Abstract
Many aspects of synaptic development, plasticity, and neurotransmission are critically influenced by NMDA-type glutamate receptors (NMDARs). Moreover, dysfunction of NMDARs has been implicated in a broad array of neurological disorders, including schizophrenia, stroke, epilepsy, and neuropathic pain. Classically, NMDARs were thought to be exclusively postsynaptic. However, substantial evidence in the last 10 years demonstrates that NMDARs also exist presynaptically, and that presynaptic NMDA receptors (preNMDARs) modulate synapse function and have critical roles in plasticity at many synapses. Here we review current knowledge of the role of preNMDARs in synaptic transmission and plasticity, focusing on the neocortex. We discuss the prevalence, function, and development of these receptors, and their potential modification by experience and in brain pathology.
Keywords: Presynaptic NMDA Receptors, timing-dependent long-term depression, spike timing-dependent plasticity, cortex, development
Introduction
NMDA-type receptors (NMDARs) are ionotropic glutamate receptors that act as non-specific cation channels which are permeable to sodium, calcium, and potassium. These receptors are hetero-tetrameric transmembrane proteins which contain two NR1 (NMDA receptor 1) and two NR2 or NR3 subunits (Dingledine and others 1999). NMDARs exhibit a voltage-dependent block by extracellular magnesium, causing them to be outwardly rectifying; however, this block varies in strength depending on the type of NR2 or NR3 subunit(s) expressed (Cull-Candy and others 2001; Monyer and others 1992; Sasaki and others 2002). NMDARs were first discovered as postsynaptic receptors at glutamatergic synapses, and have since been shown to be involved in many aspects of synaptic transmission, dendritic integration, synaptic and neuronal maturation, and plasticity throughout the brain. Given the diverse roles of NMDARs, it is not surprising that NMDAR dysfunction is thought to contribute to neurological and psychiatric disorders, including neurodegenerative conditions, stroke, epilepsy, neuropathic pain, and schizophrenia (Cull-Candy and others 2001; Kristiansen and others 2007; Lau and Zukin 2007; Meldrum 1994). As a result, NMDARs are targets for many therapeutic drugs (Chen and Lipton 2006).
Recently, NMDARs have joined the list of ionotropic receptors that can also function presynaptically (briefly discussed below). Presynaptic NMDARs (preNMDARs) may be molecularly and pharmacologically distinct from classical postsynaptic NMDARs. PreNMDARs share some functions with postsynaptic NMDARs such as involvement in long-term synaptic depression (LTD) (although by a distinct mechanism than postsynaptic NMDARs). PreNMDARs also have some novel functions such as regulation of presynaptic release probability and short-term plasticity. It is possible that some of the cellular- and network-level functions previously attributed to postsynaptic NMDARs are, in fact, mediated by preNMDARs.
Here we summarize the growing understanding of cortical preNMDARs. In an effort to elucidate the possible mechanisms by which preNMDARs modulate synaptic function, we briefly compare cortical preNMDAR activity with preNMDAR function at other central synapses (see Table 1 and How do preNMDARs regulate release?). We argue that without knowing the specific contribution of preNMDARs versus postsynaptic NMDARs to neurotransmission and plasticity, it is impossible to understand NMDAR regulation of cortical development, or to rationally design pharmacotherapies for NMDAR-related diseases. Specific involvement of preNMDAR in diseases is now being considered, including in epilepsy (Yang and others 2006) and fetal alcohol spectrum disorder (Valenzuela and others 2008).
Table 1.
Effects of endogenous or exogenous preNMDAR activation on synapses
| CNS region | Synaptic projection | NR2 Subunit | Spont. release | Evoked release | Plasticity | Ages | Refs |
|---|---|---|---|---|---|---|---|
| Visual cortex (V1) | L4 to L2/3 | nd | Promotes | nd | req. for tLTD | P7–20; lost @ P23 | (Corlew and others 2007; Li and Han 2007) |
| Ex. inputs to L4 pyr. | nd | Promotes | nd | nd | P7–20; lost @ P23 | (Corlew and others 2007) | |
| L5 pyr. to L5 pyr. | NR2B | Promotes | Promotes | req. for tLTD | P7–21; lost @ P23 | (Corlew and others 2007; Sjöström and others 2003) | |
| S1 | L4 to L2/3 | NR2B | Promotes | Promotes | req. for tLTD | P13–21 | (Bender and others 2006; Brasier and Feldman 2008; Rodríguez-Moreno and Paulsen 2008) |
| Entorhinal cortex | Ex. inputs to L2 pyr. | NR2B | Promotes | No change | nd | nd | (Berretta and Jones 1996; Woodhall and others 2001) |
| Ex. inputs to L5 pyr. | NR2B | Promotes | nd | nd | Lost by 5 months | (Woodhall and others 2001; Yang and others 2006) | |
| Hippocampus | CA3 to CA1 | NR2D/NR2B | Promote (<P5) | Promotes | nd | NR2D loss at P5 | (Mameli and others 2005; Suarez and Solis 2006; Suarez and others 2005) |
| Ex. inputs to granule cells | NR2B | Promotes | nd | nd | P10–22 | (Jourdain and others 2007) | |
| Lat. Amygdala | Cortical inputs | nd | nd | nd | req. for LTP | P21–28 | (Humeau and others 2003) |
| Cerebellum | Parallel fiber to Purkinje | nd | nd | Suppresses | req. for LTD | P18–26 | (Casado and others 2000, Casado and others 2002) |
| Interneuron to Purkinje | nd | Promotes | Promotes | nd | P11–14 | (Duguid and Smart 2004; Glitsch and Marty 1999) | |
| Spinal cord | Substance P afferents | nd | Directly triggers release | nd | Adult | (Liu and others 1997) | |
| Dorsal horn ex. afferents | nd | nd | Suppresses | nd | P6–12 | (Bardoni and others 2004) | |
Abbreviations: CNS, central nervous system; Ex, excitatory (glutamatergic); pyr, pyramidal neurons; L, cortical layer; Lat, lateral; nd, no data; req, required; S1, primary somatosensory cortex; Spont, spontaneous
Synapse regulation by presynaptic ligand-gated ion channels
Many neurotransmitters activate presynaptic receptors that modulate presynaptic function. Some of these presynaptic receptors are metabotropic receptors (e.g., GABAB receptors and metabotropic glutamate receptors) that can modulate neurotransmitter release via second messenger systems, affect presynaptic voltage-sensitive calcium channels, and/or directly alter the release machinery itself. Other presynaptic receptors are ligand-gated ion channels, including GABAA receptors, glycine receptors, kainate receptors, and most recently, NMDARs (Engelman and MacDermott 2004; Khakh and Henderson 2000; MacDermott and others 1999; Pinheiro and Mulle 2008). Particularly well characterized among these are the presynaptic kainate receptors, which are present at several synapse classes in neocortex and hippocampus (Contractor and others 2001; Lauri and others 2006; Pinheiro and others 2007; Sun and Dobrunz 2006), and the presynaptic glycine receptors, which are present at the Calyx of Held in the auditory brainstem (Awatramani and others 2005; Trussell 2002; Turecek and Trussell 2001). Activation of presynaptic ionotropic receptors generally enhances synaptic release, while presynaptic metabotropic receptors typically decrease release probability (Pinheiro and Mulle 2008; Vitten and Isaacson 2001), although this trend is not universal (Bardoni and others 2004; Casado and others 2000; Casado and others 2002). Heterogeneity in presynaptic receptor expression across synapses, including preNMDAR expression, contributes to the diversity of synapse function and plasticity in the CNS.
Physiological evidence for preNMDARs in cerebral cortex
Physiological evidence that preNMDARs regulate spontaneous synaptic transmission has been found at a wide array of cortical synapses. The first evidence was at glutamatergic synapses in layer 2 (L2) of entorhinal cortex, where bath application of the NMDAR antagonist APV decreased the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs), even when postsynaptic NMDARs had been previously blocked intracellularly. This suggested that non-postsynaptic, likely presynaptic NMDARs normally act to enhance spontaneous neurotransmitter release (Berretta and Jones 1996) (Box 1). Subsequently, NMDAR agonists were shown to enhance mEPSC frequency in both L2 and L5 of entorhinal cortex. This effect was blocked by the NR2B subunit-specific antagonist ifenprodil, suggesting that the relevant preNMDARs contain NR2B (Woodhall and others 2001). Similar effects of preNMDARs were subsequently observed at excitatory synapses in L2/3, L4, and L5 of the rodent primary visual cortex (Corlew and others 2007; Li and Han 2007; Li and others 2008; Sjöström and others 2003), CA1 of the hippocampus (Mameli and others 2005), dentate gyrus (Jourdain and others 2007), entorhinal cortex (Yang and others 2006), and in L2/3 of somatosensory cortex (Bender and others 2006; Brasier and Feldman 2008). In addition to the aforementioned studies in the neocortex and hippocampus, there is also compelling evidence for preNMDARs function in the spinal cord (Bardoni and others 2004; Liu and others 1997), cerebellum (Casado and others 2000; Casado and others 2002; Duguid and Smart 2004 ; Glitsch and Marty 1999 ; reviewed in Duguid and Sjöström 2006), and amygdala (Humeau and others 2003). Therefore, there is an abundance of evidence that preNMDARs function widely throughout the central nervous system and may qualify as a general mechanism of modulating spontaneous neurotransmitter release.
Box 1.
In addition to affecting spontaneous release, it is now clear that preNMDARs also modulate evoked (action potential-driven) transmitter release. For example, blockade of presumptive preNMDARs decreases unitary synaptic responses between L5 pyramidal cells in primary visual cortex (Sjöström and others 2003) (Fig. 1) and at synapses between L4 and L2/3 pyramids (L4-L2/3 synapses) in primary somatosensory cortex (Bender and others 2006; Brasier and Feldman 2008). Paired pulse facilitation increases and coefficient of variation (CV)−2 decreases during this effect, reflecting decreased presynaptic release probability. These experiments were performed with postsynaptic NMDARs blocked, indicating that the relevant NMDARs that regulate evoked release are non-postsynaptic, presumably preNMDARs. PreNMDAR modulation of evoked release is only evident during high-frequency burst stimulation in L5 of primary visual cortex, but occurs with sparse stimulation (two action potentials at 33 ms interspike interval) at L4-L2/3 synapses in primary somatosensory cortex (Brasier and Feldman 2008; Sjöström and others 2003). These data indicate that preNMDARs are active during conditions of modest synaptic activity in acute slices, and this endogenous activation of preNMDARs helps to maintain higher probabilities of evoked neurotransmitter release, compared to release probability when preNMDARs are blocked. Thus, preNMDARs enhance the probability of both spontaneous and evoked neurotransmitter release.
