Abstract
Background. Vascular calcification is highly prevalent in persons with chronic kidney disease (CKD) and predicts cardiovascular disease (CVD) events. Matrix Gla protein (MGP) is a potent inhibitor of vascular calcification, and lower levels of its precursor—uncarboxylated MGP (ucMGP)—are associated with vascular calcification and atherosclerosis. Whether mild to moderate decrements in kidney function are associated with lower serum ucMGP is unknown.
Methods. In a cross-sectional study among 842 outpatients with stable CVD, estimated glomerular filtration rate (eGFR), serum cystatin-C and urine albumin-to-creatinine ratio (ACR) were measured and serum ucMGP levels were determined by ELISA. Multivariate linear regression evaluated the association of each kidney function measure with serum ucMGP levels.
Results. The mean eGFR was 76 ± 23 mL/min/1.73 m2, and 186 subjects (22%) had moderate CKD (eGFR <60 mL/min/1.73 m2). The mean ± SD ucMGP level was 3289 ± 1177 nM. In unadjusted analysis, each 10 mL/ min/1.73 m2 lower eGFR was associated with 101 nM lower ucMGP level. This association was only minimally attenuated in final multivariate models wherein each 10 mL/ min/1.73 m2 lower eGFR was associated with 79 nM lower ucMGP (95% confidence interval [CI]; 44 to 115; P < 0.001) after adjustment for age, sex, race, body mass index, blood pressure, smoking, hypertension, diabetes; and serum albumin, calcium, phosphorus, and fetuin-A levels. Similarly, in models adjusted for identical covariates, each 0.1 mg/L higher cystatin-C was associated with 39 nM lower ucMGP (95% CI 23 to 55; P < 0.001). In contrast, no significant association was observed between ACR and ucMGP in either unadjusted or adjusted analyses (adjusted P = 0.17). All associations were similar among subjects with or without diabetes (P-values for interaction > 0.50).
Conclusions. Among outpatients with stable CVD, a reduced glomerular filtration rate is associated with a decreased serum ucMGP level. In contrast, ACR is not associated with ucMGP levels. Whether ucMGP is a useful marker of vascular calcification and CVD event risk in persons with CKD deserves future study.
Keywords: atherosclerosis, chronic kidney disease, matrix Gla protein, vascular calcification
Introduction
Chronic kidney disease (CKD) is a strong risk factor for cardiovascular disease (CVD) mortality [1–4] and affects ∼13% of the US population [5]. At each stage of CKD, the risk of CVD mortality is several times higher than the risk of progression to end-stage renal disease (ESRD) [1,6,7]. Despite intensive investigation, the mechanisms responsible for this strong association remain unknown [8]. One candidate mechanism may be accelerated vascular calcification, which is highly prevalent in CKD and independently predicts CVD events [9–17]. Recent research has demonstrated that vascular calcification is an actively regulated process and therefore may be modifiable [18,19]. Thus, understanding mechanisms of vascular calcification will provide novel insights into this disease process and may ultimately identify new therapeutic targets to prevent CVD among persons with CKD.
Matrix γ-carboxyglutamate (Gla) protein (MGP) is a potent inhibitor of vascular calcification [20]. MGP knockout mice develop severe aortic calcification and die at ∼6 weeks of age due to spontaneous aortic rupture [21]. Vascular calcification has been described in human Keutel syndrome, which is caused by MGP gene loss-of-function mutations [22]. MGP exerts its effects on vascular calcification directly, through inhibition of calcium crystal formation in conjunction with other calcification inhibitors such as fetuin-A, and indirectly, by influencing transcription factors that inhibit vascular cell differentiation to an osteoblast-like phenotype [23–25].
The activation of MGP occurs by post-translational carboxylation, a vitamin K-dependent process that is inhibited by warfarin [20]. Treatment of rats with warfarin results in vascular calcification [26] and diets enriched with vitamin K ameliorate the effect [27]. Recent studies have demonstrated that lower serum levels of uncarboxylated MGP (ucMGP)—the precursor to the active carboxylated MGP protein—are associated with vascular calcification and atherosclerosis [28,29]. In addition, persons with ESRD have lower ucMGP levels as compared to healthy controls [28–30]. It is not known, however, whether persons with less severe decrements in kidney function also have lower ucMGP levels compared to persons with normal kidney function. If so, ucMPG may be a novel factor linking CKD and vascular calcification. To that end, we evaluated the association of several measures of kidney function with serum ucMGP levels among 842 outpatients with stable CVD who had a range of kidney function from normal to moderate CKD. We hypothesized that more advanced CKD would be associated with lower serum ucMGP levels.
