Abstract
Activation of sphingosine kinase/sphingosine-1-phosphate (SK/S1P)-mediated signalling has been recognized as critical for cardioprotection in response to acute ischaemia/reperfusion injury. Incubation of S1P with cultured cardiac myocytes subjected to hypoxia or treatment of isolated hearts either before ischaemia or at the onset of reperfusion (pharmacologic pre- or postconditioning) results in reduced myocyte injury. Synthetic agonists active at S1P receptors mimic these responses. Gene-targeted mice null for the SK1 isoform whose hearts are subjected to ischaemia/reperfusion injury exhibit increased infarct size and respond poorly either to ischaemic pre- or postconditioning. Measurements of cardiac SK activity and S1P parallel these observations. Ischaemic postconditioning combined with sphingosine and S1P rescues the heart from prolonged ischaemia. These observations may have considerable relevance for future therapeutic approaches to acute and chronic myocardial injury.
Keywords: Sphingosine kinase, Cardioprotection, Ischaemic preconditioning, Ischaemic postconditioning, Sphingosine-1-phosphate, Ischaemia/reperfusion injury, Enzyme regulation
1. Introduction
‘Subtle as Sphinx … ’.
William Shakespeare, Love’s Labour’s Lost, Act IV, Scene iii.
The above quote illustrates one of the transformations of the myth of the Sphinx. In Greek lore, the Sphinx was a beast that strangled (‘sphingo’ = strangle) travellers unable to answer a riddle posed by the Sphinx. Oedipus solved the riddle, whereupon the Sphinx committed suicide. These two attributes of the Sphinx, entwining the victim and the enigma or riddle, were contained in the original scientific description of sphingolipids. These compounds were first extensively characterized by a German neurochemist, J.L.W. Thudichum, in the late 19th century, who named the chemical backbone of sphingolipids for their enigmatic ‘Sphinx-like’ properties. They were also identified as sheathing (‘strangling’) nerves. However, interest remained confined to a small number of investigators until relatively recently when the pathophysiological importance of sphingolipids became evident.
Among the most prominent of these lipid mediators is sphingosine-1-phosphate (S1P), which is formed when one or both isoforms of the enzyme sphingosine kinase (SK) catalyses the binding of a phosphate group to sphingosine. The latter is derived from the ubiquitous membrane lipid sphingomyelin (Figure 1). The SK/S1P pathway functions as a checkpoint in many cellular signalling cascades. Functions initiated or determined by signals activated by this pathway are critical for cell motility, cytoskeletal organization, vasculogenesis, cell growth, lymphoid trafficking, and immune function. These properties of SK/S1P signalling have led to extensive studies in cancer, immune, and inflammatory responses.
Figure 1.
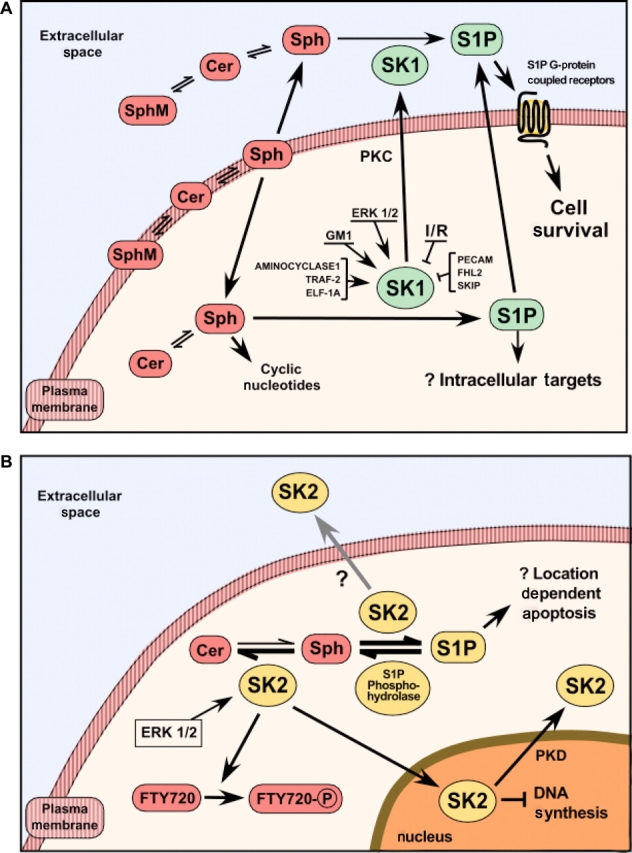