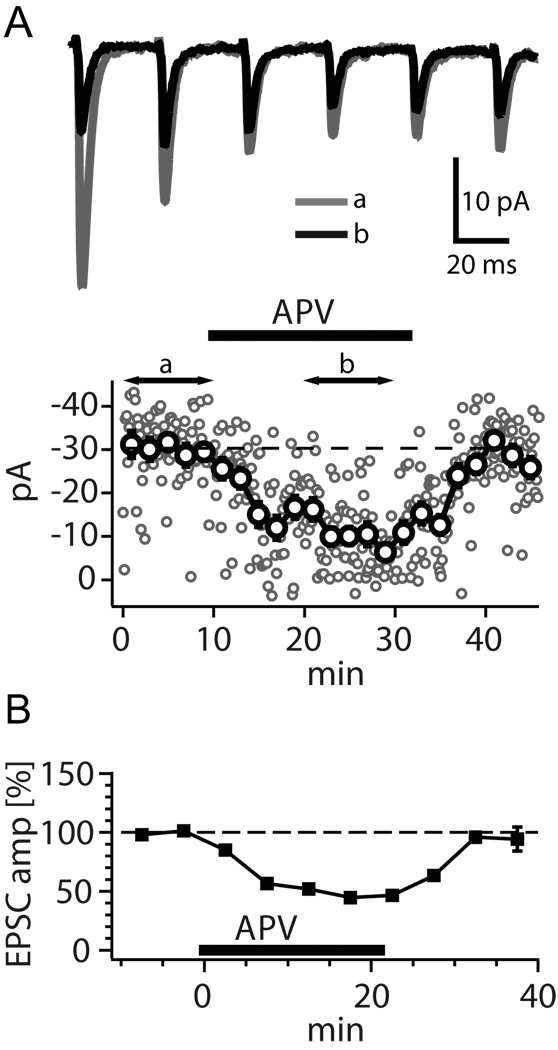
Fig. 1. PreNMDARs can enhance action potential-evoked neurotransmission.
Evidence that preNMDARs modulate evoked transmitter release between pairs of L5 pyramidal neurons in primary visual cortex. (A) With postsynaptic NMDARs blocked by voltage-clamping the postsynaptic neuron at −90mV, preNMDAR blockade decreases EPSC amplitude and short-term synaptic depression. Sample recording showing averaged responses before (a) and during (b) application of APV. (B) APV wash-in reversibly decreased responses to 30 Hz spiking between pairs of L5 neurons recorded in voltage-clamp at −90 mV. Reproduced with permission from Sjöström and others (2003).
Anatomical evidence for presynaptic NMDARs in cerebral cortex
The physiological findings described above indicate that non-postsynaptic, presumably presynaptic, NMDARs exist, which influence transmitter release. While presynaptic localization of NMDARs is the simplest explanation for the effects on presynaptic transmitter release, it remains possible that the relevant receptors are actually on another nearby neuron or glial cell, which signals to the presynaptic terminal via an unknown mechanism. Thus, an important line of evidence indicating that putative preNMDARs are actually localized on presynaptic terminals comes from electron microscopy (EM) studies indicating that NMDAR subunits are physically present at cortical presynaptic terminals (Box 2). In 1994, Aoki and colleagues observed immunoperoxidase labeling of the NMDAR subunit NR1 in axons and presynaptic terminals in primary visual cortex from 30-day old (P30) rats (Aoki and others 1994). Sparse labeling for the NR2B subunit has also been observed in presynaptic terminals from neocortex of 5–6 month-old rats (Charton and others 1999; DeBiasi and others 1996). Using immunogold labeling, NMDAR protein has also been found in presynaptic terminals of the hippocampal dentate gyrus (Jourdain and others 2007). Together these studies confirm the presence of NMDAR protein at presynaptic cortical terminals. In addition to the neocortex (Aoki and others 1994; Charton and others 1999; Corlew and others 2007; DeBiasi and others 1996; Fujisawa and Aoki 2003) and hippocampus (Charton and others 1999; Jourdain and others 2007; Siegel and others 1994), preNMDARs have also been observed using EM in the spinal cord (Liu and others 1994; Lu and others 2005) and amygdala (Farb and others 1995; Pickel and others 2006).
Box 2.
In EM studies, NMDAR immunostaining in presynaptic terminals is often sparse, and substantially less intense than postsynaptic staining. This suggests a relatively low number of preNMDARs, at least in rats > P25, when most EM studies have been performed. (See Developmental regulation of preNMDARs, below, for evidence that preNMDAR expression is substantially higher in young animals.) Another reason for sparse staining may be selective expression of preNMDARs at specific classes of excitatory terminals. Consistent with this idea, physiological evidence reveals functional preNMDARs at ascending L4-L2/3 synapses onto L2/3 pyramidal cells in somatosensory cortex, but not at horizontal, L2/3-L2/3 synapses onto the same postsynaptic neurons, or on local L4-L4 synapses made by the same presynaptic neurons (Brasier and Feldman 2008) (Fig. 2). This input- and target-specific expression of preNMDARs parallels synapse-specific expression of other presynaptic metabotropic and ionotropic receptors (Scanziani and others 1998; Sun and Dobrunz 2006), and may be a general mechanism for establishing synapse-selective release properties. The molecular mechanisms by which preNMDARs and other presynaptic receptors are targeted to a specific subset of terminals are not known, and there appear to be differences in preNMDAR expression between cortical regions (Brasier and Feldman 2008; Corlew and others 2007).
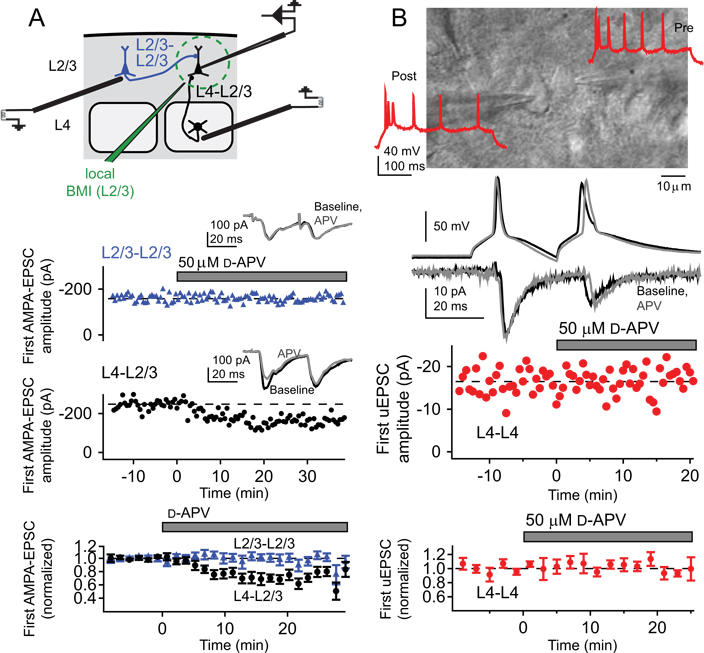
Fig. 2. Synapse-specific expression of preNMDARs.
PreNMDARs decrease synaptic strength and increase paired-pulse ratios at layer (L) 4-L2/3 synapses, but not at L2/3-L2/3 synapses or at L4-L4 synapses in the rodent somatosensory cortex. (A) Recording set-up for these experiments. Top: Representative experiment testing the effect of D-APV on AMPA-EPSCs evoked on the L2/3 cross-columnar pathway (L2/3-L2/3) and on L4-L2/3 inputs to the same postsynaptic cell. Amplitude of the first AMPA-EPSC on each pathway. Insets: Pairs of AMPA-EPSCs before (black) and during (grey) D-APV application. Bottom: Mean effect of D-APV on first AMPA-EPSC amplitude for L2/3-L2/3 inputs (triangles) and simultaneously measured L4-L2/3 inputs (circles). Bars represent population means (* = p < 0.05). (B) Differential interference contrast image of example synaptically coupled L4 excitatory cells, with regular-spiking pattern for these cells. Postsynaptic EPSCs elicited by a pair of presynaptic spikes before (black) and after (grey) 50µM D-APV application for the regular-spiking pair above. Lack of effect of D-APV on amplitude of the first uEPSC for one representative cell pair. Mean effect of D-APV application on first uEPSC amplitude. Reproduced with permission from Brasier and Feldman (2008).
How do preNMDARs regulate release?
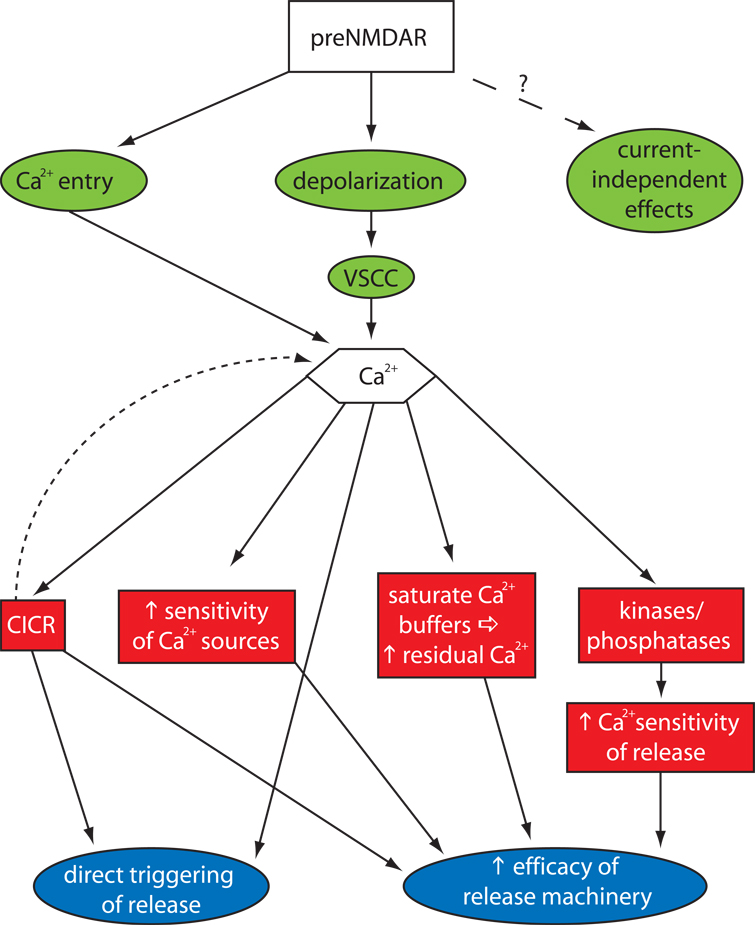
NMDARs generate depolarization and are permeant to calcium, either of which could enhance synaptic release probability via calcium-mediated signaling pathways. Which of these pathways is most relevant, and what downstream signaling events lead to modulation of release, are not known. Many potential mechanisms exist, and it is even possible that preNMDARs signal through an unknown, non-voltage dependent, non-calcium dependent mechanism. Here we consider several broad signaling motifs that may be involved in preNMDAR-mediated enhancement of transmitter release (Fig. 3).
Fig. 3. Schematic of possible mechanisms for preNMDAR-enhancement of neurotransmitter release.