Subjects and methods
Study participants
The Heart and Soul Study is an observational study designed to investigate the influence of psychosocial factors on progression of CVD [31,32]. Briefly, participants were recruited from outpatient clinics in the San Francisco Bay Area if they met one of the following inclusion criteria: (i) history of myocardial infarction; (ii) angiographic evidence of >50% stenosis in one or more coronary vessels; (iii) evidence of exercise-induced ischaemia by treadmill or nuclear testing; (iv) history of coronary revascularization or (v) documented diagnosis of coronary artery disease by an internist or cardiologist. Participants were excluded if they were not able to walk one block, had a myocardial infarction within the past 6 months or were likely to move out of the area within 3 years. The study protocol was approved by the Institutional Review Boards of participating institutions and all participants provided written informed consent.
Between September 2000 and December 2002, a total of 1024 participants enrolled and underwent a daylong baseline study appointment that included a medical history interview, a physical examination and a comprehensive health status questionnaire. Fasting (12 h) serum samples were obtained and frozen at −70°C. Subjects for whom frozen serum was not available for ucMGP measurement (n = 182, 18%) were excluded from this analysis, resulting in a final sample size of 842 subjects for this study. Kidney function was similar among subjects with and without ucMGP measurements (Table 1).
Table 1.
Comparison of kidney function in persons with or without available uncarboxylated matrix Gla protein (ucMGP) measurements
| Available ucMGP | No ucMGP | ||
|---|---|---|---|
| measurements | measurements | ||
| (N = 842) | (N = 182) | P-value | |
| MDRD eGFR | |||
| Mean (SD) (mL/min/ 1.73 m2) | 76 (23) | 75 (24) | 0.51 |
| Cystatin-C | |||
| Mean (SD) (mg/L) | 1.19 (0.51) | 1.24 (0.77) | 0.27 |
| Urine albumin-to- creatinine | |||
| Median (IQR) (mg/g) | 10 (6–20) | 9 (6–17) | 0.08 |
Kidney function
Serum creatinine was determined by the Jaffe reaction and was combined with age, sex and race to estimate glomerular filtration rate (eGFR) by the four-variable Modification of Diet in Renal Disease Study equation [33]. Serum cystatin-C concentrations were measured using a BNII nephelometer (Dade Behring, Inc., Deerfield, IL, USA) with a particle-enhanced immunonephelometric assay (N Latex Cystatin-C, Dade Behring, Inc.) [34]. The assay range was 0.195–7.330 mg/L; the intra-assay coefficient of variation was <2.8%, and the inter-assay coefficient of variation was <3.1%. Urine albumin and creatinine were measured by nephelometry and the Jaffe method, respectively, and urine albumin-to-creatinine ratios (mg albumin/g creatinine) were calculated.
Uncarboxylated matrix Gla protein
ucMGP was measured by competitive enzyme linked immunosorbent assay (ELISA) at the VitaK BV, Maastricht, The Netherlands) as previously described [28]. In brief, anti-ucMGP (VitaK BV, Maastricht, The Netherlands) was coupled to a microtitre plate via polyclonal rabbit anti-mouse IgG (Dako, Heeverlee, Belgium). After stringent washing, 5 μL of serum sample or standard was mixed with tracer (biotinylated peptide consisting of residues 35–54 in human MGP), transferred to the microtitre plate and incubated overnight at 4°C. After washing, the plate was incubated with streptavidine peroxidase (Zymed, Breda, The Netherlands) and stained with TMB (KPL, Gennep, The Netherlands) after washing. The process was stopped by adding H2SO4, and the plate was read at 450 nm. The lower limit of detection was 98 nM, and the intraassay coefficient of variation was 6% and the inter-assay coefficient was 11.4%.
Other participant characteristics and laboratory tests
Age, sex, race/ethnicity and smoking status were determined by questionnaire. Alcohol use was measured by use of the AUDIT-C questionnaire [35], with a score of ≥4 used to define regular alcohol use. Weight and height were measured at the baseline study physical examination. Body mass index (BMI) was calculated (weight [kg]/height [meters]2). Medical history of hypertension, diabetes, myocardial infarction, angioplasty, coronary bypass and heart failure was all determined by questionnaire. Fasting blood was drawn for the measurement of albumin, calcium, phosphorus, glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride and C-reactive protein concentrations using standard clinical chemistry analysers. Serum fetuin-A was measured with a BNII nephelometric assay as described in detail elsewhere [36].
