Abstract
Diacylglycerol acyltransferase (DGAT) could be a rate limiting step in triglyceride (TG) synthesis as it is the final step in this pathway. As such, between depot differences in DGAT activity could influence regional fat storage. DGAT activity and in vitro rates of direct free fatty acid (FFA) storage were measured in abdominal subcutaneous and omental adipose tissue samples from 12 non-obese (BMI < 30kg/m2) and 23 obese men and women (BMI > 30kg/m2) undergoing elective surgery. DGAT activity was greater in omental than in abdominal subcutaneous adipose tissue from non-obese patients (2.0 ± 0.9 vs. 0.9 ± 0.3 pmol/min/mg lipid, respectively, P = 0.003), but not from obese patients (1.4 ± 0.6 vs. 1.7 ± 0.7 pmol/min/mg lipid, respectively, P = 0.10). DGAT activity per unit adipose weight was negatively correlated with adipocyte size (p<0.01) and positively correlated with direct FFA storage in omental (p<0.001) but not in abdominal subcutaneous fat. Tissue DGAT activity varies as a function of adipocyte size, but this relationship differs between visceral and abdominal subcutaneous fat in obese and non-obese humans. Our results are consistent with the hypothesis that inter-individual variations in DGAT activity may be an important regulatory step in visceral adipose tissue FFA uptake/storage.
Keywords: DGAT, FFA, triglyceride, regional fat, enzyme assay, body composition, adipocyte size
Introduction
Humans accumulate fat to different degrees in visceral, lower body and upper body subcutaneous depots. Besides the differences in body fat depots between non obese and obese men and women, fat distribution is known as an important predictor for cardio-metabolic risk (1, 2). A greater proportion of visceral fat is associated with increased risk for hypertension, dyslipidemia, diabetes and the metabolic syndrome (3). Unfortunately, the detailed mechanism(s) by which some fat depots expand to a greater extent than others is still unexplained, although mounting evidence suggests that defects in lipolysis cannot account the for greater net fat retention in the major depots (4–6). The alternative explanation is that differences in fatty acid storage dictate why some individuals gain more upper body/visceral fat than lower body/subcutaneous fat.
There are significant regional differences in meal fatty acid storage between visceral and subcutaneous depots in men and women (7–9). For example, in non-obese adults visceral fat stores meal fatty acids ~ 40% more efficiently on a gram per gram basis than abdominal subcutaneous fat (9), making it easy to imagine how visceral fat could sometimes expand at the expense of other depots. The patterns of fatty acid storage between leg, omental and upper body subcutaneous adipose depots appear unique (9, 10), suggesting that adipocyte factors regulating uptake or intracellular processing of fatty acids play different key roles in different depots. The exact mechanism(s) by which some depots better compete for circulating triglycerides (9) or free fatty acids (11) is not well defined, however.
Adipose tissue lipoprotein lipase (LPL) activity predicts regional meal fatty acid storage in some populations (12), but not others (9), making it difficult to attribute all regional fatty acid storage differences tot this enzyme. In addition, there are regional differences in direct FFA storage (10, 11), which are clearly independent of LPL activity. Any number of processes could limit the transition of extracellular fatty acids to adipocyte triglyceride. First, the fatty acids must pass through the plasma membrane, either by a flip-flop mechanism (13) or via facilitated transport (14). Once inside the fatty acids must be acylated (15), followed by a series of steps that eventually culminate in the synthesis of triglyceride. In this regard, DGAT, the final step in triglyceride synthesis, is a candidate for regulating this process. Increasing or decreasing DGAT activity in mice results in coordinate changes in fat cell size (16), implying that high DGAT activity can create a favorable gradient for uptake and storage of circulating fatty acids. In this study we tested the hypotheses that regional differences in adipose tissue DGAT activity are present in obese and non-obese humans and that DGAT activity correlates with direct FFA storage rates.
Methods and Procedures
Subjects
Samples (1–4 g) of surgical waste adipose tissue were collected from 35 patients undergoing elective abdominal surgery from March to June 2007 at St. Mary’s Hospital, Mayo Clinic (Rochester, Minnesota). The patients were 35 to 75 years old. Patients with acute, intra-abdominal inflammatory conditions were not included. Adipose tissue samples from abdominal subcutaneous (epigastric) region and the peripheral portion of the greater omentum were obtained during the operations. In some cases the surgeon judged that there was insufficient subcutaneous fat that could be reasonably removed and regarded as surgical waste. This protocol was approved by the Mayo Clinic Institutional Review Board.