There are three potential signals (green) emanating directly from the preNMDAR that could modulate release: direct Ca2+ entry, depolarization leading to the opening of VSCCs, and current-independent effects. The two ultimate mechanisms (blue) for expression of increased release are direct triggering of release by Ca2+ signals, or indirect signaling that changes the probability of release. Signaling to one of these ends could be carried out directly by Ca2+, or by several different intermediates (red): an amplified Ca2+ signal through calcium-induced calcium release (CICR), kinases/phosphatases that alter future Ca2+ entry, or increased Ca2+ -sensitivity of release machinery.
Direct depolarization of the terminal
It may be that preNMDARs act by causing depolarization of the presynaptic terminal. For example, at the Calyx of Held, activation of presynaptic ionotropic glycine receptors causes subthreshold depolarization of the terminal, which results in modest calcium influx through voltage-sensitive calcium channels (VSCCs), which in turn increases the probability of release to a subsequent action potential (Awatramani and others 2005). Such depolarization of the presynaptic terminal may be a general mechanism by which presynaptic ionotropic receptors, including preNMDARs, enhance release probability (Engelman and MacDermott 2004).
Calcium influx through preNMDARs
Calcium influx through preNMDARs, rather than depolarization, could be the initial trigger for modulation of release. Recently, preNMDAR enhancement of spontaneous miniature inhibitory post-synaptic current (mIPSC) frequency at synapses onto cerebellar Purkinje cells has been shown to be independent of VSCCs, consistent with a direct effect of calcium through preNMDARs (Glitsch 2008). However, whether cortical preNMDARs act independent of VSCCs remains unknown.
The end result of either preNMDAR-mediated depolarization of the terminal or direct calcium entry would be an increase in presynaptic Ca2+. This Ca2+ may then act directly on release machinery, for example by activating synaptotagmin and triggering vesicle fusion. Alternatively, Ca2+ may act to increase release probability via one of several more indirect pathways. First, Ca2+ may enhance the function of other calcium sources to facilitate subsequent transmitter release. For example, increasing the association of presynaptic vesicle proteins with VSCCs could prime transmitter-containing vesicles so that subsequent VSCC activation more efficiently triggers transmitter release (Catterall 1998; Kim and Catterall 1997). Second, the Ca2+ signal could act by partially saturating endogenous calcium buffers, which can lead to an increase in free calcium when VSCCs open during a subsequent action potential (Felmy and others 2003). Third, many protein kinases and phosphatases depend on calcium and could be activated by preNMDARs to modify function of various presynaptic ion channels or biochemical pathways, even including modifications of the release machinery itself. In support of this idea, postsynaptic NR2B–containing NMDARs have been found to closely associate with calcium/calmodulin-dependent protein kinase II (Strack and others 2000); if such association exists for preNMDARs, calcium-dependent kinase activity would be even more plausible. Indeed, preNMDARs at the cerebellar parallel fiber to Purkinje synapse have been proposed to activate presynaptic NO synthase and trigger an anterograde NO signal which controls LTD at this synapse (Casado and others 2000; Casado and others 2002), although whether the relevant NMDARs are truly presynaptic has been questioned (Shin and Linden 2005). Finally, indirect signaling may occur through Ca2+ release from internal stores. Evidence for this pathway is found in presynaptic inhibitory terminals onto cerebellar Purkinje cells, where calcium influx through preNMDARs triggers calcium-induced calcium release (CICR) via presynaptic ryanodine receptors, resulting in depolarization-induced potentiation of inhibition (Duguid and Smart 2004). For a summary of preNMDAR action in the central nervous system, see Table 1.
The dizzying array of possible mechanisms by which preNMDARs might alter neurotransmission at different central synapses highlights the diverse roles they could play. Which putative mechanisms are involved in their function at different synapses is likely to be an important question over the next few years. Whether similar mechanisms act at different synapses or whether each class of preNMDAR-expressing synapse has a unique set of mechanisms that underlie the enhancement of transmitter release is a crucial question to understanding the general principles that regulate synaptic transmission.
Endogenous activation of preNMDARs
To conduct ionic current, most NMDAR subtypes require glutamate binding, binding of glycine (or D-serine) at the glycine site, and depolarization to relieve voltage-dependent magnesium block. How these requirements are met for preNMDARs is not yet clear. The first issue is what is the source of glutamate that activates preNMDARs? Cortical preNMDARs contain the NR2B subunit (Brasier and Feldman 2008; Li and others 2008; Sjöström and others 2003; Woodhall and others 2001; Yang and others 2006), which confers high affinity for glutamate (Laurie and Seeburg 1994; Priestley and others 1995). This opens the possibility that low ambient levels of glutamate might tonically activate cortical preNMDARs. Such tonic activation has been observed for postsynaptic NMDARs in slice preparations (Cavelier and Attwell 2005; Le Meur and others 2007; Sah and others 1989), but see (Herman and Jahr 2007). Tonic activation of preNMDARs by ambient glutamate is indicated by studies in which NMDAR antagonists decreased mEPSC frequency in slices in which all spiking activity was blocked by TTX (Berretta and Jones 1996; Corlew and others 2007; Li and Han 2007; Li and others 2008; Sjöström and others 2003; Woodhall and others 2001). These findings demonstrate that ambient glutamate, present in slices without action potential-evoked release, is sufficient to functionally activate preNMDARs. Notably, the ability of preNMDARs to modify mEPSC frequency is apparent at physiological temperatures, but is absent at room temperature unless glutamate concentration is enhanced by high-frequency synaptic activity or blockade of excitatory amino acid transporters (Bender and others 2006; Brasier and Feldman 2008; Cavelier and Attwell 2005). This suggests that ambient glutamate concentration or the sensitivity of preNMDAR modulation of release is regulated by temperature. Importantly, preNMDAR enhancement of release is not saturated by ambient glutamate because application of NMDAR agonists enhances mEPSC frequency (Brasier and Feldman 2008; Woodhall and others 2001). These data suggest that activity-dependent changes in local glutamate concentration might dynamically regulate preNMDAR activation, and therefore release probability.
The cellular source of glutamate for preNMDARs remains unclear. PreNMDARs only contribute to evoked release at L5 pyramidal cell synapses when the presynaptic cell fires bursts, suggesting that preNMDARs act as autoreceptors for glutamate that builds up with release from the presynaptic terminal (Sjöström and others 2003). As such, preNMDARs may help maintain a high release probability in the face of continuous firing. Presynaptic kainate receptors act in a similar autocrine fashion to depolarize terminals and enhance transmitter release (Sun and Dobrunz 2006). Unlike presynaptic kainate receptors, whose effect on release gradually increases with subsequent spikes within a burst, preNMDARs affect transmitter release even during the first action potential in a burst (Sjöström and others 2003). This difference may owe to the comparatively low affinity for glutamate of presynaptic kainate receptors versus NR2B–containing NMDARs (Pinheiro and others 2007). Glutamate for preNMDAR activation can also arise from postsynaptic dendritic release of glutamate, which allows preNMDARs to modulate GABAergic synapses in the cerebellum (Duguid and Smart 2004).
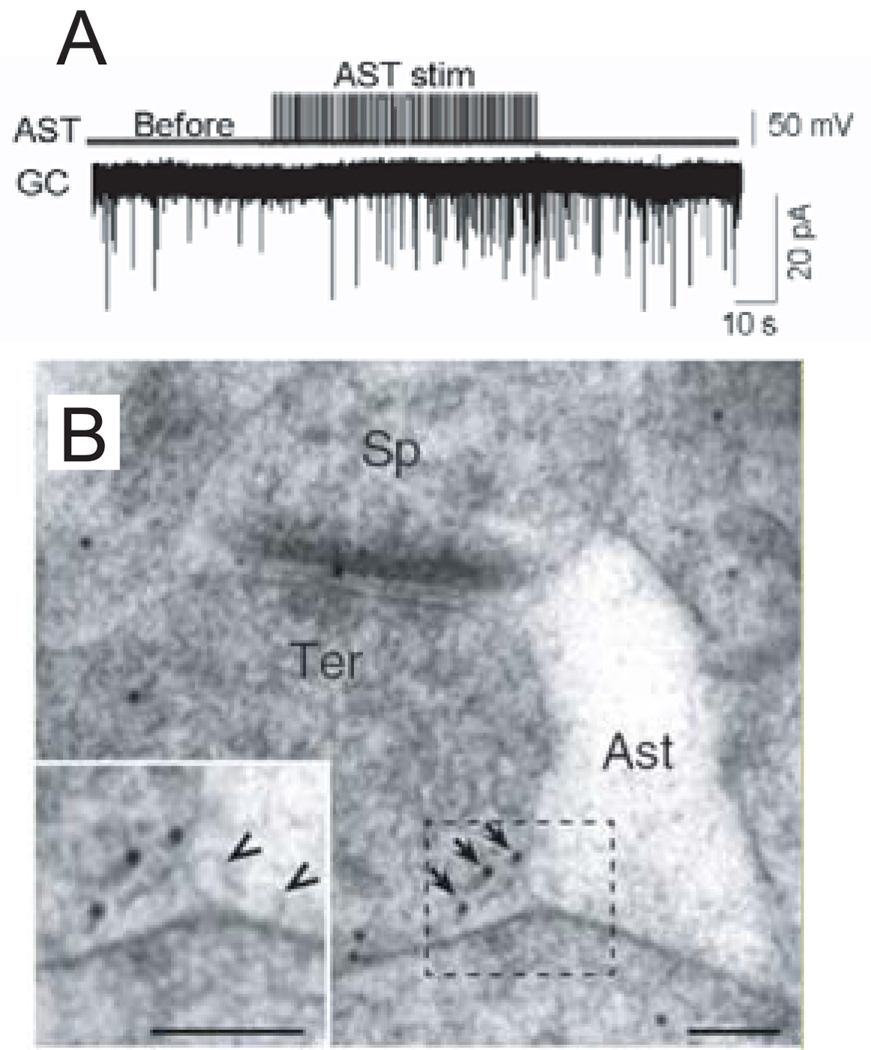
Glia may also play a key role in dynamically regulating glutamate concentration for preNMDAR activation. Astrocytes have recently been shown to contain glutamate vesicles which undergo exocytosis when stimulated by prostaglandins, tumor necrosis factor-alpha, or metabotropic glutamate receptor (mGluR) agonists (Bezzi and others 1998; Bezzi and others 2001; Bezzi and others 2004). In the hippocampal perforant path to granule cell synapse, whole-cell stimulation of synaptically associated astrocytes increases mEPSC frequency (Jourdain and others 2007) (Fig 4A). This effect was prevented when astrocytic exocytosis was blocked or when preNMDARs were blocked with NR2B–selective antagonists. Furthermore, immuno EM revealed NR2B–containing preNMDARs in the extrasynaptic portion of excitatory perforant path terminals, closely apposed to the vesicles of synaptically associated astrocytes (Jourdain and others 2007) (Fig 4B). These findings strongly suggest that dynamic glial release of glutamate contributes to activation of preNMDARs. It will be vital to test whether other preNMDARs (including in neocortex) are regulated by a similar mechanism.
Fig. 4. Glutamate released from astrocytes can regulate neurotransmitter release.