Statistical analysis
We categorized participants by tertiles of serum ucMGP levels and compared baseline characteristics across tertiles using analysis of variance (ANOVA) or the Kruskal–Wallis test for continuous variables and the chi-squared test or Fisher's Exact test for categorical variables. Next, we evaluated the association of each measure of kidney function with serum ucMGP levels using linear regression analysis. Sequential models were developed. Model 1 was unadjusted; model 2 adjusted for age, sex and race and model 3 adjusted for age, sex, race and all variables that were associated with ucMGP levels in bivariate analysis (P < 0.05). Urine albumin-to-creatinine was positively skewed and was therefore log-transformed to approximate a normal distribution. Urine albumin-to-creatinine results are provided as ‘per doubling’. Last, we created a multiplicative interaction term (ucMGP × diabetes) to determine whether or not observed associations were similar among subjects with or without diabetes. This candidate effect modifier was selected a priori on the basis of previously published results [36,37]. All analyses were performed using Stata Statistical Software, version 9.2 (College Station, TX, USA).
Results
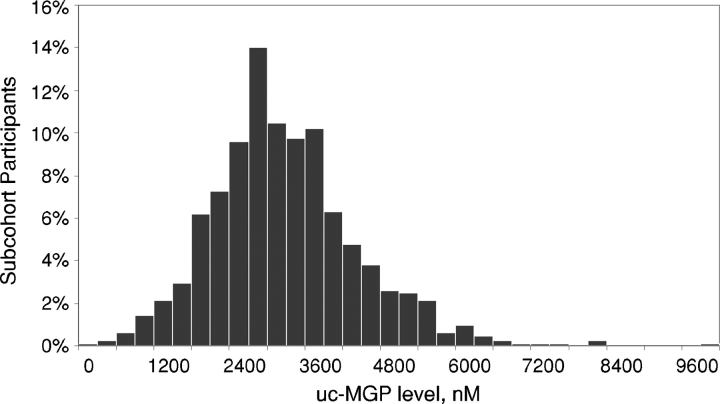
Among the 842 study participants, the mean age was 67 ± 11 years, 81% were male, 40% were non-white and 26% had diabetes mellitus. The mean eGFR was 76 ± 23 mL/min/1.73 m2, and 186 subjects (22%) had moderate CKD (eGFR < 60 mL/min/1.73 m2) [38]. The mean cystatin-C level was 1.19 ± 0.51 mg/L. The median albumin-to-creatinine ratio was 10 mg/g (interquartile range 6–20 mg/g), and 130 subjects (19%) had microalbuminuria (urine albumin-to-creatinine ≥ 30 mg/g) [38]. The mean ± SD ucMGP level was 3289 ± 1177 nM, and its distribution was approximately normal among the study participants (Figure 1). As compared to subjects in the lowest ucMGP tertile, those with higher ucMGP levels were younger, were more likely to drink alcohol and smoke, had higher BMI and were more frequently diabetic (Table 2). Prior CVD history was similar across ucMGP groups. Subjects with higher ucMGP also had higher diastolic blood pressure and serum albumin, calcium, phosphorus, fetuin-A, glucose, total cholesterol, and triglyceride levels. The relation of ucMGP with other candidate determinants of vascular calcification is shown in Table 3. Direct correlations were observed between ucMGP with calcium, phosphorus, and fetuin-A and were of modest strength, with the strongest of these observed with fetuin-A (r = 0.27, P < 0.01).
Fig. 1.
Distribution of serum uncarboxylated matrix Gla protein among 842 participants with coronary artery disease.
Table 2.
Baseline measures by uncarboxylated matrix Gla protein (ucMGP) tertiles
| ucMGP (nM) | ||||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| Characteristics | <2766 (n = 282) | 2766–3650 (n = 280) | >3650 (n = 280) | P-value |
| Demographics | ||||
| Age (year) | 71 (63–79) | 68 (60–76) | 64 (56–71) | <0.001 |
| Male sex, no. (%) | 239 (85) | 227 (81) | 219 (78) | 0.14 |
| Race | 0.56 | |||
| Caucasian, no. (%) | 180 (64) | 176 (60) | 159 (57) | |
| African American, no. (%) | 42 (15) | 47 (17) | 47 (17) | |