Regulation and function of sphingosine kinase-1 (SK1). (A) The diagram depicts some of the negative and positive regulators of SK1 activity and the synthesis and location of the SK1/S1P pathway either present in the heart or derived experimentally. A key feature of this pathway is the conversion of the ubiquitous membrane lipid sphingomyelin by several sphingomyelinases manufactured intracellularly that act both at the membrane level and extracellularly. This results in the generation of ceramide that is deacylated by neutral ceramidase, yielding sphingosine, which is then converted to sphingosine-1-phosphate (S1P) by the action of SK1. (B) A second isoform of SK (SK2) is also responsible for S1P generation. The location of S1P generation within the cell may determine its effect, but the outcomes of such location-based effects have not been determined. The recycling pathway for the conversion of sphingosine into pro-apoptotic ceramide is dependent on SK2, acting in concert with S1P phosphohydrolase 1. As shown in the figure, SK2 (like SK1) is activated by ERK 1/2 and is also responsible for phosphorylation of the immunomodulator FTY720. SK2 resides in the nucleus and its export is dependent on the activity of protein kinase D.
An initial key paper reported that activation or inhibition of this pathway can determine cell fate by altering the balance between ceramide, which is pro-apoptotic, and S1P, which activates pro-survival signalling1 (Figure 1). In the cardiovascular system, considerable attention has focused on SK/S1P effects in endothelial and smooth muscle cells, both during development and in vitro.2–4 Thus, recent reports have implicated an SK isoform, sphingosine kinase-1 (SK1), in endothelial cell proliferation and angiogenesis,5 migration stimulated by hypoxia via hypoxia-inducing factors 1α and 2α (HIF-1α and HIF-2α),6 and permeability.7,8 Activation of SK1 is also responsible for the function of vascular endothelium as an important source of plasma S1P.9,10 These aspects of SK are covered in an accompanying review in this Spotlight issue by Igarashi and Michel.
Until recently, there were few data about the role of SK/S1P signalling responses in cardiac myocytes or in the heart. In addition, there was no published information regarding outcomes after alterations in this lipid pathway during oxidative stress such as acute or chronic ischaemia or ischaemia/reperfusion injury. Indeed, most recent reviews contain scant or no information on cardiac SK/S1P signalling under either normal or stress conditions,11–14 and none are devoted to cardioprotection. Because recent published reports have shown that SK and S1P are important cardioprotective mediators, it is now apparent that these molecules (or their synthetic analogues) may harbour therapeutic potential for both acute and chronic myocardial injury.
2. Sphingosine kinase-1
Sphingosine kinase was originally purified from rat kidney.15 The purified enzyme had a molecular mass of 49 kDa, which corresponds to the subsequently determined size of SK1. Murine SK1 was then cloned16 and showed a high degree of homology with the human enzyme.17,18 Both human and murine SK1 displayed signals in the heart and specifically phosphorylated d-erythro-sphingosine.16–18 Pitson et al.19 subsequently identified a unique nucleotide-binding site for SK1. This group also showed that both SK1 and SK2 bind calmodulin.20 Disruption of calmodulin-binding ablated agonist-induced translocation of human SK1 from the cytoplasm to the plasma membrane, without any effect on phosphorylation or catalytic activation of the enzyme.20 More recently, Park et al.21 cloned a rat SK1 that harbours an N-terminal extension, termed long-rSK1. Deduced rat and human amino acid sequences display high homology and their wide tissue expression includes the heart.
