Abstract
The insulin-like growth factor (IGF) family consists of ligands (IGF-I, IGF-II, insulin), several receptors (including IGF-1R), and six binding proteins (IGFBP-1 through IGFBP-6). Members of this family regulate key cellular activities and they also play an important role in the development and progression of both adult and childhood cancers. Binding of a ligand to the receptor leads to its activation, followed by signal transduction along several pathways. In some childhood malignancies, IGF-1R can be activated by endocrine, autocrine, or paracrine mechanisms. Although mutations in IGF-1R have not been identified, this signaling pathway is upregulated in many childhood cancers. These findings have led to the development of a host of IGF-1R signaling modulators that are currently being tested in clinical trials. This review explores the role of IGF-1R in a range of childhood malignancies.
Keywords: IGF-1R, Pediatric malignancy, Molecular targeting, Therapeutic antibody
LIGANDS AND BINDING PROTEINS
Three ligands are involved in insulin-like growth factor (IGF) signaling: IGF-I, IGF-II, and insulin. The latter has been reviewed extensively and is not discussed here. IGF-I and IGF-II form the basis of signaling in the IGF pathway. Epidemiological studies first suggested that high levels of IGF-I correlated with a higher risk for cancer [1, 2]. Conversely, patients who have a congenital deficiency of IGF-I seem to be protected from the development of malignancies [3]. Modulation of IGF family members in various animal models, using genetic manipulation or pharmaceutical treatment, has supported this hypothesis and also extended its role to disease progression and tumor spread [4]. IGF-II is involved in normal growth, and higher levels of IGF-II in tumors and cancer cell lines suggest its importance in carcinogenesis [5–8].
Both of these ligands exist as complexes in the circulatory system, bound to one of six IGF binding proteins (IGFBP-1 to IGFBP-6) [9]. IGFBP-3, in conjunction with a third molecule, acid labile subunit, forms a complex that accounts for the majority of circulating IGF [1, 4, 9]. IGFBPs have a higher affinity for IGF than their cognate receptors, suggesting that they sequester IGF from the receptor. However, other models indicate that the binding proteins may potentiate IGF activity, either by extending its half-life in circulation or by binding to certain molecules on the cell surface, thus providing a reservoir of available IGF to the cell [4, 9].
IGF RECEPTORS
Three receptors form six distinct receptor combinations: IGF receptors type 1 and type 2 (IGF-1R and IGF-2R), insulin receptors A and B (IR-A and IR-B), and hybrid receptors (IGF-1R/IR-A and IGF-1R/IR-B) [10]. The possible role of insulin and hybrid receptors in human cancers has been reviewed [11]. IGF-2R preferentially binds IGF-II [5, 11]. However, IGF-2R lacks an intracellular kinase domain, precluding its ability to mediate cell signaling [4, 10]. This also helps explain the finding that loss of IGF-2R results in increased tumorigenicity, presumably by increasing the availability of IGF-II to bind to IGF-1R [12]. The remainder of this review focuses on IGF-1R.
All of the IGF receptors have significant homology, thus resulting in structural similarity and the possibility of signaling crosstalk [10]. IGF-1R is a tetrameric receptor consisting of two α-subunits and two transmembrane β-subunits that are linked by disulfide bonds [13, 14]. The α-subunits are extracellular and bind IGF. Each transmembrane β-subunit contains an intracellular tyrosine kinase domain.
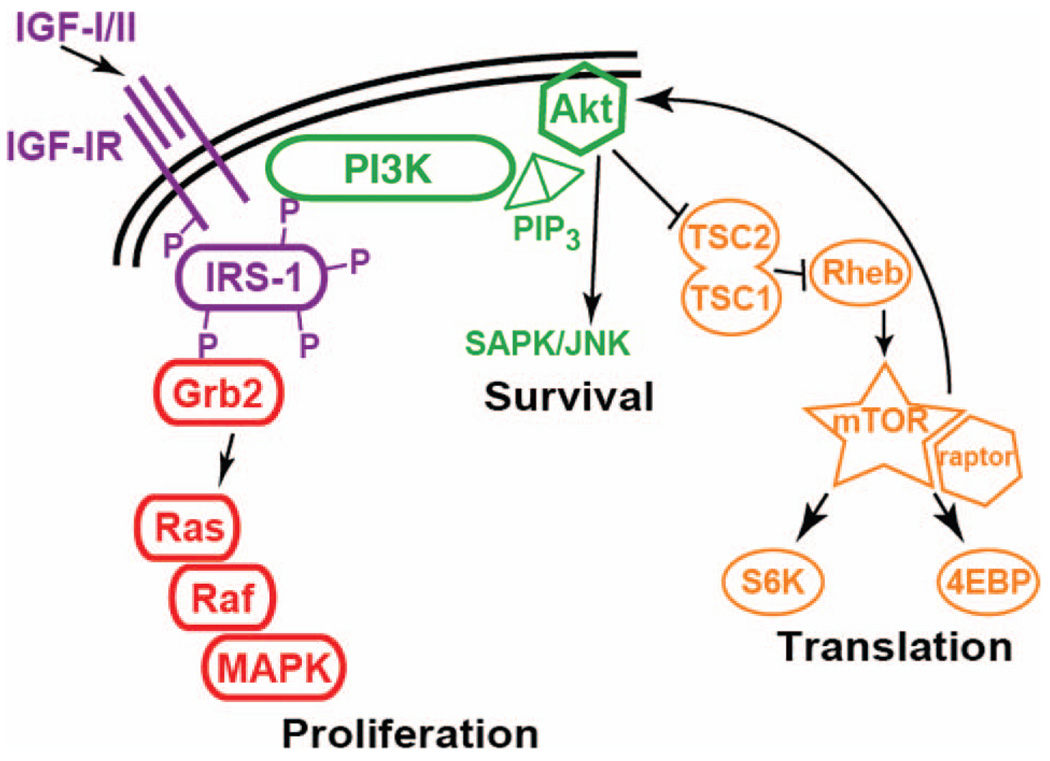
Binding of IGF-I or IGF-II ligand to IGF-1R leads to phosphorylation of three key tyrosine residues in the kinase domain, leading to phosphorylation of downstream substrates [13, 14]. In addition, phosphorylation of additional tyrosine residues in other areas of the β-subunit provide “docking sites” that allow for the recruitment of adaptor proteins [14]. Insulin receptor substrate family members are some of the many adaptor proteins that are known to have an important role in IGF-1R signaling [15]. Phosphorylation of adaptor proteins leads to binding of additional proteins, allowing for signal transduction along several specific pathways (Fig. 1). Some of the key pathways and their endpoints include phosphorylation of mitogen-activated protein kinase (MAPK) and a subsequent increase in proliferation, activation of phosphatidylinositol 3′ kinase (PI3K), leading to decreased apoptosis, and modulation of mammalian target of rapamycin (mTOR), resulting in translational adaptation [13–15].