(A) Electrical stimulation (stim) of an astrocyte (AST) increases miniature excitatory synaptic currents recorded in a granule cell (GC) in the dentate gyrus of the hippocampus. (B) Electron micrographs showing NR2B gold particles in extrasynaptic membranes (arrows) of nerve terminals (Ter) making asymmetric synapses with dendritic spines (Sp) in the dentate molecular layer and an associated astrocytic process (Ast). Inset shows at higher magnification NR2B particles apposed to an astrocytic process that contains synaptic like microvesicles (arrowheads). Scale bars, 100 nm. Reproduced with permission from Jourdain and others (2007).
A second issue is whether preNMDARs are functionally regulated by availability of glycine or other ligands at the glycine binding site. Glycine binding is required for preNMDAR function, because blockade of the glycine binding site fully prevents the effects of preNMDARs on mEPSCs in primary visual cortex. However, exogenous application of D-serine (a selective agonist for the glycine site) does not enhance mEPSC frequency (Li and Han 2007). Thus, unlike the postsynaptic NMDAR, the glycine site on preNMDARs may be saturated under physiological conditions, which may be due in part to the relatively high affinity NR2B–containing NMDARs have for glycine (Kew and others 1998). In addition to glycine and D-serine, preNMDAR function is modulated by other amino acids. Taurine, an endogenous analogue of glycine, has been found to increase the preNMDAR-mediated enhancement of Schaffer collateral fiber volley amplitude without affecting the postsynaptic NMDAR-mediated enhancement of field EPSC slope (Suarez and Solis 2006).
A third issue is the source of depolarization for preNMDAR activation. The fact that preNMDAR blockade alters mEPSC frequency when spikes are blocked by TTX (Berretta and Jones 1996; Brasier and Feldman 2008; Corlew and others 2007; Li and Han 2007; Li and others 2008; Sjöström and others 2003) indicates that preNMDARs actively enhance release even when depolarization from sodium spikes is absent. This could indicate that (i) resting potential in terminals is sufficiently depolarized to partially relieve Mg2+ block of preNMDARs, (ii) preNMDARs may exhibit less voltage-dependence than classical postsynaptic NMDARs, or (iii) preNMDAR modulation of release may not require current flow. Although a relatively depolarized resting potential is a possibility because the presynaptic terminal is a small, high input resistance compartment that would be readily depolarized by small local excitatory currents, this idea is speculative and, at least in the Calyx of Held terminals where it can be measured, the resting membrane potential is close to −80mV (Awatramani and others 2005; Duguid and Smart 2004). It is possible that preNMDARs lack a voltage-activated component, as this has been observed in some receptor subtypes. In particular, heteromeric NMDAR channels containing the obligatory NR1 subunit with either NR2C, NR2D, NR3A, or NR3B subunits (perhaps in addition to NR2B subunits) would be expected to exhibit less basal magnesium block compared to postsynaptic NMDARs composed primarily of NR1 with NR2A and/or NR2B (Cull-Candy and others 2001; Monyer and others 1992; Sasaki and others 2002). Notably, preNMDARs in CA1 hippocampus of very young (<P5) rats have been suggested to contain NR2D subunits (Mameli and others 2005). Thus, determining the molecular composition of preNMDARs will be crucially important to understanding their voltage dependence and their function under physiological conditions.
The function of preNMDARs might also be subject to neuromodulation. In hippocampal slices from <P5 rats, the excitatory neurosteroid pregnenolone sulfate increases mEPSCs frequency. This effect is blocked by bath application of NMDAR antagonists, including the NR2C/NR2D–selective antagonist PPDA, but not by selective blockade of postsynaptic NMDARs. An endogenous pregnenolone sulfate-like neurosteroid was found to be released by postsynaptic depolarization and to enhance preNMDAR function (Mameli and others 2005), raising the possibility that preNMDARs may be an important site of neuromodulatory control of release probability and network excitability.
Role in LTD
NMDARs are required for many forms of synaptic plasticity, including long-term potentiation (LTP) and depression (LTD) (Bliss and Collingridge 1993; Collingridge and Bliss 1995; Malenka and Bear 2004). While postsynaptic NMDARs are well established to trigger induction of classical forms of LTP and LTD, as best defined at CA3-CA1 excitatory synapses in hippocampus, recent studies indicate that preNMDARs, rather than postsynaptic NMDARs, mediate at least one prominent form of plasticity, spike timing-dependent LTD (tLTD), at some synapses.
Spike timing-dependent plasticity (STDP) is a physiologically realistic form of bidirectional synaptic plasticity in which LTP or LTD is induced in response to the precise timing between presynaptic spikes (and the excitatory postsynaptic potentials (EPSPs) they elicit) and postsynaptic spikes (Dan and Poo 2006; Magee and Johnston 1997; Markram and others 1997). Spike timing-dependent LTP (tLTP) is induced when presynaptic spikes precede postsynaptic spikes by < 20 ms, while tLTD is induced when presynaptic spikes follow postsynaptic spikes by up to 20–50 ms. STDP occurs throughout the neocortex (Egger and others 1999; Feldman 2000; Froemke and Dan 2002; Sjöström and others 2001) and has functional properties that may underlie development, plasticity, and competition within sensory maps. In particular, tLTD may underlie deprivation- and experience-induced weakening of sensory responses during receptive field plasticity (Allen and others 2003; Celikel and others 2004; Dan and Poo 2006).
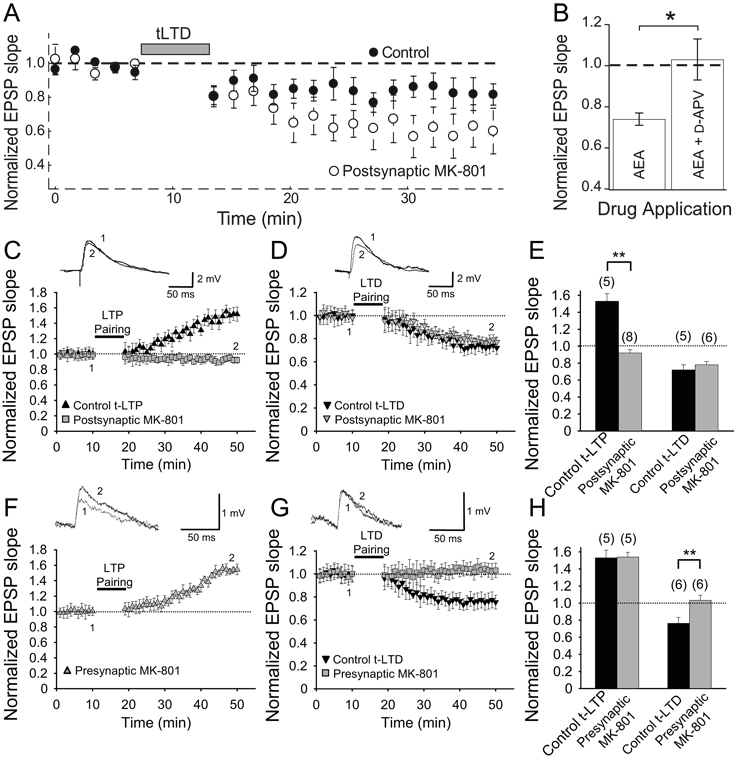
NMDAR activation is necessary for most forms of cortical STDP. At several synapses in young rodents, including L4-L2/3 synapses in somatosensory and visual cortex, and synapses between L5 pyramidal neurons in visual cortex, the induction of tLTD has been found to specifically require preNMDARs, but not postsynaptic NMDARs (Bender and others 2006; Corlew and others 2007; Rodríguez-Moreno and Paulsen 2008; Sjöström and others 2003) (Fig. 5). This was elegantly shown at unitary L4-L2/3 synapses in mouse somatosensory cortex, using dual whole-cell recordings of synaptically connected L4 and L2/3 neurons. Postsynaptic or presynaptic NMDA receptors were selectively blocked by internal application of the NMDAR pore blocker MK-801 into the pre- or postsynaptic neuron. tLTD was completely blocked by presynaptic MK-801, but was unaffected by postsynaptic MK-801. This indicates that LTD involved presynaptic, but not postsynaptic, NMDARs, and rules out the possibility that glial NMDARs are required in tLTD (Rodríguez-Moreno and Paulsen 2008 ).
Fig. 5. The induction of some forms of long-term depression (LTD) requires preNMDARs.
(A,B) Extracellularly evoked L4-L2/3 EPSPs in somatosensory cortex. (A) In L4-L2/3 somatosensory cortex connections, robust timing-dependent LTD can be induced in control experiments (closed circles) by repetitively pairing postsynaptic action potentials followed closely in time with an EPSP (pairing protocol is indicated by gray bar). The LTD is observed even when postsynaptic NMDARs are blocked with postsynaptic internal MK-801 (open circles), but not when APV is bath applied (not shown). (B) Bath-applying the endocannabinoid agonist AEA induces a lasting LTD at L4-L2/3 synapses (indicated by a reduction of EPSP slope, normalized to baseline). Prior blockade of pre- and post-synaptic NMDARs with bath applied D-APV blocks this AEA-induced LTD (no reduction in EPSP slope from baseline), while postsynaptic NMDAR blockade with MK-801 does not block AEA-induced LTD (not shown). (C–H) Synaptically coupled L4-L2/3 excitatory pairs in somatosensory cortex. (C) Postsynaptic MK-801 (grey) completely blocks induction of timing-dependent long-term potentiation (tLTP), while control (no MK-801) neurons (black) exhibit normal LTP. Inset, EPSP before (1) and 30 min after (2) the LTP pairing protocol. (D) Postsynaptic MK-801 did not block tLTD. Symbols and traces are presented as in C. (E) Summary of C, D. (F) During paired recordings, presynaptic MK-801 did not block the induction of tLTP. (G) Presynapitic MK-801 completely blocks tLTD. Symbols and traces are presented as in C. (H) Summary of F, G. Control tLTP refers to values obtained using extracellular stimulation. (E,H) The numbers of slices are shown in parentheses. All error bars are s.e.m. * = p < 0.05, ** = p < 0.01, Student’s t-test. (A,B) Reproduced with permission from Bender and others (2006), (C–H) reproduced with permission from Rodríguez-Moreno and Paulsen (2008).
PreNMDAR-dependent tLTD requires postsynaptic calcium elevation and, in the somatosensory cortex at least, activation of postsynaptic group I mGluRs, and is expressed as a reduction in the probability of neurotransmitter release, implicating a retrograde signal (Bender and others 2006; Nevian and Sakmann 2006). This retrograde signal involves the well-established endocannabinoid retrograde signaling pathway, which mediates many forms of short- and long-term synaptic plasticity (Chevaleyre and others 2006), because postsynaptic synthesis of endocannabinoids and activation of presynaptic cannabinoid type 1 (CB1) receptors are required for tLTD (Bender and others 2006; Sjöström and others 2003). Thus, tLTD at these synapses involves both preNMDAR and CB1 signaling (Bender and others 2006; Nevian and Sakmann 2006; Sjöström and others 2003).
