SUMMARY
Recent studies have identified genes and core pathways that are altered in human glioblastoma. However, the mechanisms by which alterations of these glioblastoma genes singly and cooperatively transform brain cells remain poorly understood. Further, the cell-of-origin of glioblastoma is largely elusive. By targeting a p53 in-frame deletion mutation to the brain, we show that p53 deficiency provides no significant growth advantage to adult brain cells, but appears to induce pleiotropic accumulation of cooperative oncogenic alterations driving gliomagenesis. Our data show that accumulation of a detectable level of mutant p53 proteins occurs first in neural stem cells in the subventricular zone (SVZ) and that subsequent expansion of mutant p53-expressing Olig2+ transit-amplifying progenitor-like cells in the SVZ-associated areas initiates glioma formation.
SIGNIFICANCE
Glioblastoma is the most malignant form of astrocytic gliomas and the most common primary brain cancer in adults. The poor prognosis of glioblastoma emphasizes the urgent need for a greater understanding of disease pathogenesis. We demonstrate that p53 deficiency can cooperate with diverse mitogenic signaling pathways to induce malignant glioma. For example, inactivation of the Nf1 tumor suppressor, activation of mitogen-activated protein kinase, or activation of phosphatidylinositol-3-OH kinase pathways are not essential, but can promote p53-mediated glioma formation. Furthermore, expression of mutant p53 proteins is identified as a marker for glioma cells in all stages. Analysis of brain cells with a detectable level of mutant p53 expression provides important insights into the role of neural stem cells and transit-amplifying progenitors in p53-mediated gliomagenesis.
Key words: p53, tumor suppressor gene, malignant astrocytic glioma, glioblastoma, medulloblastoma, stem/progenitor cells, neural stem cells
INTRODUCTION
Glioblastoma (Grade IV malignant astrocytic glioma), also known as glioblastoma multiforme (GBM), is the most frequent and aggressive neoplasm among human primary brain tumors (Furnari et al., 2007; Louis et al., 2007). There are two subtypes of GBM. Primary GBM arises de novo with no evidence of pre-existing lesions whereas secondary GBM develops from lower-grade, albeit malignant, i.e., Grade II or III gliomas. Despite distinctive clinical courses and differing molecular lesions, primary and secondary GBMs share the same histopathological and clinical features, most notably a high propensity to diffusely infiltrate normal brain parenchyma and resistance to virtually all current therapies. Consequently, GBM is one of the most deadly human cancers with a median survival that has remained at 12 months for over the past two decades (Furnari et al., 2007; Louis et al., 2007).
Recent studies have identified genes and core pathways that are altered in human GBM (Ohgaki et al., 2004; Parsons et al., 2008; TCGA Research Network, 2008). Mutations in the components of the p53 tumor suppressor pathway have been identified in the majority of human primary GBM, approximately 30 to 40% of which have mutations in the p53 gene (Parsons et al., 2008, TCGA Research Network, 2008). Furthermore, frequencies of p53 mutations are high and similar among lower-grade malignant gliomas and secondary GBMs, suggesting an important role of p53 gene defects in early stages of glioma development (Ohgaki et al., 2004). Consistently, individuals with Li-Fraumeni syndrome, who carry germline p53 mutations, are predisposed to development of astrocytic gliomas (Louis et al., 2007). However, the mechanisms by which p53 deficiency transforms normal brain cells remain poorly understood.
One critical challenge to understand the GBM pathogenesis is to identify the cell-of-origin of this disease. The cell-of-origin in most human cancers remains unknown as human tumors are typically presented at the terminal stages of the disease and thus do not provide a window to study this important question. Recent studies demonstrated that a number of brain cancers, including GBM, are driven and sustained by a subset of stem cell-like cells that exhibit the cellular characteristics of normal stem cells, including self-renewal and multipotency (Galli et al., 2004; Hemmati et al., 2003; Singh et al., 2004). However, whether a normal stem cell, a progenitor cell, or even a fully differentiated cell is the cell-of-origin for glioma stem cells remains largely unknown (Sanai et al., 2005; Stiles and Rowitch, 2008). In the adult brain, multipotent neural stem and progenitor cells are spatially restricted in two stem cell niches: the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampal dentate gyrus (Merkle and Alvarez-Buylla, 2006). Genetic studies using murine glioma models and imaging analysis from a clinical study provide evidence that some GBMs may arise from the SVZ stem cell niche (Alcantara Llaguno et al., 2009; Lim et al., 2007; Zhu et al., 2005a). At the cellular level, neural stem cells in the adult SVZ (type B cells or SVZ-B) give rise to a highly proliferative cell population, transit-amplifying progenitor cells (SVZ-C cells), which then differentiate into two lineage-restricted progenitor cells, neuroblasts (SVZ-A cells) and oligodendrocyte precursor cells (SVZ-OPC) (Hack et al., 2005; Menn et al., 2006). Because of a lack of reliable markers for glioma cells, particularly at early stages of tumor development, the role of the various SVZ cell populations in gliomagenesis remains undefined.
In this study, we develop a murine glioma model in which an in-frame p53 deletion mutation is specifically targeted into the nervous system and use it to investigate the role of neural stem cells and transit-amplifying progenitors in p53-mediated gliomagenesis.
RESULTS
Neural-specific p53 targeting strategy
To investigate the role of p53 in gliomagenesis, we employed two transgenic mouse strains to target a p53 mutation into the nervous system. The first one expresses Cre under the control of the human glial fibrillary acidic protein (GFAP) promoter (hereafter, hGFAP-cre). The hGFAP-cre transgene is specifically expressed in radial glial cells during embryonic development and in mature astrocytes of the postnatal brain (Malatesta et al., 2003; Zhuo et al., 2001). Because radial glial cells are the primary multipotent neural stem/progenitor cell populations in the developing brain, hGFAP-cre-mediated gene deletion will be transmitted to all the progeny of radial glial cells including neurons, glia, and adult neural stem cells (Merkle and Alvarez-Buylla, 2006). Thus, the hGFAP-cre-mediated neural-specific gene inactivation permits assessment of the relative tumor susceptibility or “tumor competence” of neural stem cells, progenitor cells, and mature glia in the adult brain (Figure S1). The second strain harbors a p53 conditional allele (p53flox) (Lin et al., 2004). Cre-mediated recombination of this p53flox allele results in an in-frame deletion of exons 5 and 6 (Figure 1A), which encode a significant portion of the p53 DNA binding domain (DBD) (Cho et al., 1994; Kitayner et al., 2006). Consistently, it has been previously shown that this p53 in-frame deletion mutant (hereafter, p53ΔE5-6) lacks transcriptional function (Lin et al., 2004). Thus, the p53ΔE5-6 allele represents a DBD-deficient loss-of-function allele.
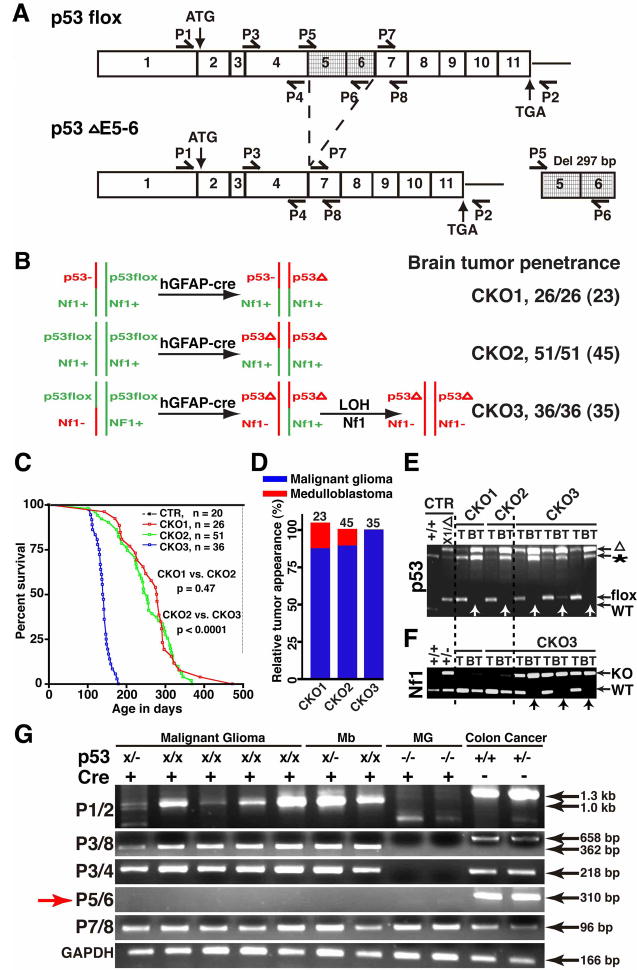
Figure 1. Construction and characterization of the CKO1, CKO2 and CKO3 mice.