Figure 1.
Downstream targets of IGF-1R. The binding of IGF-I or IGF-II to IGF-1R results in autophosphorylation of tyrosine residues in the receptor. This leads to activation of the kinase domain and also provides the docking sites necessary for adaptor proteins to bind, thereby beginning a diverse cascade of intracellular signaling. Pathways that have been well described include Grb2, which then signals though Ras, Raf, and ultimately MAPK, leading to cell proliferation. Additionally, recruitment of PI3K results in cell survival by diminishing apoptotic signals. More recently, investigators have shown that the mTOR pathway is important in regulating protein translation, and that inhibition of mTOR results in a feedback loop, leading to an increase in Akt phosphorylation.
Abbreviations: 4EBP, eukaryotic translation initiation factor 4E binding protein; Grb2, growth factor receptor-bound protein 2; IGF-1R, insulin-like growth factor receptor 1; IRS, insulin receptor substrate; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PIP3, phosphatidylinositol 3,4,5-triphosphate; PI3K, phosphatidylinositol 3′ kinase; Rheb, Ras homolog enriched in brain; SAPK/JNK, stress-activated protein kinase/c-Jun NH2-terminal kinase; SGK, ribosomal S6 kinase; TSC, tuberous sclerosis complex.
In normal cells, tyrosine kinase receptor activity is tightly regulated, allowing for homeostatic growth. In tumor cells, these same molecules are activated, either by mutation, chromosomal translocation, abnormal stimulation (autocrine, endocrine, or paracrine), or loss of genomic imprinting. For the IGF pathway, dysregulation of the latter two appears to be most relevant. There is considerable evidence for a key role of IGF signaling in many pediatric malignancies. Specifics for each tumor type are discussed in subsequent sections.
STRATEGIES TO TARGET IGF-1R
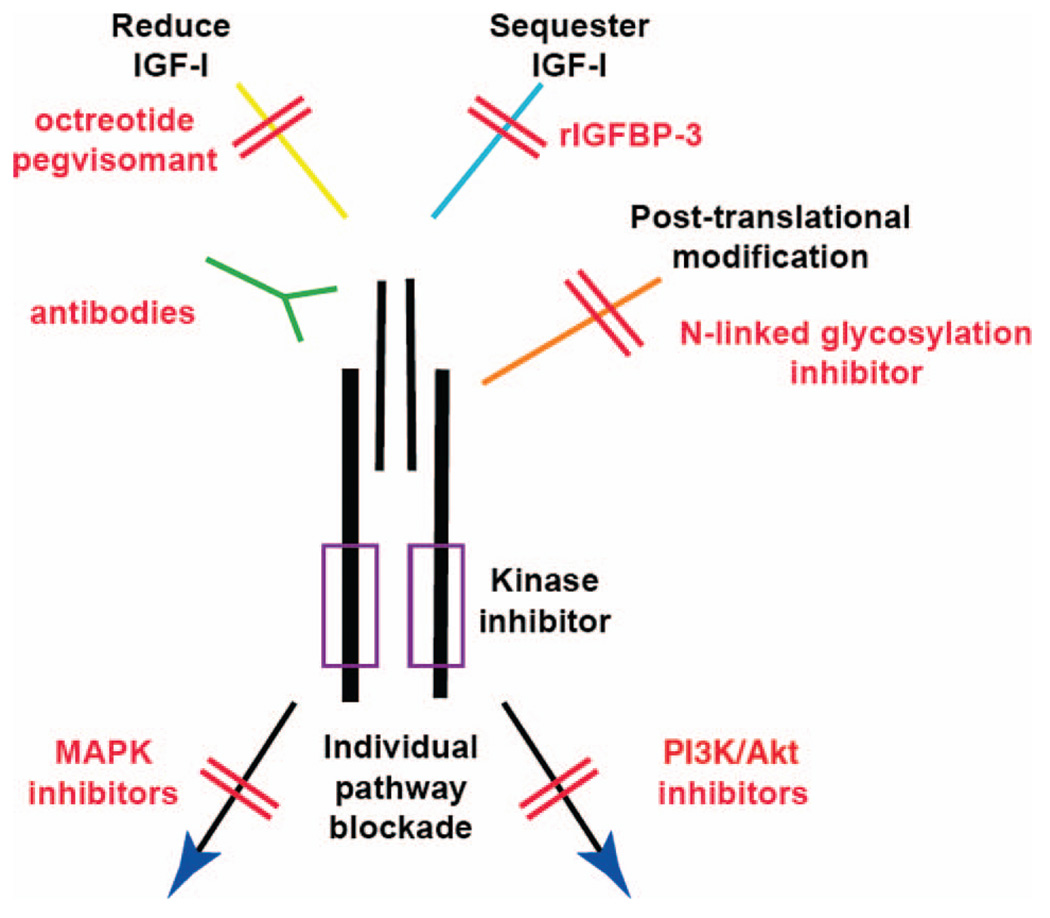
The IGF-1R signaling pathway can be inhibited at many points, from regulation of ligand to blockade of cellular signaling components (Fig. 2). There are several important points that must be considered to determine which tumor types are best suited for targeting. First, the tumor must express IGF-1R. With current technology, quantification of the number of receptors on the tumor is difficult. However, advances using sandwich enzyme-linked immunosorbent assay technology are being made to reliably quantitate expression. For rhabdomyosarcoma, a recent report shows a high correlation between the number of IGF-1R molecules on the surface of cells and response to its inhibition in mice [16]. Second, reliance upon IGF-1R should be shown, including demonstration of an intact signaling pathway and susceptibility of the tumor to its blockade. Ideally, an IGF-1R–directed pharmaceutical agent would be antitumorigenic when administered as a single agent. However, as the pathways that are affected by IGF-1R blockade in different tumor types are identified, rational therapeutic combinations can be investigated.
