Introduction
Systemic inflammation is a characteristic feature of metabolic syndrome and cardiovascular (CV) disease. One common marker used to define systemic inflammation is the plasma level of C-reactive protein (CRP) [1]. Studies by Ridker et al. have shown that subjects with elevated plasma CRP levels have an increased risk for CV death [2,3]. More recent studies have shown that an elevated CRP level may also increase the risk for CV events in patients with chronic kidney disease (CKD) [4]. Furthermore, an elevation in CRP also increases the risk for progression of kidney disease in subjects with CKD [5]. In addition, a number of therapeutic agents such as aspirin [6], statins [7,8], angiotensin converting enzyme (ACE) inhibitors [9] and antioxidants [10] have been reported to both reduce CRP levels and improve CV outcomes, thereby suggesting that reducing inflammation may provide a novel means for treating kidney disease. Therefore, understanding the mechanisms driving the inflammatory response, how it may mediate renal disease progression and how to prevent or treat this response is of great interest.
Mechanisms responsible for the systemic inflammatory response in subjects with metabolic syndrome
One of the primary precursors for diabetes, hypertension and CV disease is the metabolic syndrome. The metabolic syndrome refers to a condition associated with insulin resistance in which three of five signs must be present, including abdominal obesity, impaired fasting glucose, elevated blood pressure, hypertriglyceridaemia and low HDL cholesterol [11]. In addition to the classic five signs mentioned above, the metabolic syndrome is associated with a number of other conditions, including microalbuminuria [12], fatty liver [13], endothelial dysfunction [14], hyperuricaemia [15] and systemic inflammation [16]. While not all of these signs are necessarily present, they frequently coexist and appear to be the primary harbinger for the development of type 2 diabetes and CV disease [16,17]. In addition, the presence of metabolic syndrome has recently been recognized as a risk factor for CKD [18,19].
The aetiology of insulin resistance and the metabolic syndrome involves both environmental and genetic factors. The observation that the metabolic syndrome has been associated with the introduction of Western life style to various indigenous populations [20] coupled with its rapid rise suggests the possibility that the introduction of Western diet could be involved in its pathogenesis. In this regard, fructose is a simple sugar that is a component of table sugar (sucrose) as well as of high fructose corn syrup (HFCS), which is a sweetener used primarily in the USA. Fructose consumption has increased markedly worldwide over the last two centuries and its acceleration in intake during the last several decades correlates closely with the rise in obesity and metabolic syndrome [21]. Perhaps most concerning is the fact that the administration of fructose can induce most of the features of metabolic syndrome in both animals and humans [22,23]. This suggests that fructose intake could have a role in the development of metabolic syndrome.
Fructose as a means for inducing systemic inflammation and renal damage progression
Several studies have also documented that fructose can induce inflammation in experimental animals. For example, we recently have reported that fructose can induce the expression of the leukocyte adhesion protein, ICAM-1, in human aortic endothelial cells [24]. Unlike glucose, fructose rapidly induced both ICAM-1 mRNA and protein expression, and this occurred with concentrations of fructose (1 mM) that are readily achieved in humans following ingestion of a large fructose-based meal [21]. The mechanism was shown to be associated with a reduction in eNOS and endothelial NO production and could be partially prevented by administration of an NO donor. Additional evidence of the relevance of the finding was the demonstration that a diet in which 20% of the energy was provided as fructose could increase circulating ICAM-1 levels and induce ICAM-1 expression in glomerular and peritubular capillaries in the rat kidney [24]. This is clinically relevant as some studies have reported that the adolescents can easily ingest 15–20% of their energy intake as fructose [25]. Consistent with the studies in experimental animals, Stanhope et al. have recently reported that the administration of a high fructose, but not high glucose-based diet to overweight humans, resulted in an 8% increase in circulating ICAM-1 levels [26].
Fructose can also induce the expression of the chemokine, monocyte chemoattractant protein-1 (MCP-1), in several cell types, including human proximal tubular epithelial cells [27] and human aortic endothelial cells [24]. MCP-1 is recognized as one of the key chemokines in atherosclerosis and is also considered one of the most important chemokines mediating the inflammatory response in CKD. For example, our group recently reported that rats with remnant kidneys develop progressive CKD in association with local MCP-1 expression and intrarenal macrophages accumulation, and that systemic overexpression of IL-10 can block the MCP-1 response and slow renal progression [28]. Similarly, in the same rat model, we have recently slowed the progression of kidney damage by overexpressing angiostatin, a mediator able to block MCP-1 production and its inflammatory response [29].
