Abstract
Background. Glomerulomegaly has been associated with an increased risk of renal disease. Few reports have investigated the heterogeneity of glomerular size within kidneys and associated risk factors. This study measured the individual glomerular volume (IGV) of 720 non-sclerotic glomeruli in kidneys of adult West African males, and investigated associations of IGV with age, total glomerular (nephron) number and body surface area (BSA).
Methods. IGVs were determined in the kidneys of 24 Senegalese males from two age groups (12 subjects aged 20– 30 years and 12 subjects aged 50–70 years). Subjects were randomly chosen at autopsies performed at Le Dantec Hospital in Dakar. Volumes of 30 glomeruli per subject were determined using the disector/Cavalieri stereological method.
Results. IGVs ranged from 1.31 × 106 μm3 to 12.40 × 106 μm3 (a 9.4-fold variation). IGV varied up to 5.3-fold within single kidneys. The trimmed range of IGV within subjects (10th to 90th percentile of IGV) was directly correlated with median glomerular size. The mean and standard deviation (SD) of IGV did not differ significantly between age groups or between subjects with higher (≥1.78 m2) and lower BSA (<1.78 m2). In older subjects the SD of IGV was significantly and directly correlated with BSA. Kidneys with less than 1 million nephrons had significantly larger mean IGV than kidneys with more than 1 million nephrons, and the trimmed range of IGVs within subjects was inversely correlated with total glomerular number.
Conclusion. There was a considerable variation in IGV within kidneys of Senegalese males at autopsy. The heterogeneity of IGV was increased in association with low nephron number and increased BSA, with more pronounced effects in older subjects.
Keywords: glomerular size, glomerular volume, heterogeneity, nephron number, Senegal
Introduction
Glomerular volume varies widely in humans. A 5.2-fold range of mean glomerular volume has been observed in studies of human kidneys obtained at autopsy, with values from 3.29 to 16.99 μm3 × 106 in adults without any history of renal disease [1,2].
Glomerular hypertrophy has been associated with increased body surface area (BSA) [1,2], with hypertension in Germans and African Americans [3,4] and with low nephron numbers in a number of autopsy populations [1,2]. Renal disease is also associated with changes in glomerular volume. Glomerular hypertrophy is a feature of focal segmental glomerulosclerosis (FSGS), diabetic nephropathy, membranous glomerulonephritis (GN), hypertension and obesity-related nephropathy [5,6].
While glomerular hypertrophy may be beneficial for renal function in the short term, it appears to be detrimental in the longer term. Enlarged glomeruli are thought to be at an increased risk of sclerosis, a consequence of glomerular hyperperfusion and elevated glomerular capillary pressure. Furthermore, glomerulomegaly (large glomeruli) is particularly common in populations at a high risk of renal disease such as Australian Aborigines and Pima Indians [7–9].
Given the focal nature of glomerulosclerosis and many renal diseases, it would be reasonable to expect that glomerular hypertrophy, frequently preceding glomerulosclerosis, would also occur in a non-uniform manner within the glomerular population of ‘non-diseased’ kidneys. Little is known about the extent to which glomerular enlargement is homogeneous or heterogeneous in the normal kidney or in disease settings. This is because most studies have determined mean values for glomerular area or volume and provide no information on the heterogeneity of glomerular volumes within each kidney. Two recent exceptions are reports of glomerular volume in white and black Americans. In the first study, a report of individual glomerular volume (IGV) in African Americans and US whites [10], we found larger glomeruli in the outer cortex of older subjects (51–69 years) compared to younger subjects (20–30 years) and in subjects with a BSA >2.11 m2. The second study by Zimanyi et al. describes IGV in US whites and African Americans with low and high nephron numbers [11]. In whites, those with high nephron number had smaller IGVs and a smaller range of IGV than those with low nephron number, while in African Americans, high nephron number was not associated with small IGV.
In order to evaluate the extent of the heterogeneity of IGV and the characteristics associated with the increased heterogeneity of IGV within normal kidneys, we have analysed kidneys collected at autopsy in a developing population. Dakar, the capital of Senegal in West Africa, is a population in nutritional and epidemiological transition. Body weight and chronic diseases such as hypertension and diabetes are increasing, but have not yet reached the levels of Westernized nations [12]. Total glomerular number in kidneys of Senegalese Africans from Dakar is very similar to that in African Americans from South-East USA, a population sharing common West African ancestry, but mean glomerular volume was much lower in the Senegalese Africans [13,14].
The present study determined IGV in kidneys of younger and older Senegalese African males from Dakar. The relationships of age, BSA and nephron number with the heterogeneity of IGV were investigated.