Figure 2.
Many opportunities to block IGF-1R activity.
Abbreviations: IGF, insulin-like growth factor; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3′ kinase; rIGFBP, recombinant insulin-like growth factor binding protein.
Reduction of Circulating IGF-I
The IGF pathway begins at the level of the hypothalamus and the release of growth hormone–releasing hormone and somatostatin [1, 4]. In the pituitary gland, these two factors promote or inhibit the secretion of growth hormone (GH) into the circulation. GH is the primary determinant of IGF-I synthesis and expression, most of which occurs in the liver. However, many physiological factors, such as nutrient deprivation, can also cause GH-independent production of IGF.
Excitement for targeting IGF-I grew in the 1980s after experiments in rats showed that hypophysectomy resulted in complete abrogation of osteosarcoma metastases, suggesting an endocrine effect on the metastatic cascade [17]. This led to a phase I study of octreotide pamoate long-acting release (OncoLar®; Novartis, East Hanover, NJ), a somatostatin analog that inhibits GH secretion, thereby decreasing IGF-I levels [18]. Patients with metastatic osteosarcoma treated with OncoLar did not have a longer survival duration. However, only 50% inhibition of circulating IGF-I was obtained in these patients. Similar results were seen when OncoLar was given in combination with chemotherapy to treat pet dogs with primary osteosarcoma [19]. Unfortunately, multiple attempts to pursue this in preclinical models have been hampered by the inability to further reduce the concentration of circulating IGF levels to those obtained with hypophysectomy.
Pegvisomant is a pegylated growth hormone receptor (GHR) antagonist that binds GHR but does not activate downstream signaling [20]. It is currently indicated for the treatment of acromegaly, in which patients have elevated GH levels, increased GHR activation, and overproduction of IGF-I. Clinical pharmacodynamic studies revealed that daily dosing reduced the mean total IGF-I concentration by 62%.
These findings continue to demonstrate the major downfall of this approach, which is the inability to completely suppress levels of circulating IGF-I. Another theoretical concern is that, although IGF-I reduction may be effective in mice, it is unclear if these findings will extend to humans, who have high levels of IGF-II [5]. Innovative approaches to decrease IGF or increase IGFBP are necessary to obtain suppression to therapeutically relevant low concentrations. The relative contributions of IGF-I and IGF-II to tumorigenesis have been difficult to study in murine models because of the lack of IGF-II in adult mice [5]. However, the use of specific IGF-I and IGF-II antibodies will aid in distinguishing the importance of these two ligands in the autocrine setting using animal models [21].
DNA/RNA Inhibitors
Laboratory researchers have used various methods of DNA or RNA interference to downregulate transcription and translation of target genes with a high degree of success. In rat glioblastoma cells, inhibition of IGF-I transcription using a triple-helix strategy led to a dramatic reduction in tumor formation [22]. Similar results were obtained using antisense RNA targeting of IGF-1R [23]. A host of other interference techniques are available and the value of these methods in the laboratory cannot be understated [24]. Efforts to make these reagents effective in humans must concentrate on decreasing their susceptibility to degradation, increasing their uptake by cells, and developing innovative techniques to allow for preferential uptake by tumor cells.
Small Molecule Kinase Inhibitors
Small molecule tyrosine kinase inhibitors interfere with the tyrosine kinase domain’s ability to phosphorylate target molecules, halting the propagation of intracellular signaling. NVP-AEW541 was the first of the small molecule IGF-1R kinase inhibitors [25]. Of three pediatric sarcomas tested, Ewing’s sarcoma cells were the most sensitive to the effects of this compound [26]. Ewing’s sarcoma xenograft-bearing mice that were treated with NVP-AEW541 had delayed tumor formation. In addition, the rate of growth was markedly reduced when the compound was given in combination with vincristine [26].
When tested in neuroblastoma models, treated cells had inhibition of proliferation and induction of apoptosis [27]. Treatment of mice in vivo showed regression of established tumors, with no signs of microvascularization, which was in marked contrast to control mice that had a rich network of blood vessels [27]. This effect may have been mediated through Akt, because treatment resulted in a reduction of phosphorylated Akt, but less so for MAPK [27]. Another small molecule IGF-1R kinase inhibitor, ADW742, also induced downregulation of Akt and mTOR, but not MAPK [28]. These findings suggest that preclinical testing should examine the ability to potentiate IGF-1R blockade with MAPK inhibition. In addition, although both of these compounds have a much higher affinity for IGF-1R than IR or other tyrosine kinases, it remains to be seen whether this specific affinity will be seen in patients.
Antibodies Targeting IGF-1R
Early studies using the murine IGF-1R–neutralizing antibody αIR3 showed a reduction in proliferation in vitro and tumor growth in vivo using various tumor models [29, 30]. However, the development of clinically applicable IGF-1R–specific antibodies was limited until humanized and fully human versions could be produced. Recently, many pharmaceutical companies have developed therapeutic antibodies that specifically target IGF-1R [31]. One of these, SCH717454, was tested in the Pediatric Preclinical Testing Program, a consortium of laboratories that test the efficacy of pharmaceutical agents against a panel of pediatric cell lines and xenografts [32]. SCH717454-treated mice had a longer event-free survival duration in 20 of 35 solid tumor xenograft models. This included tumor regression in Ewing’s sarcoma and osteosarcoma models and intermediate activity against glioblastomas, neuroblastomas, rhabdomyosarcomas, and rhabdoid tumors [32].
