensity value, and this ratio was the primary imaging variable for the study [(WMH pixel total/total brain volume pixel total) × 100]. This method provides a ratio of hyperintensity load relative to the total amount of brain tissue.
Data Analysis
Descriptive statistics were generated to summarize clinical characteristics (e.g., age, education, Mini-Mental State Examination score). Medical history variables were summarized as frequencies, and cardiac output was correlated with history of hypertension and current systolic and diastolic blood pressure. Because the WMH variables were positively skewed, the periventricular, subcortical, and total WMH ratio data were log-transformed before conducting analyses. To test the hypothesis that cardiac output would be associated with subcortical WMHs, partial correlations were generated, adjusting for participant age and history of hypertension, both of which have been linked to the presence and progression of WMHs.23,24 For the secondary analyses, an age-by–cardiac output interaction term was calculated, and partial correlations were generated between the WMH variables and the interaction term, adjusting for history of hypertension. Significance for all analyses was set a priori at α = 0.05.
RESULTS
Clinical Characteristics
Demographic and clinical characteristics are provided in Table 1. The most common clinical characteristic in the sample was a history of hypertension, which was present in more than 80% of the participants. Cardiac output was not correlated with a history of hypertension (correlation coefficient (r) = −0.02, P = .89), current systolic blood pressure (r = −0.23, P = .18), or current diastolic blood pressure (r = −0.16, P = .36).
Partial Correlations for Cardiac Output and WMHs
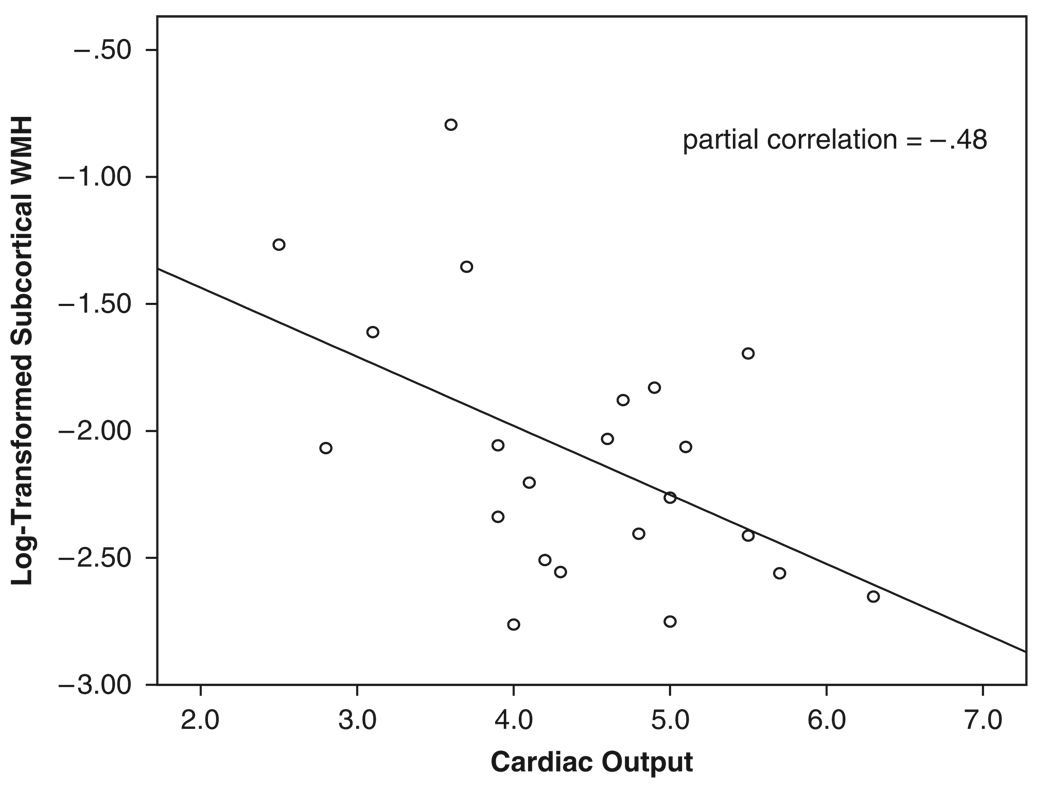
Partial correlations, adjusting for age and history of hypertension, revealed no significant association between cardiac output and total WMHs (r = −0.07, P = .77) or periventricular WMHs (r = 0.07, P = .77). In contrast, a significant association was observed between cardiac output and sub-cortical WMHs (r = −0.48, P = .03) such that, as cardiac output decreased, subcortical WMHs increased (Figure 2).
Figure 2.
Scatterplot relating cardiac output and subcortical white matter hyperintensities (WMHs). WMH values are based on log-transformation, so WMH burden increases as log-transformed values approach 0.
Partial Correlations for Cardiac Output–by-Age Interaction and WMHs
Partial correlations, adjusting for history of hypertension, revealed no significant association between the age-by–cardiac output interaction term and total WMHs (r = 0.09, P = .69) or periventricular WMHs (r = 0.23, P = .32). In contrast, a significant association was observed between the age-by–cardiac output interaction term and subcortical WMHs (r = −0.47, P = .03).