Within 2 years of cloning SK1, the Spiegel group reported the cloning of a second isoform, SK2.22 These two known isoforms of SK each contain several splice variants.23 As noted above, mouse, rat, and human SK1 exhibit substantial homology. SK2 is highly homologous to SK1 but has ∼240 additional amino acids located at the N terminus and in the centre of the enzyme, thereby accounting for its larger molecular mass.24,25 Moreover, the genes encoding these isoforms are localized on different chromosomes.12 Genetic deletion of both isoforms results in foetal death from severe bleeding and inadequate vasculogenesis.26 In contrast, mice null for either SK1 or SK2 develop normally and appear unremarkable in the basal state.27,28 Myogenic differentiation of C2C12 myoblasts also depends on the activity of SK1.29,30
Sphingosine kinase activity responds to stimulation by a variety of G-protein-coupled receptor agonists including muscarinic agonists, histamine and S1P itself, agonists at receptor tyrosine kinases (PDGF, EGF, VEGF, TGF-α, and TGF-β), immunoglobulin receptor cross-linking, interleukins, oestrogen (for a review, see reference Alemany et al.12), and activators of PKCε.31 Both TNF-α and phorbol ester [which stimulates protein kinase C (PKC)] phosphorylate SK1 at serine225, a reaction that is mediated by ERK 1/2.32 The TNF-α effect requires binding by TNF receptor-associated factor-2.33 This pathway appears to exhibit a complex interaction because it has also been reported that TNF-α activation of ERK 1/2 is itself dependent on SK activation.34
Other interacting proteins that stimulate SK include δ-catenin/neutral plakophilin-related armadillo repeat protein,35 aminocyclase 1,36 and eukaryotic elongation factor 1A.37 Reported inhibitory interacting proteins are SKIP (SK1-inteacting protein),38 PECAM-1 (platelet endothelial adhesion molecule-1),39 and FHL2/SLIM3, a Lim-only factor.40 An interacting factor that is a putative adaptor molecule is RPK118.41 Additional regulators include phospholipase D and possibly calcium.42,43 In vitro evidence also has identified cellular export of SK, which may account for substantial enzyme activity in both mouse and human blood.44,45 A number of these interactions are depicted in Figure 1A.
More recently and highly pertinent to acute ischaemia/reperfusion injury is the observation that reactive oxygen species (ROS) generated by the action of monoamine oxidase A on serotonin contained in platelets is responsible for the degradation of SK1.46 Conversely, in endothelial cells, hypoxia increases SK1 mRNA, protein levels, and activity that are dependent on HIF-1α and HIF-2α.47 Thus, the balance of hypoxia and ROS levels (and other factors as yet unidentified) could serve to regulate SK1 activity during oxidative stress in the heart (see below).
Numerous in vitro studies have demonstrated that SK1 promotes cell survival. These include an early report in cultured rat cardiac fibroblasts in which the monoganglioside GM-1, which activates SK via PKC, protected against apoptosis induced by the PKC inhibitor staurosporine and by C2-ceramide.31 These investigators also showed that GM-1 induced the synthesis of S1P, an effect that was partially blocked by the SK inhibitor N, N-dimethylsphingosine (DMS).31 The latter has recently been shown to be predominantly an inhibitor of SK1.48 In addition to SK inhibitors, subsequent work has utilized dominant negative SK1 and small-interfering RNA. Thus, knockdown of SK1 by the latter caused cell cycle arrest in MCF-7 cancer cells and induced apoptosis.49 Use of siRNA to target SK1 also has shown that the ability of adiponectin to induce cardioprotective cyclooxygenase-2 in neonatal rat myocytes is dependent on SK1.50
Except for the earlier study by Cavallini et al.,31 SK responses have not previously been studied in cardiac fibroblasts. Total SK activity is over 10-fold higher in cardiac fibroblasts than in adult mouse cardiac myocytes.51 In cardiac fibroblasts isolated from SK1 null mice, SK activity was greatly reduced indicating that SK1 is the major isoform expressed in these cells. Experiments showed that activation of endogenous SK1 serves a dual regulatory function: it is required for optimal cardiac fibroblast proliferation but is a negative modulator of proinflammatory responses, such as IL-1β-mediated activation of iNOS and subsequent NO production during hypoxia.51
Endogenous SK1 is an important regulator of intracellular ceramide levels (Figure 1). Downregulation of SK1 results in enhanced ceramide synthesis and its accumulation in mitochondria, which may be key in initiating mitochondrial events leading to cell death.49 However, evidence that either S1P or SK reside in mitochondria is sparse. In human leukaemia cells, Cuvillier and Levade52 concluded that S1P is upstream of mitochondria. In contrast, Dindo et al.53 recently reported that a novel C16-ceramide derivative induced selective upregulation of S1P in the mitochondria of SW403 human colon carcinoma cells. The authors suggested that this implied the presence of mitochondrial SK activity, which is selectively activated by treatment with exogenous long-chain ceramides. SK activity in heart mitochondria also has been characterized in response to ischaemia/reperfusion injury and ischaemic preconditioning (IPC) (see below).