Methods
Preparation of the cytosolic and microsomal fractions
The adipose tissue samples for DGAT activity were cut from the whole tissue sample, frozen immediately in liquid nitrogen and stored at −70° C until assayed. Samples from all subjects were run in the same assay. For the assay, ~500 mg of subcutaneous and omental fat were weighed and put on ice. 1.5 ml Standard Homogenization Buffer (TAE buffer) with anti-proteases (255 mM sucrose, 20 mM Tris-HCl, pH 7.5, 1 mM EDTA, anti-protease tablets from Roche, Indianapolis, IN) was added and the sample was homogenized with a rotor stator. The homogenate was centrifuged at 1000 g for 10 min at 4°C and the supernatant (without the fat cake) was centrifuged further at 16,000 g for 20 min. The supernatant containing the post-mitochondrial supernatant, hereafter referred to as the cytosolic fraction, was then transferred to a new tube. An aliquot was used to measure protein content using the BCA protein assay (Pierce; Rockford, IL, USA) and the remainder was used to measure DGAT activity. During this process all the lipid from the adipocytes was collected, pooled and finally weighed. For the experiments in which we measured both cytosolic and microsomal DGAT activity, a portion of the cytosol fraction was further centrifuged at 100,000 g for 60 min at 4 °C to obtain the microsomal fraction. The pellet from this centrifugation was diluted with 200 µl TAE buffer and saved for the same measurement as cytosolic fraction.
Measurement of DGAT activity
We used a modification of DGAT activity assay according to Coleman (17). We conducted preliminary experiments to assure the incubation time we selected (see below) was in the linear portion of the reaction curve. Briefly, we placed the following assay components into a 5 ml glass tube: 65 µl Tris/MgCl2/albumin mixture, water (55 µl) to make a final volume of 200 µl, 30 µl protein (about 5 µg), 20 µl of 2.0 mM 1,2 dioleoylsn-glycerol (Sigma-Aldrich D0138), and 30 µl [14C]palmitoyl-CoA (0.2 mM, 5 mCi/mmol) (Sigma P9804). The total reaction volume was 200 µl. The [14C]palmitoyl-CoA was added last to start the reaction followed by gentle, brief vortexing. 30 µl STE buffer was added as blank control. After 10 min of incubation at 30 °C in a shaking (80/min) water bath the reaction was stopped by adding 1 ml heptane and 0.5ml water. The solution was vortexed and spun at 800 g for 5 min, after which the upper layer was transferred completely to a new clean glass tube. Two ml of an alkaline ethanol solution was added, the mixture vortexed well, and spun at 800 g for 5 min. Half (650 µl) of the upper layer (heptane phase) was pipetted into a scintillation vial and counted using a liquid scintillation counter. The DGAT activity was calculated on the basis of pmol TG synthesized/min/mg cytosolic protein, and then converted to pmol TG synthesized/µg lipid/min using the weights of the tissue, the lipid and protein content of the tissue and cytosol. Because we measured DGAT activity per unit lipid weight of adipose tissue and we measured fat cell size (µg lipid/cell) we also calculated DGAT activity per cell. The assays were done duplicate or triplicate.
Measurement of direct FFA storage
We used a modification of the methods of Edens et al (18) to measure direct FFA storage by adipose tissue fragments. Briefly, 1–3 g of fresh adipose tissue was placed in a 50 ml polypropylene conical tube containing 25 ml of pre-warmed medium 199 (Invitrogen 11043-023, Invitrogen, Carlsbad, California) and transported to the laboratory in a thermos at 37 °C. The adipose samples were then minced coarsely into 10–20 mg pieces and placed in a 20ml polypropylene vial with 10 ml pre-incubation buffer (Krebs-Henseleit bicarbonate buffer with 4% BSA and 10 mM glucose). After pre-incubation for 1 h at 37 °C in a shaking water bath (80 rpm), 5~6 pieces (50–70 mg total) were removed, washed with warm saline and transferred into a 20ml glass vial. In that vial we placed 3 ml of the same incubation medium mixed with albumin-bound 0.5 mM palmitic acid and [9, 10 - 3H] palmitic (final specific activity = 0.5 mCi/mmol) for measurement of direct FFA storage and 14C-labeled mannitol (0.4 µM, 61mCi/mmol) as an extracellular marker. The tissue fragments were incubated in a shaking water bath (80 rpm) at 37 °C for 1 or 2 h.
At the end of the incubation period, 100 µl of the buffer was pipetted into a scintillation vial to measure [3H] and [14C] radioactivity and the tissue fragments were placed immediately in 15 ml of chloroform/methanol (2:1, v:v) to extract total lipids. The samples were incubated in a cold room for at least 2 days, after which we added 3.75mls of 0.88% KCL aqueous solution. The lipid layer was isolated by centrifugation, dried, weighed and counted for 3H. We also collected the non-lipid layer, which was dried and counted for 14C. We reasoned that the presence of 14C in the adipose lipid would indicate contamination of the sample with extracellular fluid, which would also contain extracellular [3H]palmitic acid. To avoid overestimating the direct storage of FFA in adipose lipids, the ratio of [3H]/[14C] of buffer and the SA [14C]mannitol were used to correct the [3H] SA of adipose lipid for possible extracellular [3H]palmitic acid. The adipose lipid SA (dpm/g) was measured to less than 2% counting error.