Subjects and methods
IGVs were estimated in the kidneys of 24 Senegalese Africans: 12 males aged 20–30 years and 12 males aged 50–70 years. Subjects were randomly selected from cases coming to coronial autopsy at Hôpital Aristide Le Dantec in Dakar between 2003 and 2005. The right kidney from each subject was perfusion-fixed with 10% formalin and sent whole to Monash University, Melbourne, Australia, for analysis. Kidney collection and analysis was undertaken with permission of next of kin and in accordance with the standards of the Standing Committee on Ethics in Research Involving Humans (SCERH) at Monash University (project approval numbers 2002/204 and 2006/753).
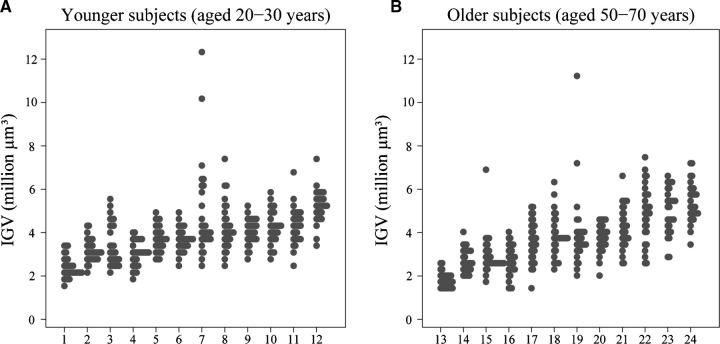
All subjects died of non-renal causes. Ten out of the 12 young subjects died of trauma, with one case of pneumopathy and one of asphyxia (subjects 7 and 8, respectively; Figure 1A, Table 1). Half of the older subjects died from trauma (road accidents, workplace accident), while two died of myocardial infarct (subjects 14 and 21, respectively; Figure 1B, Table 2) and the other four died of non-renal illnesses such as anaemia and hepatitis, pneumonia, malnutrition and infection, and laryngeal cancer (subjects 18, 19, 24 and 13, respectively; Figure 1B, Table 2).
Fig. 1.
(A and B) IGVs in each subject by the age group (30 glomeruli per subject). Subjects ranked according to median glomerular volume. Each column represents a subject and each dot represents an individual glomerulus.
Table 1.
Mean, median and range of IGV and physical characteristics of younger subjects (aged 20–30 years)
| Age | Height | Body | BSA | Kidney | Total | Mean IGV | Median IGV | Min IGV | Max IGV | Fold | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | (years) | (cm) | weight (kg) | (m2) | Weight (g) | nephron number | (μm3 × 106) | (μm3 × 106) | (μm3 × 106) | (μm3 × 106) | variation |
| 1 | 28 | 162 | 60 | 1.65 | 140 | 1 394 010 | 2.40 | 2.25 | 1.52 | 3.47 | 2.28 |
| 2 | 27 | 172 | 80 | 1.97 | 170 | 1 408 254 | 3.12 | 3.05 | 2.12 | 4.25 | 2.01 |
| 3 | 23 | 175 | 63 | 1.75 | 138 | 1 484 020 | 3.42 | 3.07 | 2.30 | 5.51 | 2.39 |
| 4 | 29 | 170 | 70 | 1.83 | 90 | 676 658 | 3.03 | 3.10 | 1.91 | 4.15 | 2.17 |
| 5 | 23 | 180 | 80 | 2.01 | 180 | 1 026 588 | 3.68 | 3.64 | 2.69 | 4.79 | 1.78 |
| 6 | 28 | 165 | 59 | 1.65 | 190 | 1 070 763 | 3.68 | 3.69 | 2.32 | 4.87 | 2.10 |
| 7 | 29 | 170 | 65 | 1.75 | 225 | 835 123 | 4.82 | 3.98 | 2.57 | 12.39 | 4.82 |
| 8 | 30 | 168 | 65 | 1.75 | 150 | 1 475 674 | 4.23 | 4.00 | 2.47 | 7.25 | 2.94 |
| 9 | 25 | 175 | 85 | 2.05 | 200 | 1 426 601 | 4.06 | 4.01 | 2.81 | 5.14 | 1.82 |
| 10 | 20 | 163 | 58 | 1.62 | 102 | 684 506 | 4.25 | 4.18 | 2.77 | 5.81 | 2.10 |
| 11 | 26 | 172 | 75 | 1.90 | 140 | 978 026 | 4.32 | 4.41 | 2.38 | 6.76 | 2.84 |
| 12 | 25 | 175 | 70 | 1.85 | 125 | 839 609 | 5.22 | 5.25 | 3.45 | 7.46 | 2.16 |
| Mean | 26 | 171 | 69 | 1.82 | 154 | 1 108 319 | 3.85 | 3.72 | 2.44 | 5.99 | 2.45 |
| SD | 3 | 5 | 9 | 0.14 | 40 | 314 331 | 0.79 | 0.78 | 0.49 | 2.37 | 0.83 |
Subjects are ranked according to median glomerular size (as in Figure 1A).