| Other, no. (%) | 60 (21) | 65 (23) | 73 (26) | |
| Regular alcohol use, no. (%) | 70 (25) | 76 (27) | 94 (34) | 0.06 |
| Regular tobacco use, no. (%) | 44 (16) | 61 (22) | 66 (24) | 0.05 |
| Body mass index (kg/m2)a | 27 (24–30) | 27 (25–31) | 29 (26–33) | <0.001 |
| Medical history | ||||
| Hypertension, no. (%) | 193 (69) | 211 (75) | 200 (72) | 0.26 |
| Diabetes, no. (%) | 57 (20) | 74 (27) | 91 (33) | 0.01 |
| Myocardial infarction, no. (%) | 159 (57) | 141 (51) | 147 (53) | 0.33 |
| Angioplasty, no. (%) | 105 (38) | 108 (39) | 115 (41) | 0.64 |
| Coronary bypass, no. (%) | 107 (38) | 108 (39) | 91 (33) | 0.30 |
| Heart failure, no. (%) | 45 (16) | 54 (19) | 52 (19) | 0.57 |
| Medication use | ||||
| Anticoagulant/thrombolytic, no. (%) | 20 (7) | 20 (7) | 23 (8) | 0.85 |
| Measurements | ||||
| SBP (mmHg) | 132 (22) | 135 (22) | 133 (20) | 0.10 |
| DBP (mmHg) | 72 (10) | 75 (12) | 76 (11) | <0.001 |
| Albumin (g/dL)a | 3.8 (3.6–4.0) | 3.9 (3.7–4.1) | 4.0 (3.8–4.2) | <0.001 |
| Calcium (mg/dL) | 9.4 (0.5) | 9.5 (0.5) | 9.6 (0.5) | <0.001 |
| Phosphorus (mg/dL)a | 3.6 (3.2–3.9) | 3.7 (3.3–4.0) | 3.7 (3.3–4.1) | 0.02 |
| Fetuin-A (g/L)a | 0.60 (0.52–0.68) | 0.64 (0.57–0.73) | 0.68 (0.60–0.79) | <0.001 |
| Glucose (fasting) (mg/dL)a | 104 (95–117) | 108 (99–123) | 111 (101–137) | <0.001 |
| Cholesterol (mg/dL)a | 163 (143–186) | 169 (145–191) | 181 (157–210) | <0.001 |
| HDL (mg/dL) | 47 (14) | 45 (14) | 46 (14) | 0.34 |
| Triglycerides (mg/dL)a | 88 (63–136) | 111 (79–157) | 130 (87–207) | <0.001 |
| CRP (mcg/dL)a | 2.27 (1.00–4.92) | 2.21 (0.84–5.43) | 2.32 (1.01–4.97) | 0.95 |
CRP, C-reactive protein; DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol; SBP, systolic blood pressure.
Data are presented as mean (SD) unless otherwise specified.
aMedian (intra-quartile range).
Table 3.
Correlation of serum uncarboxylated matrix Gla protein (ucMGP), fetuin-A, calcium and phosphorus
| ucMGP | Fetuin-A | Calcium | Phosphorus | |
|---|---|---|---|---|
| ucMGP | 1 | |||
| Fetuin-A | 0.2716 | 1 | ||
| Calcium | 0.1874 | 0.1631 | 1 | |
| Phosphorus | 0.1021 | 0.0904 | 0.1591 | 1 |
Pearson correlation coefficients showing levels of correlation between measured mediators of calcification (P < 0.01 each measure).
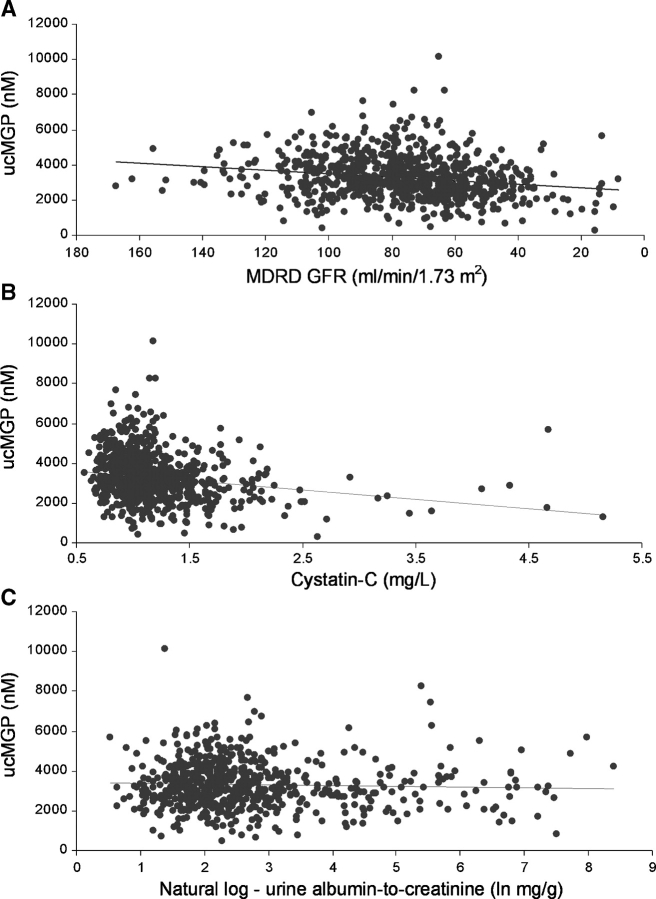
In unadjusted analysis, each 10 mL/min/1.73 m2 lower eGFR was associated with 101 nM lower ucMGP levels (Table 4 and Figure 2A). This association was minimally attenuated and remained strongly statistically significant with adjustment for age, sex and race as well as in the final multivariate model. eGFR in isolation accounted for ∼4% of the variance in ucMGP levels, whereas the multivariable model collectively accounted for 20% of its variance. Results were similar when cystatin-C was evaluated as the measure of kidney function (Table 4 and Figure 2B). In the final adjusted model, each 0.1 mg/L increase in cystatin-C was associated with a 39 nM lower ucMGP level. In contrast, we observed no significant association between the albumin-to-creatinine ratio and serum ucMGP levels in either unadjusted or multivariable adjusted models (Table 4 and Figure 2C). All associations were similar among subjects with or without diabetes (P-values for interaction >0.50).
