Abstract
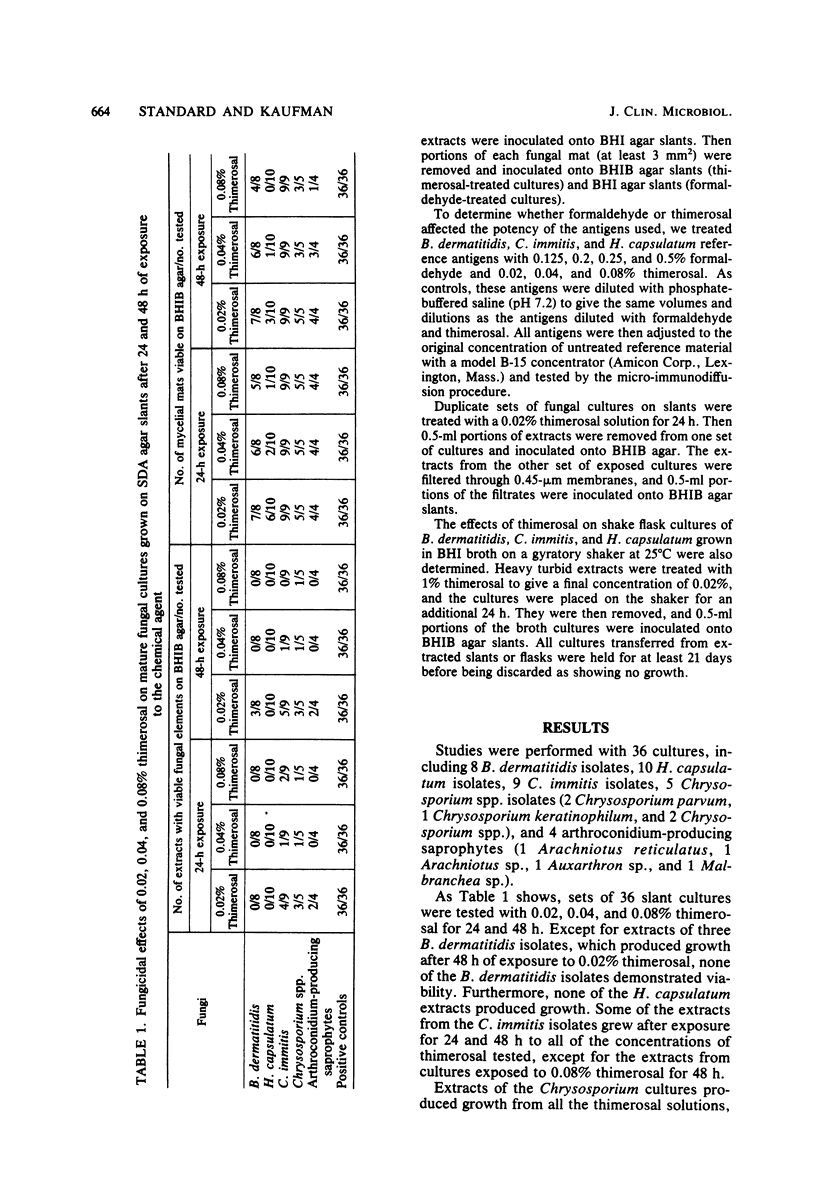
Currently, exoantigen test procedures for identifying mycelial form cultures of pathogenic molds require that the fungi being extracted be treated with thimerosal to render them safe for handling. Recent studies have demonstrated that thimerosal may not be fungicidal. In view of these reports, we investigated the effects of thimerosal and formaldehyde on a variety of exoantigen preparations. Mature mycelial form fungal cultures, including cultures of Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum and morphologically similar fungi, were grown on Sabouraud dextrose agar slants and treated with 0.02, 0.04, and 0.08% thimerosal for 24 and 48 h and with 0.2 and 0.5% formaldehyde for 24 and 48 h. We found that 0.5% formaldehyde killed all of the fungi studied, whereas 0.2% formaldehyde permitted the growth of only one fungus; 0.02, 0.04, and 0.08% thimerosal were fungistatic. Furthermore, 0.2 and 0.5% formaldehyde and 0.08% thimerosal affected certain antigens adversely. For those investigators who prefer to use 0.02% thimerosal and to work with sterile extracts, we recommend that the procedure be modified, and we advocate sterilization of extracts by passage through membrane filters.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deighton F. J., Hall N. K., Larsh H. W. Merthiolate treatment of pathogenic fungi. J Clin Microbiol. 1979 Aug;10(2):144–146. doi: 10.1128/jcm.10.2.144-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., Standard P. Improved version of the exoantigen test for identification of Coccidioides immitis and Histoplasma capsulatum cultures. J Clin Microbiol. 1978 Jul;8(1):42–45. doi: 10.1128/jcm.8.1.42-45.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Falcone R. G., Boone C. J. Effect of thimerosal on the whole yeast phase antigen of Histoplasma capsulatum. Mycopathol Mycol Appl. 1969 Jan 29;37(1):1–14. doi: 10.1007/BF02051325. [DOI] [PubMed] [Google Scholar]
- Scalarone G. M., Levine H. B. Deficient resistance to Coccidioides immitis following intravenous vaccination. I. Distribution of spherules after intravenous and intramuscular doses. Sabouraudia. 1971 Jul;9(2):81–89. [PubMed] [Google Scholar]
- Standard P. G., Kaufman L. Immunological procedure for the rapid and specific identification of Coccidioides immitis cultures. J Clin Microbiol. 1977 Feb;5(2):149–153. doi: 10.1128/jcm.5.2.149-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standard P. G., Kaufman L. Specific immunological test for the rapid identification of members of the genus Histoplasma. J Clin Microbiol. 1976 Feb;3(2):191–199. doi: 10.1128/jcm.3.2.191-199.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



