Abstract
Infiltration of activated monocytes into the brain is a prerequisite for the development of various neurological disorders such as HIV associated dementia, multiple sclerosis and other inflammatory processes. In these pathologies the chemokine SDF-1α (CXCL12) is over-expressed and might attract monocytes into the central nervous system. We demonstrate here that SDF-1α stimulates migration of monocytes through its receptor CXCR4, and decreases monocyte adherence to surfaces coated with ICAM-1, a ligand for β2 integrins. SDF-1α also decreases monocyte adherence to brain microvascular endothelial cells (BMVEC) that are activated with TNF-α, IL-1β or recombinant envelope glycoprotein from HIV-1, which increase BMVEC expression of ICAM-1. The decreased adherence is linked to down-regulation on monocytes of the activation-dependent epitope of the β2 integrin LFA-1 by SDF-1α. Knockdown of Lyn in monocytes using siRNA decreases SDF-1α-mediated migration and prevents its inhibition of monocyte attachment to ICAM-1 and activated BMVEC. Thus, in SDF-1α-stimulated monocytes, Lyn acts as a positive regulator of migration, and a negative regulator of adhesion to BMVEC through the LFA-1 integrin. These results provide a novel Lyn-mediated signaling mechanism for the regulation of monocyte movement at the blood-brain barrier.
Keywords: monocyte, SDF-1α, Lyn, LFA-1, CXCR4, ICAM-1, gp120
INTRODUCTION
Infiltration of activated monocytes into the brain is involved in the development of several neurological disorders including HIV associated dementia (HAD), multiple sclerosis, and others. In HAD, for example, HIV-1 infection within the brain is necessary but not sufficient for progression of disease, and the massive movement of activated monocytes and macrophages into the CNS are believed to secrete toxic metabolites and lead to neuronal injury (1–3). In addition, the blood-brain barrier (BBB) is often altered in inflammatory diseases and is implicated in their pathogenesis (4). Proinflammatory cytokines such as TNF-α and interleukins, as well as viral proteins such as the HIV-1 envelope glycoprotein gp120, have been shown to contribute to BBB dysfunction (5–7). Moreover, astrocytes and microglia have been shown to produce chemokines and cell migration-inducing cytokines which appear to attract peripheral blood mononuclear cells across BBB into the brain.
The chemokine stromal derived factor-1α (SDF-1α) is one of the mediators expressed at increased levels in the central nervous system of patients with HAD and other neuroinflammatory disorders (8–12). SDF-1α has been shown to play roles in ontogeny, leukocyte migration and the pathogenesis of inflammatory diseases, and induces intracellular signaling through its receptor CXCR4. SDF-1α may recruit monocytes into the CNS but the mechanism by which this chemokine couples to the signaling cascades and modulates monocyte attachment and movement at the BBB remains unclear. We now report that the Src family kinase Lyn is a critical mediator that relays suppressing signals from the chemokine receptor CXCR4 to β2 integrins in human monocytes. Moreover, we further demonstrate that Lyn mediates the monocyte chemotactic response to SDF-1α. The net result of SDF-1α/CXCR4/Lyn signaling is a decrease in monocyte attachment on activated brain microvascular endothelial cells and an increase in monocyte migration towards SDF-1α gradient. Our present results provide new insights into the modulation of SDF-1α/CXCR4 signaling in monocyte mobilization and migration across the blood brain barrier.
MATERIALS and METHODS
Monocytes
Blood was obtained from healthy donors by leukopheresis. Monocytes were isolated and purified by counter current elutriation leading to 99% purity. To verify the quality of the monocyte preparations, FACS analysis was performed to assess the surface markers CD14, CD19, CD3 and CD4. Monocytes were kept in RPMI supplemented with L-glutamine and 10% FBS. For adhesion assays monocytes were stained with Calcein according to the manufacturer’s protocol (Invitrogen). Briefly, 12.5µM Calcein was added to a cell suspension followed by incubation for 30 minutes at room temperature. The cells were washed twice before use in adhesion assays. All experiments were carried out using cells from a minimum of three different donors.
Brain endothelial BB19 cells
The human brain capillary endothelial BB19 cell line was a generous gift of Drs. J. Joseph (NIH) and J. Nelson (Oregon Health Sciences University). This cell line was derived from primary brain microvascular endothelial cells (BMVEC) immortalized through transformation with the E6 and E7 genes of the human papilloma virus type 16 (13, 14). BB19 cells were cultured in endothelial basal medium (EBM) supplemented with growth factors (BBE, hydrocortisone, hEGF, FBS and gentamicin/amphotericin-B; Cambrex, Lonza) and were utilized for a maximum of 6 passages. Cells were seeded in T-75 flasks precoated with 2µg/cm2 fibronectin at a density of 7,500 cells/cm2 and cultured at 37ºC with 5% CO2 and saturated humidity. Cell culture medium was changed every two days. Cells attached to the cell culture surface within one day and started to grow after the second day of culture. The cells were passaged every five to six days or when approximately 80% confluent. To activate the endothelial cells IL-1β, TNF-α (5ng/ml) or gp120 (1µg/ml) were added to appropriate wells for 4 hours at 37ºC in a CO2 incubator.