Table 4.
Association of kidney function with the uncarboxylated matrix Gla protein (ucMGP)
| Coefficient of | 95% Confidence | |||
|---|---|---|---|---|
| regression (ß) | interval | P-value | R2 | |
| Change in ucMGP per 10 mL/min/1.73 m2 decrease in estimated glomerular filtration rate | ||||
| Unadjusted | −101 | −135 to −67 | <0.001 | 0.039 |
| Adjusted for age, sex and race | −71 | −107 to −35 | <0.001 | 0.073 |
| Multivariable adjusteda | −79 | −115 to −44 | <0.001 | 0.197 |
| Change in ucMGP per 0.1 mg/L increase in cystatin-C | ||||
| Unadjusted | −47 | −62 to −32 | <0.001 | 0.041 |
| Adjusted for age, sex and race | −38 | −53 to −23 | <0.001 | 0.082 |
| Multivariable adjusteda | −39 | −55 to −23 | <0.001 | 0.200 |
| Change in ucMGP per doubling of urine albumin/creatinineb | ||||
| Unadjusted | −26 | −71 to 20 | 0.27 | 0.002 |
| Adjusted for age, sex and race | −23 | −68 to 21 | 0.30 | 0.068 |
| Multivariable adjusteda | −33 | −82 to 15 | 0.17 | 0.173 |
aAdjusted for age, sex, race, body mass index (BMI), blood pressure, albumin, smoking status, calcium, phosphorus, hypertension, diabetes and fetuin-A.
bData were obtained after natural log-transformation; Beta from naturally log-transformed data was multiplied by 0.693 so as to represent the predicted change in albumin/creatinine per doubling of ucMGP.
Fig. 2.
Association of MDRD eGFR (2A), cystatin-C (2B) and urine albumin-to-creatinine ratio (2C) with serum ucMGP.
Discussion
We found that mild to moderate decrements in glomerular filtration rate were associated with lower serum ucMGP levels in persons with stable CVD. In contrast, urine albumin-to-creatinine levels were not associated with serum ucMGP levels. Prior studies have demonstrated that subjects with atherosclerosis and cardiac valve calcification have lower ucMGP levels than healthy controls. Thus, serum ucMGP may ultimately prove useful as a marker of the presence and severity of vascular calcification in persons with CKD.
Although our cross-sectional study results cannot determine the direction of association between kidney function and ucMGP, we believe it is unlikely that decreased kidney clearance directly leads to lower ucMGP levels in this study. Like creatinine, most substances accumulate in serum, rather than decline, as a direct result of decreased GFR, and MGP is nearly absent from the urine of normal individuals [39], because it complexes in blood with larger molecules [25]. Furthermore, while MGP may be extracted from the circulation by the kidney, as shown by simultaneous renal vein and artery sampling, the rate of extraction is independent of GFR across mild to moderate stages of CKD [39].
We propose three potential pathways that may account for the association between decreased GFR and lower serum ucMGP. Firstly, metabolic or genetic abnormalities associated with kidney dysfunction may suppress ucMGP production. For example, vitamin D deficiency is highly prevalent in CKD [40] and suppresses ucMGP production [41]. Transforming growth factor beta (TGF-β) is up-regulated in certain forms of CKD [42] and down-regulates vascular smooth muscle cell MGP transcription [41]. Polymorphisms in the promoter region of the MGP gene, which alter affinity for the transcriptional factor complex AP-1, influence MGP expression in humans [43]. Compared to healthy individuals, these same polymorphisms that associate with lower MGP expression are more prevalent among those on dialysis [44].
Secondly, predicated on the development of severe vascular calcification in MGP knockout mice, it is possible that low ucMGP may be causally related to the development of vascular calcification. Associated haemodynamic changes and vascular stiffness, in turn, may contribute to kidney dysfunction.
Finally, it is possible that reduced kidney function may directly lead to vascular calcification, which, when abundant, may decrease serum ucMGP because ucMGP has affinity for hydroxyapatite deposited within the vasculature [26,45]. Prior studies have demonstrated that serum MGP levels decline when arteries calcify in animal models and that lower ucMGP levels are associated with vascular calcification and atherosclerosis in select human populations [26,28,29].