Such CB1- and preNMDAR-dependent, presynaptically expressed tLTD is common in neocortex, at least during early postnatal development (Corlew and others 2007), but is not universal (e.g., Froemke and others 2005). Currently, there is no explanation for why some forms of LTD are preNMDAR-dependent while others are postsynaptic NMDAR-dependent. In addition to tLTD, it is tempting to speculate that more forms of preNMDAR-dependent plasticity exist, but have not been discovered due to assumptions, based on analogy to CA3-CA1 synapses in hippocampus, that postsynaptic NMDARs generally mediate LTP and LTD. The prevalence of multiple, distinct forms of LTD throughout the brain, including preNMDAR-CB1 LTD, and the recent discovery of preNMDAR-dependent LTP in the amygdala (Humeau and others 2003), indicate that plasticity at CA3-CA1 synapses may not be canonical and underscore the need to test specifically for pre- versus post-synaptic NMDAR involvement in specific forms of synaptic plasticity.
Role of preNMDARs in CB1/preNMDAR-dependent LTD
How might preNMDARs contribute to tLTD? Coincidence detection for STDP, and for Hebbian plasticity generally, is widely posited to be performed by postsynaptic NMDARs (Dan and Poo 2006). In one standard STDP model, presynaptically released glutamate strongly activates postsynaptic NMDAR currents when release is rapidly followed by depolarization from postsynaptic spikes, leading to calcium influx that is supralinear compared to glutamate release or postsynaptic spikes alone. This supralinear calcium is thought to drive LTP induction. In contrast, post-leading-pre spike order drives sublinear calcium influx, leading to LTD (Koester and Sakmann 1998; Shouval and Kalantzis 2005). While this model may hold at synapses that exhibit postsynaptic NMDAR-dependent STDP, calcium from postsynaptic NMDARs is not required to drive tLTD at synapses which exhibit preNMDAR/CB1-dependent tLTD (Bender and others 2006; Corlew and others 2007; Duguid and Sjöström 2006; Sjöström and others 2003). Thus, at these synapses, postsynaptic NMDARs cannot perform coincidence detection for tLTD, and the induction of tLTD must involve a separate coincidence detection mechanisms than those for tLTP (Bender and others 2006; Nevian and Sakmann 2006). Induction of tLTD at these synapses may involve preNMDARs and CB1 receptors (Duguid and Sjöström 2006; Sjöström and others 2003).
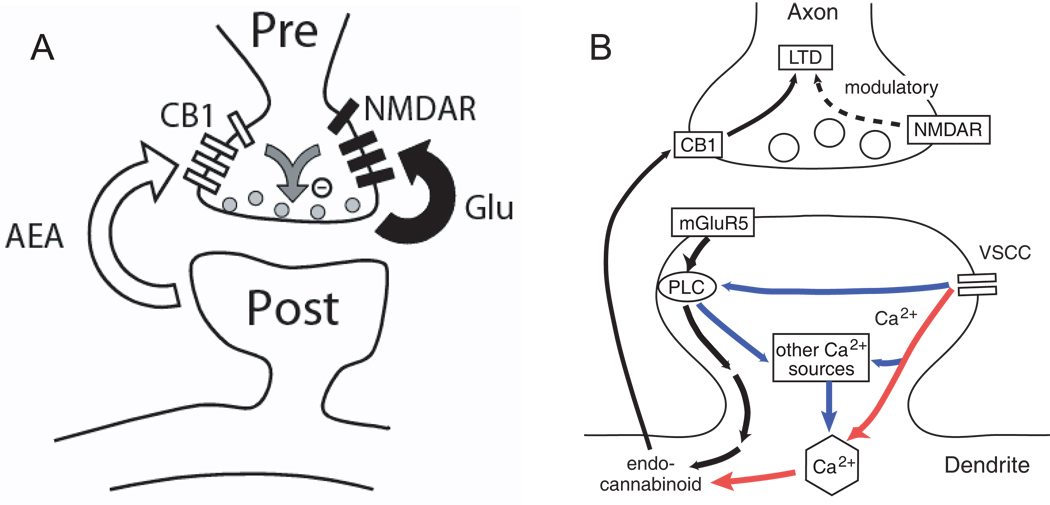
Whether and how preNMDARs contribute to coincidence detection for preNMDAR-dependent, CB1-dependent tLTD is debated. In one hypothesis, presynaptic spikes provide glutamate and depolarization to activate preNMDARs, postsynaptic spikes evoke endocannabinoid release to activate CB1 receptors, and coincident activation of preNMDARs and CB1 receptors triggers tLTD (Duguid and Sjöström 2006; Sjöström and others 2003) (Fig. 6A). Consistent with this model, exogenous cannabinoid agonists induce tLTD at synapses between L5 pyramidal cells in visual cortex, and this effect is blocked when preNMDARs are blocked by APV, indicating that coincident activation of CB1 receptors and preNMDARs is required for this form of synapse weakening. Also consistent with this model, prolonging the half-life of endogenously released cannabinoids broadens the timing window for tLTD within the STDP rule (Sjöström and others 2003). However, a potential difficulty with this model is that endocannabinoid synthesis, release, and retrograde signaling must occur with ~10 ms precision, which may be faster than possible for endocannabinoid signaling (Heinbockel and others 2005). Another difficulty is that, at least at L4-L2/3 synapses in primary somatosensory cortex, tLTD can be induced even when preNMDARs are blocked during post-leading-pre spike pairing, indicating that these receptors do not participate in coincidence detection for tLTD (Bender and others 2006).
Fig. 6. Two models showing preNMDAR involvement in the coincidence detection of spike timing-dependent LTD.
(A) It has been proposed in L5 of visual cortex (Sjöström and others 2003) that postsynaptic action potentials trigger the release of endocannabinoids in a mechanism similar to short-term depression caused during depolarization-induced suppression of inhibition. When presynaptic activity follows this cannabinoid release in a short time window, it coincidently activates preNMDARs and presynaptic CB1 receptors to trigger LTD induction. Reproduced with permission from Sjöström and others 2003. (B) Schematic for a possible postsynaptic coincidence detector for CB1-mediated tLTD. Black, pathway for mGluR-dependent cannabinoid synthesis. Red, pathway for VSCC- and calcium-dependent cannabinoid synthesis. Purple, potential synergistic pathways that increase cannabinoid production in response to appropriately timed pre- and postsynaptic spikes. Presynaptic NMDARs play a modulatory role in LTD in this hypothesis. Schematic based on data in Bender and others 2006.
An alternative hypothesis is that preNMDARs do not contribute to millisecond-scale coincidence detection of pre- and postsynaptic activity during tLTD induction, but rather act on a slower time scale to modulate tLTD induction. In this model, rapid coincidence detection for tLTD occurs through a separate, postsynaptic mechanism. One such mechanism is suggested by findings at L4-L2/3 synapses in primary somatosensory cortex, where tLTD requires postsynaptic VSCCs and group I mGluRs, both of which are upstream of endocannabinoid synthesis (Bender and others 2006) (Fig. 6B). Calcium and mGluR activation are known to drive endocannabinoid synthesis, and joint activation of these pathways greatly facilitates endocannabinoid synthesis and cannabinoid-dependent plasticity (Chevaleyre and others 2006; Hashimotodani and others 2005). According to this model, presynaptic spikes provide glutamate to activate mGluRs, postsynaptic spikes drive calcium entry through VSCCs, and appropriately timed, near-coincident activation of these two pathways leads to synergistic endocannabinoid synthesis and release. The retrograde endocannabinoid signal then instructs the presynaptic terminal to express LTD. While acute blockade of preNMDARs during post-pre spike pairing does not block tLTD at this synapse, persistent blockade of preNMDARs for many minutes prior to pairing does block tLTD (Bender and others 2006). This suggests that preNMDAR activity is required on long time scales for tLTD induction, but not for rapid coincidence detection during pairing. This model does not predict strong synapse specificity for tLTD, since post-pre pairing at one synapse would generate cannabinoid signals that could diffuse retrogradely to neighboring synapses and drive heterosynaptic LTD. In contrast, the model proposed by Sjöström et al. (2003) predicts that only those presynaptic terminals which are active within milliseconds of postsynaptic activation would experience coincident activation of preNMDARs and CB1 receptors, and would undergo LTD. Future studies need to distinguish between these distinct tLTD models.
Developmental regulation of preNMDARs role in neurotransmission and plasticity
Despite the large body of evidence reviewed above, preNMDARs are not found at all synapses, in all brain areas, in all studies. Some explanation is offered by a growing body of evidence indicating that the function of preNMDARs decreases dramatically over development. One of the first suggestions that preNMDARs are regulated over development came from a study in the CA1 region of the hippocampus, where the neuromodulatory effects of pregnenolone sulfate on preNMDARs was observed only during a brief window of postnatal rodent development (<P5) (Mameli and others 2005). While this effect might arise at any point along the neuromodulatory pathway, it was hypothesized that the loss of function arose from a decline in preNMDAR function due to a developmental loss of NR2D–containing preNMDARs. The presence of NR2D permits NMDARs to function at hyperpolarized potentials and, thus, could account for the ability of preNMDARs to be tonically active in the absence of strong depolarization (Mameli and others 2005). The developmental loss of NR2D might then increase voltage-dependence of preNMDARs, effectively disabling their function. In support of this interpretation, while preNMDARs may facilitate tonic transmitter release at the CA3-CA1 synapse only in young (<P5) mice (Mameli and others 2005) they may continue to alter evoked transmitter release at older ages (Suarez and Solis 2006; Suarez and others 2005). Thus, with a change in the subunit composition of preNMDARs, their ability to participate in spontaneous and evoked transmitter release might change.
Several studies now indicate that preNMDAR function attenuates with development, although the developmental timing of this attenuation varies in different regions of the brain. For example, unlike the early loss of preNMDAR function observed in the hippocampus (Mameli and others 2005), preNMDAR function in entorhinal cortex L5 pyramidal neurons is not lost until late in development (Yang and others 2006). Specifically, preNMDARs enhance the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) at 5 weeks of age in the entorhinal cortex, but this effect is absent by 5 months (Yang and others 2006). Although the time course for the developmental loss of preNMDARs is different between the hippocampus and entorhinal cortex, a change in preNMDAR subunit composition may underlie the loss of preNMDAR function in both regions (Mameli and others 2005; Yang and others 2006). To examine this possibility in the entorhinal cortex, the investigators took advantage of the observation that preNMDARs, but not postsynaptic NMDARs, are thought to contain the NR2B subunit in L5 pyramidal neurons at 5 weeks of age. Accordingly, the NR2B agonist Ro25–6981 could effectively reduce the frequency of sEPSCs at 5 weeks of age but not at 5 months of age, indicating that NR2B–containing preNMDARs were no longer contributing to the spontaneous release probability.