Table 2.
Mean, median and range of IGV and physical characteristics of older subjects (aged 50–70 years)
| Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Height | Body | BSA | Kidney | nephron | Mean IGV | Median IGV | Min IGV | Max IGV | Fold | |
| Subject | (years) | (cm) | weight (kg) | (m2) | Weight (g) | number | (μm3 × 106) | (μm3 × 106) | (μm3 × 106) | (μm3 × 106) | variation |
| 13 | 56 | 166 | 60 | 1.66 | 95 | 970 364 | 1.81 | 1.73 | 1.36 | 2.49 | 1.83 |
| 14 | 51 | 163 | 57 | 1.61 | 150 | 1 764 421 | 2.68 | 2.62 | 1.95 | 3.90 | 2.00 |
| 15 | 53 | 163 | 65 | 1.73 | 120 | 1 037 201 | 2.90 | 2.67 | 1.77 | 6.99 | 3.95 |
| 16 | 70 | 165 | 70 | 1.80 | 200 | 1 179 763 | 2.69 | 2.70 | 1.31 | 4.07 | 3.09 |
| 17 | 56 | 168 | 60 | 1.67 | 106 | 871 328 | 3.59 | 3.59 | 1.50 | 5.22 | 3.48 |
| 18 | 50 | 168 | 52 | 1.55 | 128 | 652 027 | 3.79 | 3.66 | 2.34 | 6.27 | 2.68 |
| 19 | 50 | 170 | 85 | 2.03 | 140 | 1 157 257 | 3.94 | 3.67 | 2.10 | 11.18 | 5.33 |
| 20 | 50 | 168 | 72 | 1.84 | 140 | 940 797 | 3.77 | 3.85 | 1.91 | 4.62 | 2.42 |
| 21 | 51 | 175 | 80 | 1.98 | 160 | 926 549 | 4.21 | 4.14 | 2.44 | 6.53 | 2.67 |
| 22 | 70 | 160 | 92 | 2.06 | 134 | 536 171 | 4.89 | 4.99 | 2.47 | 7.38 | 2.99 |
| 23 | 64 | 170 | 90 | 2.09 | 150 | 953 694 | 4.99 | 5.11 | 2.73 | 6.68 | 2.44 |
| 24 | 50 | 170 | 60 | 1.68 | 170 | 993 153 | 5.40 | 5.21 | 3.57 | 7.25 | 2.03 |
| Mean | 56 | 167 | 70 | 1.81 | 141 | 998 560 | 3.72 | 3.66 | 2.12 | 6.05 | 2.91 |
| SD | 8 | 4 | 14 | 0.19 | 28 | 302 639 | 1.07 | 1.10 | 0.65 | 2.24 | 0.98 |
Subjects are ranked according to median glomerular size (as in Figure 1B).
Estimation of individual glomerular volume
A single block (10 × 10 × 1 mm) containing full thickness cortex from the medulla to the capsule was cut from the mid-hilar region of each kidney and processed to glycolmethacrylate. Glycolmethacrylate was chosen in preference to paraffin to minimize tissue shrinkage. The tissue block was exhaustively sectioned at 10 μm using a Leica DM2165 Supercut rotary microtome, with every second section collected for analysis.
Sections were stained with periodic acid Schiff (PAS) and projected onto a table top grid (1 cm × 1 cm) using an Olympus BH-2 microscope at a final magnification of 312×.
The renal cortex was divided into three evenly spaced zones: outer (superficial glomeruli), mid and inner (juxtamedullary glomeruli) cortex. Outer cortical glomeruli were within 3–4 glomerular diameters of the capsule, while juxtamedullary glomeruli were within 3–4 glomerular diameters of the corticomedullary junction. The volumes of 10 glomeruli in each zone (30 per subject) were measured using the disector/Cavalieri principle. Glomeruli were selected for measurement in the order they first appeared in the serial sections (disector sampling [15]). The area of each glomerular profile of the selected glomeruli was determined by point counting (using a 1 cm square grid). IGV was obtained using the Cavalieri principle by multiplying the sum of the profile areas for each individual glomerulus by the section thickness and the sampling fraction. On average, 11 glomerular profiles (ranging from 8 to 21) were measured for each glomerulus. Only non-sclerotic glomeruli were measured. The examination of serial sections from each glomerulus meant that we could be sure that the glomeruli measured were not sclerotic.