Phase I and phase II trials using many of these antibodies are under way. To date, there have been very few serious side effects resulting from treatment. Hyperglycemia, when present, has been mild and has been seen in only some of the antibodies tested [33–36]. Because of the important role of the IGF pathway in normal growth, there is concern about the impact of IGF blockade in patients who are still growing. Details of the tumor types that are currently being pursued in clinical trials are provided below. Unfortunately, in these tumor types, many of the patients are teenagers or children. The hypothetical concern of disruption of normal growth must be considered, but it must also be weighed against the pressing issue of tumor progression. The only way to assess the impact of IGF-1R–directed therapy on normal growth is to monitor young patients who have been treated with the antibody for a prolonged period of time over the course of their growth period. On the positive side, this would indicate that the patient is responding, or not progressing, on therapy.
There are eight IGF-1R antibodies that are currently being tested in clinical trials [31]. Data have not emerged suggesting that one antibody is more effective than another. Slight differences in whether they are fully human or humanized, their relative affinities to the IGF-1R and IR, and their ability to block the binding of either IGF-I or IGF-II ligand have not resulted in markedly different side effects or tolerability, but could lead to differences in clinical activity [33–36].
Encouragingly, these trials have also produced several clinical responses, mostly in patients with Ewing’s sarcoma [35, 36]. Reports of these responses, though limited, have generated enthusiasm to continue and expand both the number of patients and also the tumor types studied. A list of trials that are actively recruiting patients is accessible at http://www.clinicaltrials.gov. A search using the specific name of the antibody will reveal additional information for studies that are open (AMG-479, AVE-1642, BIIB-022, CP-751-871, IMC-A12, MK-0646, R-1507, SCH-717454).
IGF-1R IN CHILDHOOD MALIGNANCIES: RATIONALE FOR THERAPEUTIC TARGETING
Ewing’s Sarcoma Family of Tumors
Ewing’s sarcoma, peripheral primitive neuroectodermal tumor, and Askin tumor form a group of tumors, collectively termed Ewing’s sarcoma family of tumors (ESFT). These tumors are characterized by specific chromosomal translocations that cause the N-terminus of EWS to be fused to the C-terminus of one member of the ETS family of transcription factors, most commonly FLI1 [37]. Expression of the fusion product has been implicated in oncogenesis.
The role of IGF-1R signaling in ESFT has been evaluated extensively. EFST cell lines express IGF-1R and secrete IGF-I, and IGF-1R–blocking antibodies interrupt this autocrine loop [38, 39]. The prevalence of IGF-1R expression is very high, with six of six cell lines and eight of eight clinical samples positive for expression [38]. The EWS-FLI1 oncoprotein requires the presence of IGF-1R in order to transform murine fibroblasts [40]. Additionally, some evidence indicates that relapse-free survival may correlate with the ratio of serum IGFBP-3 to IGF-I [41]. In support of this argument, EWS-FLI1 directly reduces the expression and secretion of IGFBP-3 whereas exogenous IGFBP-3 inhibits the growth of ESFT cells [42]. There is also evidence that pathways downstream of IGF-1R, including PI3K/Akt and MAPK, are activated and are vital to ESFT cell survival [43]. Inhibitors of both PI3K and MAPK cause growth arrest in ESFT cells, and blockade of PI3K leads to greater sensitivity to doxorubicin [43, 44]. Systematic sequencing of the IGF-1R gene in ESFT patient samples to determine if there are activating mutations is proceeding. Another mechanism that may be associated with IGF pathway activation is the loss of imprinting of the IGF-II allele [45].
The successful treatment of xenografts with IGF-1R inhibitory antibodies demonstrates that IGF-1R blockade might prove therapeutic for patients with ESFT [30, 32]. Small molecule kinase inhibitors have also reduced IGF-1R activity in ESFT cells and reduced tumor growth in xenografts, either alone or in combination with chemotherapy [25, 28]. Overall, the preclinical data suggest that targeting IGF-1R in patients with ESFT should be beneficial. The finding that some ESFT patients have had sustained clinical remissions in phase I trials using various IGF-1R antibodies has provided excitement for continued exploration using these agents [35, 36].
Rhabdomyosarcoma
Rhabdomyosarcoma is the most common soft tissue sarcoma of childhood, arising from developing cells that form striated muscle. IGF-II is involved in normal muscle growth, and Northern blot analysis of tumor biopsy specimens from patients with both alveolar and embryonal rhabdomyosarcoma demonstrated high levels of IGF-II mRNA expression [6]. This suggested the possibility that upregulation of IGF-II plays a role in the unregulated growth of these tumors. Support for this hypothesis came from the finding that rhabdomyosarcoma cell lines also secrete IGF-II, which then binds to IGF-1R, resulting in autocrine growth proliferation and increased cell motility [46].
Genetic mutations in the IGF pathway have not been detected to date. However, epigenetic changes leading to loss of imprinting (LOI) of the IGF-II locus, resulting in over-expression of IGF-II, have been identified [47]. In addition, the PAX3–FKHR translocation that characterizes alveolar rhabdomyosarcomas transactivates the IGF-1R promoter, thus providing further evidence that the IGF pathway plays an important role in the progression of rhabdomyosarcoma [48]. All rhabdomyosarcoma cell lines show some level of IGF-1R expression, although they differ by as much as 30-fold based on quantitative protein analysis, and five of nine tumor samples tested express IGF-1R [16]. Initial testing of one of the monoclonal IGF-1R antibodies in the Pediatric Preclinical Testing Program revealed an intermediate level of activity against two of four rhabdomyosarcoma xenografts [32]. These findings, in addition to many others, provided the rationale for trials of IGF-1R antibody in patients with rhabdomyosarcoma.