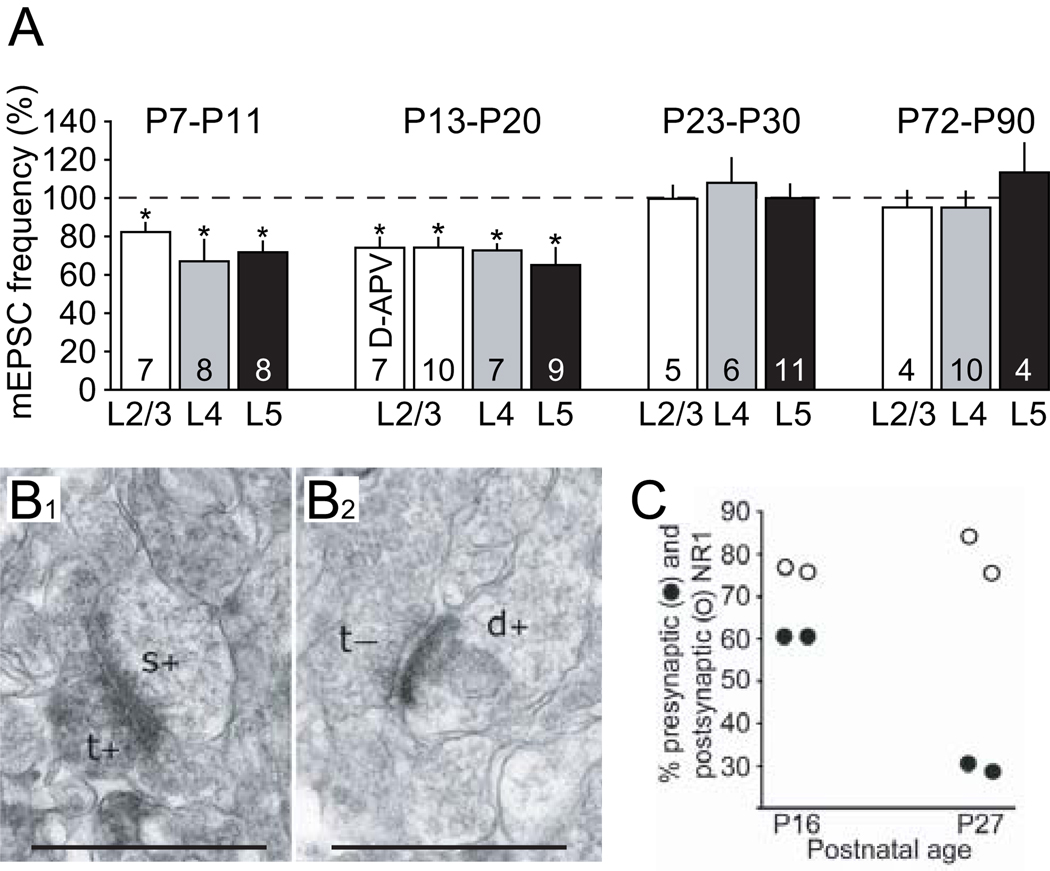
A profound developmental reduction in preNMDAR functions has been observed in the primary visual cortex (Corlew and others 2007), suggesting that this might be a general feature of preNMDAR expression. In the mouse primary visual cortex, the ability of preNMDARs to enhance spontaneous release probability onto cells in L2/3, L4, and L5 is completely lost at 3 weeks of age (Fig. 7). Consistent with this observation, there is a dramatic reduction in the anatomical expression of terminals containing preNMDARs; while roughly 60% of presynaptic terminals contain the obligatory NMDAR subunit NR1 in L2/3 asymmetric (excitatory) synapses at P16, only 30% do so at P27. While there are several possible explanations for the total loss of preNMDAR functions in spontaneous release without the complete loss of expression at P27, one parsimonious explanation is that the reduction in preNMDAR expression is also coupled to a change in preNMDAR subunit composition. Such an observation would be consistent with the mechanisms suggested for the developmental loss of preNMDARs in entorhinal cortex and hippocampus. Moreover, a role for preNMDARs in supporting evoked neurotransmitter release in the adult neocortex has not been tested to date.
Fig. 7. Evidence for a developmental reduction in preNMDAR functions.
(A) D,L-APV (or D-APV where indicated) strongly reduced AMPAR-mediated mEPSC frequency from baseline in L2/3, L4, and L5 pyramidal cells in the visual cortex of mice at P7–20 but not older mice. Sample sizes are given within the bars. (B) Immuno-electon microscopy for the obligatory NR1 subunit of the NMDAR reveals a developmental decrease in presynaptic, but not postsynaptic, NR1. Electron micrograph in L2/3 of visual cortex of a (B1) P16 mouse, demonstrating an NR1-positive terminal (t+) forming a synapse onto a NR1 positive spine (s+) and (B2) from a P27 mouse, demonstrating an unlabeled terminal (t-) forming a synapse onto a labeled dendrite (d+). Scale bar 250 nm. (C) Scatter plot from four mice (2 at each age) quantifying the selective loss of presynaptic, but not postsynaptic, NR1 over development. Reproduced with permission from Corlew and others (2007).
What is the physiological outcome of a developmental decrease in preNMDARs? Neurotransmitter release probability decreases significantly during early development, causing some synapses to switch with age from displaying a depressing response with pairs or bursts of stimulation to a facilitating response (Bolshakov and Siegelbaum 1995; Choi and Lovinger 1997; Dekay and others 2006; Pouzat and Hestrin 1997; Reyes and Sakmann 1999). The timing for these observed changes in presynaptic function coincides roughly with the developmental decrease in preNMDAR function in the hippocampus (Mameli and others 2005), entorhinal cortex (Yang and others 2006), and visual cortex (Corlew and others 2007). Therefore, a reduction in preNMDAR function might contribute to a developmental decrease in release probability, which may be a general property of early circuit formation. Synaptic terminals may require a high release probability to facilitate synapse formation during early synaptogenesis (Rumpel and others 2004). When synaptic connections become more stable, the need for that high release probability may decrease as connections can be strengthened and weakened by changes on the postsynaptic side.
As presynaptic and postsynaptic receptor properties change with development, synaptic plasticity mechanisms may be forced to adapt to these changes (Frenkel and Bear 2004; He and others 2006; Jo and others 2006; Nosyreva and Huber 2005; Yashiro and others 2005; Yasuda and others 2003). The mechanisms of synaptic plasticity cannot be studied without reference to development: even the pre- versus postsynaptic locus induction and expression of LTP and LTD can vary with development (Corlew and others 2007; Crozier and others 2007; Nosyreva and Huber 2005). As preNMDARs are lost during development of the L4-L2/3 pathway in visual cortex, there is a developmental switch from the involvement of preNMDARs to postsynaptic NMDARs in LTD (Corlew and others 2007). Thus, while preNMDARs can contribute to the properties of neurotransmission and act as coincidence detectors for tLTD induction during early life, the role of postsynaptic NMDARs in tLTD increases as that of preNMDARs diminishes.
Activity-dependent and disease-induced changes in preNMDARs
While postsynaptic NMDAR expression and function are sensitive to experience-driven changes in neural activity levels (Carmignoto and Vicini 1992; Hestrin 1992; Monyer and others 1994; Philpot and others 2001), it was only recently that similar activity dependence has been observed for preNMDARs. For example, in cultured cerebellar GABAergic neurons, where preNMDARs may act as heteroreceptors for glutamate to increase GABA release, the developmental loss of preNMDARs can be accelerated by treatment with NMDA (Fiszman and others 2005). While there may be several mechanisms that underlie this activity-dependent change in preNMDAR function, it is possible that changes in activity levels may alter the subunit composition of preNMDARs and/or their expression at the synapse (due to differential receptor trafficking). In support of this idea, pharmacological blockade of NMDARs in vivo rapidly increases the presence of presynaptic NR2A subunits, and decreases that of NR2B subunits, in both postsynaptic spines and presynaptic terminals of adult rat visual cortex (Aoki and others 1994; Fujisawa and Aoki 2003). The time scale (<30 min) of these changes suggest an activity-dependent modification in preNMDAR trafficking, and changes in activity levels may uniquely alter the trafficking of the various NMDAR subtypes.
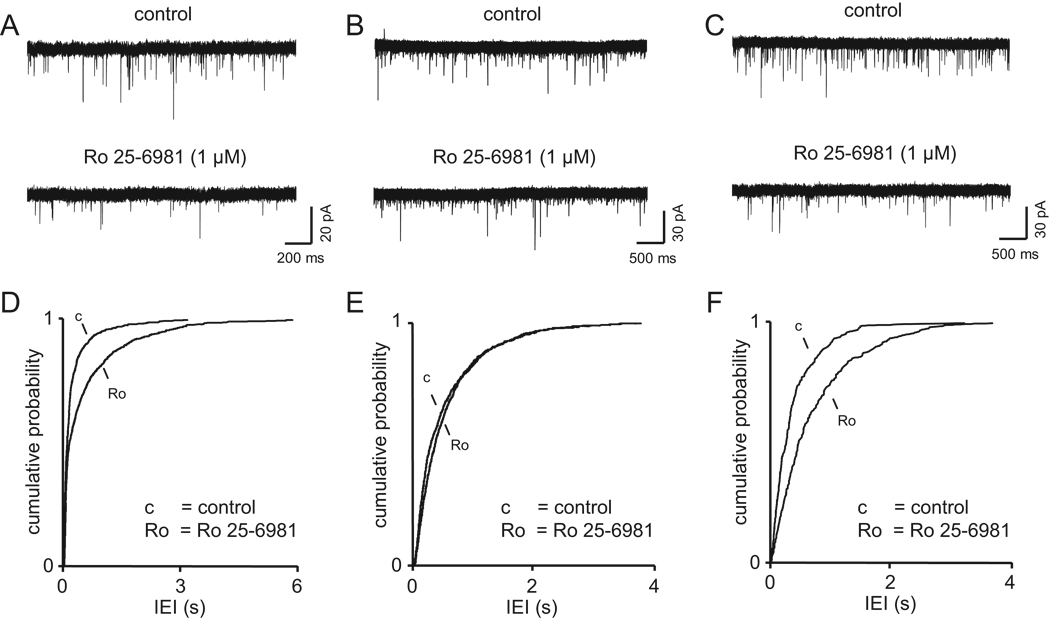
If modulating activity in vivo similarly alters expression and function of preNMDARs, then changes in preNMDAR function could occur during, or contribute to, neurological disorders such as epilepsy that involve large-scale changes in network activity and excitation. Chronic changes in neural activity levels in epileptic patients have been shown to affect NMDAR function and expression (Avanzini and Franceschetti 2003; Dalby and Mody 2001; Morimoto and others 2004). However, few studies have considered the possibility that preNMDARs may be affected. One recent study in rodents indicates that epileptic activity could affect the function of preNMDARs. Specifically, preNMDARs were reinstated, or their normal developmental down-regulation prevented, in the adult entorhinal cortex following 2–4 weeks of lithium-pilocarpine treatment to induce seizures (Yang and others 2006). In these mice, the recurrent seizure activity recovered a high frequency of sEPSCs that could be decreased by the NR2B–specific antagonist Ro 25–6981. Littermate controls with no seizures had no increase in sEPSCs, and the frequency of sEPSCs in these control mice was not affected by Ro 25–6981 application (Yang and others 2006) (Fig. 8). These data indicate that the ability of preNMDARs to enhance spontaneous transmitter release was enhanced in a seizure model, although it has not yet been determined whether this increase was causal to, or a consequence of, increased neural activity. In support of the idea that preNMDARs may be involved in some forms of epilepsy, it is interesting to note that gabapentin, a drug prescribed to treat epilepsy, may act to decrease neurotransmitter release via preNMDARs (Suarez and others 2005).