Estimation of total glomerular number
Total glomerular (and thereby nephron) number was estimated for the 24 subjects using the physical disector/fractionator combination [15]. The right kidney was bisected, and one half, chosen at random, was sliced into 4 mm slices. One in four slices was sampled using a random number from 1 to 4. The slices were cut into smaller pieces and further randomly sampled to give between 8 and 15 tissue blocks, which were processed to glycolmethacrylate and sectioned exhaustively at 20 μm. Sections were stained with PAS.
In accordance with the disector technique, systematically sampled section pairs (10/11th) were used to count glomeruli. The fundamental principle of the technique is to count glomeruli at a unique point, for example, when a glomerulus first appears or disappears in serial sections. By counting at a unique point, assumptions and biases involving size, size distribution, shape and orientation of glomeruli are not required. Glomeruli were counted in a known fraction of the kidney and basic calculations were used to calculate the total number in the whole kidney. These unbiased methods have been previously described [1,15].
Glomerulosclerosis
A wedge of tissue containing full thickness cortex and medulla was taken from the mid-hilar region of each kidney adjacent to the block used for estimation of IGV. Tissue was embedded in paraffin, sectioned at 4 μm and stained with PAS. The number of sclerosed glomeruli present in a single section was expressed as the percentage of all glomeruli examined in the section (100 glomeruli). All sclerosed glomeruli observed were globally sclerosed and followed the pattern of ischaemic obsolescence.
Statistical analysis
Statistical analyses were performed using Stata, Prism and SPSS. Comparisons between the two age groups were performed using an unpaired Student's t-test or a Mann–Whitney test for non-parametric data. Linear regression was used to analyse relationships between parametric variables, and Spearman rank correlation was used for variables not displaying normal distribution. Relationships of variables on mean IGV in the various zones of the kidney were analysed using a generalized linear model in SPSS. The variation in IGV within subjects is expressed as standard deviation (SD) and trimmed range (10th–90th percentile). The latter was used to exclude extreme values when making comparisons.
Results
Heterogeneity of IGV
A 9.4-fold variation in glomerular volume was observed in the 720 non-sclerotic glomeruli measured in the 24 kidneys, with a range of 1.31 × 106 to 12.40 × 106 μm3. A considerable variation in glomerular volume was also observed within single kidneys. The greatest range in IGV observed within a subject was 5.3-fold (2.10–11.18 × 106 μm3) in a 50-year-old male, while the smallest range (1.8-fold) was observed in a 23-year-old male (2.69–4.79 × 106 μm3) (Tables 1 and 2, Figure 1A and B).
Overall enlargement of glomeruli was associated with increasing heterogeneity of IGV. The SD of IGVs within subjects tended to increase with increasing median IGV (r = 0.393, P = 0.058) (Table 3), while correlation of the trimmed range of IGV (representing the range of glomerular size in the majority of glomeruli, but excluding outliers) to median IGV was even stronger (r = 0.571, P = 0.004) (Table 3).
Table 3.
Pairwise correlations between variation in IGV (SD and trimmed range) and median IGV, BSA, total nephron number and glomerulosclerosis in all 24 subjects
| Variation | Median | BSA | Nephron | Glomerulosclerosis | |
|---|---|---|---|---|---|
| in IGV | IGV | number | |||
| SD | r | 0.393 | 0.187 | −0.264 | 0.168 |
| P | 0.058 | 0.383 | 0.213 | 0.433 | |
| Trimmed | r | 0.571 | 0.174 | −0.418 | 0.330 |
| Range | P | 0.004 | 0.416 | 0.042 | 0.115 |
Significant correlations are indicated in bold.
Mean and range of IGV
By a cortical zone.