(A) The exon structure of the p53flox and deleted p53ΔE5-6 (Del) alleles and RT-PCR strategy. Positions for the primers, P1-8, are illustrated. (B) Schematic drawing of the genetic configurations of CKO1-3 mice. All the mutant mice analyzed developed brain tumors including those in early stages. The number in parenthesis shows the number of mutant mice that developed high-grade brain tumors. (C) Survival curves of control and CKO1-3 mutant mice. “p” value was obtained from Kaplan/Meier Survival Test. (D) The percentage of malignant astrocytic gliomas and medulloblastomas observed in the mutant mice with high-grade brain tumors. Of note, one of 23 CKO1 mice developed both malignant glioma and medulloblastoma. (E, F) Genomic DNAs isolated from the tails (T) and brain tumors (BT, arrows) of CKO1-3 mice were subjected to PCR analysis for the p53 (E) and Nf1 alleles (F). WT, wild-type allele; flox, p53flox allele; “*”, p53 pseudogene; “Δ”, deleted p53 allele; KO, Nf1 knockout allele. (G) RT-PCR analysis showing an aberrant transcript, lacking exons 5 and 6, produced from the mutant alleles in brain tumors including malignant glioma and medulloblastoma (Mb). The p53 full-length cDNAs were amplified from colon cancers of the Smad3−/− mice, serving as a positive control; the p53-null cDNAs from malignant gliomas (MG) of the hGFAP-cre+;p53KO/KO;Nf1flox/flox; mice were used as a negative control. Of note, the p53-null allele produces a truncated transcript lacking exons1 to 6.
P53 deficiency drives tumor formation in the adult brain
To study tumorigenic activity of the p53ΔE5-6 mutation in the brain, we crossed the hGFAP-cre line with the p53flox mouse to generate hGFAP-cre+;p53flox/+ mice. We monitored 10 hGFAP-cre+;p53flox/+ mice over 12 months. These mice were healthy and behaved indistinguishably from the wild-type littermates. Moreover, we found no evidence of tumor formation in the brain of these aged mice (data not shown). We then generated hGFAP-cre+;p53flox/KO (hereafter referred to as conditional knockout 1, CKO1) and hGFAP-cre+;p53flox/flox (CKO2) mouse strains (Figure 1B). As we have previously shown that inactivation of p53 followed by Nf1 loss efficiently induces malignant gliomas with complete penetrance (Zhu et al., 2005a), we introduced a heterozygous Nf1 mutation to the p53ΔE5-6 mutant background and generated a third mutant strain with the genotype of hGFAP-cre+;p53flox/flox;Nf1KO/+ (CKO3). In CKO3 brains, p53-deficient cells are expected to inactivate the Nf1 wild-type allele to undergo p53/Nf1-mediated gliomagenesis whereas p53-deficient cells in the CKO1/2 brains could potentially cooperate with diverse oncogenic alterations to drive glioma formation (Figure 1B).
Both CKO1 and CKO2 mice were healthy, fertile, and indistinguishable from their control littermates within the first 6 months after birth (Figure 1C). However, 85% of CKO1 (22/26) and 84% of CKO2 (43/51) mice analyzed in this study developed neurological symptoms including tremor, seizure, ataxia or lack of balance requiring sacrifice between ages of 6 and 14 months (Figure 1C). Each of the CKO1 and CKO2 mice with neurological deficits exhibited enlarged brains with evidence of high-grade brain tumors. The remaining CKO1 and CKO2 mice were sacrificed because of the presence of tumors outside the central nervous system (CNS). These non-CNS tumors were diagnosed as soft-tissue sarcomas, likely arising due to hGFAP-cre expression in cells outside the CNS (data not shown). A portion of the CKO1 (1/4) and CKO2 (2/8) mice with non-CNS sarcomas also exhibited enlarged brains with high-grade tumors. Together, nearly 90% of both CKO1 (23/26) and CKO2 (45/51) mutant mice developed high-grade brain tumors (Figure 1B), most of which (20/23 of CKO1 and 40/45 of CKO2) were histopathologically characterized as malignant gliomas with astrocytic characteristics and the remainders were medulloblastomas (Figure 1D). Because CKO1 and CKO2 mice exhibited essentially the same brain tumor phenotypes (Figure 1B–D), they will be presented together as the p53ΔE5-6 mutant mice.
Mutant p53ΔE5-6 expression marks p53-deficient brain tumor cells
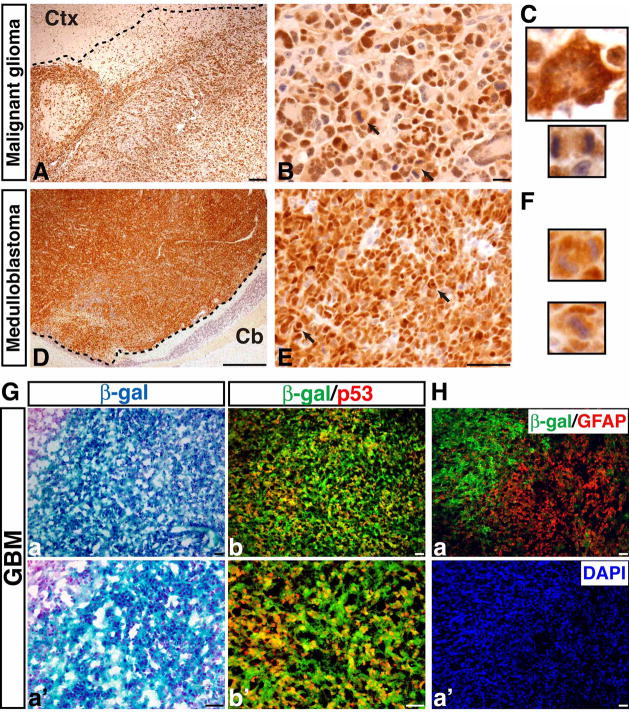
To determine whether Cre-mediated p53 deletion drives tumor formation, we first performed PCR analysis on genomic DNA of brain tumors isolated from p53ΔE5-6 mutant mice (n = 6) and found that the p53flox allele had been completely converted to the p53ΔE5-6 allele (Figure 1E). RT-PCR analysis revealed that the p53ΔE5-6 transcript was the only p53 transcript that could be detected in these tumors (n = 7) (Figure 1G). We further cloned and sequenced the p53 cDNAs and genomic DNAs derived from these brain tumors and found no somatic mutation other than the expected deletion of exons 5 and 6 (see methods). Similar to human tumors, all the p53ΔE5-6 brain tumors expressed high levels of mutant p53 proteins (Figures 2A–F, 3Cb, b′). The p53 nuclear immunostaining was very specific for p53ΔE5-6 brain tumor cells, as no labeling was observed in the normal brain cells, including those carrying one or two p53ΔE5-6 alleles, or in the p53-null malignant astrocytic gliomas (Table S1) (Zhu et al., 2005a). In addition, we crossed the p53ΔE5-6 mutant mice (CKO1 and CKO2) to a mouse strain harboring the Rosa26-LacZ reporter (R26R-LacZ), whose expression requires Cre-mediated recombination (Soriano, 1999). Nearly all the SVZ stem and progenitor cells as well as Olig2-expressing progenitors and oligodendrocytes in the corpus callosum of the resulting mice could readily be labeled by β-galactosidase (β-gal) expressed from the R26R-LacZ (Figure S1B, S1C). Accordingly, all the p53ΔE5-6 brain tumors analyzed extensively expressed high levels of β-gal (Figure 2Ga, a′). Furthermore, almost all the p53ΔE5-6-positive brain tumor cells exhibited high levels of β-gal expression (Figure 2Gb, b′). It is worth noting that 3 of 5 p53ΔE5-6 brain tumors analyzed contained a subpopulation population of tumor cells with no or low β-gal staining, which often exhibited high levels of GFAP and hGFAP-cre expression (Figure 2H and data not shown). This is consistent with the previous observations that the R26R-LacZ is not expressed in most GFAP-expressing mature astrocytes, despite their expression of hGFAP-cre (Figure S1C) (Malatesta et al., 2003). Taken together, these studies demonstrate that brain tumors observed in this model arose from p53-deficient cells, which can be specifically marked by a detectable mutant p53 protein expression.
Figure 2. P53ΔE5-6 brain tumors specifically express a detectable level of mutant p53 proteins.
Representative examples showing p53ΔE5-6-positive malignant astrocytic gliomas (A, B) and medulloblastomas (D, E). The dashed lines roughly mark the border of the tumors and surrounding brain tissues because of the infiltrating nature of these tumors (A, D). Arrows in (B) and (E) point to mitotic figures in malignant gliomas and nuclear molding in medulloblastomas, respectively. (C) High magnification views of a p53ΔE5-6-positive multinucleated giant cell (upper panel) and mitotic figure (bottom panel) in a malignant glioma. (F) High magnification views of a p53ΔE5-6-positive mitotic figure (upper panel) and nuclear molding (bottom panel) in a medulloblastoma. Ctx, cerebral cortex; Cb, cerebellum. (G) Adjacent sections from p53ΔE5-6 brain tumors harboring the R26R-LacZ transgene were stained with X-gal (a, a′) and anti-β-gal/anti-p53 antibodies (b, b′). All the p53ΔE5-6-positive tumor cells exhibited β-gal expression indicating p53 deficiency. (H) A small population of tumor cells expressed little or no β-gal, but had high levels of GFAP expression (a). Tumor nuclei were counterstained with DAPI (a′). Scale bar: 50 μm.