IGFBP-6 is unique among the other binding proteins because of its IGF-II binding specificity [9]. IGF-II has higher affinity for IGFBP-6 than for IGF-1R, suggesting that IGFBP-6 can sequester IGF-II from binding to the receptor. Various results support this hypothesis, including the finding that overexpression of IGFBP-6 in the RH30 rhabdomyosarcoma cell line resulted in a marked delay in tumor growth in nude mice [49]. The time to tumor formation was further prolonged when IGFBP-6 – overexpressing mice were also treated with the rapamycin analog CCI-779. All of the rapalogues inhibit mTOR, which is downstream of IGF-1R (Fig. 1). This finding is not surprising because treatment of both RH30 and RD cells with rapamycin induces feedback activation of Akt through an IGF-1R–dependent mechanism [50]. These results suggest that modulation of multiple components of the IGF pathway may be more effective as antitumor therapy.
Osteosarcoma
The peak incidence of osteosarcoma occurs during adolescence, corresponding to both the growth spurt and peak concentrations of circulating GH and IGF-I [2]. This epidemiological correlation led to the hypothesis that high levels of IGF-I play an important role in the pathogenesis of osteosarcoma, which was supported by a host of preclinical data: (a) osteosarcoma cells express functional IGF-1R on the cell surface [51], (b) exogenous IGF-I stimulates osteosarcoma cells to proliferate [51], (c) IGF-I–dependent growth can be inhibited using monoclonal antibodies or antisense oligonucelotides against IGF-1R [51], (d) treatment of mice using a humanized anti-IGF-1R antibody resulted in tumor regression in two osteosarcoma xenograft models [32], and (e) the majority of osteosarcoma patient samples express IGF ligands and 45% express IGF-1R [52].
As discussed previously, interest in studying IGF and osteosarcoma began in the early 1980s after experiments in rats showed that hypophysectomy resulted in complete abrogation of osteosarcoma metastases [17]. Unfortunately, clinical trials attempting to reduce circulating IGF-I levels did not lead to longer survival [18, 19]. However, the question of whether this was a result of the inability to completely suppress IGF-I levels, or whether endocrine-mediated suppression was ineffective, was left unanswered. Both the techniques that had been successful in mice, surgical resection of the pituitary gland and plasmid overexpression of IGFBPs, were not practical in humans. The lack of reagents therefore resulted in waning of interest in this area. Advances in the IGF field could help answer this question though. Anti–IGF-II fully human monoclonal antibodies that have high affinity to IGF-II with no crossreactivity were developed recently [21]. The combination of endocrine IGF suppression along with treatment with anti-IGF antibodies may be proven to be effective for two reasons. First, the antibodies could bind residual endogenous IGF in the circulation. Second, the antibodies could also bind autocrine IGF produced by the tumor [51, 53]. Even if complete suppression does not affect either the primary or metastatic settings, these types of experiments would provide a definitive answer as to the utility of endocrine IGF suppression.
Gastrointestinal Stromal Tumors
Gastrointestinal stromal tumors (GISTs) are rare tumors that occur late in life and are almost always associated with activating mutations in the cell surface receptors KIT or platelet-derived growth factor receptor A [54]. Not surprisingly, treatment with the tyrosine kinase inhibitors (TKIs) imatinib and sunitinib has resulted in marked prolongation of survival in nonresectable and metastatic patients [55, 56]. Pediatric patients also develop GISTs, but they rarely have the characteristic mutations (therefore, termed wild-type) and they do not seem to respond to TKI therapy [54]. GISTs, in general, and also in the wild-type setting, are unresponsive to chemotherapy. In addition, even when resected, there is a high rate of recurrence. All these factors point to the need for alternative therapies. Almost all samples from wild-type GISTs have markedly higher levels of IGF-1R than those from KIT-mutated GISTs [57, 58]. Unfortunately, wild-type KIT cell lines and xenograft models are not available, precluding the ability to test potentially therapeutic agents in the preclinical setting. Based on IGF-1R expression profiles, and the futility of standard treatment, efforts are under way to initiate a trial with IGF-1R antibody for the treatment of pediatric or wild-type GIST patients with IGF-1R antibody.
Wilms’ Tumor
In early studies of IGF in childhood cancer, radioligand binding assays demonstrated relatively high levels of IGF-II in the pediatric kidney neoplasm Wilms’ tumor [59, 60]. LOI of the IGF-II gene and high levels of IGF-II expression have been reported in approximately half of all Wilms’ tumor samples [61, 62]. More importantly, examination of other imprinted genes showed no signs of LOI, supporting the epigenetic specificity of IGF-II LOI in Wilms’ tumors [63]. IGF-1R mRNA levels have been shown to be sixfold higher in Wilms’ tumors than in normal adjacent kidney tissue [64]. Inactivation of the WT1 tumor suppressor gene is a key step in the etiology of a subset of Wilms’ tumors. In addition to being overexpressed in Wilms’ tumors, the levels of IGF-1R correlate inversely with the levels of WT1 [65, 66]. Furthermore, expression of WT1in WT1-deficient cells results in a significant decrease in levels of IGF-1R mRNA [66]. Recent reports show that there is a significant correlation between a higher IGF-1R copy number and a shorter relapse-free survival time, as determined by comparative genomic hybridization [67]. The cumulative evidence strongly supports the development of IGF-1R–targeted therapy, particularly in a subset of Wilms’ tumors that appear to be more clinically aggressive.
Other Pediatric Tumors
Neuroblastoma is an embryonal tumor that typically arises in cells that eventually make up the adrenal medulla. IGF-II mRNA was increased in two of eight neuroblastoma samples and work using cell lines demonstrated that it acted as an autocrine growth factor through IGF-1R, which was expressed uniformly in all the samples [8]. In situ hybridization further revealed that, even when IGF-II was not expressed in tumor cells, it was detectable in cells of surrounding nonmalignant tissues, implicating a paracrine mechanism for tumorigenesis [68]. These results were the first to implicate IGF-II as both an autocrine and a paracrine growth factor and also showed that the growth regulatory pathways used by neuroblastomas were similar to those in precursor cell types.