Reagents
Recombinant TYBE gp120 was provided by Dr. R. Doms (University of Pennsylvania). Recombinant LAV gp120 was obtained from the National Institutes of Health AIDS Research and Reference Reagent proteins. Other reagents were AMD3100 (Sigma), Calcein (Invitrogen), SDF-1α (PeproTech Inc.), PP2 (Calbiochem) and recombinant human ICAM-1 or VCAM-1 (R&D Systems).
Adhesion assay
BB19 cells were seeded in 96 well plates at 5×104 cells per well and grown for 2 to 3 days at 37ºC in CO2 incubator with fresh media added after 24 hours. When indicated, the endothelial cells were activated as described above. The cells in each well were then washed twice with PBS Ca+Mg+. Calcein labeled monocytes, cultured with or without SDF-1α (200ng/ml) were added to the desired wells and incubated for 45 minutes at 37ºC. The non-adherent cells were washed away and plates were read with a fluorescence plate reader using 485/530nm excitation/emission filter sets. Average percent adhesion was calculated as: [(RFU after wash) / (RFU before wash)] X 100. For adhesion assays using recombinant proteins, the 96 well plates were coated with recombinant human ICAM-1 or recombinant human VCAM-1 or purified BSA overnight at 4ºC. Calcein labeled monocytes were incubated in the recombinant protein-coated wells for 90 minutes at 37°C. The rest of the procedure was followed as described above for endothelial cell adhesion assays.
Migration assay
1×105 monocytes cultured in RPMI with 2% FBS were placed in the upper chamber of a Transwell (Costar). The lower chamber was filled with media containing SDF-1α (). Dose response analysis demonstrated a typical U-shaped curve with maximal migration at 200ng/ml (data not shown) and that concentration was therefore chosen to most effectively carry out the inhibitory studies as well as other experiments. Monocytes were allowed to migrate through the polycarbonated membrane for 2 hours at 37ºC. The cells in the lower chamber were collected and counted by FACS at top speed for 1 minute. The chemotactic index (CI) was determined using the formula: CI = (number of cells migrated in the presence of SDF-1α) / (number of cells migrated in the absence of SDF-1α).
Transendothelial migration
BB19 cells were seeded on 6.5mm diameter and 5µm pore size polycarbonate microporous membranes of Transwell chambers (Costar) that were precoated with human fibronectin. 100µl of EC medium were added to the top chamber and 600µl were added to the bottom chamber. After 4 days of culture, the monolayer was assessed for transendothelial electrical resistance using EVOM voltmeter (World Precision Instruments) which showed a resistance pattern in the range of ~60 Ohms, consistent with prior studies of solute transport (14). Prior to the transendothelial migration the media was removed and the inserts were transferred to a new 24 well plate containing 600µl of fresh RPMI supplemented with 2% FBS. 100µl of monocytes (1×105) were added on the monolayer. After 4 hours the media in the bottom chamber containing migrated monocytes were collected and counted by FACS. Also, monocytes attached to the bottom of the Transwells were stained with Diff Quik and counted under the microscope. The chemotactic index was calculated as described above.
siRNA transfection
Double stranded RNA oligonucleotides were purchased from Dharmacon, Inc., Lafayette, Colorado, USA. Four short interfering RNAs targeting human Lyn as well as four nonspecific scrambled siRNA (negative control) were designed by Dharmacon. Double stranded RNA was resuspended at a final concentration of 20µM in 1x siRNA universal buffer. Aliquots were kept at −80ºC. Monocytes were transfected with a nucleofector device type I using Human Monocyte Nucleofection kits and protocols, all provided by Amaxa, Inc. In brief, freshly isolated monocytes were suspended in Human Monocyte Nucleofector solution at a final concentration of 6×106 monocytes / 100µl. For each transfection 100µl monocytes solution was subsequently mixed with 5µg of siRNA and transferred into Amaxa-certified cuvettes. Cells were pulsed using the program Y-01 of the nucleofector-I. Immediately after transfection, 1ml of pre-warmed media was added to the cells. The transfected monocytes were transferred to one well of a 12-well plate using specific pipette tips as provided in the Amaxa kit. The transfected monocytes were allowed to recover for 48 hours in a humidified 37ºC / 5% CO2 incubator. Such a protocol resulted in approximately 80% cell survival.