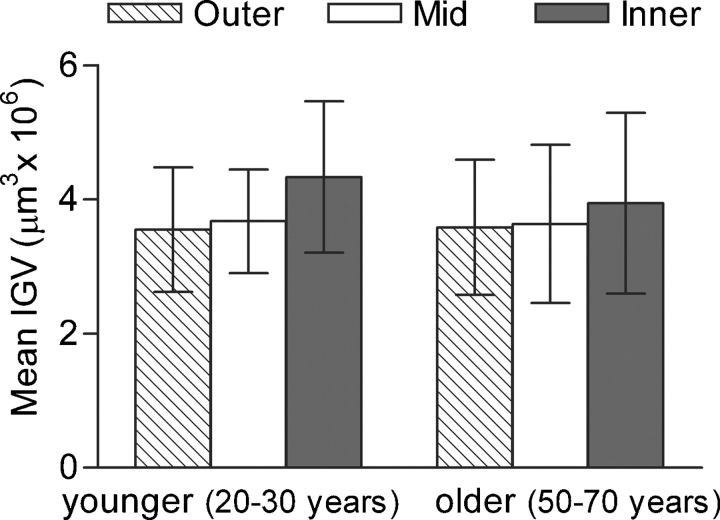
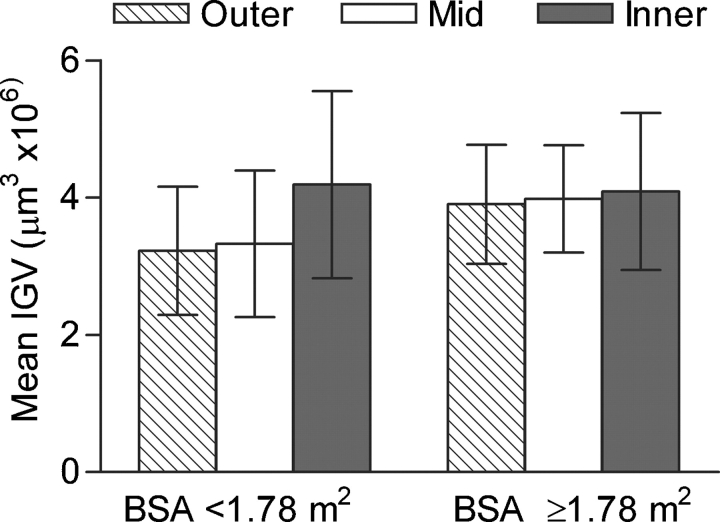
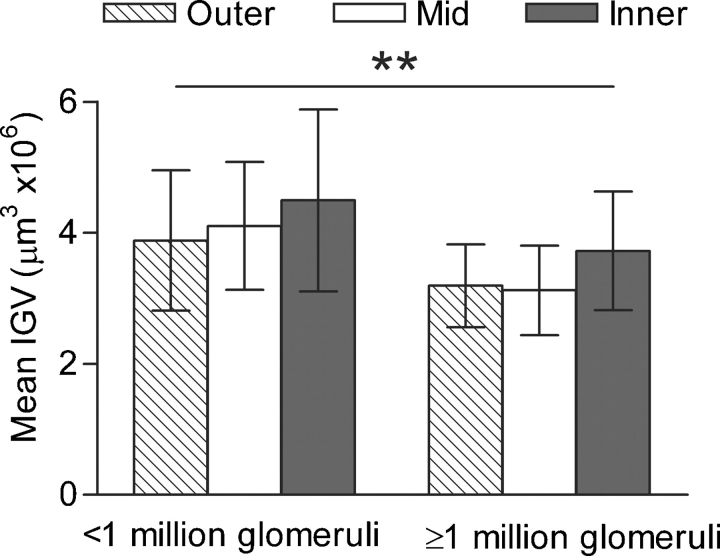
There were no significant differences in the mean IGV of each cortical zone in either age group (Figure 2), or by BSA (Figure 3) or nephron number (Figure 4). In all 24 subjects, SD of IGV was greater in inner cortical glomeruli than mid-cortical glomeruli by repeated-measures ANOVA (1.56 ± 0.59 and 1.08 ± 0.21 μm3 × 106, P = 0.003), but not different to outer cortical glomeruli (1.16 ± 0.23 μm3 × 106).
Fig. 2.
Mean (SD) of IGV by a cortical zone by age group.
Fig. 3.
Mean (SD) of IGV by a cortical zone by BSA category.
Fig. 4.
Mean (SD) of IGV by a cortical zone by nephron number. Mean IGV was significantly greater in subjects with >1 million nephrons (P = 0.002).
By age.
The physical characteristics of the subjects in each age group were very similar (Tables 1 and 2). Height (P = 0.093), body weight (P = 0.820), BSA (P = 0.924) and kidney weight (P = 0.366) were not significantly different. Total glomerular number was also similar (20–30 years, 1 108 319 ± 314 331; 50–70 years, 998 560 ± 302 639; P = 0.393). Mean IGV was similar for each age group, 3.85 ± 0.79 μm3 × 106 in younger subjects (Table 1) compared to 3.72 ± 1.07 μm3 × 106 in older subjects (Table 2) (Figure 2) (P = 0.746). There was also no significant difference between age groups in the range of IGV [SD of IGV (P = 0.620); trimmed range of IGV (P = 0.446)]. However, the relationship of increasing heterogeneity of IGV with increasing median IGV was observed in older males but not in younger males (Table 4).
Table 4.
Pairwise correlations between the variation in IGV (SD and trimmed range) and median IGV, BSA and total nephron number by age group
| Younger subjects | Older subjects | ||||||
|---|---|---|---|---|---|---|---|
| Variation in IGV | Median | BSA | Nephron | Median | BSA | Nephron | |
| IGV | number | IGV | number | ||||
| SD | r | 0.252 | −0.229 | −0.201 | 0.571 | 0.625 | −0.321 |
| P | 0.430 | 0.473 | 0.532 | 0.052 | 0.029 | 0.310 | |
| Trimmed | r | 0.228 | −0.353 | −0.213 | 0.816 | 0.546 | −0.576 |
| Range | P | 0.477 | 0.261 | 0.506 | 0.001 | 0.066 | 0.050 |
Significant correlations are indicated in bold.