Measurement of visceral and subcutaneous fat by CT
If an abdominal computed tomography (CT) from the pre-operative period was available we measured the subcutaneous and visceral adipose tissue area at the L2–3 level (19).
Measurement of adipocyte size
Adipocyte size was assessed using a modification of the approach of Di Girolamo et al (20). Briefly, using digital photographs of cells recovered from digested adipose tissue, the area of at least 300 fat cells was measured using an automated program (21) and used to calculate cell size.
Measuring mRNA for DGAT1
Fragments of the adipose tissue samples were washed and flash frozen until extraction. RNA was extracted using RNeasy lipid tissue mini kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA using a high capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Quantitative RT-PCR was performed on an ABI 7900 PCR machine using primer and probe sets from Applied Biosystems. Calculations of relative transcript amounts were normalized to a "housekeeping"/endogenous control gene (cyclophilin A) and then reported relative to a calibrator sample.
Calculations and Statistics
Statistical analysis was performed using SPSS v15.0 for Windows (SPSS, Inc, Chicago). Values were expressed as means ± SD unless stated otherwise. Comparisons between sites (omental and abdominal subcutaneous) were made using a repeated measures ANOVA for site with terms for sex and body weight status. If significant differences were detected, specific comparisons were made using paired Student’s t test for site and comparisons between groups (male and female, obese and non-obese) were performed using an independent Student’s t test or either a Chi-squared or a Fisher’s exact test, whichever was appropriate. We performed linear regression analysis between the DGAT activity and adipocyte cell size and between DGAT activity and in vitro FFA uptake using obese and non-obese as different groups. Two-tailed P < 0.05 was accepted as statistically significant.
Results
Subject Characteristics
We obtained samples from 35 patients undergoing elective surgical procedures at Mayo Clinic. The primary surgical procedures (number of patients) were as follows: bariatric surgery - 15, revision of prior bariatric procedure - 3, hernia repair - 5, splenectomy - 2, resection of ileal capillary hemangioma - 1, exploratory celiotomy for abdominal pain - 1, repair gastroduodenal fistula - 1, pancreatic mass – a) non-malignant - 2, b) malignant - 3, resection of liver mass - 2. Table 1 provides the basic characteristics of the subjects. Men and women did not differ significantly across these variables with the exception of fasting plasma glucose concentrations. Consistent with the expected sex differences in fat distribution, the subcutaneous fat area measured by CT was greater than visceral fat area for women (P = 0.001), but not for men (P = 0.95). Abdominal subcutaneous adipocytes were larger in obese than non-obese individuals (0.98 ± 0.34 vs. 0.63 ± 0.31 µg lipid/cell, respectively, P = 0.004). Likewise, omental adipocytes were larger in obese than non-obese subjects (0.79 ± 0.27 vs. 0.54 ± 0.27 µg lipid/cell, respectively, P = 0.008).
Table 1.
Subject Characteristics
| Male N=14 |
Female N=21 |
P value | |
|---|---|---|---|
| Age (years) | 60 ± 12 | 58 ± 11 | 0.52 |
| BMI (kg/m2) | 39.1 ± 14.9 | 39.1 ± 12.5 | 0.99 |
| OB/Non-OB | 9/5 | 14/7 | 0.58 |
| Triglycerides (mg/dl) | 143 ± 37 | 183 ± 68 | 0.24 |
| Cholesterol (mg/dl) | 153 ± 38 | 171 ± 36 | 0.37 |
| Fasting glucose (mg/dl) | 114 ± 17 | 134 ± 34 | 0.02 |
| Visceral fat area (cm2) | 204 ± 124 | 136 ± 78* | 0.21 |
| SQ fat area (cm2) | 207 ± 129 | 297 ± 154 | 0.24 |
| Omental adipocyte size (µg lipid/cell) | 0.74 ± 0.20 | 0.68 ± 0.34* | 0.47 |
| SQ adipocyte size (µg lipid/cell) | 0.84 ± 0.34 | 0.85 ± 0.38 | 0.96 |
Abbreviation: BMI: Body mass index; OB: obese (BMI ≥ 30), Non-OB: non-obese (BMI<30); SQ: Subcutaneous. Quantitative variables are expressed as mean ± SD.
P values refer to male vs. female comparisons. Independent Student’s t tests were performed to compare the differences of quantitative variables between male and female groups. For categorical variables, Chi-squared with Fisher’s exact test were performed.