Western blotting
Gene silencing in monocytes transfected with either scrambled siRNA or Lyn-siRNA was analyzed by Western blotting. The cells were washed twice with cold PBS before being lysed in Reporter lysis buffer (Promega). 30µg of total proteins were loaded per well and separated on a 7% sodium dodecyl sulfate-polyacrylamide gel by electrophoresis (SDS-PAGE) for 90 minutes at 100 volts. Proteins were subsequently transferred to a nitrocellulose membrane (Bio-Rad Laboratories) overnight at 30 volts. The nitrocellulose membranes were probed with Lyn monoclonal antibody at a concentration of 1:2000 (Upstate Biotechnology). The secondary antibody was a goat-anti-rabbit antibody conjugated to horseradish peroxidase (HRP) used at a concentration of 1:5000 (Cell Signaling Technology). The proteins were revealed by chemiluminescence using the ECL kit (Amersham). For loading control, the membranes were stripped and reprobed with GAPDH antibody at a concentration of 1:2000 (Cell Signaling Technology). Lyn expression was determined relative to GAPDH, based on densitometry.
Antibodies
The antibodies used for flow cytometry and their suppliers were as follows: monoclonal antibody 24 (mAb24) for the active conformation of human β2 integrins was kindly provided by Dr. N. Hogg (London Research Institute), IgG1 control mAb (BD Pharmingen), goat F(ab’)2 antimouse IgG-PE secondary antibody (Caltag Laboratories), FITC-conjugated anti-human ICAM-1/CD54 and control IgG1 mAbs (R&D Systems). For adhesion blocking an unconjugated anti-human ICAM-1/CD54 antibody (R&D Systems) was used. For Western blotting anti-Lyn (Upstate Biotechnology) and anti-GAPDH (Cell Signaling Technology) antibodies were used.
FACS analysis
Immunofluorescence staining was performed according to standard procedures. Briefly, 2×105 cells per sample were incubated with mAb24 or control mAb at 37ºC in Mg+ buffer for 30 minutes. The cells were then washed with ice cold PBS 1% FBS, incubated with PE-conjugated goat F(ab’)2 antimouse IgG at 4ºC for 30 minutes, washed again, fixed with 1% formaldehyde and then analyzed on a FACScan flow cytometer. ICAM staining was carried out similarly except that FITC-conjugated primary antibodies were used.
RESULTS
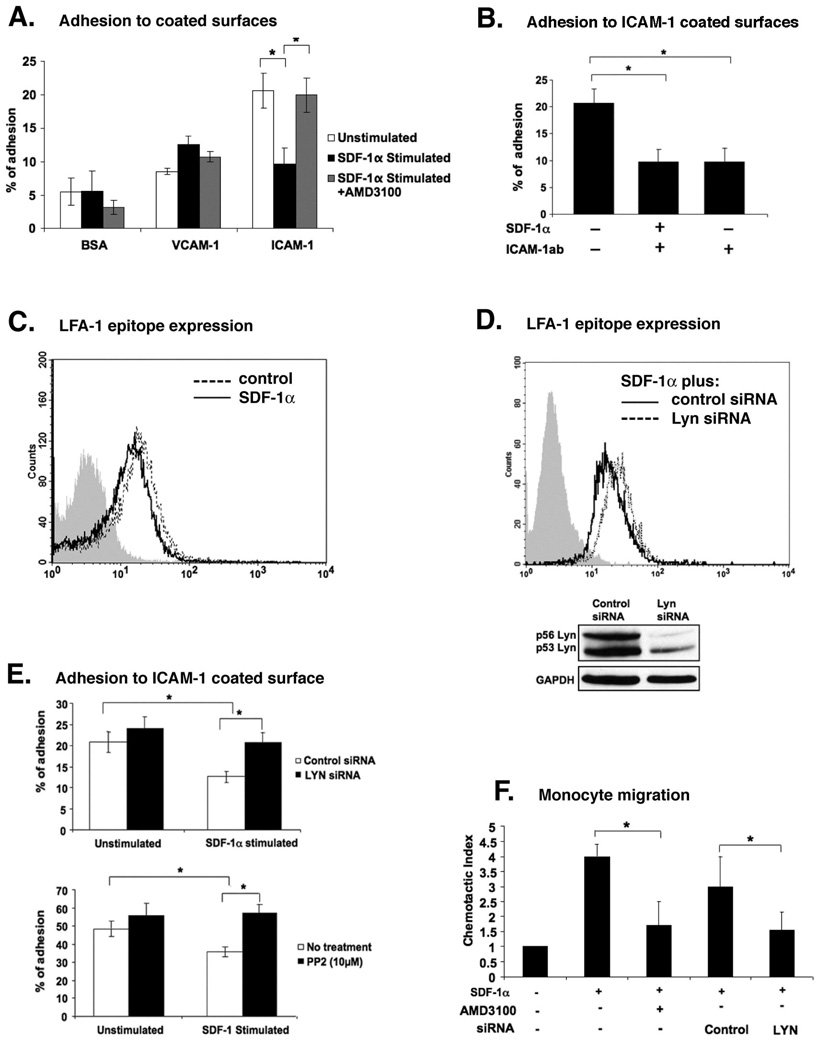
We first investigated the effect of SDF-1α on attachment of primary human monocytes to the surfaces coated with ICAM-1 (ligand for β2 integrin), VCAM-1 (ligand for β1 and β7 integrins) or BSA (control) (15). Attachment of monocytes was highest on ICAM-1 and lowest on BSA, with intermediate attachment observed for VCAM-1 (Figure 1A). Treatment of monocytes with SDF-1α suppressed the increased adhesion to ICAM-1 but had no effect on attachment to VCAM-1. The effect of SDF-1α could be reversed by pretreatment of monocytes with the specific CXCR4 antagonist AMD3100 (Figure 1A). Furthermore, incubation of anti-human ICAM-1 antibody with ICAM-1 coated surfaces prior to addition of monocytes blocked attachment, confirming specific binding to ICAM-1 (Figure 1B). Together, these data indicate that SDF-1α through its receptor CXCR4 down regulated monocytes adherence to surfaces coated with ICAM-1.