The rare pediatric tumors hepatoblastoma and retinoblastoma may also partially rely on the IGF signaling pathway. In hepatoblastoma, comparison of tumor regions with matched normal regions demonstrated higher levels as follows: IGF-I in six of 11 samples, IGF-II in nine of 11 samples, and IGF-1R in five of 11 samples [69]. In addition, levels of IGFBP-1 and IGFBP-2 were lower in nine of 11 and 10 of 11 samples. In retinoblastoma, the Y79 retinoblastoma cell line can grow in the absence of serum, suggesting that these cells produce growth factors that mediate autocrine-induced proliferative capacity [70]. Studies also showed that these cells secreted both IGF-I and IGF-II into the medium. Furthermore, αIR3, a monoclonal IGF-1R antibody, was able to markedly reduce proliferation of Y79 cells, suggesting a possible role of the IGF pathway in the pathogenesis of retinoblastoma.
TWO WORDS OF CAUTION
The small number of clinical responses in phase I trials of IGF-1R antibodies has raised hopes for the success of this therapeutic modality [35, 36]. However, the lessons we have learned from early studies with single-agent chemotherapy suggest that even successes will be met by a lack of continued utility. In many different tumors, this shortcoming was overcome with the use of multiagent combination chemotherapy. Preclinical findings using IGF-1R inhibitors have demonstrated potent antitumor activity. However, in many cases the responses have only lasted for a finite period of time. This suggests that other pathways are being recruited that compensate for IGF-1R inhibition. The factors that mediate this subversion are currently being identified. An alternative to this theory is that the initial tumor inoculum began as a heterogenous population. Cells that were susceptible to IGF-1R inhibition died out, leaving behind those that were resistant.
It is unknown if patients will experience the same recruitment of tumor-compensatory pathways or overgrowth of resistant clones. Several clinical remissions have been durable and others have resulted in tumor shrinkage followed by progression. Therefore, it is imperative that preclinical models to address these questions be developed rapidly, in order to determine why some tumors respond while others do not. It is also necessary to begin feasibility studies of the addition of IGF-1R inhibitors to chemotherapy or other biological agents, in order to sustain clinical remissions.
The second word of caution involves clinical trials. Fortunately, pediatric sarcomas are rare and recurrences that are refractory to other therapies are even rarer. The number of patients who are eligible for clinical trials is therefore small. Thus, definitive clinical testing of IGF-1R inhibitors, either alone or in combination with other agents, will require cooperation among all parties involved. Large collaborative efforts that can guarantee the enrollment of a sufficient number of patients will be needed to maximize advances in this field.
FUTURE DEVELOPMENT
The substantial number of antibodies and other inhibitors of IGF-1R will provide many clinical reagents for the treatment of patients with childhood cancers. This review suggests that the greatest impact of IGF-1R–directed therapy would be against ESFT. However, many preclinical models suggest the potential for clinical activity in a variety of pediatric tumors. The addition of IGF-1R–directed therapy holds significant promise to improve morbidity and reduce mortality from childhood cancer.
Footnotes
Disclosures
Su Young Kim: None; Jeffrey A. Toretsky: None; Daniel Scher: None; Lee J. Helman: None
Section editors Susan M. Blaney and Ross Pinkerton have disclosed no financial relationships relevant to the content of this article.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias.
REFERENCES
- 1.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: A preliminary report. Growth Horm IGF Res. 2007;17:54–57. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Yakar S, Wu Y, Setser J, et al. The role of circulating IGF-I: Lessons from human and animal models. Endocrine. 2002;19:239–248. doi: 10.1385/ENDO:19:3:239. [DOI] [PubMed] [Google Scholar]
- 5.Chao W, D’Amore PA. IGF2: Epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 2008;19:111–120. doi: 10.1016/j.cytogfr.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minniti CP, Tsokos M, Newton WA, Jr, et al. Specific expression of insulin-like growth factor-II in rhabdomyosarcoma tumor cells. Am J Clin Pathol. 1994;101:198–203. doi: 10.1093/ajcp/101.2.198. [DOI] [PubMed] [Google Scholar]
- 7.Yun K, Molenaar AJ, Fiedler AM, et al. Insulin-like growth factor II messenger ribonucleic acid expression in Wilms tumor, nephrogenic rest, and kidney. Lab Invest. 1993;69:603–615. [PubMed] [Google Scholar]
- 8.El-Badry OM, Romanus JA, Helman LJ, et al. Autonomous growth of a human neuroblastoma cell line is mediated by insulin-like growth factor II. J Clin Invest. 1989;84:829–839. doi: 10.1172/JCI114243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach LA, Headey SJ, Norton RS. IGF-binding proteins—the pieces are falling into place. Trends Endocrinol Metab. 2005;16:228–234. doi: 10.1016/j.tem.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.De Meyts P, Whittaker J. Structural biology of insulin and IGF1 receptors: Implications for drug design. Nat Rev Drug Discov. 2002;1:769–783. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- 11.Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-1 receptors in human cancer. Curr Pharm Des. 2007;13:671–686. doi: 10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- 12.O’Gorman DB, Costello M, Weiss J, et al. Decreased insulin-like growth factor-II/mannose 6-phosphate receptor expression enhances tumorigenicity in JEG-3 cells. Cancer Res. 1999;59:5692–5694. [PubMed] [Google Scholar]