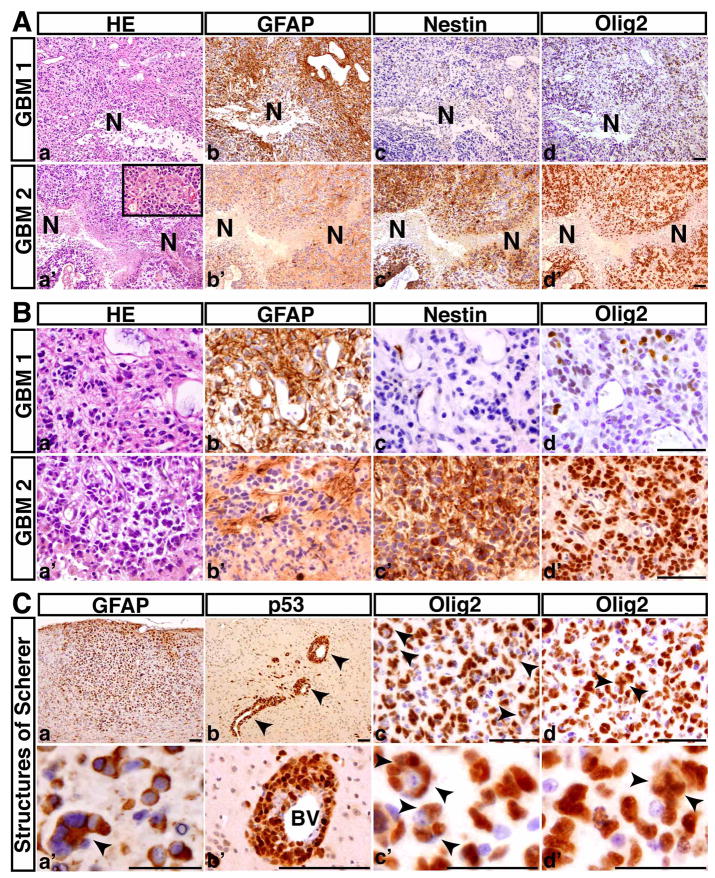
Figure 3. The p53ΔE5-6 mutant mice develop GBMs.
(A) Serial sections from two representative p53ΔE5-6 GBMs (GBM1, 2) were stained with H&E (a, a′), anti-GFAP (b, b′), anti-Nestin (c, c′), and anti-Olig2 (d, d′). N, necrosis. There are regions of coagulation necrosis (a–d), and pseudopalisades of malignant GBM cells adjacent to necrosis (a′-d′). The inset in (a′) shows microvascular proliferation in this GBM. (B) High-magnification views of (A) illustrate that both malignant cells and nuclei are highly pleomorphic. Many GBM cells have substantial cytoplasm and some have eccentric nuclei (a, a′). (C) The p53ΔE5-6 gliomas exhibit the secondary structures of Scherer: accumulation of GFAP-positive tumor cells in the subpial zone of the cerebral cortex (a, a′); perivascular satellitosis (arrowheads) (b, b′); and perineuronal satellitosis (arrowheads, c, c′). (d, d′) The examples of Olig2+ tumor cells with abnormal mitoses (arrowheads). BV, blood vessels. Scale bar, (A, B, Cad), 50 μm; (Ca′-d′), 25 μm.
CD133+ malignant astrocytic glioma and GBM
Malignant astrocytic gliomas observed in the advanced-stage p53ΔE5-6 mice diffusely infiltrated the forebrain and expressed glioma markers GFAP, Nestin, and Olig2, albeit with a high degree of heterogeneity within individual tumors and between different tumors (Figures 3A, 3B, S2, Table S2). Importantly, approximately 40% of the p53ΔE5-6 high-grade astrocytic gliomas exhibited the characteristics of human GBM, including the presence of necrosis, pseudopalisading tumor cells, and microvascular proliferation (Figure 3A). It is worth noting that regardless of the presence or absence of detectable necrosis, these malignant gliomas exhibited similarly high degrees of nuclear atypia (Figures 3B, S2B, S3), resembling human primary GBM. These tumors also exhibited the defining features of human high-grade diffuse gliomas: high levels of mitotic figures and presence of secondary structures of Scherer (Figures 2B, 3C). Furthermore, similar to human counterparts, a subpopulation of p53ΔE5-6 tumor cells expressed a glioma stem cell marker, CD133 (also known as prominin1), and the CD133+ glioma cells were often located around blood vessels and expressed p53, Nestin, and Olig2 (Figure S4) (Calabrese et al., 2007; Singh et al., 2004). Taken together, the p53ΔE5-6 mouse model accurately recapitulates both the genetics and pathology of human high-grade astrocytic gliomas.
P53 deficiency provides no significant growth advantage to adult brain cells
To investigate the mechanisms underlying p53-mediated gliomagenesis, we examined the brain of young adult mutant mice. At 2 months of age, no significant difference was identified between the control and p53ΔE5-6 brains (n = 6 for each genotype), which is consistent with the notion that p53 is dispensable for normal brain development (Donehower et al., 1992; Jacks et al., 1994). Although previous studies demonstrated that a germline p53-null mutation results in mild hyperplasia in the SVZ of the adult brain (Gil-Perotin et al., 2006; Meletis et al., 2006), we found no abnormality in the p53ΔE5-6 mutant SVZ stem cell niche with respect to the number or the density of the total SVZ cells (Figure S5). Furthermore, no significant difference in the number, the density, or the percentage of BrdU-positive proliferating cells was identified between the control and mutant brains including the SVZ region at 2 months (Figure S5M-Q). Together, these results indicate that the p53ΔE5-6 mutation does not confer a measurable growth advantage to brain cells including SVZ stem and progenitor cells in young adult mice.
P53 deficiency induces accumulation of cooperative oncogenic events
The results described above suggest that additional cooperative oncogenic alterations must occur to transform p53-deficient brain cells. To test this, we examined the status of other GBM core pathways such as Rb and receptor tyrosine kinase (RTK) signaling pathways in these tumors. Western blot analysis revealed that all the p53ΔE5-6 gliomas exhibited high levels of Cdk4 expression and about half of the tumors also had Cyclin D1 overexpression, whereas the expression of Rb and p27 was not significantly different between tumors and control forebrains (Figure 4A). These results demonstrate that deregulation of the Rb-mediated cell-cycle regulatory pathway is a universal phenomenon in these p53ΔE5-6 gliomas. Because deregulation of one of the RTKs, platelet-derived growth factor receptor (PDGFR), is often identified in human malignant gliomas with p53 mutations (Furnari et al., 2007; Louis et al., 2007), we investigated the expression of PDGFRα and its ligands in the p53ΔE5-6 gliomas. Compared to normal brains, all the p53ΔE5-6 gliomas analyzed in this study exhibited high levels of expression of PDGFRα and its two ligands, PDGF-A and PDGF-B (Figure 4B), suggesting that these tumor cells established an autocrine or paracrine stimulatory loop of the PDGF/PDGFR signaling. As in human tumors (Furnari et al., 2007), the p53ΔE5-6 malignant astrocytic gliomas at all stages analyzed (n = 8) exhibited high levels of PDGFRα expression (Figure S3), suggesting that activation of PDGFR signaling is an early event in p53-mediated gliomagenesis.
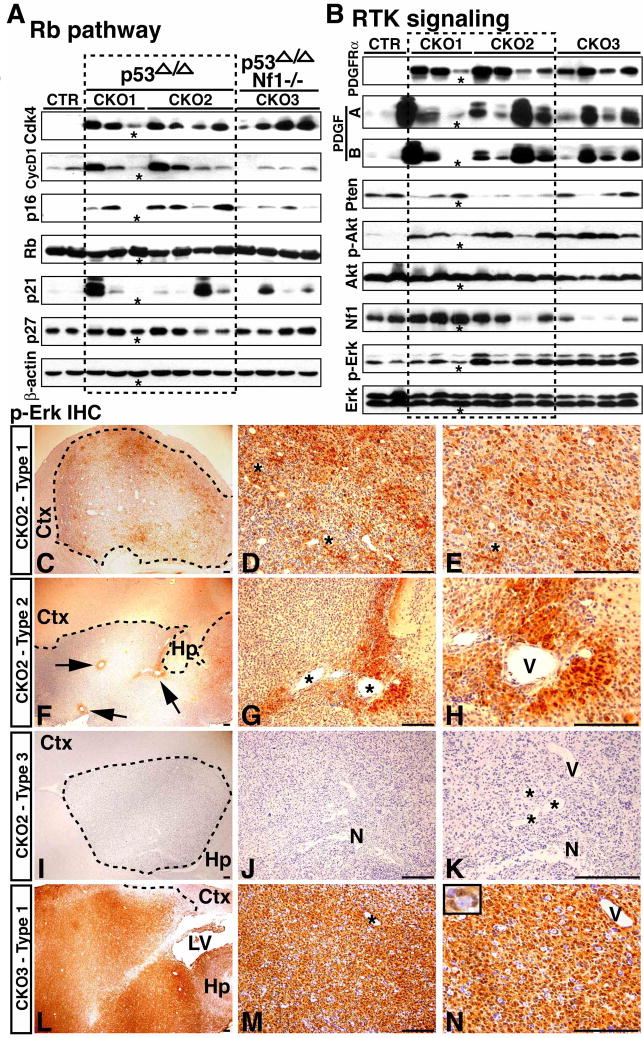
Figure 4. Molecular characterization of malignant astrocytic gliomas.