Figure 1. CXCR4-Lyn signaling axis is a positive regulator of monocyte migration and a negative regulator of LFA-1–mediated attachment to surfaces coated with ICAM-1.
(A) Effect of SDF-1α stimulation on the attachment of human monocytes to surfaces coated with ICAM-1, VCAM-1 or BSA (control) was assessed using adhesion assay. Pre-treatment of monocytes with AMD3100, a specific antagonist for the SDF-1α receptor CXCR4, was used as control. (B) Anti-ICAM-1 mAb inhibits monocyte attachment to surfaces coated with ICAM-1. ICAM-1 coated wells were pre-incubated for one hour using 25µg/ml of antibody before adding monocytes. (C) FACS analysis of β2 integrin-activation epitope expression in unstimulated (dotted line) and SDF-1α-stimulated monocytes (solid line). Cells were stained with anti-integrin mAb24, or with isotype control (gray histogram). (D) FACS analysis of β2 integrin-activation epitope expression in SDF-1α-stimulated monocytes that were transfected with either control siRNA (solid line) or Lyn siRNA (dotted line). Cells were stained with anti-integrin mAb24 or isotype control (gray histogram). Lower panel: Western blot showing the expression levels of Lyn kinase in monocytes at 48 hours following transfection with Lyn siRNA or control siRNA. (E) Attachment of SDF-1α-stimulated or unstimulated monocytes to surfaces coated with ICAM-1 at 48 hours post transfection with Lyn siRNA or control siRNA (upper panel). Attachment of PP2 treated and untreated monocytes, in the presence or absence of SDF-1α, to surfaces coated with ICAM-1 (lower panel). (F) Chemotaxis assay showing the migration of monocytes towards SDF-1α gradient. Monocytes were pre-treated with or without AMD3100 or transfected with control or Lyn siRNA. All data show means ±SEM of three independent experiments using cells from different donors, or FACS profiles or Western blots representative of three independent experiments. *p<0.05.
Since ICAM-1 is a ligand for the β2 integrin LFA-1 (16), it seemed plausible to examine the expression of an activation-dependent epitope of β2 integrin in SDF-1α-stimulated and unstimulated monocytes. Therefore, we employed FACS analysis using mAb24, which recognizes activated β2 integrins (17). Our results show that activation state dependent conformational epitope for β2 integrin expression was decreased in SDF-1α stimulated cells (Figure 1C). The Src family kinase Lyn was previously identified as a mediator of inside-out signaling pathway that relays signals from the chemokine receptor CXCR4 to β2 integrin LFA-1 in hematopoietic stem/progenitor cells (18). Therefore, we examined whether Lyn was involved in SDF-1α mediated down regulation of LFA-1 in human primary monocytes. Using siRNA approach we were able to achieve approximately 80% reduction in Lyn expression in primary human monocytes as shown by Western blot (Figure 1D, lower panel). Evaluation of β2 integrin expression in monocytes following SDF-1α stimulation showed that Lyn depletion led to increased expression of the activation state dependent conformational β2 integrin epitope (Figure 1D, upper panel). In parallel, we also tested the adherence of Lyn-depleted cells to surfaces coated with ICAM-1 (Figure 1E, upper panel). Control siRNA transfected monocytes showed down-regulation of adhesion following SDF-1α stimulation (white bars). The loss of Lyn expression in monocytes, however, prevented down-regulation of cell adherence to ICAM-1 in response to SDF-1α (black bars). To further address the question of the involvement of Lyn kinase activity in CXCR4-regulated inhibition of attachment to ICAM-1, we examined the effect of pyrazolopirimidine (PP2) on SDF-1α induced inhibition of monocytes attachment of ICAM-1 (Figure 1E, lower panel). PP2 inhibits Src family kinases (19) and has been shown to completely inhibit tyrosine phosphorylation of Lyn at the concentration of 10µM (20). Pretreatment of monocytes with PP2 prevented the down-regulation of adhesion to ICAM-1 in response to SDF-1α (black bars) as compared to the untreated monocytes (white bars). Combined, these data indicate that SDF-1α/CXCR4 signaling axis inhibits LFA-1 mediated adhesion of monocytes to ICAM-1 through Lyn kinase.