Fig. 8. Developmental and activity-dependent regulation of preNMDAR functions.
Presynaptic NR2B–containing receptors enhance spontaneous release in the entorhinal cortex of young rats and epileptic adults, but not in normal adults. (A–C) Voltage-clamp recordings of sEPSCs in a layer 5 neurons in a slice from (A) a 4-week-old rat, (B) a 5-month-old rat, and (C) and a 5-month-old epileptic rat. Blockade of NR2B receptors with Ro 25–6981 decreases the frequency of sEPSCs only in young and epileptic rats. Postsynaptic NMDARs are blocked by intracellular MK-801. (D–F) Corresponding pooled data for inter-event interval of the sEPSCs from control and Ro 25–6981 recordings. Reproduced with permission from Yang and others (2006).
NMDARs have a known role in the etiologies of many serious neurological disorders including Huntington's, Parkinson’s, stroke, schizophrenia, epilepsy, and neuropathic pain (Fan and Raymond 2007; Gogas 2006; Heresco-Levy and Javitt 1998; Liu and others 1997; Missale and others 2006; Visser and Schug 2006). However the contribution of preNMDARs to these disorders is unexplored due to the assumption that NMDARs act primarily postsynaptically. It is now clear that preNMDARs may have a very important role in synaptic transmission and plasticity, especially during very early development when many such neuropathologies are forming. With an increased understanding of the importance of preNMDAR functions, we must now consider the possibility that many global NMDAR antagonists that are being used to treat neurological disorders may have some of their therapeutic value or deleterious off-target effects by acting on preNMDARs (Suarez and others 2005). If this is the case, it suggests that better pharmacological therapies for disorders involving NMDAR dysfunction may be revealed by selectively targeting pre- versus post-synaptic NMDARs. Furthermore, preNMDARs have been found to have a direct involvement in epilepsy (Yang and others 2006) and also appear to contribute to fetal alcohol spectrum disorder via a pregnenolone-dependent mechanism (Valenzuela and others 2008). Therefore we now need a better understanding of when, where, and how preNMDARs can affect synaptic communication, plasticity, and neural network function. The molecularly distinct composition of pre- versus post-synaptic NMDARs provides the promise of a future generation of drugs which can selectively target the relevant receptors.
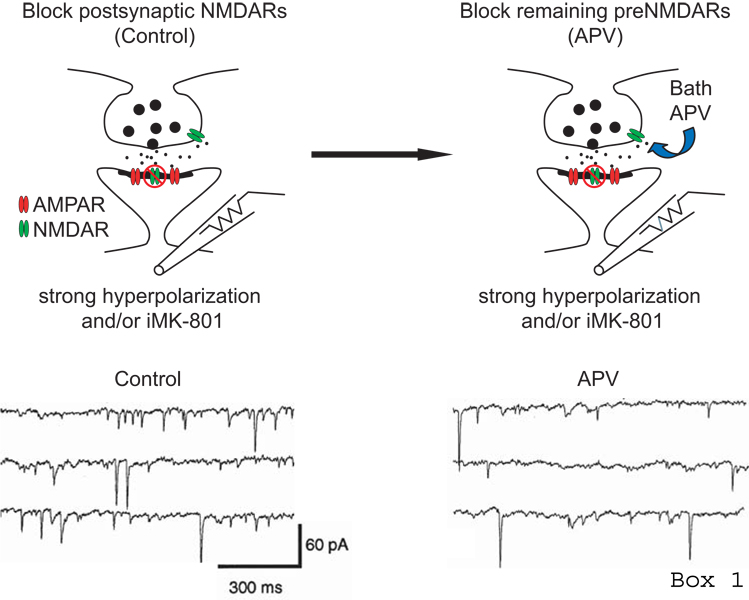
Box 1. Physiological methods to detect functional preNMDARs
Two general approaches have been used to provide evidence for the existence and function of preNMDARs: physiological and anatomical. The most common has been the physiological approach of measuring the effect of blocking preNMDARs on the frequency of neurotransmitter release, measured electrophysiologically as spontaneous or miniature excitatory postsynaptic currents (sEPSCs or mEPSCs) recorded in the postsynaptic neuron. If preNMDARs normally function to increase spontaneous release frequency, then acute blockade of preNMDARs should reversibly decrease the frequency of synaptic events. Because most pharmacological blockers of preNMDARs also block postsynaptic NMDARs, this approach is only interpretable if postsynaptic NMDARs are selectively blocked first, so that subsequent effects of NMDAR antagonists can be attributed to non-postsynaptic, presumably presynaptic NMDARs (Berretta and Jones 1996; Woodhall and others 2001; Sjostrom and others 2003; Bender and others 2006; Corlew and others 2007; Brasier and Feldman 2008). This recording paradigm is schematized (reproduced with permission from Corlew and others 2007) and an example given (reproduced with permission from Beretta and Jones 1996) to illustrate the approach. In this example, after first blocking postsynaptic NMDARs, subsequent bath application of the NMDAR antagonist of D-APV reduces the frequency of sEPSCs measured in layer 2 entorhinal cortex neurons.
Used alone, this approach has two shortcomings. First, although the NMDARs that regulate the frequency of synaptic events in this assay cannot be postsynaptic, they are only presumed to be presynaptic (though localization in the presynaptic terminal is the most parsimonious explanation for modulation of transmitter release probability). Second, because spontaneous transmitter release and action potential-evoked release can be distinct processes, whether preNMDARs modulate evoked release must be tested separately. This has been done by acutely blocking preNMDARs (again, when postsynaptic NMDARs are already selectively blocked), and testing for a decrease in evoked EPSC amplitude, coupled with changes in short-term synaptic depression or facilitation that occur characteristically when release probability is altered.
Improved tools will help better test for preNMDARs and determine their precise function. Recently, selective blockade of preNMDARs has been achieved by performing paired synaptic recordings and loading the presynaptic neuron with an NMDAR antagonist (Rodríguez and Paulsen, 2008). No other methods currently exist to selectively activate, block, or remove preNMDARs without also affecting postsynaptic NMDARs. Modern genetic and optical methods could provide such tools. For example, if preNMDARs preferentially contain specific NMDAR subunits, viral expression of siRNA or other genetic approaches could be used to selectively block expression of functional NMDARs in specific neurons (Zong and others 2005; Miskevich and others 2006; Luo and others 2008). Paired whole-cell recordings could then be used to identify differences in transmission between preNMDAR-deleted and control synapses. Another approach would be to monitor presynaptic function directly (e.g., by calcium imaging in presynaptic terminals) while selectively modulating preNMDARs by focal application of agonists or antagonists (e.g. glutamate uncaging), to test whether, and how, preNMDAR activity modulates transmitter release. These and other new approaches would enable preNMDAR prevalence and function to be examined in more detail.
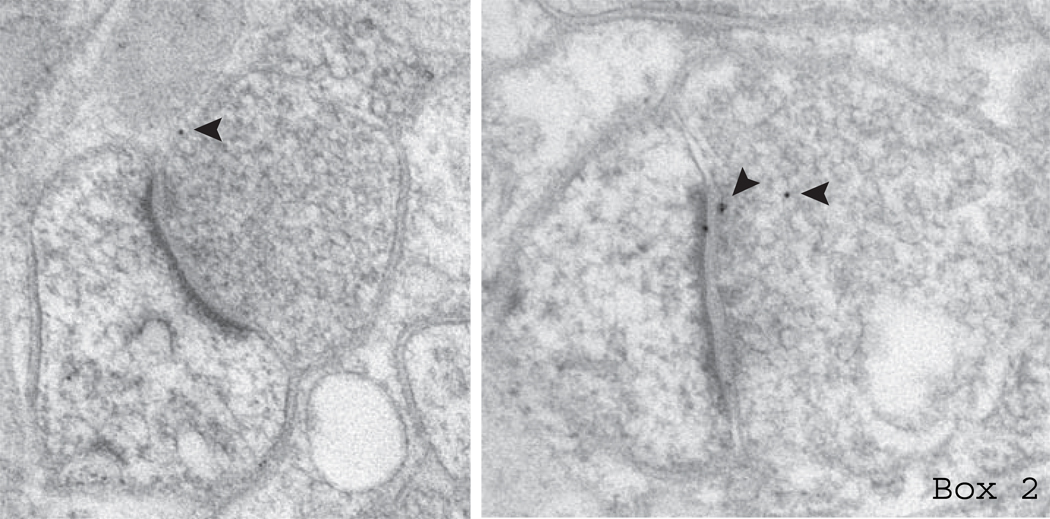
Box 2. Anatomical methods to visualize preNMDARs
In addition to electrophysiological approaches, immunoperoxidase and immunogold electron microscopy (EM) have been used to directly visualize preNMDARs. Immunoperoxidase staining is a sensitive assay in which an enzymatic reaction increases the intensity of the diaminobenzidine (DAB) reporter signal for maximum threshold of localization of NMDARs (Aoki and others 1994; Liu and others 1994; Siegel and others 1994; DeBiasi and others 1996; Charton and others 1999; Lu and others 2005; Corlew, Wang and others 2007). While immunoperoxidase EM is extremely sensitive, it suffers from technical limitations. It is very difficult to distinguish weak from strong immunoreaction. Furthermore, the visualized reaction product tends to migrate during the peroxidase reaction, degrading subcellular localization. Therefore, while presynaptic staining can be detected, exact localization within the terminal is not possible. A more precise localization method uses gold particles conjugated to the secondary antibody (immunogold EM). Below is an example of anatomical evidence for preNMDARs in L2/3 of the rodent neocortex suggested by postembedding immunogold for an antibody that recognizes both NR2A and NR2B (Figure is courtesy R. Weinberg, University of North Carolina). Presynaptic labeling with 10 nm gold particles is indicated by arrowheads. Using this method, several studies have observed expression of NMDARs on the presynaptic membrane, where they would be well-positioned to modulate neurotransmitter release (Aoki and others 2003; Fujisawa and Aoki 2003; Pickel and others 2006; Jourdain and others 2007). The relatively limited identification of preNMDARs could be due to several factors, including a low prevalence of preNMDARs compared to postsynaptic NMDARs, and age- or region-specific expression of preNMDARs.