By BSA.
Subjects in this study had a limited range of BSA, from 1.55 to 2.09 m2. Despite this, we found a tendency towards larger glomeruli in subjects with larger BSA. Median IGV (but not mean IGV) was significantly correlated with BSA (r = 0.409, P = 0.047), and the relationship was stronger (but not statistically significant) in older subjects (r = 0.537 P = 0.07).
No significant difference was observed between mean IGV in subjects with a BSA greater or less than the 50th percentile of 1.78 m2 (Figure 3), although statistical significance was approached (3.99 μm3 × 106 and 3.58 μm3 × 106, respectively, P = 0.099).
In the entire cohort, the heterogeneity of IGV did not appear to be associated with changes in BSA; however, the SD of IGV was significantly correlated with BSA in older subjects (r = 0.625, P = 0.029) (Table 4). Correlation between BSA and the trimmed range of IGV was less significant (r = 0.546. P = 0.066).
By total glomerular number.
Mean IGV tended to be inversely correlated with total glomerular number (r = −0.395, P = 0.056), so subjects with fewer glomeruli tended to have larger glomeruli. The mean number of glomeruli per kidney for the 24 subjects was 1 053 440. Subjects with fewer than 1 million nephrons in their kidney were found to have significantly larger mean IGV than those with more than 1 million nephrons (4.16 μm3 × 106 versus 3.35 μm3 × 106, P = 0.002) (Figure 4).
The heterogeneity of IGV was increased in people with fewer nephrons. The trimmed range of IGV was inversely correlated with total glomerular number in the 24 subjects (r = −0.418, P = 0.042) and in older subjects alone (r = −0.576, P = 0.0499) (Tables 3 and 4). No correlation was observed between the SD of IGV and total glomerular number.
By glomerulosclerosis.
Glomerulosclerosis increased with age (rho = 0.549, P = 0.006). The median and range of glomerulosclerosis were significantly greater in the older group: 4.71% (0–15.24%) compared to 0.77% (0–2.86%) in younger subjects (P = 0.003; range P < 0.0001). No correlations were found between the percentage of glomerulosclerosis and mean of IGV or with glomerular number or BSA.
The percentage of glomerulosclerosis was not significantly different between subjects with higher (>1.78 m2) or lower BSA (P = 0.131) or between subjects with higher (≥1 million nephrons) and lower nephron number (P = 0.988). Subjects with higher BSA had a significantly greater variation in the levels of glomerulosclerosis than those with lower BSA (F-test for equality of SD, P = 0.018).
The relationship of glomerulosclerosis with the heterogeneity of IGV differed by age. In older subjects, where glomerulosclerosis varied widely, the trend was towards a positive correlation (r = 0.505, P = 0.094), with greater heterogeneity of IGV in subjects with more glomerulosclerosis. Surprisingly in younger subjects, where the extent of glomerulosclerosis was low, glomerulosclerosis was significantly and inversely correlated with the trimmed range of IGV (r = −0.610, P = 0.035).
Discussion
This is one of the few studies to use unbiased techniques to determine IGV and the first to do so in people from a developing country. We found a large range in volume of the 720 glomeruli measured (9.4-fold) and a considerable range within kidneys (up to 5.3-fold). The variation in IGV, of a similar magnitude, was previously observed in the kidneys of African and Caucasian Americans, where the range between subjects (12-fold) and within single kidneys (up to 8-fold) was even larger [10].
Our study demonstrated a positive correlation between median IGV and the trimmed range of IGV in these 24 Senegalese males. The increased heterogeneity in glomerular size in those with enlarged glomeruli may indicate that enlargement occurs at different times and/or rates within individual glomeruli. Our findings suggest that increased BSA and low glomerular number are associated with this hypertrophy.
The trimmed range of IGV was increased in subjects with lower nephron number. Autopsy studies of kidneys of Australians [1,16], Americans [1,2,4], Germans [3] and Senegalese Africans [17] have similarly demonstrated strong inverse correlations between mean glomerular volume and total glomerular number. It has been suggested that glomeruli enlarge to compensate for the reduced glomerular filtration surface area associated with lower glomerular numbers [18]. Non-uniform enlargement of glomeruli associated with low total nephron number may be responsible for the increased heterogeneity in glomerular size within these subjects. However, it should be noted that the SD of IGV, which takes into account the extreme outliers in IGV, was not correlated with total nephron number. Thus, glomerular enlargement associated with low nephron number appears to affect a large proportion of glomeruli but may not account for the extreme enlargement observed in certain glomeruli.