We then examined whether SDF-1α could elicit chemotactic response in monocytes. Concomitant increase in the chemotactic index towards SDF-1α gradient was observed (Figure 1F) which could be blocked by treatment with AMD3100. Furthermore, the selective depletion of Lyn by siRNA potently inhibited SDF-1α induced cell migration (Figure 1F). We also observed inhibition of migration towards SDF-1α following treatment with PP2 (data not shown). Together, these results support the role of SDF-1α as a positive regulator of monocytes migration and a negative regulator of monocyte adhesion via the Lyn kinase.
CXCR4 also serves as an entry coreceptor for X4 strains of HIV-1 but in addition to supporting viral entry, CXCR4 ligation by the HIV-1 envelope glycoprotein gp120 can activate intracellular signals in macrophages and trigger inflammatory mediator release (21, 22). We hypothesized that X4 gp120 might also elicit chemotaxis in monocytes through CXCR4-induced signaling pathway. Interestingly, we failed to see any increase in chemotaxis of monocytes in response to recombinant X4 gp120 or in response to whole virions, nor did X4 gp120 affect the attachment of monocytes to ICAM-1 or VCAM-1 (data not shown).
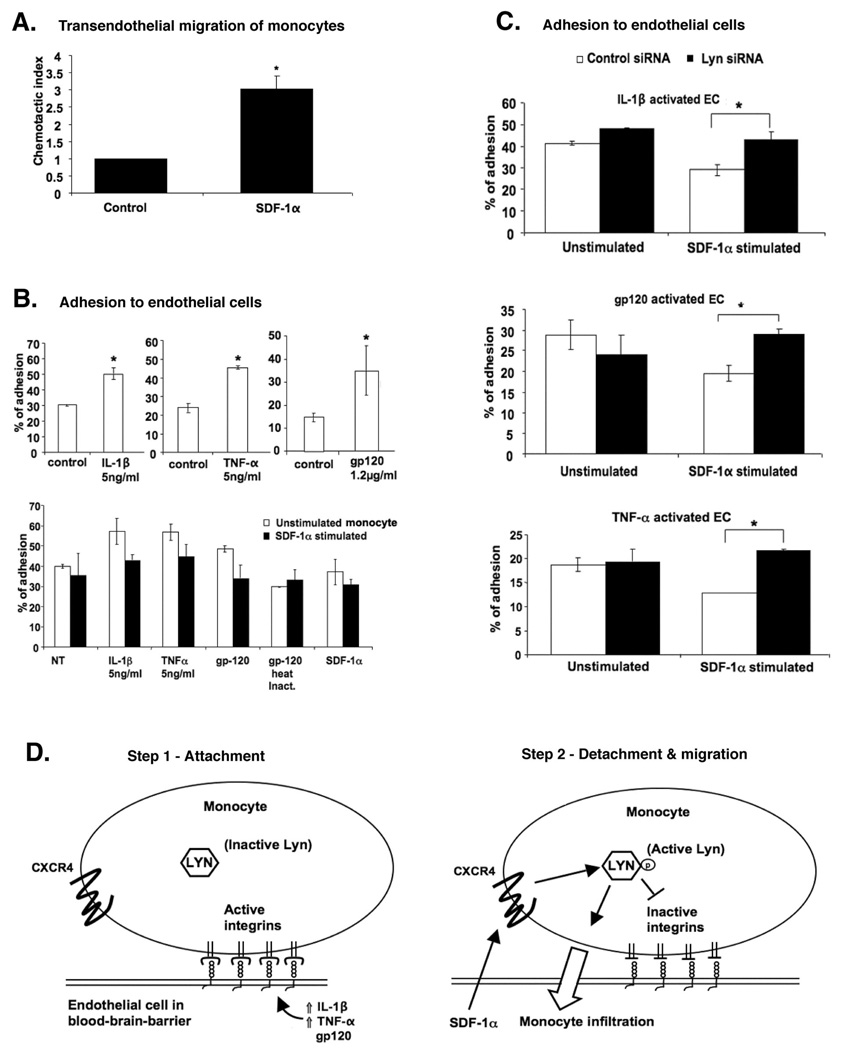
To further extend the data obtained above, we then tested the transendothelial migration of monocytes across a layer of BMVEC, utilizing the transformed BB19 cell line. Monocytes were allowed to migrate across the endothelial monolayers for 4 hours in the presence of SDF-1α. Approximately 3-fold increase in the migration of monocytes across endothelial monolayers was observed in response to SDF-1α gradient (Figure 2A). Next we examined the adhesion of monocytes onto endothelial cells. It has been shown that expression of ICAM-1 is up-regulated on the surface of BMVEC cells upon activation with pro-inflammatory cytokines or with recombinant HIV-1 gp120 (23, 24). In agreement with the data obtained using purified substrate, untreated monocytes showed increased attachment on the activated BB19 cells by approximately 2-fold as compared to the unactivated endothelial cells (Figure 2B, upper panel). Stimulation of monocytes with SDF-1α decreased attachment to activated BMVEC (Figure 2B, lower panel). As control, monocytes attachment to SDF-1α-treated BMVEC was also analyzed, but SDF-1α activation of BMVEC did not effect the attachment of monocytes (data not shown). We then showed that the inhibition of attachment resulting from monocyte treatment with SDF-1α was clearly due to the activation of Lyn kinase, as cells depleted of Lyn by siRNA did not show a reduction of adhesion to BMVEC following SDF-1α treatment (Figure 2C). This indicates that Lyn, when activated by SDF-1α through CXCR4, acts as a negative regulator of monocytes attachment on activated BMVEC that express high level of ICAM-1. These results are consistent with the data obtained with the purified ICAM-1 substrate (Figure 1).
Figure 2. CXCR4-Lyn signaling inhibits attachment of monocytes to activated BB19 brain microvascular endothelial cells and promotes transendothelial migration towards SDF-1α.