The possibility that fructose may accelerate renal damage through its proinflammatory effects was recently investigated. We pair-fed rats with remnant kidneys a high fructose, a high glucose (starch) or a control diet for 8 weeks. Interestingly, the high glucose diet was similar to the control diet whereas the administration of a high fructose diet caused rapid worsening of proteinuria, renal function and more severe glomerulosclerosis [27]. We also documented a marked increase in intrarenal MCP-1 expression associated with intrarenal macrophage accumulation [27].
The mechanism by which fructose causes MCP-1 expression has been studied. Fructose is unique among sugars in that it is taken up in cells by specific transporters (Glut 5) and then metabolized initially by fructokinase (KHK) to fructose-1-phosphate. Since the first report in 1968 [30], it is well known that the phosphorylation of fructose by KHK rapidly depletes liver ATP and inorganic phosphates (Pi) and this is a consequence of the sequestration of phosphates in the phosphorylated intermediary metabolites such as fructose 1-P and glyceraldehyde-3-phosphate. This makes Pi unavailable for the oxidative phosphorylation of ADP to ATP, so that ATP cannot be restored. Moreover, unlike in glycolysis, where glucokinase and phosphofructokinase are inhibited by their products and ATP, KHK is not regulated by negative feedback and so ATP depletion cannot be prevented. ATP and Pi are important inhibitors of 5′nucleotidase and AMP-deaminase, the key enzymes involved in the degradation of AMP to uric acid [30]; in ATP and Pi depleted conditions, the inhibition of these enzymes is removed and their activity is enhanced resulting in increased degradation of AMP to hypoxanthine, with subsequent transformation of the latter to uric acid by xanthine oxidoreductase (XOR) which catalyses the final step of purine catabolism [31].
As mentioned above, fructose increases intracellular concentration of uric acid, which is subsequently released into the extracellular environment. Indeed, the administration of fructose to humans result in an increase in serum uric acid [32,33]. Fructose intake correlates with serum uric acid levels and in turn uric acid levels both predict [34] and are associated with [35] metabolic syndrome. As discussed below, there is increasing evidence that the rise in uric acid associated with fructose, and observed in the metabolic syndrome, may have an important role in driving systemic inflammation and the progression of renal damage.
Uric acid, MCP-1 expression, and renal progression
Uric acid has historically been considered an antioxidant and as a result has been thought by many to be potentially beneficial in CV disease [36]. Uric acid can scavenge peroxynitrite and iron-based radicals [37]. Indeed, Ames et al. suggested that uric acid may function as a key antioxidant molecule that protects against the oxidative stress associated with ageing and cancer [36].
Nevertheless, whensoluble uric acid was added to cultured rat vascular smooth muscle cells, a marked increase in MCP-1 mRNA and protein was observed [38]. The mechanism was shown to involve uptake of uric acid via an organic anion transport system with stimulation of both mitogen activated protein (MAP) kinases and stimulation of the nuclear transcription factors NF-Kappa B and AP-1 [38,39]. An upregulation of the proinflammatory COX-2 was also shown, as well as of various growth factors including PDGF [38]. More recently uric acid was also shown to stimulate the local renin–angiotensin system and to induce oxidative stress as noted by a rise in hydrogen peroxide and 8-isoprostane in vascular smooth muscle cells [40]. Interestingly, blocking oxidative stress with N-acetylcysteine was found to block uric acid stimulated MCP-1 production [38]. How uric acid is stimulating oxidative stress in these cells remains unclear. However, it may be via the stimulation of NADPH oxidase, as we have demonstrated that uric acid can stimulate NADPH oxidase in murine adipocytes [41].
We also examined whether uric acid induced MCP-1 production might occur in the kidney. We found that uric acid potently induced MCP-1 expression in human proximal tubular epithelial cells [42]. In rats with acute renal failure experimentally induced with cisplatin, we found that an acute raising of uric acid concentration could induce a significant increase of renal injury [43]. Indeed, hyperuricaemic animals had a worse renal inflammation and greater intrarenal MCP-1 expression [43]. We also examined the effect of hyperuricaemia in rats with remnant kidneys [44]. Hyperuricaemic rats also showed markedly worse renal function and glomerulosclerosis, and this was also associated with a greater increase of intrarenal inflammation. While we did not examine the expression of MCP-1 in the kidneys of these rats, we did retrospectively find an elevation in serum MCP-1 that was prevented with allopurinol treatment [44].
Additional evidence for the proinflammatory effects of uric acid was demonstrated in human vascular smooth muscle and endothelial cells [45,46]. In these cells we found that uric acid, at concentrations observed in humans, can induce CRP mRNA and protein expression and reduce local nitric oxide generation [46].