Higher BSA in adults has also been associated with glomerular enlargement [6,19]. Severe cases can progress to obesity-related nephropathy, a disease inducing nephrotic or sub-nephrotic proteinuria and FSGS, and characterized by glomerulomegaly in 100% of cases [6]. The range of BSA in our sample of randomly selected males from autopsy cases in Dakar was narrow, varying only 1.3-fold in the 24 subjects. Despite the limited BSA range compared to studies of American males, the SD of IGV was significantly correlated to BSA in the 12 older subjects. The relationship between BSA and the trimmed range of IGV in these subjects was not as powerful as the relationship between BSA and the SD of IGV (which includes extreme values), suggesting BSA or possibly an undetermined secondary variable linked to BSA could be associated with the extreme enlargement observed in a small number of glomeruli within our sample.
Age was not associated with significant changes in the mean values or the range of IGV in these urban West African males. However, relationships between median IGV, total nephron number, BSA and the range of IGV were stronger in older subjects than in younger ones, which suggests that the drivers of glomerular hypertrophy establish their effects over time, and that older subjects have possibly been exposed to the hypertrophic stimuli for a longer duration. The relationship of glomerulosclerosis with the range of IGV also differed by age. The fact that the more intuitive, positive correlation was observed in older subjects may reflect an emerging longer term consequence of glomerular hypertrophy in these older males.
Although the present study focused on glomerular volume in West African males, it is likely that relationships of nephron number and BSA with glomerular volume in Senegalese females would be similar to those in males. Sex is not an independent determinant of glomerular volume [20], and in our previous study of 28 Senegalese from Dakar, mean glomerular volume of Senegalese females was not different to that of males [14]. However, further study is needed to confirm the relationships with glomerular volume in women.
No zonal differences in IGV were found between the two age groups in this study. These findings contrast with those of Samuel et al. [10] who used the same measurement techniques to measure IGVs in African and Caucasian Americans males. Samuel et al. found that young Americans (aged 20–30 years), like Senegalese, did not display zonal differences in mean IGV. However, in contrast to the present study, older Americans (aged 51–69 years) had significantly larger outer cortical (superficial) glomeruli than inner cortical glomeruli. The gradient of glomerular size increased from inner to outer cortical zones [10].
Differences in glomerular size between different cortical regions of the kidney have been recognized in fetal and infant kidneys, but controversy surrounds the persistence of this zonal size difference into adulthood. Nephrogenesis during kidney development involves reciprocal molecular signalling between cells of the metanephric mesenchyme and epithelial cells at the tips of the ureteric branches [21,22]. Inner cortical (juxtamedullary) nephrons form first, while the most superficial outer cortical nephrons form last. In fetal kidneys, juxtamedullary glomeruli have been reported to be larger than outer cortical glomeruli [23]. Studies by Fetterman et al. [24] using maceration methods and by Souster and Emery [23] who measured the glomerular profile area sectioned through the hilum found that zonal size differences disappeared at 16 months or by 3 years of age. In contrast, Moore et al. [25] studied glomerular area in 114 children up to the age of 16 years and reported that inner cortical glomeruli were persistently larger than outer cortical glomeruli at all ages. In the present study, we found no significant zonal differences in mean IGV in either younger or older adult subjects. However, in the younger age group, in the lower BSA category and in subjects with more than 1 million nephrons, inner cortical (juxtamedullary) glomeruli tended to be larger (not statistically significant) than outer and mid-cortical glomeruli. If the association between increasing age and larger BSA and enlarged outer cortical glomeruli found in the kidneys of Americans [10] is true of other populations, then it is possible that within this sample of Senegalese we are seeing the beginnings of outer and mid-cortical glomerular enlargement in the older and higher BSA subjects.
In conclusion, findings in this Senegalese population confirm the characteristics and relationships that have been described in other populations. The benefit of examining the Senegalese population is that it provides a clear view of these relationships in a more homogeneous population and is a yardstick against which profiles in other, more heterogeneous groups and groups in different stages of cultural transition may be compared. The relationships observed between total glomerular number, BSA and median glomerular size suggest that influences on glomerular growth in later life are important contributors to the heterogeneity of IGV. Increased glomerular volume and increased heterogeneity of glomerular volume were associated with increased BSA and decreased nephron number, particularly in older Senegalese males in this urban West African sample.
Acknowledgments
The authors would like to acknowledge Sue Connell and Julie Hickey for their assistance sectioning kidney tissue. PhD scholarship funding for B. McNamara was provided by an Australian Postgraduate Award (APA) and a Monash University, Faculty of Medicine Dean's Excellence Award. Partial support was provided by grants from the National Institutes of Health NIH 1 RO1 DK065970-01, NIH Center of Excellence in Minority Health 5P20M00534-02 and National Health and Medical Research Council (NHMRC) Program Grant 502009.