(A) Transendothelial migration assay showing the migration of monocytes towards SDF-1α gradient through monolayers of unstimulated BB19 cells. (B) Attachment of unstimulated or SDF-1α-stimulated monocytes to BB19 cells activated with IL-1β, TNF-α or HIV-1 gp120 was performed as described in Materials and Methods. (C) Attachment of SDF-1α-stimulated or control monocytes to activated BB19 was carried out 48 hours after transfection with either Lyn siRNA or control siRNA. Values in panels A-C represent means ± SEM of three independent experiments using cells from different donors; *p<0.05. (D) A two-step model for the increased infiltration of monocytes across inflamed BBB in response to SDF-1α. Left: Upregulation of ICAM-1 expression on BMVEC due to activation of these cells with IL-1β, TNF-α or gp120 promotes arrest and firm attachment of circulating monocytes via β2 integrin/ICAM-1 interactions. Right: SDF-1α activates Lyn through CXCR4 on monocytes. Lyn activation medates monocyte chemotaxis; in addition, β2 integrin-mediated adhesion is transiently destabilized through inside-out signaling, thereby loosening tight monocyte attachment and facilitating transendothelial migration through blood-brain barrier.
DISCUSSION
The present study indicates that the Src family kinase Lyn is an important part of a regulatory network that couples SDF-1α/CXCR4-induced monocyte chemotactic signals with downregulation of β2 integrin/LFA-1-dependent adhesion to activated brain microvascular endothelial cells (Figure 2D). SDF-1α triggers monocyte chemotaxis and decreases β2 integrin-mediated attachment to activated BB19 BMVEC, whereas blockade of Lyn expression with siRNA in monocytes results in increased attachment and decreased monocyte movement following SDF-1α stimulation. Thus, we demonstrate that these cellular effects are associated with Lyn-dependent inside-out LFA-1 integrin signaling. Consistent with these findings, it has been previously shown that Lyn can relay signals from CXCR4 to LFA-1 in normal hematopoietic cells (18) and that the BCR-ABL oncoprotein can disrupt this signaling pathway in malignant leukemic progenitors (25).
Our results suggest a mechanism by which SDF-1α could play an important role in attracting monocytes into the CNS. The chemokine SDF-1α is over-expressed in astrocytes and neurons in patients with HAD and certain other neurological disorders (8–11). Moreover, in many neuroinflammatory processes, over-expression of the pro-inflammatory cytokines IL-1β and TNF-α is seen as well (3, 26). Stimulation of microvascular brain endothelial cells with these pro-inflammatory cytokines, as well as with HIV-1 gp120, results in the increased expression of adhesion molecules as ICAM-1 on these cells (23, 24). We confirmed that IL-1β, TNF-α and gp120 increase ICAM-1 expression on the surface of BB19 cells (data not shown). More importantly, even though donor variation led to differences in the absolute level of monocyte adhesion (eg; Fig 2B versus 2C), endothelial cell activation consistencly enhanced monocyte adhesion through pathways that were regulated by SDF-1α and Lyn.