Autophagy is a cellular process responsible for the degradation of long-lived proteins and organelles.54 Normally it occurs at a low level, but is activated by cellular stress. In the heart, as in other cells and organs, autophagy may be either beneficial or detrimental, depending on its extent and timing. Autophagy associated with survival is regulated in part by SK1,55,56 while cell-death-associated autophagy is promoted by ceramide.37 As originally proposed by Cuvillier et al.,1 this represents an example of the sphingolipid ‘rheostat’ at work. Lavieu et al.56 have suggested that autophagy may be a key mechanism by which sphingolipids control cell fate, but evidence for this possibility in the heart is not yet available.
Underlying the anti-apoptotic effects of SK1 is its function as the terminal step in the intracellular synthesis of S1P. There is considerable evidence that intracellular S1P is then exported to activate pro-survival signalling pathways in an autocrine and/or paracrine manner, and this phenomenon (‘inside-out signalling’)12,57 has been described in cardiac myocytes.58 A counterintuitive function of SK1 has recently been proposed in which apoptosis induces SK1 expression to release S1P that serves a ‘come-and-get-me’ signal for scavenger cells to engulf them in order to prevent necrosis.59 The data were obtained in Jurkat and U937 leukaemia cells and required massive apoptosis to elicit this phenomenon.
3. Sphingosine kinase-2
As noted earlier, SK2 was cloned shortly after SK1.22 It was found to have a much larger molecular mass, but like SK1 its expression resulted in elevated S1P levels. Robust expression was present in murine heart.22 Amino acid sequence analysis revealed the same conserved domains as were found in SK1.22 Like long SK1 present in the human heart,21 SK2 has an N-terminal extended form resulting in an isoform that has 36 more amino acids expressed in human tissues compared with mouse.60 Another similarity is that both SK1 and SK2 are activated by ERK 1/2-mediated phosphorylation.32,61
Despite these similarities and even though it catalyses the production of S1P, SK2 has opposing actions to SK1.62 Thus, SK2 inhibits cell growth and enhances apoptosis, in part by regulating ceramide levels. Downregulation of SK2 reduced conversion of sphingosine to ceramide, while downregulation of SK1 increased it.62 As noted by Spiegel and Milstien,63 sphingosine is not synthesized de novo; rather it is derived from cleavage of ceramide by ceramidases. Sphingosine thus produced can be re-utilized for synthesis of ceramide and complex sphingolipids or phosphorylated to form S1P.63 This recycling pathway for the conversion of sphingosine into pro-apoptotic ceramide is dependent on SK2, but not SK1, acting in concert with S1P phosphohydrolase 164 (Figure 1B).
Other effects of SK2 relating to apoptosis may be its nuclear localization resulting in inhibition of DNA synthesis65,66 or its BH3 domain.67 In addition, SK2 contains a unique nuclear export sequence that can be phosphorylated by protein kinase-D to facilitate exit of SK2 from the nucleus.68 In keeping with the designation of sphingolipids as ‘Sphinx-like’ or enigmatic,69 it nevertheless remains a mystery why SK2 is pro-apoptotic despite being responsible for S1P synthesis. Thus, SK1 null mice, which lack SK2, exhibit normal cardiac function until stressed (see below). In these mice, tissue S1P levels are normal but serum levels are reduced by half.27 One explanation of these observations has been the location of S1P generated by SK isoforms. When the normally anti-apoptotic SK1 form was targeted to the endoplasmic reticulum of NIH 3T3 fibroblasts, apoptosis ensued.62 The authors suggested that the differences between pro-survival SK1 and pro-apoptotic SK2 are related in part to distinct subcellular localizations and spatially restricted production of S1P.62 However, such localization has not been demonstrated in cardiac cells or in vivo in any organs.