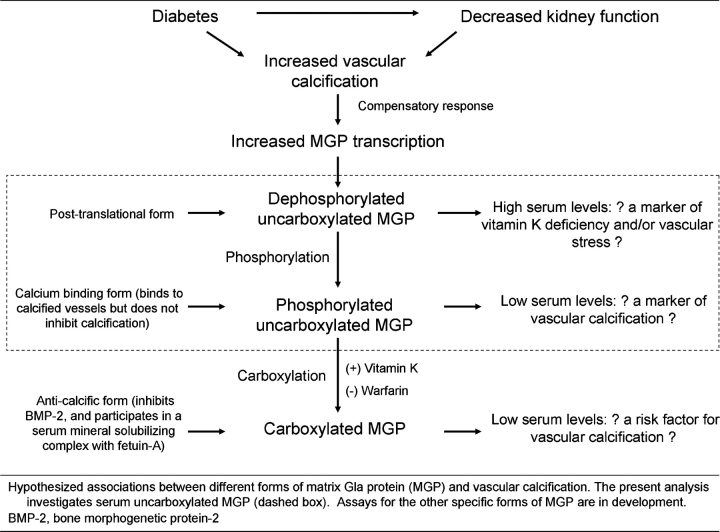
Future studies with repeated measures of ucMGP, kidney function and vascular calcification are required to differentiate between these possibilities but it is our hypothesis that this final possibility is most likely. Two important post-translational modifications of matrix Gla protein exist: (1) a carboxylation step which is dependent on vitamin K and inhibited by warfarin, and (2) phosphorylation of serine residues within the protein [46]. Carboxylation has been shown to be necessary for MGP's ability to inhibit the ‘pro-osteoblastic’ transcription factor BMP-2 [23,24] and is also necessary for binding to a circulating fetuin-A complex, which may be important to solubilize circulating calcium crystals [25]. Phosphorylated ucMGP likely retains its affinity for calcium and it has been shown to colocalize in the vessel walls of calcified arteries [45], potentially depleting serum levels in persons with vascular calcification. The present analysis uses an assay that detects both the phosphorylated and un-phosphorylated forms of ucMGP, so competing influences of (1) upregulated MGP transcription in response to vascular stress and (2) the absorptive forces of already calcified vessels will likely dictate serum ucMGP levels (Figure 3). It is likely that the latter factor will be dominant in the setting of vascular calcification, as most MGP produced is in a phosphorylated state [46]. New assays that can distinguish the phosphorylation status of ucMGP are being produced and may provide important insights into future research.
Fig. 3.
Different forms of the matrix Gla protein and their hypothesized association with vascular calcification.
The association of CKD with ucMGP has implications for prior and future studies evaluating ucMGP as a marker of vascular calcification or CVD events. Because there was no adjustment for severity of CKD in prior studies, it is possible that the association of ucMGP with vascular calcification reported previously [28] may reflect residual confounding by kidney disease. On the basis of our results, future studies evaluating the association of ucMGP with vascular calcification should adjust for the severity of CKD to determine if the associations remain. Furthermore, a number of other novel regulators of vascular calcification have been identified, including fetuin-A [47], fibroblast growth factor 23 [48] and pyrophosphate [49]. Because these factors likely work in concert, future studies evaluating the associations of ucMPG with vascular or valvular calcification may benefit from the measurement of other regulators simultaneously to determine their independent and potential additive effects. Because several of these novel inhibitors appear not only to influence vascular calcification, but may also influence insulin resistance [50–52], future studies should also evaluate whether associations are similar among persons with or without diabetes.
The strengths of this study include the measurement of ucMGP rather than total MGP. The association of lower serum ucMGP with atherosclerosis or vascular calcification has been consistent [28,29] whereas the association of total serum MGP with vascular calcification has provided conflicting results [53–56]. Additional strengths include the relatively large study population, availability of several markers of kidney function and other molecules involved in regulation of vascular calcification and measurement of multiple potential confounding variables.
The manuscript also has important limitations. Firstly, all participants had prevalent CVD and most were older men. Results may differ in younger persons, women and persons without CVD. Dietary vitamin K intake was not measured and warfarin use was infrequent in our study sample. Frozen serum was not available for ucMGP measurement among 18% of Heart and Soul participants. However, kidney function was similar in subjects with and without available ucMGP data. The study participants had moderate CKD, at most, and our results may not generalize to persons with advanced stage CKD. However, prior studies have demonstrated that ucMGP are also lower in persons with ESRD compared to healthy controls [28–30]. We cannot exclude that the observed associations may reflect residual confounding by unmeasured factors. However, to explain the strength of associations observed here, any such factor would have to be strongly linked with both moderate CKD and serum ucMGP levels.