P=0.001, paired-samples T test was used to compare the difference between visceral fat and SQ fat in men and women separately.
Microsomal and cytosolic adipocyte DGAT activity
DGAT activity was measured in both microsomal and cytosolic fractions of eight different adipose tissue samples and expressed as picomoles of TG synthesized per minute per mg adipose tissue lipid. There was no difference in DGAT activity (paired t-test) measured using these two approaches and excellent agreement (R2=0.975, P < 0.001) between the values with a slope that was not different than 1.0 and an intercept not different than 0. Because the preparation of the cytosolic fraction is simpler we elected to use this approach.
DGAT activity in abdominal subcutaneous and omental adipose tissue
During the sample processing we were unable to accomplish a complete recovery of lipid from 3 omental adipose samples and thus could not determine DGAT activity per mg adipose lipid. Subcutaneous adipose was not obtained from 3 patients and we were unable to measure DGAT activity per mg adipose lipid for 8 of the subcutaneous samples due to an insufficient quantity of subcutaneous tissue.
There were no differences in the DGAT activity between omental and abdominal adipose tissue in men (1.74 ± 0.82 vs. 1.32 ± 0.63 pmol•min−1•mg lipid−1, P = 0.19, n = 9) or women (1.61 ± 0.72 vs. 1.43 ± 0.74, P = 0.47, n = 14). In contrast, DGAT activity in omental adipose tissue was greater than that in abdominal subcutaneous adipose tissue from non-obese subjects (2.0 ± 0.9 vs. 0.9 ± 0.3 pmol•min−1•mg lipid−1, P = 0.003, n = 9), whereas the values were not different in tissues from obese subjects (1.4 ± 0.6 vs. 1.7 ± 0.7 pmol•min−1•mg lipid−1, omental vs. subcutaneous, P = 0.098, n = 14). There was no effect of diabetic status or fasting blood sugar on the results.
DGAT activity and adipocyte size
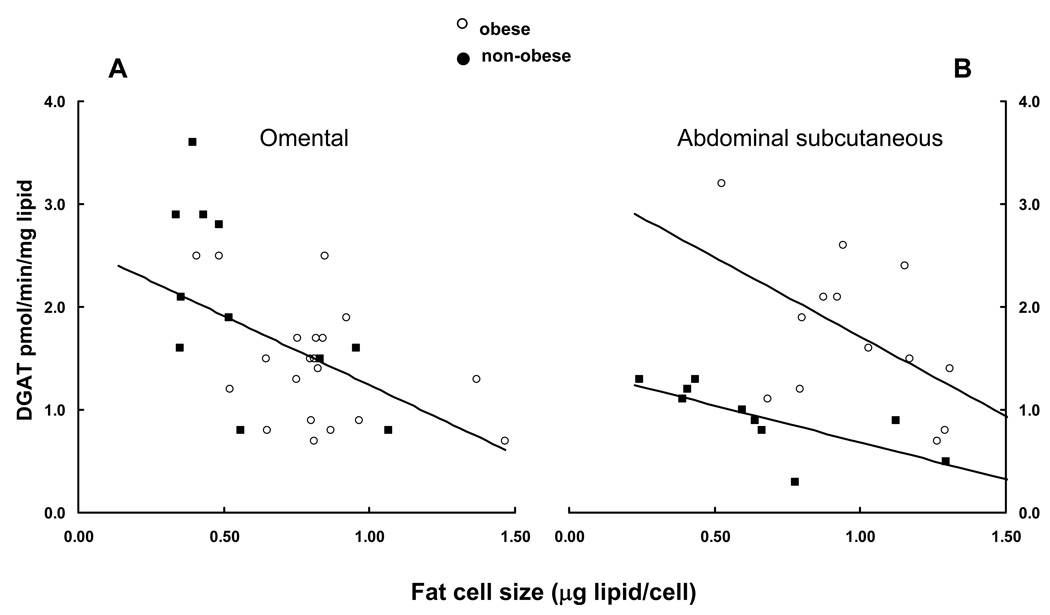
There was a negative relationship between omental adipocyte size and DGAT activity when expressed relative to adipose lipid (Figure 1A, r = −0.59, P = 0.0004, n = 32), with no evident distinction between obese and non-obese subjects. In contrast, for abdominal subcutaneous adipose tissue, the negative relationship between adipocyte size and DGAT activity was shifted such that DGAT activity was twice as great per mg lipid at any given cell size in obese compared with non-obese adipose tissue. The correlation between adipocyte size and DGAT activity was significant for the non-obese (r = −0.73, P = 0.017, n = 10) and of borderline significance for the obese (Figure 1B, r = −0.524, p=0.066, n = 13).
Figure 1.