Summary
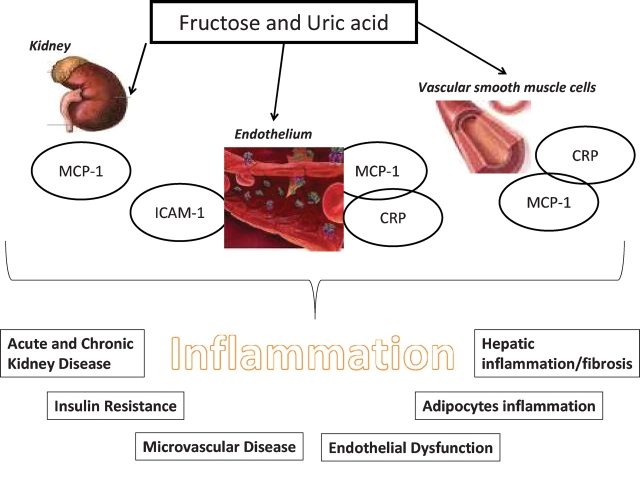
In conclusion, there is increasing evidence that systemic inflammation may increase the risk for CV outcomes and the progression of renal disease. One of the key driving forces for the systemic inflammatory response appears to be the presence of the metabolic syndrome. In turn, recent studies suggest that excessive intake of fructose may have a key role in inducing the metabolic syndrome and that it may be due to the unique role of this sugar to induce ATP depletion and uric acid generation. Furthermore, it appears that the fructose- and uric acid-mediated effects may involve the induction of leukocyte adhesion proteins (ICAM-1) and chemokines (MCP-1) that act in combination with oxidative stress and endothelial dysfunction to accelerate the renal lesion. This may theoretically be important not simply in CKD, but also in acute kidney injury. It may also represent an important mechanism by which fructose or uric acid may cause microvascular inflammation, metabolic syndrome and insulin resistance (Figure 1). Thus, these studies suggest that we may be able to prevent and to slow the progression of renal disease by a variety of novel strategies, including dietary reduction of fructose, lowering of uric acid or treating downstream events including inflammatory proteins (MCP-1, ICAM-1) or the signalling pathways driving their response.
Fig. 1.
Potential role of inflammatory molecules in fructose/uric acid-related events.
Acknowledgments
This work was supported by Italian Society of Nephrology Grant (P.C.), by Italian Ministry of Health (Ex 56 grant, L.G.), the NIH HL-68607 (R.J.J.), the Gatorade foundation (Y.S.), the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (R01-2008-000-10845-0).
Conflict of interest statement. R.J.J., Y.S. and T.N. are listed as inventors on patent applications by the University of Florida related to the role of fructose in hypertension and metabolic syndrome. Dr Johnson has also written a book on fructose for the lay public (Rodale Press, 2008).
References
- 1.Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–443. [PubMed] [Google Scholar]
- 2.Albert CM, Ma J, Rifai N, et al. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 4.Soriano S, González L, Martín-Malo A, et al. C-reactive protein and low albumin are predictors of morbidity and cardiovascular events in chronic kidney disease (CKD) 3–5 patients. Clin Nephrol. 2007;67:352–357. doi: 10.5414/cnp67352. [DOI] [PubMed] [Google Scholar]
- 5.Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int. 2001;59:407–414. doi: 10.1046/j.1523-1755.2001.059002407.x. [DOI] [PubMed] [Google Scholar]
- 6.Hovens MM, Snoep JD, Groeneveld Y, et al. Effects of aspirin on serum C-reactive protein and interleukin-6 levels in patients with type 2 diabetes without cardiovascular disease: a randomized placebo-controlled crossover trial. Diabetes Obes Metab. 2008;10:668–674. doi: 10.1111/j.1463-1326.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 7.Asher J, Houston M. Statins and C-reactive protein levels. J Clin Hypertens. 2007;9:622–628. doi: 10.1111/j.1524-6175.2007.06639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voleti B, Agrawal A. Statins and nitric oxide reduce C-reactive protein production while inflammatory conditions persist. Mol Immunol. 2006;43:891–896. doi: 10.1016/j.molimm.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Napoli M, Papa F. Angiotensin-converting enzyme inhibitor use is associated with reduced plasma concentration of C-reactive protein in patients with first-ever ischemic stroke. Stroke. 2003;34:2922–2929. doi: 10.1161/01.STR.0000099124.84425.BB. [DOI] [PubMed] [Google Scholar]
- 10.Patrick L, Uzick M. Cardiovascular disease: C-reactive protein and the inflammatory disease paradigm: HMG-CoA reductase inhibitors, alpha-tocopherol, red yeast rice, and olive oil polyphenols. A review of the literature. Altern Med Rev. 2001;6:248–271. [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program Third Report of the National Cholesterol Education Program (NCEP) on Detection and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 12.Klausen KP, Parving HH, Scharling H, et al. The association between metabolic syndrome, microalbuminuria and impaired renal function in the general population: impact on cardiovascular disease and mortality. J Intern Med. 2007;262:470–478. doi: 10.1111/j.1365-2796.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- 13.Khashab MA, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease as a component of the metabolic syndrome. Curr Gastroenterol Rep. 2008;10:73–80. doi: 10.1007/s11894-008-0012-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim JA, Montagnani M, Koh KK, et al. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa T, Tuttle KR, Short RA, et al. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 16.Calabro P, Yeh ET. Intra-abdominal adiposity, inflammation, and cardiovascular risk: new insight into global cardiometabolic risk. Curr Hypertens Rep. 2008;10:32–38. doi: 10.1007/s11906-008-0008-z. [DOI] [PubMed] [Google Scholar]