In the absence of endothelial cells to which monocytes may attach (Figure 1F), or if endothelial cells are present and not activated (Figure 2A), SDF-1α acts as a chemoattractant. If endothelial cells are present and activated, increased monocyte adherence will occur (Figure 2B). In this setting, for SDF-1α to be effective in recruiting monocytes, it is likely that both chemoattraction and detachment from the activated endothelial cells are necessary. We suggest that in the setting of inflammation and SDF-1α-mediated chemoattraction, whereby circulating blood monocytes attach through the LFA-1 integrin to activated BMVEC expressing high levels of ICAM-1, the SDF-1α/CXCR4/Lyn-mediated down-regulation of β2 integrin affinity represents one mechanism by which monocytes are aided in their migration across the brain microvascular endothelial barrier. When Lyn is activated through SDF/CXCR4 activation, β2 integrin-mediated monocyte adhesion is transiently destabilized through inside-out signaling, thereby loosening attachments and facilitating movement across the endothelial cell barrier. Of note, signaling through chemokine receptors has been suggested to result in preferential localization of activated Src kinases to the leading edge of the cells (27), likely explaining the observation that only a small decrease in the expression of activation dependent epitope LFA-1 was detected by FACS in our experiments.
In addition to SDF-1α, multiple other chemokines are overexpressed in neuroinflammatory disorders including MCP-1, MIP-1β, and others (28, 29), and pathogenic roles are supported by studies using animal and in vitro model systems (30–32). Whether the mechanism identified here, by which Lyn mediates SDF-1α/CXCR4 regulation of monocyte attachment and migration, is involved in responses to other chemokines will require further study. Similarly, the precise molecular mechanism that links Lyn to the conformational activativity of LFA-1 in SDF-1α/CXCR4-stimulated monocytes remains to be defined. Inside-out regulation of integrins is typically mediated through Rap 1 regulation of talin binding to the integrin cytoplasmic tail, but the specific upstream linkages can differ among various agonists that regulate inside-out integrin affinity (33–35)(36). Finally, the reason that X4 gp120 failed to modulate adhesion and migration in primary human monocytes is uncertain, but is consistent with previous observations by others (37, 38).
Based on these observations, we propose a two phase model for the regulation of monocyte movement across the BBB in response to SDF-1α in the setting of neuroinflammatory disorders (Figure 2D). In the first phase, the monocytes circulating in the blood may become firmly attached through the LFA-1 integrin to the activated BMVEC expressing high levels of ICAM-1. In the second phase, SDF-1α activation of CXCR4 leads to decreased ICAM-1-binding activity of monocyte LFA-1 through inside-out signaling mediated by Lyn kinase, along with monocyte chemotaxis towards the SDF-1α gradient, enabling migration across the blood-brain barrier into brain.
Acknowledgments
This work was supported by NIH grants R01MH61139 and P01NS27405 (to R. Collman) and R01CA108552 (to A. Ptasznik).
We thank Dr. N. Hogg (London Research Institute) for mAb24, Dr. R. Doms (University of Pennsylvania) for providing recombinant gp120, and J. Joseph (NIH) and J. Nelson (Oregon Health Sciences University) for BB19 cells. We also thank the Immunology and Viral/Molecular Cores of the Penn Center for AIDS Research for valuable assistance.