DGAT activity per mg adipose lipid is plotted vs. fat cell size for omental (panel A) and abdominal subcutaneous (panel B) adipose tissue. There was a significant correlation (r = −0.59, P = 0.0004) between omental DGAT activity and fat cell size for the combined group. Subcutaneous DGAT activity was correlated with fat cell size for non-obese (r = −0.73, P = 0.017) and obese (r = −0.524, p=0.066) subjects, but the relationships differed significantly.
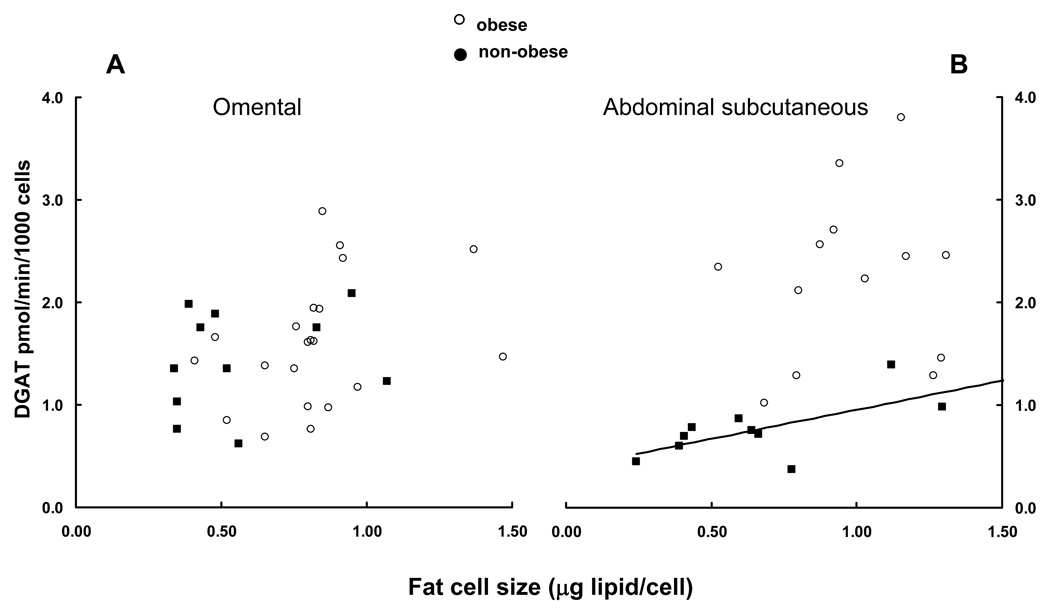
DGAT activity per 1000 cells increased significantly (r = 0.51, P = 0.01, n = 10) as a function of cell size (Figures 2 A and B) only for subcutaneous cells from non-obese persons.
Figure 2.
DGAT activity per 1000 cells is plotted vs. fat cell size for omental (panel A) and abdominal subcutaneous (panel B) adipose tissue. DGAT activity per 1000 abdominal subcutaneous cells and fat cell size were significantly correlated (r = 0.51, P = 0.01) only for non-obese.
DGAT activity and direct FFA storage rates
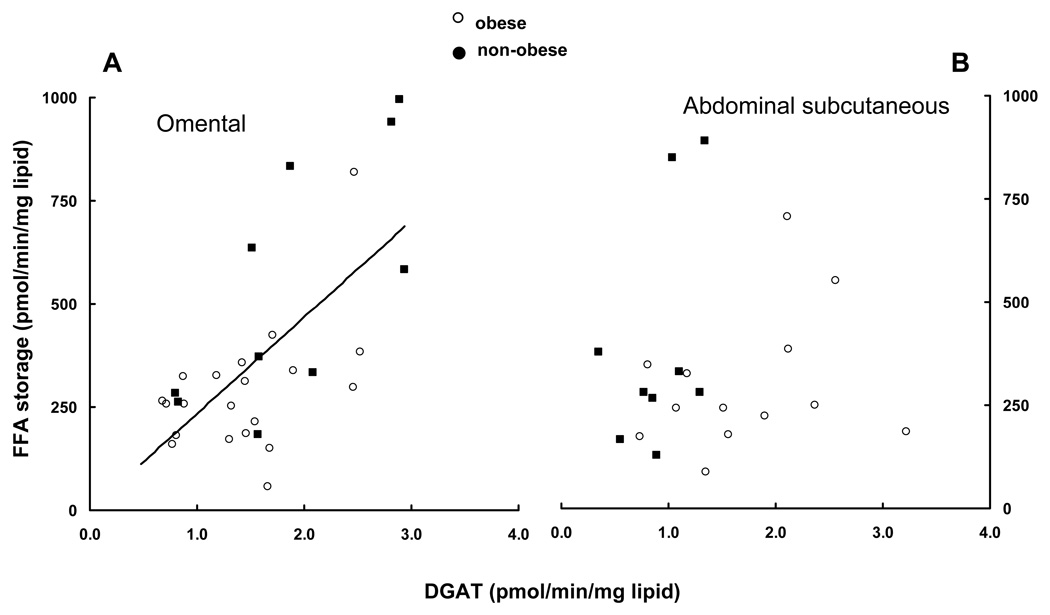
Direct FFA storage in omental and abdominal subcutaneous adipose tissue fragments averaged 388 ± 259 and 323 ± 200 pmol•min−1•mg lipid−1, respectively (P = 0.12, n = 30). For omental tissue, DGAT activity correlated positively with FFA storage (Figure 3A, r = 0.66, P < 0.0001, n = 30), and samples from obese and non-obese patients showed the same pattern. For abdominal subcutaneous adipose tissue, however, there was no significant relationship between FFA storage and DGAT activity for obese, non-obese or the combined data set (n = 22).