- 13.Adams TE, Epa VC, Garrett TPJ, et al. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57:1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler AA, Yakar S, Gewolb IH, et al. Insulin-like growth factor-I receptor signal transduction: At the interface between physiology and cell biology. Comp Biochem Physiol B Biochem Mol Biol. 1998;121:19–26. doi: 10.1016/s0305-0491(98)10106-2. [DOI] [PubMed] [Google Scholar]
- 15.Dearth RK, Cui X, Kim HJ, et al. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle. 2007;6:705–713. doi: 10.4161/cc.6.6.4035. [DOI] [PubMed] [Google Scholar]
- 16.Cao L, Yu Y, Darko I, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68:8039–8048. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollak M, Sem AW, Richard M, et al. Inhibition of metastatic behavior of murine osteosarcoma by hypophysectomy. J Natl Cancer Inst. 1992;84:966–971. doi: 10.1093/jnci/84.12.966. [DOI] [PubMed] [Google Scholar]
- 18.Mansky PJ, Liewehr DJ, Steinberg SM, et al. Treatment of metastatic osteosarcoma with the somatostatin analog OncoLar: Significant reduction of insulin-like growth factor-1 serum levels. J Pediatr Hematol Oncol. 2002;24:440–446. doi: 10.1097/00043426-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Khanna C, Prehn J, Hayden D, et al. A randomized controlled trial of octreotide pamoate long-acting release and carboplatin versus carboplatin alone in dogs with naturally occurring osteosarcoma: Evaluation of insulin-like growth factor suppression and chemotherapy. Clin Cancer Res. 2002;8:2406–2412. [PubMed] [Google Scholar]
- 20.Yin D, Vreeland F, Schaaf LJ, et al. Clinical pharmacodynamic effects of the growth hormone receptor antagonist pegvisomant: Implications for cancer therapy. Clin Cancer Res. 2007;13:1000–1009. doi: 10.1158/1078-0432.CCR-06-1910. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y, Zhu Z, Xiao X, et al. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Mol Cancer Ther. 2006;5:114–120. doi: 10.1158/1535-7163.MCT-05-0252. [DOI] [PubMed] [Google Scholar]
- 22.Resnicoff M, Sell C, Rubini M, et al. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994;54:2218–2222. [PubMed] [Google Scholar]
- 23.Rininsland F, Johnson TR, Chernicky CL, et al. Suppression of insulin-like growth factor type I receptor by a triple-helix strategy inhibits IGF-I transcription and tumorigenic potential of rat C6 glioblastoma cells. Proc Natl Acad Sci U S A. 1997;94:5854–5859. doi: 10.1073/pnas.94.11.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putral LN, Gu W, McMillan NAJ. RNA interference for the treatment of cancer. Drug News Perspect. 2006;19:317–324. doi: 10.1358/dnp.2006.19.6.985937. [DOI] [PubMed] [Google Scholar]
- 25.García-Echeverría C, Pearson MA, Marti A, et al. In vivo antitumor activity of NVP-AEW541—a novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 26.Scotlandi K, Manara MC, Nicoletti G, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65:3868–3876. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 27.Tanno B, Mancini C, Vitali R, et al. Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clin Cancer Res. 2006;12:6772–6780. doi: 10.1158/1078-0432.CCR-06-1479. [DOI] [PubMed] [Google Scholar]
- 28.Martins AS, Mackintosh C, Martin DH, et al. Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clin Cancer Res. 2006;12:3532–3540. doi: 10.1158/1078-0432.CCR-05-1778. [DOI] [PubMed] [Google Scholar]
- 29.Pollak MN, Polychronakos C, Richard M. Insulinlike growth factor I: A potent mitogen for human osteogenic sarcoma. J Natl Cancer Inst. 1990;82:301–305. doi: 10.1093/jnci/82.4.301. [DOI] [PubMed] [Google Scholar]
- 30.Scotlandi K, Benini S, Nanni P, et al. Blockage of insulin-like growth factor-I receptor inhibits the growth of Ewing’s sarcoma in athymic mice. Cancer Res. 1998;58:4127–4131. [PubMed] [Google Scholar]
- 31.Feng Y, Dimitrov DS. Monoclonal antibodies against components of the IGF system for cancer treatment. Curr Opin Drug Discov Devel. 2008;11:178–185. [PubMed] [Google Scholar]
- 32.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 33.Rodon J, Patnaik A, Stein M, et al. A phase I study of q3W R1507, a human monoclonal antibody IGF-1R antagonist in patients with advanced cancer. J Clin Oncol. 2007;25 18 suppl:3590. [Google Scholar]
- 34.Higano CS, Yu EY, Whiting SH, et al. A phase I, first in man study of weekly IMC-A12, a fully human insulin like growth factor-1 receptor IgG1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol. 2007;25 18 suppl:3505. [Google Scholar]
- 35.Tolcher AW, Rothenberg ML, Rodon J, et al. A phase I pharmacokinetic and pharmacodynamic study of AMG 479, a fully human monoclonal antibody against insulin-like growth factor type 1 receptor (IGF-1R), in advanced solid tumors. J Clin Oncol. 2007;25 18 suppl:3002. [Google Scholar]
- 36.Atzori F, Tabernero J, Cervantes A, et al. A phase I, pharmacokinetic (PK) and pharmacodynamic (PD) study of weekly (qW) MK-0646, an insulin-like growth factor-1 receptor (IGF1R) monoclonal antibody (MAb) in patients (pts) with advanced solid tumors. J Clin Oncol. 2008;26 15 suppl:3519. [Google Scholar]
- 37.Owen LA, Lessnick SL. Identification of target genes in their native cellular context: An analysis of EWS/FLI in Ewing’s sarcoma. Cell Cycle. 2006;5:2049–2053. doi: 10.4161/cc.5.18.3213. [DOI] [PubMed] [Google Scholar]
- 38.Scotlandi K, Benini S, Sarti M, et al. Insulin-like growth factor I receptor-mediated circuit in Ewing’s sarcoma/peripheral neuroectodermal tumor: A possible therapeutic target. Cancer Res. 1996;56:4570–4574. [PubMed] [Google Scholar]