Both the synthetic sphingosine analogue FTY720 and the putative SK inhibitor dimethylsphingosine inhibit primarily the SK1 form, but can activate the SK2 form at low substrate concentrations in rat heart.48 SK2 is also necessary to phosphorylate and thus activate the synthetic immunomodulator FTY720.70–74 When phosphorylated, this drug acts as an agonist for S1P1–5 receptors.71 FTY720 is being used in clinical trials to prevent renal transplant rejection and reduce relapses in patients with multiple sclerosis.75,76
4. Sphingosine kinase in cardioprotection
In neonatal rat cardiac myocytes, initial observations suggested that exogenously applied S1P enhanced cardiac myocyte survival during hypoxia.77 Subsequent work employed cultured adult mouse cardiac myocytes58,78 for hypoxia studies that serve as a model for in vivo conditions resulting from coronary artery occlusion.79
As noted above, the monoganglioside GM-1 enhanced the survival of cardiac fibroblasts subjected either to PKC inhibition or C2-ceramide treatment.31 GM-1 also increased S1P levels, an effect abrogated by the SK inhibitor DMS.31 Using isolated adult mouse hearts (Langendorff technique), Jin et al.80 reported that exogenous S1P and GM-1 separately induced substantial resistance to ischaemia–reperfusion injury in wild-type mouse hearts as determined by haemodynamic and infarct size measurements. Similar experiments were reported by Lecour et al.81 in isolated rat heart. The importance of the pro-survival kinase PKCε was emphasized by experiments in which GM-1 proved to be ineffective in PKCε null hearts. In addition, GM-1, but not exogenous S1P, stimulated translocation of activated PKCε to myocyte particulate fractions. Nevertheless, exogenously administered S1P was effective both in isolated PKCε null hearts subjected to ischaemia/reperfusion injury80 and in isolated cardiac myocytes from these hearts subjected to hypoxia.58 These experiments also provided evidence for the postulate that PKCε is a critical upstream modulator of SK activity and endogenous S1P production in cardiac tissue. Consistent with this hypothesis, it was shown using PKCε peptide agonists and antagonists and targeted deletion of the PKCε gene that PKCε activation is essential for cardioprotection induced by IPC.82
Subsequently, the hypothesis that SK activation mediates IPC in isolated mouse hearts was tested.83 Results showed that IPC sufficient to reduce infarction size in wild-type hearts increased SK localization and activity in tissue membrane fractions. Interestingly, IPC triggered SK translocation to tissue membrane fractions in PKCε null hearts but did not enhance enzymatic activity or decrease infarction size after ischaemia–reperfusion.83 As noted above, DMS, the endogenous sphingolipid generated by N-methylation of sphingosine, inhibited tissue SK activity. As predicted, 10 µM DMS pretreatment abolished IPC-induced cardioprotection in wild-type hearts.83
In contrast to a moderate concentration of DMS (10 μM), low DMS concentrations (0.3–1.0 μM) enhanced cytosolic SK activity.84 DMS at a low concentration also stimulated translocation of activated PKCε to tissue particulate fractions and reduced cardiac ischaemia–reperfusion injury. Importantly, low-concentration DMS effects were abolished in PKCε null hearts, and SK1 was found to co-immunoprecipitate with activated PKCε phosphorylated at serine729. In addition, low-concentration DMS induced translocation of total Akt from Triton-insoluble fractions to the cytosol and increased activated Akt phosphorylated at serine473.
Another example of the concentration dependence of molecules usually considered to be inhibitory in the SK/S1P pathway is sphingosine, the immediate precursor of S1P. Although there is abundant evidence that sphingosine is toxic to cells, including cardiac myocytes,85,86 Vessey et al.87 recently reported that at lower, more physiologic (submicromolar) concentrations, sphingosine was cardioprotective in isolated Langendorff perfused rat hearts subjected to ischaemia/reperfusion injury. Unlike S1P, sphingosine-induced cardioprotection appears to be mediated by cyclic nucleotide (protein kinases A and G)-dependent pathways.87 At the higher concentrations usually employed (e.g. 5 µM), sphingosine proved to be cardiotoxic.
In prior work, the study of the SK/S1P pathway had been hampered by complex SK assays that required thin-layer chromatography and high-performance liquid chromatography. We reported a rapid solvent-extraction-based radioassay exhibiting excellent sensitivity.88 This assay has made it feasible to perform numerous time point assays that are necessary when non-saturating substrate concentrations and interfering enzymes are present in microsomal and mitochondrial fractions. Fractionation of cytosolic SK activity by gel filtration chromatography yielded two peaks of activity.88 Using antibody studies, we identified these clearly separated enzymes as SK2 and SK1, respectively. When tested with the classic SK inhibitor DMS, the activity of SK2 was unaffected by concentrations as high as 20 µM. Consistent with this observation, DMS was only a partial inhibitor of total cytosolic SK activity.48 Also SK2 was not inhibited by the immunomodulator FTY720. As noted earlier, SK1 was efficiently inhibited by both DMS and FTY720.48 Thus, prior experiments in other cells and tissues in which DMS was used as inhibitor of SK may require reinterpretation.