Figure 3.
FFA storage into adipose lipid is plotted vs. DGAT activity for omental (panel A) and abdominal subcutaneous (panel B) adipose tissue. Omental DGAT activity was significantly correlated with FFA storage (r = 0.66, P < 0.0001), but abdominal DGAT activity was not.
DGAT activity vs. DGAT1 mRNA
We could not detect a relationship between omental DGAT activity and omental DGAT1 mRNA transcript levels (r = 0.18, P = 0.32, n = 31) or between abdominal subcutaneous DGAT activity and abdominal subcutaneous DGAT1 mRNA transcript levels (r = −0.36, P = 0.10, n = 22). Likewise, the pattern of mRNA expression (omental:subcutaneous) was unrelated to the relative activity of DGAT in these two depots.
Discussion
These studies were conducted to determine if regional (omental vs. abdominal subcutaneous) differences in adipose tissue DGAT activity exist in humans and whether DGAT activity correlates with direct FFA storage in a tissue specific manner. To this end, we collected samples from 35 patients with a wide range of body fat who were undergoing elective abdominal surgery. Fat cell size and FFA uptake were measured using fresh tissue, while DGAT activity was measured from all samples in one assay. We found that DGAT activity/mg adipose lipid correlated negatively with fat cell size, but correlated positively with FFA storage. There were no sex differences in regional DGAT activity, however, regional differences were found in samples from non-obese adults. These findings suggest that DGAT could be a rate limiting step for fatty acid storage in some adipose tissue depots, potentially contributing to the differential partitioning of fatty acids between omental and subcutaneous adipose tissue.
To our knowledge, this is the first study to test for regional differences in DGAT activity in human adipose tissue. As the final step in TG synthesis, DGAT could help to regulate regional fat accumulation. For example, greater omental DGAT activity could accelerate fatty acid shunting into TG, thereby creating a favorable extracellular-to-intracellular concentration gradient compared with abdominal subcutaneous fat. We reported recently that in vivo FFA storage in omental fat is more efficient on a gram-per-gram basis than storage in abdominal subcutaneous fat (10). The greater rates of FFA storage in omental compared with subcutaneous fat in the present in vitro study were similar to our in vivo results (10), but a different pattern than was reported by Bower et al (22), who found greater TG synthesis in subcutaneous fat. The discrepancy could be due to differences in the tracer used (FFA vs. glucose) or the means of data expression (per unit lipid weight vs. per 106 cells). The results of the present study suggest that inter-individual variations in DGAT activity should be considered as a possible etiologic factor in explaining inter-individual differences in visceral fatty acid storage.
DGAT activity decreased as a function of fat cell size when expressed per mg adipose tissue lipid, but not when expressed per 1000 cells, similar to the pattern for hormone-sensitive lipase activity (23). The discrepancy between the two modes of data expression can be explained by the greater number of cells per gram of adipose tissue if the adipocytes are smaller. Both approaches to data expression provide insights into adipose physiology regulation. This study was designed to address whether DGAT enzyme activity differs between depots, because some fat depots take up and store fatty acids better on a per unit mass basis than do others (7, 11, 12, 24, 25). Because fat cell size of omental and subcutaneous depots differs, expressing activity per cell would not allow us to understand whether one depot might be at a competitive advantage for TG synthesis relative to the mass of tissue. The mass of adipose tissue depots can now be routinely measured using DEXA and imaging techniques (19). Likewise, tracers can be used to measure meal-derived fatty acid or FFA storage per gram of tissue (7, 11, 24, 25). By providing DGAT activity results both as a function of tissue mass and adipocyte number, we hope investigators are able to consider the separate questions of whether differences in DGAT activity may relate to differences in whole tissue storage of fatty acids as well as whether DGAT activity is modulated as a function of adipocyte size.