- 39.Yee D, Favoni RE, Lebovic GS, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation: A potential autocrine growth factor. J Clin Invest. 1990;86:1806–1814. doi: 10.1172/JCI114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toretsky JA, Kalebic T, Blakesley V, et al. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 41.Toretsky JA, Steinberg SM, Thakar M, et al. Insulin-like growth factor type 1 (IGF-1) and IGF binding protein-3 in patients with Ewing sarcoma family of tumors. Cancer. 2001;92:2941–2947. doi: 10.1002/1097-0142(20011201)92:11<2941::aid-cncr10072>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 42.Prieur A, Tirode F, Cohen P, et al. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. 2004;24:7275–7283. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benini S, Manara MC, Cerisano V, et al. Contribution of MEK/MAPK and PI3-K signaling pathway to the malignant behavior of Ewing’s sarcoma cells: Therapeutic prospects. Int J Cancer. 2004;108:358–366. doi: 10.1002/ijc.11576. [DOI] [PubMed] [Google Scholar]
- 44.Toretsky JA, Thakar M, Eskenazi AE, et al. Phosphoinositide 3-hydroxide kinase blockade enhances apoptosis in the Ewing’s sarcoma family of tumors. Cancer Res. 1999;59:5745–5750. [PubMed] [Google Scholar]
- 45.Zhan S, Shapiro DN, Helman LJ. Loss of imprinting of IGF2 in Ewing’s sarcoma. Oncogene. 1995;11:2503–2507. [PubMed] [Google Scholar]
- 46.El-Badry OM, Minniti C, Kohn EC, et al. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1:325–331. [PubMed] [Google Scholar]
- 47.Zhan S, Shapiro DN, Helman LJ. Activation of an imprinted allele of the insulin-like growth factor II gene implicated in rhabdomyosarcoma. J Clin Invest. 1994;94:445–448. doi: 10.1172/JCI117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayalon D, Glaser T, Werner H. Transcriptional regulation of IGF-I receptor gene expression by the PAX3-FKHR oncoprotein. Growth Horm IGF Res. 2001;11:289–297. doi: 10.1054/ghir.2001.0244. [DOI] [PubMed] [Google Scholar]
- 49.Gallicchio MA, van Sinderen M, Bach LA. Insulin-like growth factor binding protein-6 and CCI-779, an ester analogue of rapamycin, additively inhibit rhabdomyosarcoma growth. Horm Metab Res. 2003;35:822–827. doi: 10.1055/s-2004-814153. [DOI] [PubMed] [Google Scholar]
- 50.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 51.Kappel CC, Velez-Yanguas MC, Hirschfeld S, et al. Human osteosarcoma cell lines are dependent on insulin-like growth factor I for in vitro growth. Cancer Res. 1994;54:2803–2807. [PubMed] [Google Scholar]
- 52.Burrow S, Andrulis IL, Pollak M, et al. Expression of insulin-like growth factor receptor, IGF-1, and IGF-2 in primary and metastatic osteosarcoma. J Surg Oncol. 1998;69:21–27. doi: 10.1002/(sici)1096-9098(199809)69:1<21::aid-jso5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 53.Raile K, Hoflich A, Kessler U, et al. Human osteosarcoma (U-2 OS) cells express both insulin-like growth factor-I (IGF-I) receptors and insulin-like growth factor-II/mannose-6-phosphate (IGF-II/M6P) receptors and synthesize IGF-II: Autocrine growth stimulation by IGF-II via the IGF-I receptor. J Cell Physiol. 1994;159:531–541. doi: 10.1002/jcp.1041590317. [DOI] [PubMed] [Google Scholar]
- 54.Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: Management of patients with gastrointestinal stromal tumor (GIST)—update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5 suppl 2:S1–S31. [PubMed] [Google Scholar]
- 55.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 56.Demetri GD, van Oosteram AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 57.Agaram NP, Laquaglia MP, Ustun B, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janeway KA, Liegl B, Harlow A, et al. Pediatric KIT wild-type and platelet-derived growth factor α-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res. 2007;67:9084–9088. doi: 10.1158/0008-5472.CAN-07-1938. [DOI] [PubMed] [Google Scholar]
- 59.Reeve AE, Eccles MR, Wilkins RJ, et al. Expression of insulin-like growth factor-II transcripts in Wilms’ tumour. Nature. 1985;317:258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- 60.Scott J, Cowell J, Robertson ME, et al. Insulin-like growth factor-II gene expression in Wilms’ tumour and embryonic tissues. Nature. 1985;317:260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- 61.Rainier S, Johnson LA, Dobry CJ, et al. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- 62.Ogawa O, Eccles MR, Szeto J, et al. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms’ tumour. Nature. 1993;362:749–751. doi: 10.1038/362749a0. [DOI] [PubMed] [Google Scholar]
- 63.Bjornsson HT, Brown LJ, Fallin MD, et al. Epigenetic specificity of loss of imprinting of the IGF2 gene in Wilms tumors. J Natl Cancer Inst. 2007;99:1270–1273. doi: 10.1093/jnci/djm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gansler T, Allen KD, Burant CF, et al. Detection of type 1 insulinlike growth factor (IGF) receptors in Wilms’ tumors. Am J Pathol. 1988;130:431–435. [PMC free article] [PubMed] [Google Scholar]
- 65.Werner H, Re GG, Drummond IA, et al. Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993;90:5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Werner H, Shen-Orr Z, Rauscher FJ, 3rd, et al. Inhibition of cellular proliferation by the Wilms’ tumor suppressor WT1 is associated with suppression of insulin-like growth factor I receptor gene expression. Mol Cell Biol. 1995;15:3516–3522. doi: 10.1128/mcb.15.7.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natrajan R, Reis-Filho JS, Little SE, et al. Blastemal expression of type I insulin-like growth factor receptor in Wilms’ tumors is driven by increased copy number and correlates with relapse. Cancer Res. 2006;66:11148–11155. doi: 10.1158/0008-5472.CAN-06-1931. [DOI] [PubMed] [Google Scholar]
- 68.El-Badry OM, Helman LJ, Chatten J, et al. Insulin-like growth factor II-mediated proliferation of human neuroblastoma. J Clin Invest. 1991;87:648–657. doi: 10.1172/JCI115042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gray SG, Eriksson T, Ekström C, et al. Altered expression of members of the IGF-axis in hepatoblastomas. Br J Cancer. 2000;82:1561–1567. doi: 10.1054/bjoc.1999.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giuliano M, Vento R, Lauricella M, et al. Role of insulin-like growth factors in autocrine growth of human retinoblastoma Y79 cells. Eur J Biochem. 1996;236:523–532. doi: 10.1111/j.1432-1033.1996.00523.x. [DOI] [PubMed] [Google Scholar]