In addition to signal transduction assays for studies of S1P receptor subtype function cited above,58,78 the time course of SK activity in adult rat hearts subjected to ischaemia/reperfusion injury and preconditioning has been reported.89 Cytosolic SK activity declined dramatically during ischaemia and did not recover upon reperfusion (Figure 2A). These findings paralleled effects on left ventricular developed pressure (LVDP). IPC reduced the decrease in enzyme activity during ischaemia by half, and upon reperfusion activity returned to normal. LVDP recovered to 79% of control values and infarct size was reduced. IPC restored SK activity almost to normal during reperfusion (Figure 2A). Parallel effects were observed in mitochondria from the same hearts.89
Figure 2.
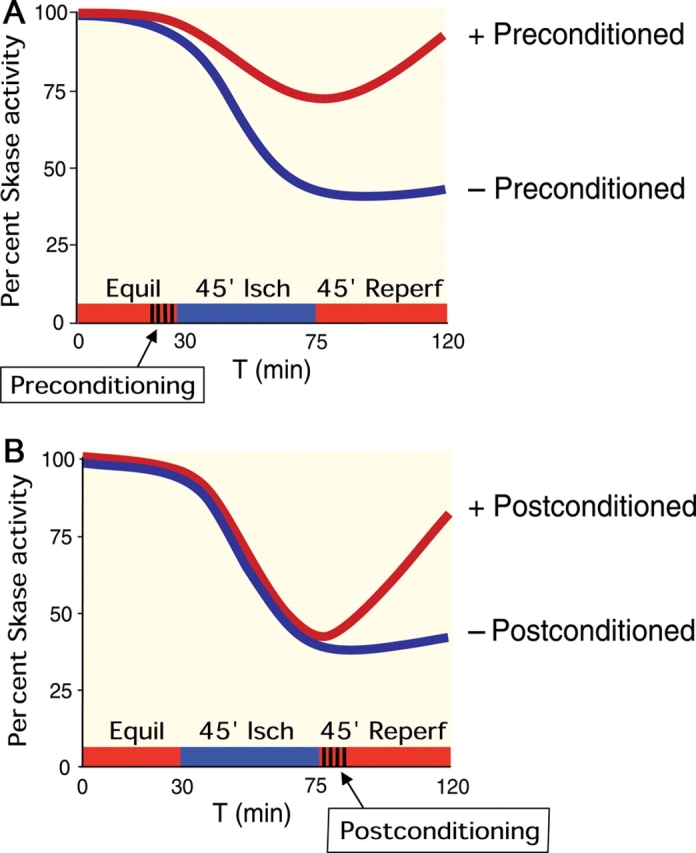
Time course of sphingosine kinase (SK) activity in pre- and postconditioning. (A) Schematic graphs showing the time course of SK activity in response to ischaemia and reperfusion in isolated hearts that had been subjected to ischaemic preconditioning or not. Compared with non-preconditioned hearts, preconditioned hearts had a smaller decline in SK activity and recovered to near normal levels during reperfusion. Adapted from data in Vessey et al.89 (B) Schematic graphs showing the time course of SK activity in response to ischaemia and reperfusion in isolated hearts that were subjected to ischaemic postconditioning or not. Non-postconditioned hearts exhibited a marked decline in SK activity during ischaemia, which did not recover during reperfusion. In contrast, postconditioned hearts, while displaying a similar reduction in SK activity during ischaemia, recovered substantially during reperfusion. Adapted from data in Jin et al.100
In these experiments,89 total S1P in cardiac tissue was quantified by LC/MS/MS (liquid chromatography) followed by tandem mass spectrometry.90 In control hearts, S1P content declined from baseline following both ischaemia and reperfusion. Preconditioned hearts had higher S1P levels after ischaemia/reperfusion relative to control hearts. Treatment of non-preconditioned hearts at reperfusion (pharmacologic postconditioning) with 100 nM S1P improved recovery of LVDP. Thus, maintenance of SK activity resulting higher S1P levels is critical for recovery from ischaemia/reperfusion injury. In this connection, the activity of S1P phosphatases and lyase has not been reported during experiments involving ischaemia/reperfusion injury in the heart.