- 17.Greenfield JR, Campbell LV. Relationship between inflammation, insulin resistance and type 2 diabetes: ‘cause or effect’? Curr Diabetes Rev. 2006;2:195–211. doi: 10.2174/157339906776818532. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 19.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 20.Ko GT, Chan JC. Burden of obesity—lessons learnt from Hong Kong Chinese. Obes Rev. 2008;9(Suppl 1):35–40. doi: 10.1111/j.1467-789X.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 21.Gaby AR. Adverse effects of dietary fructose. Altern Med Rev. 2005;10:294–306. [PubMed] [Google Scholar]
- 22.Elliott SS, Keim NL, Stern JS, et al. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 23.Hwang IS, Ho H, Hoffman BB, et al. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- 24.Glushakova O, Kosugi T, Roncal C, et al. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol. 2008;19:1712–1720. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dwyer JT, Evans M, Stone EJ, et al. Child and Adolescent Trial for Cardiovascular Health (CATCH) Cooperative Research Group Adolescents’ eating patterns influence their nutrient intakes. J Am Diet Assoc. 2001;101:798–802. doi: 10.1016/s0002-8223(01)00198-5. [DOI] [PubMed] [Google Scholar]
- 26.Stanhope KL, Griffen SC, Bair BR, et al. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–1203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gersch MS, Mu W, Cirillo P, et al. Fructose but not dextrose accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol. 2007;293:F1256–F1261. doi: 10.1152/ajprenal.00181.2007. [DOI] [PubMed] [Google Scholar]
- 28.Mu W, Ouyang X, Agarwal A, et al. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol. 2005;16:3651–3660. doi: 10.1681/ASN.2005030297. [DOI] [PubMed] [Google Scholar]
- 29.Mu W, Long DA, Ouyang X, et al. Angiostatin overexpression is associated with an improvement in chronic kidney injury by an anti-inflammatory mechanism. Am J Physiol Renal Physiol. 2009;296:F145–F152. doi: 10.1152/ajprenal.90430.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mäenpää PH, Raivio KO, Kekomäki MP. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968;161:1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- 31.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 32.Stirpe F, Della Corte E, Bonetti E, et al. Fructose-induced hyperuricaemia. Lancet. 1970;2:1310–1311. doi: 10.1016/s0140-6736(70)92269-5. [DOI] [PubMed] [Google Scholar]
- 33.Gao X, Qi L, Qiao N, et al. Intake of added sugar and sugar-sweetened drink and serum UA concentration in US men and women. Hypertension. 2007;50:306–312. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 34.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. Br Med J. 2008;336:309–312. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442–447. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muraoka S, Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol. 2003;93:284–289. doi: 10.1111/j.1600-0773.2003.pto930606.x. [DOI] [PubMed] [Google Scholar]
- 38.Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 39.Kang DH, Han L, Ouyang X, et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol. 2005;25:425–433. doi: 10.1159/000087713. [DOI] [PubMed] [Google Scholar]
- 40.Corry DB, Eslami P, Yamamoto K, et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26:269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 41.Sautin YY, Nakagawa T, Zharikov S, et al. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 42.Cirillo P, Gersch MS, Mu W, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. doi: 10.1681/ASN.2008060576. 2009 Jan 21 [E-pub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roncal CA, Mu W, Croker B, et al. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2007;292:F116–F122. doi: 10.1152/ajprenal.00160.2006. [DOI] [PubMed] [Google Scholar]
- 44.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 45.Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 46.Kang DH, Park SK, Lee IK, et al. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]