(A) Western blot analysis of the Rb pathway. Protein extracts from normal forebrain tissues, p53ΔE5-6 gliomas (CKO1, 2) and p53ΔE5-6;Nf1−/− gliomas (CKO3) were analyzed by Western blot analysis using antibodies against components in the G1/S cell cycle regulatory circuits. β-actin was used as a loading control. (B) Western blot analysis of the RTK and mitogenic signaling pathways on the same samples analyzed in (A). A glioma sample marked by “*” was found contaminated with a significant amount of normal tissues. Sections of malignant gliomas marked by dash lines from CKO2 (C-K) and CKO3 (L-N) mice were stained with an anti-p-Erk antibody. Based upon the number and pattern of p-Erk-positive cells, the p53ΔE5-6 gliomas are highly heterogeneous and can be classified into three types: (1) Type 1 tumors have extensive pErk staining (C-E), (2) Type 2 tumors only have a small number of p-Erk-positive cells that are typically found associated with blood vessels (F-H), and (3) Type 3 tumors exhibit no p-Erk staining (I-K). “N” in (J, K) labels necrosis. In contrast, the p53ΔE5-6;Nf1−/− gliomas are relatively homogeneous and exhibit extensive and intense p-Erk staining (L-N). Ctx, cerebral cortex; Hp, hippocampus; LV, lateral ventricle; “V” and “*”, blood vessels. The inset in (N) shows an example of p-Erk-positive cells encircling a neuron. Scale bar, 100 μm.
We then investigated two mitogenic signaling pathways, the phosphatidylinositol-3-OH kinase (PI3K) and mitogen-activated protein kinase (MAPK), which are major effectors of RTK signaling. The expression of a major negative regulator of the PI3K pathway, the Pten tumor suppressor, was either lost or significantly reduced in over 80% of the p53ΔE5-6 gliomas (Figure 4B). Consistently, the majority of the p53ΔE5-6 gliomas exhibited activation of the PI3K signaling pathway evidenced by increased levels of phosphorylated Akt (p-Akt) (Figures 4B, S6A-L). As a negative regulator of Ras oncoprotein, NF1 tumor suppressor gene was previously found overexpressed in human sporadic GBMs (Gutmann et al., 1996). Consistently, compared to normal brains, most p53ΔE5-6 gliomas exhibited increased expression of Nf1 (Figure 4B). However, one of 7 (14%) p53ΔE5-6 gliomas analyzed exhibited loss of Nf1 expression, which agreed well with more recent large-scale genomic studies that NF1 is mutated in approximately 15% of human GBMs (Parsons et al., 2008, TCGA Research Network, 2008). Accordingly, only half of the p53ΔE5-6 gliomas exhibited activation of the MAPK signaling pathway (Figure 4B, 4C-K). Together, these results indicate that the p53ΔE5-6 mutation can induce accumulation of downstream oncogenic alterations in Rb and RTK signaling pathways, which drive gliomagenesis. Importantly, these results validate molecular fidelity of the p53ΔE5-6 model to human GBM.
Nf1 inactivation promotes p53-mediated malignant glioma formation
Despite that Nf1 inactivation or activation of PI3K and MAPK signaling pathways appears not essential for p53-mediated gliomagenesis, all the CKO3 mice (hereafter, p53ΔE5-6;Nf1−/−) analyzed (n = 36) developed malignant astrocytic gliomas with significantly shorter latency compared to the p53ΔE5-6 mutant mice (Figure 1B–D). Strikingly, nearly 70% of malignant gliomas observed in p53ΔE5-6;Nf1−/− mice exhibited histopathological features of human GBM, such as necrosis (Figure S7). Molecular analysis confirmed that p53ΔE5-6;Nf1−/− gliomas arose from p53 (Figure 1E) and Nf1 double deficient cells, as evidenced by invariable loss of the Nf1 wild-type allele (arrows, Figure 1F). Compared to the p53ΔE5-6 gliomas, the p53ΔE5-6;Nf1−/− gliomas exhibited relatively similar molecular alterations between individual tumors as well as in cells within a tumor. For example, all the p53ΔE5-6;Nf1−/− gliomas exhibited consistent activation of MAPK and PI3K/Akt pathways (Figures 4B, 4L-N, S6M-R). At histopathological levels, unlike the p53ΔE5-6 tumors, the p53ΔE5-6;Nf1−/− gliomas exhibited a relatively homogeneous expression pattern of lineage markers. (Figure S7, Table S2). Thus, these results demonstrate that Nf1 inactivation, as one of cooperative oncogenic alterations, albeit not essential, can rapidly promote p53ΔE5-6-mediated gliomagenesis.
Expression of mutant p53ΔE5-6 proteins marks the earliest-stage glioma cells
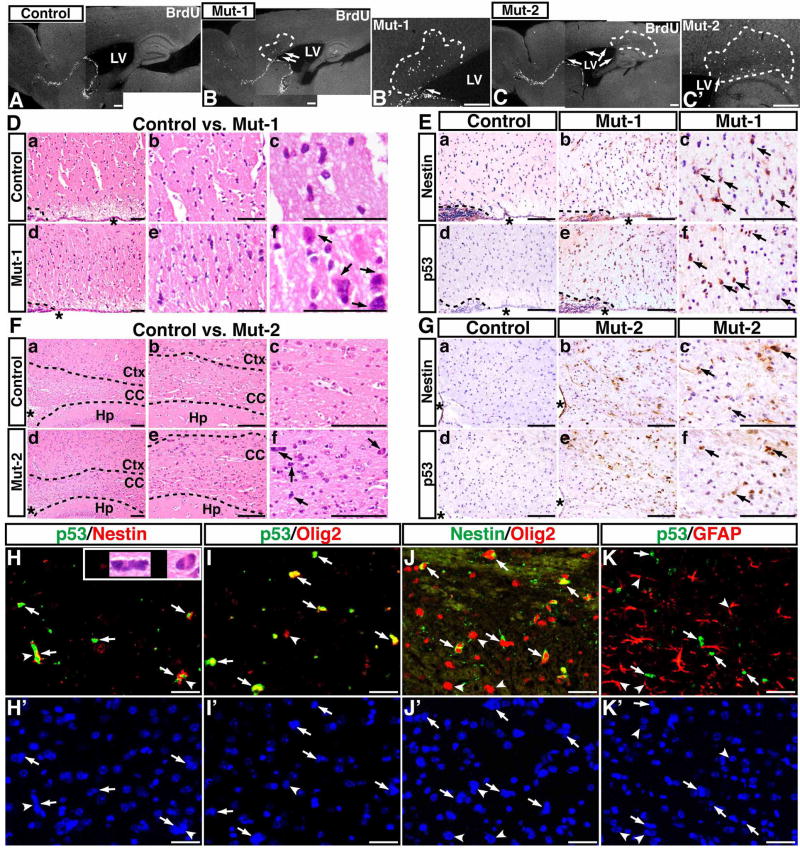
The molecular and histological studies of p53ΔE5-6 and p53ΔE5-6;Nf1−/− gliomas suggest that acquired cooperative oncogenic alterations can influence the histological features of advanced-stage malignant gliomas. Alternatively, the highly histological heterogeneity of p53ΔE5-6 malignant gliomas could result from distinct cell(s)-of-origin. To distinguish these two possibilities, we first sought to identify the putative earliest-stage glioma cells (for simplicity, referred to as glioma precursor). In the normal adult brain, particularly after 4 months of age, virtually all the proliferating cells are restricted to the SVZ or RMS (Figure 5A). This extremely low proliferation background permits the use of BrdU immunostaining as a sensitive assay to identify glioma precursors. Based upon the time course of tumor development in p53ΔE5-6 mice (Figure 1C), we studied the brain of 10 mutant mice at ages of 4 to 4.5 months and found that all of them exhibited abnormal proliferation in the portion of the corpus callosum immediately adjacent to the SVZ or RMS (Figure 5B, C). Specifically, 4 of these 10 p53ΔE5-6 brains had small clusters of abnormal proliferating cells, 3 of which were identified in the corpus callosum immediately adjacent to the SVZ (Figure 5B′, C′) and one was observed in the olfactory bulb (OB) (Figure 8D). By analyzing the serial sections of these 4 brains, we confirmed that the identified proliferating clusters were the only proliferating cell cluster present in these brains. Thus, these results indicate that the proliferating clusters constitute the initially expanding glioma precursors. Consistently, many cells in the proliferating cell clusters exhibited a high degree of nuclear atypia (arrows, Figure 5D, F), a property reminiscent of malignant glioma cells. In addition to the cells in the adult neurogenic niches, glioma precursors were the only cells in mutant brains that expressed Nestin, which is never found in the normal corpus callosum (Upper panels, Figure 5E, 5G). Together, we conclude that glioma precursors in most mutant brains were Nestin-expressing (Nestin+) cells located in the corpus callosum immediately adjacent to the SVZ.