DISCUSSION
Systemic blood flow, measured according to cardiac output, was inversely associated with WMHs contiguous to the subcortical nuclei independent of age and history of hypertension. In contrast, cardiac output was not associated with total or periventricular WMHs. Acknowledging that these preliminary data are cross-sectional and cannot determine causality, the current findings highlight a potentially important relationship between systemic blood flow and presence of WMHs contiguous to the subcortical nuclei.
These findings extend previous work by others employing animal and human models that collectively showed a relationship between reduced cerebral blood flow and WMHs.25–28 In a mouse model, one study25 reported that chronic cerebral hypoperfusion induced the development and progression of white matter lesions (WMLs) with relative preservation of gray matter. Another study26 documented subcortical cerebral perfusion differences between asymptomatic older adults with WMLs and those without WMLs. In a sample with a broader age range, a third study27 reported a modest inverse association between white matter perfusion and deep and subcortical hyperintensities. Previous research relating periventricular WMHs to cerebral blood flow in patients with dementia yielded nonsignificant findings.29 The data from the current study augment these previous findings by indicating that systemic blood flow is similarly associated with subcortical white matter integrity but not periventricular WMHs.
The present data also extend previous work that reported relations between systemic hypoperfusion and impairments in neuropsychological components mediated by frontal-subcortical circuitry (i.e., executive dysfunction, including problems with sequencing and planning).13 In light of the reciprocal white matter pathways connecting frontal and subcortical structures,14 subcortical white matter damage may mediate the cardiac output and executive dysfunction association previously reported. Replication and extension of these findings are needed to elucidate these relations.
The present data, in conjunction with previous work, lead to several preliminary conclusions. First, cerebrovascular degeneration and the chronicity of reduced cardiac output may exacerbate the susceptibility of subcortical white matter integrity to alterations in blood flow in older adults. Specifically, older persons with reduced cardiac output may be at a greater risk for WMH than those with normal systemic blood flow. The observation that the interaction term (age-by–cardiac output) was associated with WMHs adjacent to the subcortical nuclei supports this conclusion, although this interpretation must be tempered by the fact that the interaction term did not consider time since onset of reduced cardiac output, which would be a more precise surrogate of chronicity. Second, these data supplement the existing literature by demonstrating that, in addition to traditional cardiovascular risk factors known to be associated with WMHs, such as blood pressure,30 cardiac output may be a relatively novel risk factor for the development or progression of WMHs and corresponding cognitive decline. The exact underlying mechanism for this association remains unclear, but possible factors include perfusion variability and differences in vascular architecture between white matter regions.
Although a primary association between systemic blood flow reductions and WMH is posited, the possibility that a secondary mechanism, unrelated to blood flow, accounts for our observation cannot be excluded. First, there are a number of maladaptive neurohumoral responses associated with cardiac output that act locally by reducing output.31 These same processes may have a maladaptive effect at a more-systemic level that includes central nervous system involvement. Second, there may be some commonality in the mechanism underlying the small-vessel disease in subcortical brain regions the small-vessel disease of the myocardium, which contributes to reductions in myocardial function, and the small-vessel disease in subcortical brain regions. Future studies are needed to determine whether this observation reflects a primary association; an epiphenomenon, such as a maladaptive neurohumoral response associated with cardiac output; or some combination of these factors.
There are several limitations to the current study that must be considered. First, the demographics and referral source of the sample may limit generalizability, because the sample was predominantly college educated, white of European descent, and significant for some form of cardiovascular disease or risk factor. The sample size was small, which precludes more-rigorous statistical analyses and raises concern that insufficient power precluded additional relations from being detected, although the effect sizes observed suggest that the nonsignificant findings were likely unrelated to insufficient statistical power. Multiple statistical analyses were also employed to test the hypotheses, which may have resulted in false positives. Future large-scale studies are warranted to confirm the association between systemic blood flow and subcortical WMHs and elucidate whether shared risk factors or some epiphenomenon explains the findings.
In summary, this study is among the first to report an inverse relationship between cardiac output and subcortical WMHs. These findings may be secondary to shared mechanisms, the vasculature supplying blood flow to white matter, or some yet unknown factor.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Al Ozonoff for his statistical consultation.
Financial Disclosure: This research was supported by F32-AG022773 (ALJ), K12-HD043444 (ALJ), K23-MH073416 (DFT), K23-MH065857 (RPH), R01-AG017975 (RAC), F32-HL74568 (JG), F32-AG024708 (AMB), and P30-AG 013846 (Boston University Alzheimer’s Disease Core Center).
Sponsor’s Role: This study had no sponsor.
Footnotes
A portion of these data were presented at the 34th annual meeting of the International Neuropsychological Society, Boston, Massachusetts, February 2006.