Footnotes
Conflicts
The authors declare no conflict of interest or financial interests
REFERENCES
- 1.Vazeux R, Lacroix-Ciaudo C, Blanche S, Cumont M-C, Henin D, Gray F, Boccon-Gibod L, Tardieu M. Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am.J.Pathol. 1992;140:137–144. [PMC free article] [PubMed] [Google Scholar]
- 2.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 3.Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 2005;7:1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- 4.Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Greenberg RN, Berger JR. Neuroimaging correlates of HIV-associated BBB compromise. J Neuroimmunol. 2004;157:140–146. doi: 10.1016/j.jneuroim.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Khan NA, Di Cello F, Stins M, Kim KS. Gp120-mediated cytotoxicity of human brain microvascular endothelial cells is dependent on p38 mitogen-activated protein kinase activation. J Neurovirol. 2007;13:242–251. doi: 10.1080/13550280701286531. [DOI] [PubMed] [Google Scholar]
- 6.Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 7.Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, Graves MC, Witte M, Kim KS. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol.Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 8.Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, Hunter DD, Nath A, Hedreen JC, Navia BA. SDF-1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62:617–626. doi: 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- 9.Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 10.Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39. doi: 10.1016/j.jneuroim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prudhomme JG, Sherman IW, Land KM, Moses AV, Stenglein S, Nelson JA. Studies of Plasmodium falciparum cytoadherence using immortalized human brain capillary endothelial cells. Int.J.Parasitol. 1996;26:647–655. doi: 10.1016/0020-7519(96)00027-6. [DOI] [PubMed] [Google Scholar]
- 14.Kusch-Poddar M, Drewe J, Fux I, Gutmann H. Evaluation of the immortalized human brain capillary endothelial cell line BB19 as a human cell culture model for the blood-brain barrier. Brain Res. 2005;1064:21–31. doi: 10.1016/j.brainres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 16.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 17.Stewart MP, Cabanas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- 18.Nakata Y, Tomkowicz B, Gewirtz AM, Ptasznik A. Integrin inhibition through Lyn-dependent cross talk from CXCR4 chemokine receptors in normal human CD34+ marrow cells. Blood. 2006;107:4234–4239. doi: 10.1182/blood-2005-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 20.Ptasznik A, Urbanowska E, Chinta S, Costa MA, Katz BA, Stanislaus MA, Demir G, Linnekin D, Pan ZK, Gewirtz AM. Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells. J Exp Med. 2002;196:667–678. doi: 10.1084/jem.20020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Corno M, Liu QH, Schols D, Clercq Ede, Gessani S, Freedman BD, Collman RG. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood. 2001;98:2909–2916. doi: 10.1182/blood.v98.10.2909. [DOI] [PubMed] [Google Scholar]
- 22.Lee C, Tomkowicz B, Freedman BD, Collman RG. HIV-1 gp120-induced TNF-{alpha} production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathways. J Leukoc Biol. 2005;78:1016–1023. doi: 10.1189/jlb.0105056. [DOI] [PubMed] [Google Scholar]
- 23.Stins MF, Pearce D, Cello FDi, Erdreich-Epstein A, Pardo CA, Sik Kim K. Induction of intercellular adhesion molecule-1 on human brain endothelial cells by HIV-1 gp120: role of CD4 and chemokine coreceptors. Lab Invest. 2003;83:1787–1798. doi: 10.1097/01.lab.0000107008.13321.c8. [DOI] [PubMed] [Google Scholar]
- 24.Hess DC, Bhutwala T, Sheppard JC, Zhao W, Smith J. ICAM-1 expression on human brain microvascular endothelial cells. Neurosci Lett. 1994;168:201–204. doi: 10.1016/0304-3940(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 25.Chen YY, Malik M, Tomkowicz BE, Collman RG, Ptasznik A. BCR-ABL1 alters SDF-1alpha-mediated adhesive responses through the beta2 integrin LFA-1 in leukemia cells. Blood. 2008;111:5182–5186. doi: 10.1182/blood-2007-10-117705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, Epstein LG, Gendelman HE. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: Implications for the neuropathogenesis of HIV disease. J.Exp.Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics. 2007;4:590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J.Immunol. 1998;161:6896–6903. [PubMed] [Google Scholar]
- 31.Sanders VJ, Mehta AP, White MG, Achim CL. A murine model of HIV encephalitis: xenotransplantation of HIV-infected human neuroglia into SCID mouse brain. Neuropathol Appl Neurobiol. 1998;24:461–467. doi: 10.1046/j.1365-2990.1998.00145.x. [DOI] [PubMed] [Google Scholar]
- 32.Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am.J.Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 34.Kliche S, Breitling D, Togni M, Pusch R, Heuer K, Wang X, Freund C, Kasirer-Friede A, Menasche G, Koretzky GA, Schraven B. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol Cell Biol. 2006;26:7130–7144. doi: 10.1128/MCB.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regelmann AG, Danzl NM, Wanjalla C, Alexandropoulos K. The hematopoietic isoform of Cas-Hef1-associated signal transducer regulates chemokine-induced inside-out signaling and T cell trafficking. Immunity. 2006;25:907–918. doi: 10.1016/j.immuni.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Banno A, Ginsberg MH. Integrin activation. Biochem Soc Trans. 2008;36:229–234. doi: 10.1042/BST0360229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang JM, Ueda H, Howard OM, Grimm MC, Chertov O, Gong X, Gong W, Resau JH, Broder CC, Evans G, Arthur LO, Ruscetti FW, Oppenheim JJ. HIV-1 envelope gp120 inhibits the monocyte response to chemokines through CD4 signal-dependent chemokine receptor down-regulation. J.Immunol. 1998;161:4309–4317. [PubMed] [Google Scholar]
- 38.Wahl SM, Allen JB, Gartner S, Orenstein JM, Popovic M, Chenoweth DE, Arthur LO, Farrar WL, Wahl LM. HIV-1 and its envelope glycoprotein down-regulate chemotactic ligand receptors and chemotactic function of peripheral blood monocytes. J.Immunol. 1989;142:3553–3559. [PubMed] [Google Scholar]