Figure 5. Expression of mutant p53ΔE5-6 proteins identifies the earliest-stage glioma cells.
BrdU staining was performed on sections from age-matched control (A) and two representative p53ΔE5-6 brains that contain proliferation clusters (marked by dashed lines) in the corpus callosum adjacent to the anterior SVZ (B, B′, Mut-1) and to the posterior SVZ (C, C′, Mut-2), respectively. Arrows in (B to C′) point to abnormal proliferating cells in the SVZ. LV, lateral ventricle. (D) The H&E-stained sections from similar areas of control brain (a–c) and the proliferating cluster of Mut-1 brain (d–f) were imaged and shown at three different magnifications. (E) Adjacent sections of the control and Mut-1 brains (D) were stained with anti-Nestin and anti-p53 antibodies. (F, G) Similar morphological and immunohistochemical analyses of (D, E) were performed on control brain and a proliferating cluster of Mut-2 brain. Arrows in (D, F) point to abnormal cells with atypical nuclei in mutant brains. Arrows in (E, G) point to Nestin+ and p53+ cells in mutant brains. “*” in (D to G) denotes the lateral ventricle. The dashed lines in (D, E) and (F) mark the border of the SVZ and corpus callosum, and the border of the corpus callosum and surrounding tissues, respectively. Ctx, cerebral cortex; CC, corpus callosum; Hp, hippocampus. The sections from the proliferating clusters were stained with anti-p53/anti-Nestin (H) and DAPI (H′); anti-p53/anti-Olig2 (I) and DAPI (I′); anti-Nestin/anti-Olig2 (J) and DAPI (J′); anti-p53/anti-GFAP (K) and DAPI (K′). Arrows in (H–K) point to p53ΔE5-6-positive cells. Arrowheads in (J, J′) point to Nestin−/Olig2+ cells that are most likely normal glial progenitors or oligodendrocytes. Arrowheads in (K, K′) point to the p53-negative nuclei of GFAP+ cells. The inset in (H) shows atypical nuclei of two p53+/Nestin+ cells (arrowheads). Scale bar, 25 μm.
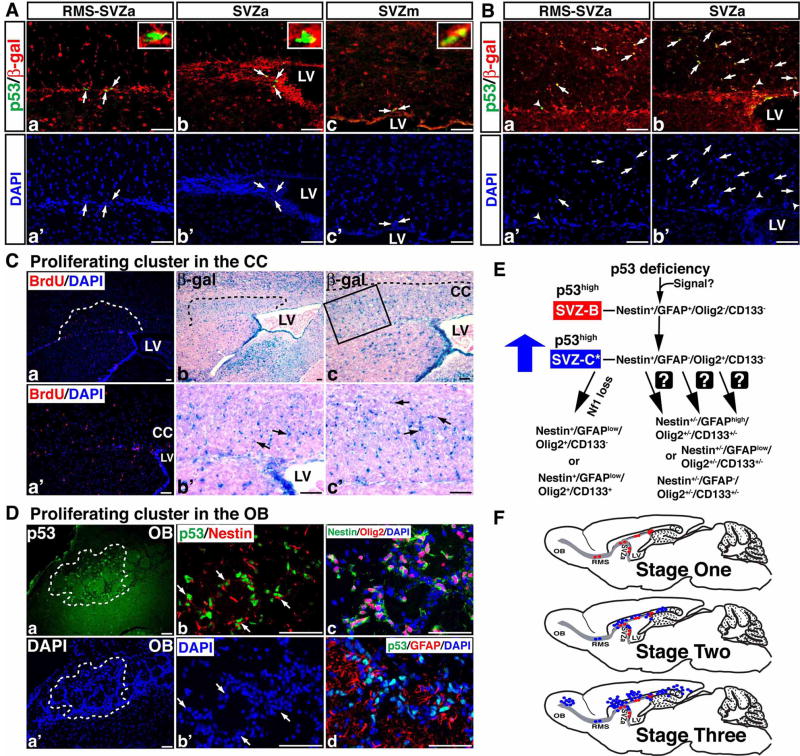
Figure 8. P53ΔE5-6-positive SVZ stem cells and glioma precursors express the R26R-LacZ.
(A) Sections from 2-month-old p53ΔE5-6 brains with the R26R-LacZ transgene were stained with anti-p53/anti-β-gal antibodies (a–c) and were counterstained with DAPI for labeling nuclei (a′-c′). All the p53ΔE5-6-positive cells (arrows) at this age express β-gal of the R26R-LacZ regardless of their location in the RMS, anterior, or medial SVZ (SVZa and SVZm). The insets in (a–c) show the high-magnification views of p53/β-gal double positive cells. (B) Two representative proliferating clusters identified in the corpus callosum adjacent to either the junction of RMS and SVZa (a, a′) or SVZa (b, b′) were sectioned and stained with anti-p53/anti-β-gal antibodies. Within these proliferating clusters, all the p53ΔE5-6-positive glioma precursors in the corpus callosum (arrows) and SVZ (arrowheads) express β-gal of the R26R-LacZ. (C) Adjacent sections of a proliferating cluster were stained by BrdU/DAPI staining (a, a′) and X-gal (b, c). (b′, c′) High-magnification views of the boxed areas shown in (b, c) reveal a group of β-gal-positive cells with atypical nuclei (arrows). LV, lateral ventricle. (D) A mutant brain contains p53ΔE5-6-positive glioma precursors in the OB (a, a′). Sections from this proliferating cluster were stained with anti-p53/anti-Nestin (b) and DAPI (b′), anti-Nestin/anti-Olig2/DAPI (c), and anti-p53/anti-GFAP/DAPI (d). Arrows in (b, b′) point to p53ΔE5-6-positive cells. (E) A model summarizes p53-mediated gliomagenesis (see Discussion for details). (F) The anatomical location of p53ΔE5-6-positive early-stage glioma precursors during tumor development. Red dots, p53ΔE5-6-positive SVZ-B stem cells; blue dots, p53ΔE5-6-positive SVZ-C*-like glioma precursors. Scale bar, 50 μm
Given that a detectable level of mutant p53ΔE5-6 proteins is exclusively identified in advanced-stage malignant glioma cells, we then sought to determine whether the expression of mutant p53ΔE5-6 proteins can specifically mark glioma precursors. We found that about 90% of the proliferating cells revealed by BrdU staining in the corpus callosum expressed a detectable level of mutant p53ΔE5-6 proteins whereas no cells exhibited such expression in the similar areas of normal brains (Bottom panels, Figure 5E, 5G). Double-immunofluorescence labeling revealed that Nestin and mutant p53ΔE5-6 proteins were almost always co-expressed in these cells (Figure 5H, H′). Furthermore, the p53ΔE5-6-positive cells expressed Olig2, but not GFAP, CD133, or markers for lineage-restricted progenitors such as PSA-NCAM (a SVZ-A cell marker) or NG2 (a SVZ-OPC cell marker) (Figure 5I, I′, 5K, K′, and data not shown). We further confirmed that all the Nestin+ cells in the proliferating clusters co-expressed Olig2 (Nestin+/Olig2+, Figure 5J, J′), demonstrating that glioma precursors are distinct from Nestin−/Olig2+ normal glial progenitors or oligodendrocytes in the corpus callosum (arrowheads, Figure 5J, J′). Taken together, these results demonstrate that the expression of mutant p53ΔE5-6 proteins specifically marks glioma precursors, which immunohistochemically resemble a subpopulation of transit-amplifying SVZ-C progenitor cells (hereafter, SVZ-C*) with the lineage marker expression pattern of Nestin+/GFAP−/Olig2+/CD133−.
To validate that the initially expanded p53ΔE5-6-positive SVZ-C*-like cells are glioma precursors, we analyzed 19 grossly healthy p53ΔE5-6 mice at older ages (6 to 9 months) along with 3 mutant mice with non-CNS sarcomas at 10 to 12 months of age. Of these 22 grossly normal p53ΔE5-6 brains, 15 exhibited no microscopically visible tumor mass (Table S3). Compared to the mutant brains at younger ages, all the brains with no microscopically visible tumor (n = 14) analyzed between ages of 6 and 10 months had markedly increased numbers of p53ΔE5-6-positive cells in the corpus callosum and adjacent areas, indicating a continuous expansion of the p53ΔE5-6-positive cellular populations during tumor development (Figure S8). In all but two of these 14 mutant brains, the p53ΔE5-6-positive cells were specifically distributed in the forebrain areas that are anatomically immediately adjacent to the SVZ-associated areas (Table S3, Figure S8). Importantly, most, if not all, of the continuously expanded p53ΔE5-6-positive cells exhibited the similar features of glioma precursors identified in younger mutant mice, which include hyperproliferation, and expression of Nestin and Olig2, but not GFAP (Figure S8 and data not shown). Together, these results suggest that the p53ΔE5-6-positive SVZ-C*-like cells in the corpus callosum are glioma precursors, whose continuous expansion underlying malignant glioma development. These observations also demonstrate that the histologically homogeneous p53ΔE5-6-positive SVZ-C*-like glioma precursors can give rise to malignant gliomas with a high degree of heterogeneity.