As an example of how one might use the information regarding DGAT activity per unit lipid weight consider the following examples. A lean individual with small omental adipocytes (~3 pmol/mg lipid/min DGAT activity) and 1 kg of visceral fat is compared with an obese individual with larger omental adipocytes (~1 pmol/min/mg lipid DGAT activity) and 6 kg visceral fat. The lean and obese individuals would be predicted to have total visceral DGAT activities of 3 and 6 µmol/min, only 2-fold differences despite 6 fold differences in fat mass. Contrast this with a lean individual with small abdominal subcutaneous adipocytes (~1 pmol/min/mg lipid DGAT activity) and 5 kg of upper body subcutaneous fat and an obese individual with medium sized adipocytes (~2 pmol/min/mg lipid DGAT activity) and 30 kg upper body subcutaneous fat. The total upper body subcutaneous DGAT activity in the lean and obese persons would be 5 and 60 ~mol/min, respectively, a 12-fold difference in triglyceride synthesis capacity.
The decrease in DGAT activity/g adipose lipid we observed as a function of cell size is not what is seen when lipoprotein lipase (LPL) activity is examined using the same approach (26). This observation appears counterintuitive. We expected that, because greater LPL activity in hypertrophic adipose tissue beds will make more fatty acids available from circulating TG, larger adipocytes will require a greater capacity to synthesize intracellular TG. Of note, however, abdominal subcutaneous adipose tissue from obese patients had greater DGAT activity at any cell size compared with tissue from non-obese patients. This capacity might preserve the ability of abdominal subcutaneous fat in obese adults to store greater amounts of fatty acids as TG. The lack of an “obesity effect” on omental DGAT activity (figure 2A) could limit the ability of visceral fat to store fatty acids derived from LPL, which could contribute to the greater spillover of fatty acids from chylomicron hydrolysis into the splanchnic FFA pool compared with the tissue beds (27).
Several limitations to these studies must be acknowledged. First, a large number of proteins and enzymes contribute to trafficking fatty acids from the extracellular environment to the final product of TG. In this study we measured only the final step in TG synthesis (DGAT). Other factors such as CD36, FATP 1 and 4, acyl-CoA synthase, and the other acyl-transferases involved in TG synthesis could be equally or more important in regulating fatty acid storage. Moreover, the amount and activity of these other proteins and enzymes might be coordinately regulated with DGAT. In this case, the association we found between DGAT activity and in vitro FFA storage may not be cause and effect, but “guilt by association”.
Nevertheless, our results are at least consistent with the hypothesis that the DGAT activity is involved in the regulation of FFA uptake/storage in adipose tissue, especially visceral fat. DGAT activity correlated positively with in vitro FFA storage rates in omental adipose tissue. The lack of a similar, strong relationship in abdominal subcutaneous adipose tissue argues that other steps may be rate limiting in that tissue bed with respect to FFA storage. Thus, to the extent that DGAT might play an important role in regulating FFA uptake, our data suggests that role is not the same in all fat depots.
We did not find a significant relationship between the abundance of DGAT1 mRNA and DGAT activity, although it has previously been reported that DGAT1 mRNA and DGAT activity increase coordinately in response to pioglitazone in humans (28). The lack of a relationship between DGAT1 mRNA and DGAT activity in our population may imply that under usual circumstances DGAT activity in humans is regulated to some extent at the post-transcriptional level. Alternatively, it is possible that the events surrounding the stress of surgery and anesthesia have rapid effects on adipocyte transcription that are not reflected in DGAT activity.
In summary, the DGAT activity does not increase as fat cell size increases. This means that DGAT activity per gram of tissue is less in depots with larger cells. In addition, this discrepancy varies between visceral and abdominal subcutaneous fat and between obese and non-obese humans. DGAT activity is associated positively with direct FFA storage as measured in vitro. Our findings are consistent with the hypothesis that DGAT may be an intracellular factor involved in the regulation of direct FFA storage. Additional studies will be required to understand whether the patterns of depot and obesity-related differences in DGAT activity we observed directly contribute to regional differences in fat storage and fat distribution in humans.
Acknowledgments
Supported by grants DK45343 and DK50456 from the U.S. Public Health Service, 7-07-DCS-03 from the American Diabetes Association, and by the Mayo Foundation. Dr. Xinguo Hou was supported by Qilu Hospital, Shandong University, China. We would like to thank Dr. Michael Kendrick for providing some of the samples for this study.
Footnotes
Disclosures.
The authors have no conflict of interest to disclose.