In conclusion, mild to moderate decrements in glomerular filtration rate are strongly associated with lower serum ucMGP levels in out-patients with stable CVD. Future studies should evaluate whether serum ucMGP may account for some of the association of CKD with vascular calcification and whether serum ucMGP levels may identify individuals at increased risk of future CVD events.
Acknowledgments
This study was supported by a Hypertension Training Grant (T32 HL007261) through the National Heart Lung and Blood Institute (BDP) and an American Heart Association Fellow-to-Faculty transition grant (JHI). The Heart and Soul Study was supported by the Department of Veterans Epidemiology Merit Review Program; the Department of Veterans Affairs Health Services Research and Development service; the National Heart Lung and Blood Institute (R01 HL079235); the American Federation for Aging Research (Paul Beeson Scholars Program); the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program) and the Ischemia Research and Education Foundation. The funding organizations had no role in the design and conduct of the study; collection, management, analysis and inter- pretation of the data; and preparation, review or approval of the manuscript.
Conflict of interest statement. None declared.
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003;325:163–167. doi: 10.1097/00000441-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Culleton BF, Wilson PW. Cardiovascular disease: risk factors, secular trends, and therapeutic guidelines. J Am Soc Nephrol. 1998;9:S5–S15. [PubMed] [Google Scholar]
- 4.Levin A, Djurdjev O, Barrett B, et al. Cardiovascular disease in patients with chronic kidney disease: getting to the heart of the matter. Am J Kidney Dis. 2001;38:1398–1407. doi: 10.1053/ajkd.2001.29275. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 8.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 9.Braun J, Oldendorf M, Moshage W, et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996;27:394–401. doi: 10.1016/s0272-6386(96)90363-7. [DOI] [PubMed] [Google Scholar]
- 10.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 11.Kramer H, Toto R, Peshock R, et al. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol. 2005;16:507–513. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 12.Merjanian R, Budoff M, Adler S, et al. Coronary artery, aortic wall, and valvular calcification in nondialyzed individuals with type 2 diabetes and renal disease. Kidney Int. 2003;64:263–271. doi: 10.1046/j.1523-1755.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 13.Qunibi WY, Abouzahr F, Mizani MR, et al. Cardiovascular calcification in Hispanic Americans (HA) with chronic kidney disease (CKD) due to type 2 diabetes. Kidney Int. 2005;68:271–277. doi: 10.1111/j.1523-1755.2005.00402.x. [DOI] [PubMed] [Google Scholar]
- 14.Russo D, Palmiero G, De Blasio AP, et al. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Tomiyama C, Higa A, Dalboni MA, et al. The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol Dial Transplant. 2006;21:2464–2471. doi: 10.1093/ndt/gfl291. [DOI] [PubMed] [Google Scholar]
- 16.Toussaint ND, Lau KK, Strauss BJ, et al. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant. 2008;23:586–593. doi: 10.1093/ndt/gfm660. [DOI] [PubMed] [Google Scholar]
- 17.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 18.Giachelli CM, Speer MY, Li X, et al. Regulation of vascular calcification roles of phosphate and osteopontin. Circ Res. 2005;96:717–722. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 20.Price PA, Fraser JD, Metz-Virca G. Molecular cloning of matrix Gla protein: implications for substrate recognition by the vitamin K-dependent gamma-carboxylase. Proc Natl Acad Sci USA. 1987;84:8335–8339. doi: 10.1073/pnas.84.23.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 22.Meier M, Weng LP, Alexandrakis E, et al. Tracheobronchial stenosis in Keutel syndrome. Eur Respir J. 2001;17:566–569. doi: 10.1183/09031936.01.17305660. [DOI] [PubMed] [Google Scholar]
- 23.Bostrom K, Tsao D, Shen S, et al. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–14052. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 24.Zebboudj AF, Imura M, Bostrom K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 2002;277:4388–4394. doi: 10.1074/jbc.M109683200. [DOI] [PubMed] [Google Scholar]
- 25.Price PA, Nguyen TM, Williamson MK. Biochemical characterization of the serum fetuin-mineral complex. J Biol Chem. 2003;278:22153–22160. doi: 10.1074/jbc.M300739200. [DOI] [PubMed] [Google Scholar]
- 26.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 27.Schurgers LJ, Spronk HM, Soute BA, et al. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–2831. doi: 10.1182/blood-2006-07-035345. [DOI] [PubMed] [Google Scholar]
- 28.Cranenburg EC, Vermeer C, Koos R, et al. The circulating inactive form of matrix Gla Protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res. 2008;45:427–436. doi: 10.1159/000124863. [DOI] [PubMed] [Google Scholar]