A minor population of adult SVZ cells express mutant p53ΔE5-6 proteins
The observations described above raise the possibility that we might be able to identify the cell-of-origin for p53ΔE5-6-positive malignant glioma cells by determining which cell type(s) in mutant brains first accumulates a detectable level of mutant p53ΔE5-6 proteins. To test this, we analyzed the brain of 11 two-month-old p53ΔE5-6 mice when there was no overt abnormality. Immunohistochemical analysis revealed that 1 of these 11 mutant brains lacked any p53ΔE5-6-positive cells and 5 of them exhibited p53 expression only in one of the two brain hemispheres. These results further confirm the notion that accumulation of a detectable p53ΔE5-6 protein expression is not an inherent property of this mutant allele, but rather a tumor-dependent event and hence may be used for identifying incipient tumor cells.
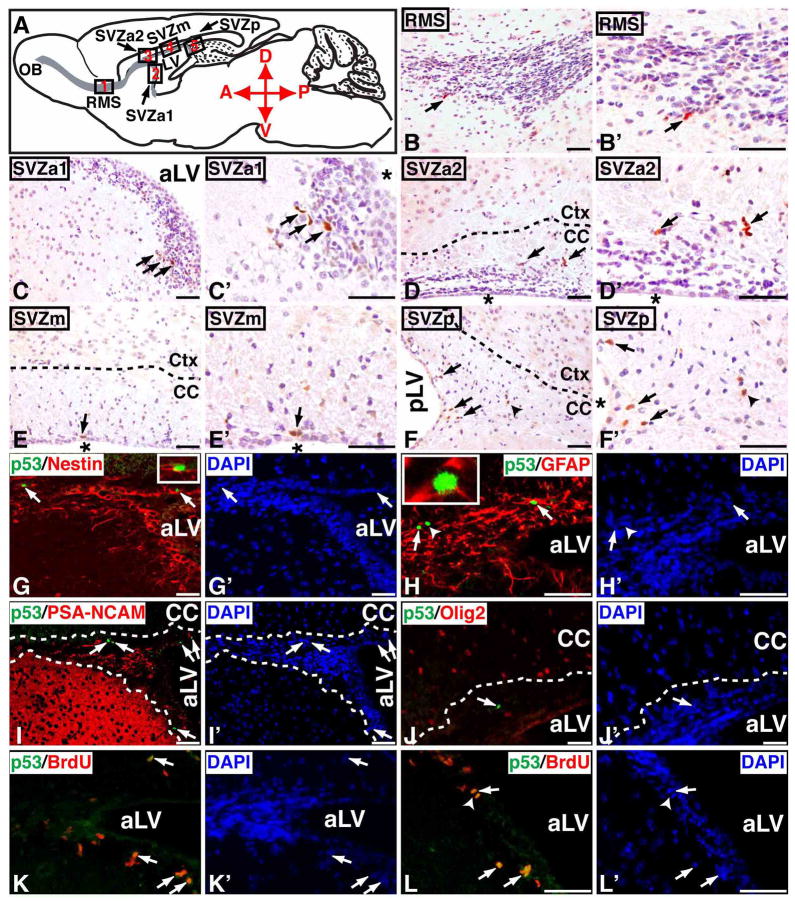
In brains with p53ΔE5-6 expression, about 80% of p53ΔE5-6-positive cells were exclusively identified in the adult SVZ niche including the anterior (SVZa1 and SVZa2), medial (SVZm), and posterior SVZ (Figure 6A, 6C-F′). Approximately 14% of p53ΔE5-6-positive cells were found in the RMS (Figure 6B, B′), and a minor population was identified in the areas of the corpus callosum just outside the SVZ (Figure 6F, F′, arrowhead). Similar to the SVZ-B stem cells, all the p53ΔE5-6-positive cells expressed Nestin, about 90% of which expressed GFAP, but not markers for lineage-restricted cells (e.g. PSA-NCAM and Olig2) (Figure 6G-J′) (Merkle and Alvarez-Buylla, 2006; Stiles and Rowitch, 2008). A small population of the p53ΔE5-6-positive cells in the SVZ were GFAP-negative, which resemble the transit-amplifying SVZ-C progenitor cells (arrowhead, Figure 6H, H′) (Merkle and Alvarez-Buylla, 2006). Together, these results suggest that a minor population of the adult SVZ cells, particularly the SVZ-B stem cells with the lineage marker expression pattern of Nestin+/GFAP+/PSA-NCAM−/Olig2−/NG2−/CD133−, are the first and the primary cell types that accumulate a detectable p53ΔE5-6 protein expression in the young adult brain. However, it is worth noting that these p53ΔE5-6-positive cells morphologically were not significantly different from the surrounding SVZ cells (Figure 6).
Figure 6. A minor population of SVZ stem and progenitor cells exhibit a detectable mutant p53ΔE5-6 protein expression in young adult brain.
(A) Schematic drawing of the sagittal plane of the adult mouse brain. Except for the dentate gyrus of the hippocampus, all the Nestin+ cells in the adult brain are restrictively located in the SVZ and RMS colored in grey. Different parts of the RMS and SVZ are shown in five designated boxed areas. At 2 months of age, most of the p53ΔE5-6-positive cells (arrows, B to F′) are identified in the adult neurogenic areas, which include the RMS (B, B′), the anterior SVZ (SVZa1, C, C′ and SVZa2, D, D′), medial SVZ (SVZm, E, E′), and posterior SVZ (SVZp, F, F′). The arrowhead in (F, F′) point to a p53ΔE5-6-positive cell in the adjacent corpus callosum. Sections of the anterior SVZ from 2-month-old p53ΔE5-6 mice were stained by anti-p53/anti-Nestin (G) and DAPI (G′); anti-p53/anti-GFAP (H) and DAPI (H′); anti-p53/anti-PSA-NCAM (I) and DAPI (I′); anti-p53/anti-Olig2 (J) and DAPI (J′); and anti-p53/anti-BrdU (K, L) and DAPI (K′, L′). Arrows in (G-L′) point to the p53ΔE5-6-positive cells in the SVZ. An arrowhead in (H, H′) points to a p53ΔE5-6-positive/GFAP-negative progenitor cell. The insets in (G, H) show the high-magnification views of the p53ΔE5-6-positive cells expressing Nestin and GFAP, respectively. Arrowheads in (L, L′) point to a p53ΔE5-6-positive cells during mitosis. aLV and pLV, anterior and posterior lateral ventricle; “*”, lateral ventricle. Scale bar, 25 μm.
To assess whether these p53ΔE5-6-positive SVZ cells manifested other tumor cell characteristics, we examined the percentage of proliferating cells in the p53ΔE5-6-positive and -negative cellular compartments. As revealed by BrdU staining, about 33% of the p53ΔE5-6-negative stem or progenitor cells were proliferating in the mutant SVZ (370/1112, n = 4 brains). Strikingly, nearly 77% of the p53ΔE5-6-positive SVZ cells were proliferating (36/47, n = 7 brains, p53high vs p53negative, p < 0.0001) (Figure 6K-L′). Because most of the p53ΔE5-6-positive cells were immunohistochemically identified as SVZ-B stem cells that are relatively a quiescent cell population (Merkle and Alvarez-Buylla, 2006), these results suggest that the expression of mutant p53ΔE5-6 proteins may identify a subpopulation of SVZ stem cells as incipient tumor cells in otherwise relatively normal brains at this age.
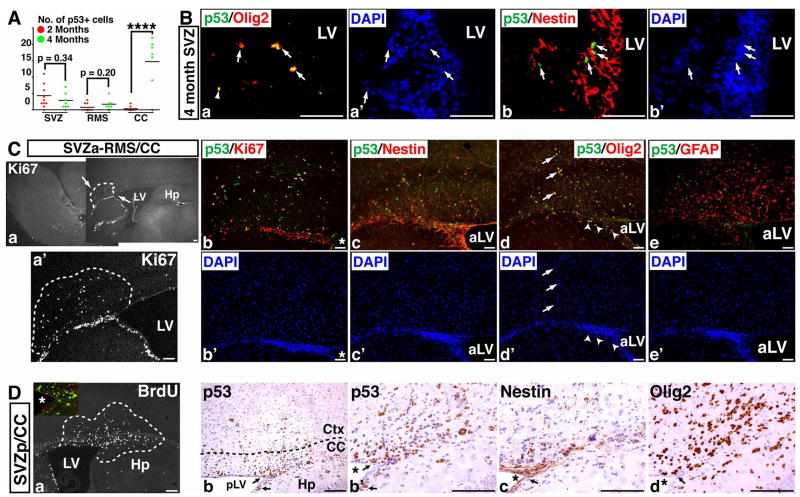
A possible lineage relationship between p53ΔE5-6-positive cell populations
The hyperproliferative phenotypes of p53ΔE5-6-positive SVZ-B stem cells in the 2-month-old brain raise the possibility that these cells are the cell-of-origin for the p53ΔE5-6-positive SVZ-C*-like glioma precursors identified in the corpus callosum of the older mice. To study a possible lineage relationship between these two p53ΔE5-6-positive cell populations, we quantified the number of p53ΔE5-6-positive cells in mutant brains at these two ages. Except for the corpus callosum, the number of p53ΔE5-6-positive cells in mutant SVZ or RMS at 4 months was not significantly increased compared to those at younger age (Figure 7A). However, in contrast to the p53ΔE5-6-positive cells that rarely expressed Olig2 in the 2-month-old SVZ, over 60% of the p53ΔE5-6-positive cells in the SVZ and RMS at 4 months acquired Olig2 expression and exhibited the same lineage markers expression pattern as that of the p53ΔE5-6-positive glioma precursors (Figure 7B). These results suggest that similar to normal stem cells, the p53ΔE5-6-positive SVZ-B stem cells can differentiate into Olig2+ SVZ-C* progenitor cells, which may function as an intermediate cell type between SVZ-B stem cells and Olig2+ SVZ-C*-like glioma precursors. In support of such transition, most of the proliferating clusters were identified in the areas immediately adjacent to the adult SVZ neurogenic niches, some of which directly involved the cells in the SVZ (Figure 7C, D). More importantly, within these proliferating clusters, the p53ΔE5-6-positive cells inside and outside the SVZ stem cell niche expressed the same lineage markers as the Olig2+ SVZ-C* progenitors regardless of the positions at the anterior SVZ (Figures 7C, S9) or posterior SVZ (Figure 7D).