References
- 1.Fox CS, Massaro JMh, U, Pou K, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulaton. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 2.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–1420. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 3.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 4.Burguera B, Proctor DN, Dietz W, Guo Z, Joyner MJ, Jensen MD. Leg FFA kinetics during exercise in men and women. Am J Physiol. 2000;278 doi: 10.1152/ajpendo.2000.278.1.E113. E113-E7. [DOI] [PubMed] [Google Scholar]
- 5.Guo ZK, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48:1586–1592. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA. Regional uptake of meal fatty acids in humans. Am J Physiol. 2003;285 doi: 10.1152/ajpendo.00220.2003. E1282-E8. [DOI] [PubMed] [Google Scholar]
- 8.Marin P, Oden B, Olbe L, Bengtsson B-A, Bjorntorp P. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab. 1996;81:1018–1022. doi: 10.1210/jcem.81.3.8772568. [DOI] [PubMed] [Google Scholar]
- 9.Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD. Meal fatty acid uptake in visceral fat in women. Diabetes. 2007;56:2589–2597. doi: 10.2337/db07-0439. [DOI] [PubMed] [Google Scholar]
- 10.Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD. Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes. 2008;57:1186–1194. doi: 10.2337/db07-0664. [DOI] [PubMed] [Google Scholar]
- 11.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56:1369–1375. doi: 10.2337/db06-1680. [DOI] [PubMed] [Google Scholar]
- 12.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol. 2006;291 doi: 10.1152/ajpendo.00196.2006. E1115-E23. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes. 1999;48:2255–2269. doi: 10.2337/diabetes.48.12.2255. [DOI] [PubMed] [Google Scholar]
- 14.Schaffer JE. Fatty acid transport: the roads taken. Am J Physiol. 2002;282 doi: 10.1152/ajpendo.00462.2001. E239-E46. [DOI] [PubMed] [Google Scholar]
- 15.Gargiulo CE, Stuhlsatz-Krouper SM, Schaffer JE. Localization of adipocyte long-chain fatty acyl-CoA synthetase at the plasma membrane. J Lipid Res. 1999;40:881–892. [PubMed] [Google Scholar]
- 16.Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV., Jr. Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 2002;51:3189–3195. doi: 10.2337/diabetes.51.11.3189. [DOI] [PubMed] [Google Scholar]
- 17.Coleman RA. Diacylglycerol acyltransferase and monoacylglycerol acyltransferase from liver and intestine. Methods Enzymol. 1992;209:98–104. doi: 10.1016/0076-6879(92)09013-s. [DOI] [PubMed] [Google Scholar]
- 18.Edens NK, Fried SK, Kral JG, Hirsch J, Leibel RL. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am J Physiol. 1993;265 doi: 10.1152/ajpendo.1993.265.3.E374. E374-E9. [DOI] [PubMed] [Google Scholar]
- 19.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–278. doi: 10.1093/ajcn/61.2.274. [DOI] [PubMed] [Google Scholar]
- 20.Di Girolamo M, Mendlinger S, Fertig JW. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971;221:850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- 21.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res. 2003;44:1795–1801. doi: 10.1194/jlr.D300001-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Bower JF, Vadlamudi S, Barakat HA. Ethnic differences in in vitro glyceride synthesis in subcutaneous and omental adipose tissue. Am J Physiol. 2002;283 doi: 10.1152/ajpendo.00225.2002. E988-E93. [DOI] [PubMed] [Google Scholar]
- 23.Reynisdottir S, Dauzats M, Thorne A, Langin D. Comparison of hormone-sensitive lipase activity in visceral and subcutaneous human adipose tissue. J Clin Endocrinol Metab. 1997;82:4162–4166. doi: 10.1210/jcem.82.12.4427. [DOI] [PubMed] [Google Scholar]
- 24.Marin P, Oden B, Bjorntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995;80:239–243. doi: 10.1210/jcem.80.1.7829619. [DOI] [PubMed] [Google Scholar]
- 25.Romanski SA, Nelson R, Jensen MD. Meal fatty acid uptake in adipose tissue: Gender effects in non-obese humans. Am J Physiol. 2000;279 doi: 10.1152/ajpendo.2000.279.2.E455. E455-E62. [DOI] [PubMed] [Google Scholar]
- 26.Votruba SB, Jensen MD. Sex differences in abdominal, gluteal, and thigh LPL activity. Am J Physiol. 2007;292 doi: 10.1152/ajpendo.00601.2006. E1823-E8. [DOI] [PubMed] [Google Scholar]
- 27.Nelson RH, Basu R, Johnson CM, Rizza RA, Miles JM. Splanchnic spillover of extracellular lipase-generated fatty acids in overweight and obese humans. Diabetes. 2007;56:2878–2884. doi: 10.2337/db07-0812. [DOI] [PubMed] [Google Scholar]
- 28.Ranganathan G, Unal R, Pokrovskaya I, et al. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J Lipid Res. 2006;47:2444–2450. doi: 10.1194/jlr.M600248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]