Despite strong corroborating evidence that DMS modulates resistance to injury by effects on SK, this agent alters PKC activity84 that could confound interpretation of experimental data. Accordingly, SK1 knockout mice have been employed in studies designed to test the hypothesis that SK1 is critical for cardioprotection conferred by IPC.91,92 After SK1 gene disruption, SK2 expression increased resulting in total cardiac SK activity half that of wild-type. Although SK1 null hearts exhibited normal haemodynamic performance under baseline conditions, contractile abnormalities and infarction were more severe after ischaemia/reperfusion than in wild-type hearts. As predicted, targeted disruption of the SK1 gene abolished IPC-induced cardioprotection91 (Figure 3). Haemodynamic responses were similarly impaired, emphasizing the connection between SK activity and cardioprotection noted above89 (Figure 2A).
Figure 3.
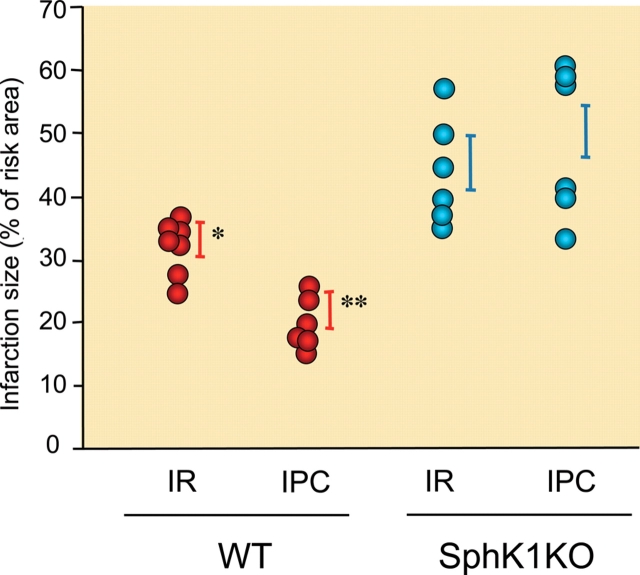
Sphingosine kinase-1 (SK1) null hearts do not respond to preconditioning. Wild-type and SK1 null mouse hearts were subjected to 50 min of global ischaemia and 40 min of reperfusion with or without ischaemic preconditioning (two cycles of 2 min of global ischaemia and 2 min of reperfusion). Infarct size (TTC staining) was expressed as a percentage of the risk area. As can be seen, SK1 null hearts do not respond to ischaemic preconditioning and infarct size is larger in these hearts compared with wild-type hearts. RA, risk area; WT, wild-type; IR, ischaemia/reperfusion; IPC, ischaemic preconditioning; SphK1KO, sphingosine kinase1 knockout. From Jin et al.91 *P < 0.05 vs. all other values; **P < 0.05 vs. all other values.
Importantly, when the index ischaemia time was reduced from 50 to 40 min, infarct size in the SK1 KO hearts declined to the level seen in the wild-type hearts subjected to ischaemia/reperfusion injury. At this reduced level of injury, IPC was still ineffective in producing cardioprotection in the KO hearts. However, exogenous S1P retained the ability to induce cardioprotection in these SK1 null hearts. Despite an increase in SK2 expression in the SK1 null hearts, infusion of DMS did not affect infarct size, suggesting that the absence of SK1 rather than the increased presence of SK2 was critical to the loss of cardioprotection in myocardium null for SK1.91
A recent study carried out in isolated rat hearts reported that previous adenoviral gene transfer of SK1 protected against haemodynamic deterioration, and reduced creatine kinase release and arrhythmias during acute ischaemia/reperfusion injury.93 When gene transfer was performed at the time of acute left anterior descending coronary artery ligation, studies 2 weeks later revealed improved left ventricular function, reduced infarct size, more neovascularization, and reduced collagen content in the treated mice.