Figure 7. A possible lineage relationship between p53ΔE5-6-positive SVZ-B stem cells and SVZ-C* progenitor-like glioma precursors.
(A) The quantification of p53ΔE5-6-positive cells in brains of mutant mice at ages of 2 months and 4 months. Each dot represents the quantification from each individual brain. p**** < 0.0001. (B) Sections from a 4-month-old mutant brain were stained with anti-p53/anti-Olig2 (a) and DAPI (a′); and anti-p53/anti-Nestin (b) and DAPI (b′), in which arrows point to p53ΔE5-6-positive cells in the SVZ. (C) One representative mutant brain has proliferating clusters (marked by dashed lines) in the corpus callosum directly associated with the anterior SVZ (a, a′). Sections from this proliferating cluster were stained with anti-p53/anti-Ki67 (b) and DAPI (b′); anti-p53/anti-Nestin (c) and DAPI (c′); anti-p53/anti-Olig2 (d) and DAPI (d′); and anti-p53/anti-GFAP (e) and DAPI (e′). Arrows and arrowheads in (d, d′) point to p53ΔE5-6-positive Olig2+ SVZ-C*-like glioma precursors located in the corpus callosum and SVZ, respectively. (D) Adjacent sections from one proliferating cluster identified in the posterior SVZ (a) were stained with anti-p53 (b, b′), anti-Nestin (c), and anti-Olig2 antibodies (d). Arrows in (b to d) point to p53ΔE5-6-positive glioma precursors inside the SVZ. The inset in (a) shows the high-magnification view of proliferating p53ΔE5-6-positive glioma precursors in the SVZ [BrdU(green)/p53(red)]. aLV and pLV, anterior and posterior lateral ventricle. “*”, lateral ventricle. Scale bar, 100 μm (Ca, Ca′, Da); 50 μm (B, Cb-e′, Db-d).
If the p53ΔE5-6-positive SVZ-B stem cells give rise to the p53ΔE5-6-positive Olig2+ SVZ-C*-like glioma precursors, both should be p53 deficient and thus can be labeled by Cre-mediated R26R-LacZ expression. To test this, we studied the R26R-LacZ expression in the p53ΔE5-6 brain. In contrast to most mature astrocytes, all the p53ΔE5-6-positive SVZ cells expressed β-gal in the mutant brains at 2 months, confirming our lineage marker analysis that these cells were SVZ stem cells or transit-amplifying progenitors (Figure 8A). Furthermore, all the p53ΔE5-6-positive glioma precursors could also be identified by β-gal expression within the proliferating clusters located in the corpus callosum of older mutant brains (Figure 8B, C). These results indicate that both p53ΔE5-6-positive SVZ-B stem cells and Olig2+ SVZ-C*-like glioma precursors are p53 deficient, supporting the notion that these neural stem and progenitor-like cells are the precursors for p53-deficient malignant glioma cells in this model. Finally, of the mutant brains identified with a proliferating cluster, one rare case contained p53ΔE5-6-positive glioma precursors in the neuronal layers of the OB (Figure 8Da, a′). Despite their presence in the migratory destination of neuroblasts, these p53ΔE5-6-positive glioma precursors expressed the same lineage markers as Olig2+ SVZ-C* progenitors (Figure 8Db-d). Thus, these observations indicate that some glioma precursors exhibit the cellular characteristics of migrating neuroblasts - another progeny of multipotent SVZ neural stem cells.
DISCUSSION
The role of p53 deficiency in gliomagenesis
This study demonstrates that a single p53 mutation can efficiently induce malignant astrocytic glioma formation in mice. In contrast, using the same hGFAP-cre driver as described here, we previously showed that the hGFAP-cre+;Nf1flox/flox mice exhibited no evidence of tumor formation in the brain, even in the p53 heterozygous background, despite that Nf1-deficient brain cells had activation of both MAPK and PI3K/Akt signaling pathways (Zhu et al., 2005a; Zhu et al., 2005b). In addition, p53 deficiency can cooperate with Nf1- or Pten-deficiency to induce malignant astrocytic gliomas in mice (Reilly et al., 2000; Zheng et al., 2008; Zhu et al., 2005a). These results reveal a central role of p53 deficiency in gliomagenesis. Surprisingly, analysis of brains of mutant mice at younger ages reveals that p53 deficiency provides no significant growth advantage to brain cells but rather appears to induce pleiotropic accumulation of cooperative oncogenic alterations in Rb and RTK pathways. One intriguing finding of this study is that a sizable fraction of p53ΔE5-6 gliomas exhibited no activation of MAPK (~50%) or PI3K/Akt (~20%) pathways. Furthermore, the majority of the p53ΔE5-6 gliomas exhibited increased instead of decreased levels of Nf1 protein expression. Similar results were also observed in human GBMs (Gutmann et al., 1996; Mawrin et al., 2003; Wang et al., 2004). Together, these observations demonstrate that p53-deficient brain cells are highly plastic with abilities to cooperate with diverse downstream oncogenic alterations to drive glioma development (Figure 8E) and raise the concern of whether inhibitors for the MAPK and PI3K/AKT pathways will be effective to treat human GBM.
Cell-of-origin for malignant astrocytic glioma
Murine models have been widely used to study the cell-of-origin of a cancer. Ideally, an oncogenic mutation can be targeted into the cells at various developmental stages of a specific cell lineage. However, this approach is often limited in the CNS due to a lack of well-defined cell-lineage specific promoters. For example, despite being one of the best characterized and the most widely used CNS stem/progenitor cell marker, Nestin is expressed not only in multipotent SVZ-B neural stem cells and SVZ-C transit-amplifying progenitors, but also in lineage-restricted SVZ-A neuroblasts in the adult brain (Doetsch et al., 1997). Moreover, if a deletion-based mutational strategy (e.g. Cre/loxP system) is employed to drive tumor formation, lineage-restricted SVZ-OPC glial progenitors and oligodendrocytes, albeit expressing no Nestin, will inherit the same mutation(s) that are targeted in Nestin+ SVZ-B and C cells (Menn et al., 2006). Thus, with currently available technologies, it is not feasible to specifically target a genetic mutation(s) in the multipotent CNS stem/progenitor cells.
Compared to currently available p53-associated glioma models, one feature of our model is that mutant p53ΔE5-6 proteins are accumulated to a detectable level in all stages of glioma cells but not in normal brain cells. Combining with proliferation, lineage marker expression and morphological analysis, this p53 marker allowed us to study glioma precursors at the single-cell level during the earliest stages of tumor development. Although advanced-stage p53ΔE5-6 gliomas are extremely heterogeneous histopathologically and molecularly, the p53ΔE5-6-positive glioma precursors are remarkably homogeneous and exhibit a lineage marker expression pattern reminiscent of Olig2+ SVZ-C* transit-amplifying progenitor cells. Moreover, despite that the p53ΔE5-6 mutation was targeted to diverse CNS cell populations throughout the brain, the p53ΔE5-6-positive glioma precursors were exclusively identified in the two brain areas – corpus callosum and OB, which are the migratory destinations of the two differentiated progeny of the multipotent SVZ stem cells. Therefore, the lineage marker expression pattern and anatomical location of glioma precursors suggest that the p53ΔE5-6-positive malignant glioma cells may arise from multipotent SVZ stem cells and/or transit-amplifying progenitors. Consistently, we demonstrate that the SVZ-B stem cells and SVZ-C progenitors are the earliest cell types that accumulate a detectable p53ΔE5-6 protein expression in the brain of younger mutant mice. Based upon these results, we propose a model for the role of neural stem cells and transit-amplifying progenitors in p53-mediated gliomagenesis: (1) the SVZ-B stem cells are most susceptible to accumulation of oncogenic alterations, as evidenced by high levels of mutant p53ΔE5-6 expression; (2) the glioma-initiating cells are the Olig2+ SVZ-C* progenitor-like cells at the earliest stages of tumor development; (3) the majority of differentiation-stalled Olig2+ SVZ-C* progenitor-like cells migrate as SVZ-OPCs to the corpus callosum, and a minor population migrate as SVZ-A neuroblasts to the RMS and OB; (4) p53ΔE5-6-positive SVZ-C*-like glioma precursors can use diverse cooperative oncogenic alterations to form high-grade gliomas with a markedly high degree of heterogeneity (Figure 8E, F).