- 29.Hermans MM, Vermeer C, Kooman JP, et al. Undercarboxylated matrix GLA protein levels are decreased in dialysis patients and related to parameters of calcium-phosphate metabolism and aortic augmentation index. Blood Purif. 2007;25:395–401. doi: 10.1159/000108629. [DOI] [PubMed] [Google Scholar]
- 30.Shroff RC, Shah V, Hiorns MP, et al. The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol Dial Transplant. 2008;23:3263–3271. doi: 10.1093/ndt/gfn226. [DOI] [PubMed] [Google Scholar]
- 31.Ix JH, Shlipak MG, Liu HH, et al. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the Heart and Soul Study. J Am Soc Nephrol. 2003;14:3233–3238. doi: 10.1097/01.asn.0000095642.25603.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruo B, Rumsfeld JS, Hlatky MA, et al. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 35.Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 36.Ix JH, Chertow GM, Shlipak MG, et al. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:2533–2539. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ix JH, Shlipak MG, Katz R, et al. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2007;50:412–420. doi: 10.1053/j.ajkd.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 38.K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 39.Rennenberg RJ, Schurgers LJ, Vermeer C, et al. Renal handling of matrix Gla-protein in humans with moderate to severe hypertension. Hypertens Res. 2008;31:1745–1751. doi: 10.1291/hypres.31.1745. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez EA, Sachdeva A, Oliver DA, et al. A Single Center Observational Study. Vitamin D insufficiency and deficiency in chronic kidney disease. Am J Nephrol. 2004;24:503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 41.Farzaneh-Far A, Weissberg PL, Proudfoot D, et al. Transcriptional regulation of matrix gla protein. Z Kardiol. 2001;90(Suppl 3):38–42. doi: 10.1007/s003920170040. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman BB, Sharma K, Ziyadeh FN. Potential role of TGF-beta in diabetic nephropathy. Miner Electrolyte Metab. 1998;24:190–196. doi: 10.1159/000057369. [DOI] [PubMed] [Google Scholar]
- 43.Farzaneh-Far A, Davies JD, Braam LA, et al. A polymorphism of the human matrix gamma-carboxyglutamic acid protein promoter alters binding of an activating protein-1 complex and is associated with altered transcription and serum levels. J Biol Chem. 2001;276:32466–32473. doi: 10.1074/jbc.M104909200. [DOI] [PubMed] [Google Scholar]
- 44.Brancaccio D, Biondi ML, Gallieni M, et al. Matrix GLA protein gene polymorphisms: clinical correlates and cardiovascular mortality in chronic kidney disease patients. Am J Nephrol. 2005;25:548–552. doi: 10.1159/000088809. [DOI] [PubMed] [Google Scholar]
- 45.Schurgers LJ, Teunissen KJ, Knapen MH, et al. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629–1633. doi: 10.1161/01.ATV.0000173313.46222.43. [DOI] [PubMed] [Google Scholar]
- 46.Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603. [PubMed] [Google Scholar]
- 47.Westenfeld R, Schafer C, Smeets R, et al. Fetuin-A (AHSG) prevents extraosseous calcification induced by uraemia and phosphate challenge in mice. Nephrol Dial Transplant. 2007;22:1537–1546. doi: 10.1093/ndt/gfm094. [DOI] [PubMed] [Google Scholar]
- 48.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lomashvili KA, Cobbs S, Hennigar RA, et al. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 50.Ix JH, Wassel CL, Kanaya AM, et al. Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008;300:182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathews ST, Rakhade S, Zhou X, et al. Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem Biophys Res Commun. 2006;350:437–443. doi: 10.1016/j.bbrc.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 52.Hesse M, Frohlich LF, Zeitz U, et al. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Braam LA, Dissel P, Gijsbers BL, et al. Assay for human matrix gla protein in serum: potential applications in the cardiovascular field. Arterioscler Thromb Vasc Biol. 2000;20:1257–1261. doi: 10.1161/01.atv.20.5.1257. [DOI] [PubMed] [Google Scholar]
- 54.Jono S, Ikari Y, Vermeer C, et al. Matrix Gla protein is associated with coronary artery calcification as assessed by electron-beam computed tomography. Thromb Haemost. 2004;91:790–794. doi: 10.1160/TH03-08-0572. [DOI] [PubMed] [Google Scholar]
- 55.O’Donnell CJ, Shea MK, Price PA, et al. Matrix Gla protein is associated with risk factors for atherosclerosis but not with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2006;26:2769–2774. doi: 10.1161/01.ATV.0000245793.83158.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schurgers LJ, Teunissen KJ, Knapen MH, et al. Characteristics and performance of an immunosorbent assay for human matrix Gla-protein. Clin Chim Acta. 2005;351:131–138. doi: 10.1016/j.cccn.2004.08.003. [DOI] [PubMed] [Google Scholar]