Conflict of interest statement. None declared.
References
- 1.Hoy WE, Douglas-Denton RN, Hughson MD, et al. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int. 2003;83(Suppl):S31–S37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 2.Douglas-Denton RN, McNamara BJ, Hoy WE, et al. Does nephron number matter in the development of kidney disease? Ethn Dis. 2006;16(Suppl 2):S2-40–S2-45. [PubMed] [Google Scholar]
- 3.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 4.Hughson MD, Douglas-Denton R, Bertram JF, et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 5.Hughson MD, Johnson K, Young RJ, et al. Glomerular size and glomerulosclerosis: relationships to disease categories, glomerular solidification, and ischemic obsolescence. Am J Kidney Dis. 2002;39:679–688. doi: 10.1053/ajkd.2002.31980. [DOI] [PubMed] [Google Scholar]
- 6.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoy WE, Mathews JD, McCredie DA, et al. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int. 1998;54:1296–1304. doi: 10.1046/j.1523-1755.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- 8.Young RJ, Hoy WE, Kincaid-Smith P, et al. Glomerular size and glomerulosclerosis in Australian aborigines. Am J Kidney Dis. 2000;36:481–489. doi: 10.1053/ajkd.2000.9788. [DOI] [PubMed] [Google Scholar]
- 9.Lemley KV. A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney Int. 2003;83(Suppl):S38–42. doi: 10.1046/j.1523-1755.63.s83.9.x. [DOI] [PubMed] [Google Scholar]
- 10.Samuel T, Hoy WE, Douglas-Denton R, et al. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol. 2005;16:3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- 11.Zimanyi M, Hoy W, Douglas-Denton R, et al. Differences in glomerular volume between African Americans and Caucasians with low or high nephron number. J Am Soc Nephrol. 2006;17:94A. Abstract Issue. [Google Scholar]
- 12.Nugent R. Chronic diseases in developing countries: health and economic burdens. Ann N Y Acad Sci. 2008;1136:70–79. doi: 10.1196/annals.1425.027. [DOI] [PubMed] [Google Scholar]
- 13.McNamara B, Diouf B, Douglas-Denton R, et al. Nephron number and glomerular volume in two populations of African origin: Senegalese Africans and African Americans; World Congress of Nephrology; Rio de Janeiro, Brazil. 2007. p. 401. T-P400-1227. [Google Scholar]
- 14.McNamara BJ, Diouf B, Hughson MD, et al. Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Transplant. 2008;23:2576–2585. doi: 10.1093/ndt/gfn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995;161:111–172. doi: 10.1016/s0074-7696(08)62497-3. [DOI] [PubMed] [Google Scholar]
- 16.Hoy WE, Hughson MD, Singh GR, et al. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70:104–110. doi: 10.1038/sj.ki.5000397. [DOI] [PubMed] [Google Scholar]
- 17.McNamara BJ,, Diouf B, Hughson MD, et al. Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Transplant. 2008;23(8):2576–2585. doi: 10.1093/ndt/gfn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner B, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(Pt 1):335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 19.Adelman RD. Obesity and renal disease. Curr Opin Nephrol Hypertens. 2002;11:331–335. doi: 10.1097/00041552-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Neugarten J, Kasiske B, Silbiger SR, et al. Effects of sex on renal structure. Nephron. 2002;90:139–144. doi: 10.1159/000049033. [DOI] [PubMed] [Google Scholar]
- 21.Clark AT, Bertram JF. Molecular regulation of nephron endowment. Am J Physiol. 1999;276:F485–F497. doi: 10.1152/ajprenal.1999.276.4.F485. [DOI] [PubMed] [Google Scholar]
- 22.Burrow CR. Regulatory molecules in kidney development. Pediatr Nephrol. 2000;14:240–253. doi: 10.1007/s004670050049. [DOI] [PubMed] [Google Scholar]
- 23.Souster LP, Emery JL. The sizes of renal glomeruli in fetuses and infants. J Anat. 1980;130:595–602. [PMC free article] [PubMed] [Google Scholar]
- 24.Fetterman GH, Shuplock NA, Philipp FJ, et al. The growth and maturation of human glomeruli and proximal convolutions from term to adulthood: studies by microdissection. Pediatrics. 1965;35:601–619. [PubMed] [Google Scholar]
- 25.Moore L, Williams R, Staples A. Glomerular dimensions in children under 16 years of age. J Pathol. 1993;171:145–150. doi: 10.1002/path.1711710212. [DOI] [PubMed] [Google Scholar]