Although the expression of mutant p53 proteins provides a sensitive tool to monitor early-stage glioma cells at the single-cell level, the extremely low number of the p53ΔE5-6-positive cells in mutant brains makes it difficult to establish a definitive lineage relationship between the morphologically normal p53ΔE5-6-positive neural stem cells and glioma precursors. However, we obtain several independent lines of evidence supporting a lineage relationship between these two p53ΔE5-6-positive cell populations. First, p53ΔE5-6-positive cells in the SVZ are the only cells that exhibit measurable tumor cell characteristics - hyperproliferation, suggesting that these cells are the incipient glioma cells in otherwise normal brains at 2 months. Second, we identify an intermediate cell type between p53ΔE5-6-positive SVZ-B stem cells and Olig2+ SVZ-C*-like glioma precursors in the 4 month-old mutant brain. These p53ΔE5-6-positive intermediate cells resemble Olig2+ SVZ-C*-like glioma precursors, but are located in the SVZ. Consistently, in some proliferating clusters, expansion of the p53ΔE5-6-positive SVZ-C*-like glioma precursors can be found in the SVZ and corpus callosum. Finally, both p53ΔE5-6-positive SVZ-B stem cells and Olig2+ SVZ-C*-like glioma precursors in the corpus callosum can be marked by Cre-mediated R26R-LacZ expression, indicating that these two cell populations are genetically p53 deficient.
Murine GBM models and clinical implication
Malignant astrocytic gliomas observed in the p53ΔE5-6 model recapitulate genetic, histopathological and molecular features of human GBM (Furnari et al., 2007; Louis et al., 2007). The utility of the p53ΔE5-6 model is further illustrated by the cooperation of the p53ΔE5-6 mutation with an Nf1 heterozygous mutation, which produces a fast-growing GBM model, albeit at the expense of loss of histopathological and molecular heterogeneity. These results suggest that cooperative oncogenic alterations influence the histological features of advanced-stage malignant glioma cells, despite arising from remarkably homogeneous progenitor-like cells. Finally, given that the Nf1 heterozygous mutation has no tumor inducing activity in the brain, the robust GBM phenotypes observed in the p53ΔE5-6;Nf1−/− mice indicate that the p53ΔE5-6 mice provide a sensitive genetic background for glioma development. With the advent of the Cancer Genome Atlas (TCGA) initiative, this important feature will be particularly valuable to validate newly identified glioma genes and to generate a series of GBM models for preclinical testing of therapies that target more specific oncogenic pathways.
EXPERIMENTAL PROCEDURES
Control and conditional CKO1, CKO2 and CKO3 mutant mice
The mutant mice that harbor a p53 null mutation (p53KO) (Jacks et al., 1994) and a floxed p53 allele (p53flox) (Lin et al., 2004) were maintained on the 129Svj/C57Bl6 hybrid genetic background. The hGFAP-cre transgenic mice were originally generated on the FVB background (Zhuo et al., 2001) and have subsequently been backcrossed to the 129Svj/C57Bl background for more than 5 generations (Zhu et al., 2005a). Therefore, CKO1 and CKO2 mice analyzed in this study were maintained on approximately 99% 129Svj/C57Bl6 (49.5%/49.5%) and less than 1% FVB hybrid background. Many CKO1 and CKO2 mice analyzed in this study were littermates. Control mice were the pool of phenotypically indistinguishable mice with genotypes of hGFAP-cre-;p53flox/KO and hGFAP-cre-;p53flox/flox. Introduction of an Nf1 heterozygous mutation (Brannan et al., 1994) to the CKO2 background generated the CKO3 mice, which shared the same genetic background as CKO1 and CKO2 mice. All mice in this study were cared for according to the guidelines that were approved by the Animal Care and Use Committees of the University of Michigan at Ann Arbor.
Histological grading of malignant astrocytic gliomas in CKO1, CKO2 and CKO3 mice
Histopathology of neoplasms that developed in these mice had to meet diagnostic criteria of the World Health Organization (WHO) for classification of tumors of the CNS (Louis et al., 2007). Tumors with neoplastic astrocyte-like cells were classified as astrocytic gliomas. Astrocytic features include extension of cellular processes from neoplastic cells. These vary from long and thick to thin and short. They are highlighted with H&E and GFAP stains. Variable nuclear shapes, particularly elongated, bent and dark staining nuclei characterize neoplastic astrocytes. Their perinuclear cytoplasm is highly variable: from imperceptible to substantial and appearing to push the nucleus to the perimeter of the cell. Detailed histopathological grading of these murine malignant astrocytic gliomas is provided in the Supplemental Materials.
Quantification and Statistical Analysis
To quantify the number of immunohistochemically labeled cells, anatomically comparable sections from control and mutant brains were visualized under 40X magnification using an Olympus BX51 microscope. Images were captured and subjected to double-blinded counting. Lengths and areas were measured by the NIH software, ImageJ. The number of cells was quantified and statistical analysis was carried out using two-tailed Student’s t-test. p < 0.05 was considered to be statistically significant. To compare the proportions from two independent cell populations and to determine if they are significantly different from one another, the number of specific cell types were quantified and subjected to z-test for proportions. These results were also confirmed by Chi-Square test. p < 0.05 was considered to be statistically significant.
Detection of the p53ΔE5-6-positive cells in the mutant brains at early stages of tumor development
The brain of the p53ΔE5-6 mice at ages of 2 months, 4 to 4.5 months, and 6 to 9 months were dissected and examined for enlargement under a dissecting microscope. Only the grossly normal mutant brains were used to study glioma cells in early stages. The mutant brains were evenly cut into four pieces along the midline (2 pieces for each brain hemisphere) and subsequently were processed for paraffin-embedded sections. Each brain was serially sectioned at sagittal planes and four 5-μm paraffin sections were put on a single slide. At 4 independent saggittal planes, we sampled a total of 1760-μm brain thickness along the midline to lateral hemisphere for each mutant brain. Serial sections were stained with an anti-p53 antibody and visualized with DAB staining. We examined p53ΔE5-6-positive cells in these sections under a light microscope (J.Y. and Y.Z.). Once we determined the restricted distribution of p53ΔE5-6-positive cells in the corpus callosum, SVZ/RMS and their associated areas of mutant brains, we focused on the brain areas that contain the SVZ and RMS of the lateral ventricle. Double-immunofluorescence labeling was performed on the adjacent sections to determine the identity of the p53ΔE5-6-positive cells.
Histological, molecular, genetic, and statistical analyses
Detailed descriptions for the experimental procedures are provided in the Supplemental Materials.
Supplementary Material
Acknowledgments
We thank L. Liu, L. Cregan, M. Best, and M. Hancock for technical assistance; members of the Zhu lab for support; Dr. L. Parada for support at early stages of the project; A. Messing for providing hGFAP-cre+ mice; C. Stiles and B. Novitch for Olig2 antibodies; and Drs. E. Fearon, S. Morrison, and L. Chang for comments. This work is supported by grants from the NIH (1R01 NS053900), Brain Tumor Society, and the Comprehensive Cancer Center and Biological Sciences Scholars Program of the University of Michigan (Y.Z.). Y.Z. is an American Cancer Society research scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues [published erratum appears in Genes Dev 1994 Nov 15;8(22):2792] Genes & Development. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Garcia-Verdugo JM, Casaccia-Bonnefil P. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26:1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Giordano MJ, Mahadeo DK, Lau N, Silbergeld D, Guha A. Increased neurofibromatosis 1 gene expression in astrocytic tumors: positive regulation by p21-ras. Oncogene. 1996;12:2121–2127. [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Kitayner M, Rozenberg H, Kessler N, Rabinovich D, Shaulov L, Haran TE, Shakked Z. Structural basis of DNA recognition by p53 tetramers. Molecular cell. 2006;22:741–753. doi: 10.1016/j.molcel.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-oncology. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Lee KF, Nikitin AY, Hilsenbeck SG, Cardiff RD, Li A, Kang KW, Frank SA, Lee WH, Lee EY. Somatic mutation of p53 leads to estrogen receptor alpha-positive and -negative mouse mammary tumors with high frequency of metastasis. Cancer Res. 2004;64:3525–3532. doi: 10.1158/0008-5472.CAN-03-3524. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumors of the Central Nervous System. Lyon: IARC; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Mawrin C, Diete S, Treuheit T, Kropf S, Vorwerk CK, Boltze C, Kirches E, Firsching R, Dietzmann K. Prognostic relevance of MAPK expression in glioblastoma multiforme. International journal of oncology. 2003;23:641–648. [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Alvarez-Buylla A. Neural stem cells in mammalian development. Curr Opin Cell Biol. 2006;18:704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science. 2008 doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects [In Process Citation] Nat Genet. 2000;26:109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. The New England journal of medicine. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain [letter] Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- TCGA Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Laboratory investigation; a journal of technical methods and pathology. 2004;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005a;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005b;132:5577–5588. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.