Acknowledgements
Special thanks to Vanessa Bender, Lee Langer, and Adam Roberts for critical readings of the manuscript. The authors’ research was supported by NRSA F31GM080162-01 (NIGMS) to R.C., an HHMI Predoctoral Fellowship to D.J.B., R01 NS046652-05 (NINDS) to D.E.F., and the Whitehall Foundation and RO1 EY018323-01 (NEI) to B.D.P‥
References
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Mong JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994;14:5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini G, Franceschetti S. Cellular biology of epileptogenesis. Lancet Neurol. 2003;2:33–42. doi: 10.1016/s1474-4422(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci. 2004;24:2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-D-aspartate autoreceptors in the entorhinal cortex. Neuroscience. 1996;75:339–344. doi: 10.1016/0306-4522(96)00301-6. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmiter release during development and long-term potentiation. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- Brasier DJ, Feldman DE. Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J Neurosci. 2008;28:2199–2211. doi: 10.1523/JNEUROSCI.3915-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Casado M, Dieudonne S, Ascher P. Presynaptic N-methyl-D-aspartate receptors at the parallel fiber-Purkinje cell synapse. Proc Natl Acad Sci U S A. 2000;97:11593–11597. doi: 10.1073/pnas.200354297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33:123–130. doi: 10.1016/s0896-6273(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol. 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci. 2004;7:534–541. doi: 10.1038/nn1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charton JP, Herkert M, Becker CM, Schroder H. Cellular and subcellular localization of the 2B–subunit of the NMDA receptor in the adult rat telencephalon. Brain Res. 1999;816:609–617. doi: 10.1016/s0006-8993(98)01243-8. [DOI] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci U S A. 1997;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci U S A. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dalby NO, Mody I. The process of epileptogenesis: a pathophysiological approach. Curr Opin Neurol. 2001;14:187–192. doi: 10.1097/00019052-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- DeBiasi S, Minelli A, Melone M, Conti F. Presynaptic NMDA receptors in the neocortex are both auto- and heteroreceptors. Neuroreport. 1996;7:2773–2776. doi: 10.1097/00001756-199611040-00073. [DOI] [PubMed] [Google Scholar]
- Dekay JG, Chang TC, Mills N, Speed HE, Dobrunz LE. Responses of excitatory hippocampal synapses to natural stimulus patterns reveal a decrease in short-term facilitation and increase in short-term depression during postnatal development. Hippocampus. 2006;16:66–79. doi: 10.1002/hipo.20132. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Duguid I, Sjöström PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol. 2006;16:312–322. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci. 2004;7:525–533. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- Egger V, Feldmeyer D, Sakmann B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat Neurosci. 1999;2:1098–1105. doi: 10.1038/16026. [DOI] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Farb CR, Aoki C, Ledoux JE. Differential localization of NMDA and AMPA receptor subunits in the lateral and basal nuclei of the amygdala: a light and electron microscopic study. J Comp Neurol. 1995;362:86–108. doi: 10.1002/cne.903620106. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Felmy F, Neher E, Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–811. doi: 10.1016/s0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Fiszman ML, Barberis A, Lu C, Fu Z, Erdelyi F, Szabo G, Vicini S. NMDA receptors increase the size of GABAergic terminals and enhance GABA release. J Neurosci. 2005;25:2024–2031. doi: 10.1523/JNEUROSCI.4980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–438. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–225. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Aoki C. In vivo blockade of N-methyl-D-aspartate receptors induces rapid trafficking of NR2B subunits away from synapses and out of spines and terminals in adult cortex. Neuroscience. 2003;121:51–63. doi: 10.1016/s0306-4522(03)00341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M, Marty A. Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J Neurosci. 1999;19:511–519. doi: 10.1523/JNEUROSCI.19-02-00511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch MD. Calcium influx through N-methyl-d-aspartate receptors triggers GABA release at interneuron-Purkinje cell synapse in rat cerebellum. Neuroscience. 2008;151:403–409. doi: 10.1016/j.neuroscience.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Gogas KR. Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol. 2006;6:68–74. doi: 10.1016/j.coph.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinbockel T, Brager DH, Reich CG, Zhao J, Muralidharan S, Alger BE, Kao JP. Endocannabinoid signaling dynamics probed with optical tools. J Neurosci. 2005;25:9449–9459. doi: 10.1523/JNEUROSCI.2078-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC. The role of N-methyl-D-aspartate (NMDA) receptor-mediated neurotransmission in the pathophysiology and therapeutics of psychiatric syndromes. Eur Neuropsychopharmacol. 1998;8:141–152. doi: 10.1016/s0924-977x(97)00050-3. [DOI] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- Jo J, Ball SM, Seok H, Oh SB, Massey PV, Molnar E, Bashir ZI, Cho K. Experience-dependent modification of mechanisms of long-term depression. Nat Neurosci. 2006;9:170–172. doi: 10.1038/nn1637. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kew JN, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci. 1998;18:1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Henderson G. Modulation of fast synaptic transmission by presynaptic ligand-gated cation channels. J Auton Nerv Syst. 2000;81:110–121. doi: 10.1016/s0165-1838(00)00111-9. [DOI] [PubMed] [Google Scholar]
- Kim DK, Catterall WA. Ca2+-dependent and -independent interactions of the isoforms of the alpha1A subunit of brain Ca2+ channels with presynaptic SNARE proteins. Proc Natl Acad Sci U S A. 1997;94:14782–14786. doi: 10.1073/pnas.94.26.14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc Natl Acad Sci U S A. 1998;95:9596–9601. doi: 10.1073/pnas.95.16.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa A, Segerstrale M, Collingridge GL, Isaac JT, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-D-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Le MeurK, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580:373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Han TZ. Glycine binding sites of presynaptic NMDA receptors may tonically regulate glutamate release in the rat visual cortex. J Neurophysiol. 2007;97:817–823. doi: 10.1152/jn.00980.2006. [DOI] [PubMed] [Google Scholar]
- Li YH, Han TZ, Meng K. Tonic facilitation of glutamate release by glycine binding sites on presynaptic NR2B–containing NMDA autoreceptors in the rat visual cortex. Neurosci Lett. 2008;432:212–216. doi: 10.1016/j.neulet.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci U S A. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CR, Willcockson HH, Phend KD, Lucifora S, Darstein M, Valtschanoff JG, Rustioni A. Ionotropic glutamate receptors are expressed in GABAergic terminals in the rat superficial dorsal horn. J Comp Neurol. 2005;486:169–178. doi: 10.1002/cne.20525. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mameli M, Carta M, Partridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci. 2005;25:2285–2294. doi: 10.1523/JNEUROSCI.3877-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500(Pt 2):409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS. The role of glutamate in epilepsy and other CNS disorders. Neurology. 1994;44:S14–S23. [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Busi C, Collo G, Spano PF. The NMDA/D1 receptor complex as a new target in drug development. Curr Top Med Chem. 2006;6:801–808. doi: 10.2174/156802606777057562. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2005;25:2992–3001. doi: 10.1523/JNEUROSCI.3652-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Colago EE, Mania I, Molosh AI, Rainnie DG. Dopamine D1 receptors co-distribute with N-methyl-D-aspartic acid type-1 subunits and modulate synaptically-evoked N-methyl-D-aspartic acid currents in rat basolateral amygdala. Neuroscience. 2006;142:671–690. doi: 10.1016/j.neuroscience.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouzat C, Hestrin S. Developmental regulation of basket/stellate cell-->Purkinje cell synapses in the cerebellum. J Neurosci. 1997;17:9104–9112. doi: 10.1523/JNEUROSCI.17-23-09104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J Neurosci. 1999;19:3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat Neurosci. 2008 doi: 10.1038/nn.2125. [DOI] [PubMed] [Google Scholar]
- Rumpel S, Kattenstroth G, Gottmann K. Silent synapses in the immature visual cortex: layer-specific developmental regulation. J Neurophysiol. 2004;91:1097–1101. doi: 10.1152/jn.00443.2003. [DOI] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Sasaki YF, Rothe T, Premkumar LS, Das S, Cui J, Talantova MV, Wong HK, Gong X, Chan SF, Zhang D, Nakanishi N, Sucher NJ, Lipton SA. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol. 2002;87:2052–2063. doi: 10.1152/jn.00531.2001. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proc Natl Acad Sci U S A. 1998;95:12004–12009. doi: 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol. 2005;94:4281–4289. doi: 10.1152/jn.00661.2005. [DOI] [PubMed] [Google Scholar]
- Shouval HZ, Kalantzis G. Stochastic properties of synaptic transmission affect the shape of spike time-dependent plasticity curves. J Neurophysiol. 2005;93:1069–1073. doi: 10.1152/jn.00504.2004. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Brose N, Janssen WG, Gasic GP, Jahn R, Heinemann SF, Morrison JH. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc Natl Acad Sci U S A. 1994;91:564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson S. Neocortical LTD via Coincident Activation of Presynaptic NMDA and Cannabinoid Receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- Suarez LM, Solis JM. Taurine potentiates presynaptic NMDA receptors in hippocampal Schaffer collateral axons. Eur J Neurosci. 2006;24:405–418. doi: 10.1111/j.1460-9568.2006.04911.x. [DOI] [PubMed] [Google Scholar]
- Suarez LM, Suarez F, Del OlmoN, Ruiz M, Gonzalez-Escalada JR, Solis JM. Presynaptic NMDA autoreceptors facilitate axon excitability: a new molecular target for the anticonvulsant gabapentin. Eur J Neurosci. 2005;21:197–209. doi: 10.1111/j.1460-9568.2004.03832.x. [DOI] [PubMed] [Google Scholar]
- Sun HY, Dobrunz LE. Presynaptic kainate receptor activation is a novel mechanism for target cell-specific short-term facilitation at Schaffer collateral synapses. J Neurosci. 2006;26:10796–10807. doi: 10.1523/JNEUROSCI.2746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Modulation of transmitter release at giant synapses of the auditory system. Curr Opin Neurobiol. 2002;12:400–404. doi: 10.1016/s0959-4388(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Partridge LD, Mameli M, Meyer DA. Modulation of glutamatergic transmission by sulfated steroids: role in fetal alcohol spectrum disorder. Brain Res Rev. 2008;57:506–519. doi: 10.1016/j.brainresrev.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006;60:341–348. doi: 10.1016/j.biopha.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Vitten H, Isaacson JS. Synaptic transmission: exciting times for presynaptic receptors. Curr Biol. 2001;11:R695–R697. doi: 10.1016/s0960-9822(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Evans DI, Cunningham MO, Jones RS. NR2B–containing NMDA autoreceptors at synapses on entorhinal cortical neurons. J Neurophysiol. 2001;86:1644–1651. doi: 10.1152/jn.2001.86.4.1644. [DOI] [PubMed] [Google Scholar]
- Yang J, Woodhall GL, Jones RS. Tonic facilitation of glutamate release by presynaptic NR2B–containing NMDA receptors is increased in the entorhinal cortex of chronically epileptic rats. J Neurosci. 2006;26:406–410. doi: 10.1523/JNEUROSCI.4413-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Corlew R, Philpot BD. Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. J Neurosci. 2005;25:11684–11692. doi: 10.1523/JNEUROSCI.4362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]