REFERENCES
- 1.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 2.Kertesz A, Black SE, Tokar G, et al. Periventricular and subcortical hyperintensities on magnetic resonance imaging. ‘Rims, caps, and unidentified bright objects’. Arch Neurol. 1988;45:404–408. doi: 10.1001/archneur.1988.00520280050015. [DOI] [PubMed] [Google Scholar]
- 3.de Leeuw FE, de Groot JC, Oudkerk M, et al. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology. 2000;54:1795–1801. doi: 10.1212/wnl.54.9.1795. [DOI] [PubMed] [Google Scholar]
- 4.de Leeuw FE, De Groot JC, Oudkerk M, et al. Aortic atherosclerosis at middle age predicts cerebral white matter lesions in the elderly. Stroke. 2000;31:425–429. doi: 10.1161/01.str.31.2.425. [DOI] [PubMed] [Google Scholar]
- 5.Lindgren A, Roijer A, Rudling O, et al. Cerebral lesions on magnetic resonance imaging, heart disease, and vascular risk factors in subjects without stroke. A population-based study. Stroke. 1994;25:929–934. doi: 10.1161/01.str.25.5.929. [DOI] [PubMed] [Google Scholar]
- 6.de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus R, Prettyman R, Cherryman G. White matter lesions on magnetic resonance imaging and their relationship with vascular risk factors in memory clinic attenders. Int J Geriatr Psychiatry. 2005;20:274–279. doi: 10.1002/gps.1283. [DOI] [PubMed] [Google Scholar]
- 8.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 9.Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord. 1998;9 Suppl 1:2–5. doi: 10.1159/000051182. [DOI] [PubMed] [Google Scholar]
- 10.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 11.Munoz DG, Hastak SM, Harper B, et al. Pathologic correlates of increased signals of the centrum ovale on magnetic resonance imaging. Arch Neurol. 1993;50:492–497. doi: 10.1001/archneur.1993.00540050044013. [DOI] [PubMed] [Google Scholar]
- 12.Saxena PR, Schoemaker RG. Organ blood flow protection in hypertension and congestive heart failure. Am J Med. 1993;94:4S–12S. [PubMed] [Google Scholar]
- 13.Jefferson AL, Poppas A, Paul RH, et al. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 15.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt R, Fazekas F, Offenbacher H, et al. Neuropsychologic correlates of MRI white matter hyperintensities: A study of 150 normal volunteers. Neurology. 1993;43:2490–2494. doi: 10.1212/wnl.43.12.2490. [DOI] [PubMed] [Google Scholar]
- 17.DeCarli C, Fletcher E, Ramey V, et al. Anatomical mapping of white matter hyperintensities (WMH): Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Mattis S. Dementia Rating Scale, Professional Manual. Odessa, FL: Psychological Assessment Resources; 1973. [Google Scholar]
- 21.Moulinier L, Venet T, Schiller NB, et al. Measurement of aortic blood flow by Doppler echocardiography: Day to day variability in normal subjects and applicability in clinical research. J Am Coll Cardiol. 1991;17:1326–1333. doi: 10.1016/s0735-1097(10)80143-3. [DOI] [PubMed] [Google Scholar]
- 22.Gunstad J, Cohen RA, Tate DF, et al. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Press. 2005;14:353–358. doi: 10.1080/08037050500364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 24.Kozachuk WE, DeCarli C, Schapiro MB, et al. White matter hyperintensities in dementia of Alzheimer’s type and in healthy subjects without cerebrovascular risk factors. Amagnetic resonance imaging study. Arch Neurol. 1990;47:1306–1310. doi: 10.1001/archneur.1990.00530120050009. [DOI] [PubMed] [Google Scholar]
- 25.Shibata M, Ohtani R, Ihara M, et al. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 26.Hatazawa J, Shimosegawa E, Satoh T, et al. Subcortical hypoperfusion associated with asymptomatic white matter lesions on magnetic resonance imaging. Stroke. 1997;28:1944–1947. doi: 10.1161/01.str.28.10.1944. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi W, Takagi S, Ide M, et al. Reduced cerebral glucose metabolism in subjects with incidental hyperintensities on magnetic resonance imaging. J Neurolog Sci. 2000;176:21–27. doi: 10.1016/s0022-510x(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 28.Marstrand JR, Garde E, Rostrup E, et al. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33:972–976. doi: 10.1161/01.str.0000012808.81667.4b. [DOI] [PubMed] [Google Scholar]
- 29.Waldemar G, Christiansen P, Larsson HB, et al. White matter magnetic resonance hyperintensities in dementia of the Alzheimer type: Morphological and regional cerebral blood flow correlates. J Neurol Neurosurg Psychiatry. 1994;57:1458–1465. doi: 10.1136/jnnp.57.12.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein IB, Bartzokis G, Hance DB, et al. Relationship between blood pressure and subcortical lesions in healthy elderly people. Stroke. 1998;29:765–772. doi: 10.1161/01.str.29.4.765. [DOI] [PubMed] [Google Scholar]
- 31.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]