It has been known for some time that the widely used anaesthetic agent isoflurane protects against organ damage. In a recent report, Kim et al.94 noted that an important mechanism of protection induced by isoflurane is activation of the SK/S1P pathway. Using an ischaemia/reperfusion model of renal injury, these investigators reported that isoflurane anaesthesia reduced the degree of renal failure and necrosis. Mice deficient in SK1 were not protected, and in wild-type mice, protection was abrogated by DMS and the S1P1 and 3 antagonist VPC2309. The authors also demonstrated that isoflurane increased SK1 mRNA in HK-2 cells.
The chemotherapeutic drug doxorubicin has well-recognized cardiotoxicity. In MCF7 human breast cancer cells, downregulation of SK2 by small-interfering RNA markedly enhanced apoptosis induced by doxorubicin.95 This occurs in part by preventing induction of anti-apoptic p21. Inhibition of SK1 also increased doxorubicin sensitivity in leukaemia cell lines.96 Whether modulation of either SK1 or SK2 can influence cardiac myocyte viability after doxorubicin treatment has not been determined.
Another area little studied is the role of SK1 in regulating glucose metabolism. Both basal and insulin-stimulated glucose uptake in C2C12 cells were enhanced when SK1 was overexpressed by adenoviral gene transfer.97 The converse occurred when SK1 activity was blocked. SK1 gene delivery significantly reduced the blood glucose level of KK/Ay diabetic mice, but had no effect in normal animals. Cardiac CK release and mitochondrial and myofibrillar disruption in diabetic mice were largely prevented in animals overexpressing SK1.97 These observations implicate SK1 in glucose regulation by mechanisms described earlier in this review.
Like IPC, ischaemic postconditioning is cardioprotective.98 This observation has recently been extended to patients undergoing percutaneous coronary interventions.99 To ascertain whether the SK/S1P pathway mediates successful postconditioning, isolated wild-type and SK1 null mouse hearts were subjected to ischaemia/reperfusion injury.100 At the onset of reperfusion, hearts selected for treatment underwent three brief 5 s cycles of postconditioning. Results were similar to the preconditioning studies cited above: haemodynamics were improved and infarct size reduced compared with untreated hearts. Postconditioning restored SK activity during reperfusion (Figure 2B). Phospho-Akt and phospho-ERK were enhanced. SK1 null hearts exhibited none of these findings. Thus, SK1 is also critical for successful ischaemic postconditioning. This observation is particularly pertinent to the mechanisms underlying acute protection from cardiac ischaemia–reperfusion injury, which involves free radical generation during reperfusion.101 However, as reported by Tritto et al.,102 low concentrations of free radicals, presumably generated by pre- and postconditioning, are involved in the activation of cardioprotective PKC. Both pre- and postconditioning activate pro-survival kinases that inhibit mitochondrial permeability transition.101,103 Accordingly, SK1, which as noted above is dependent on PKCε activation,58,80,84 should be added to the list of pro-survival kinases that substantially protect against injury inflicted by massive free radical generation during reperfusion.
S1P, low-dose sphingosine, and ischaemic postconditioning, which as noted above is SK1-mediated, are each cardioprotective.80,87,100 However, these approaches have not previously been combined to achieve cardioprotection. In a recent report, Vessey et al.104 were able to rescue isolated rat hearts from as much as 90 min of ischaemia by infusing low-dose S1P and sphingosine at the time of reperfusion combined with a novel ramped ischaemic postconditioning regimen. These observations suggest that combination therapy involving elements of the SK/S1P pathway could be effectively used in many clinical situations, including elective and emergency percutaneous coronary interventions as well as following cardiopulmonary bypass. In principle, such an approach could also be used to treat limb and cerebral ischaemia and any other organ subject to ischaemia/reperfusion injury.
5. Future directions
In summary, the Sphinx, while subtle indeed, has begun to yield many of its secrets. Elucidation of the importance of SK1 in cardioprotection and preliminary solutions to the enigma of SK2 promise to provide further clues to understanding the biological pas de deux between cell survival and demise. Of immediate therapeutic relevance is the ability of the combination of postconditioning, which is SK1-dependent, S1P, and sphingosine to rescue severely ischaemic myocardium from injury.104 Combination therapy with agonists of other receptors, such as adenosine and opioid receptors, may provide an additional fruitful avenue of investigation. Although translation of the laboratory findings described in this review to patient care is a highly desirable goal, before this can be accomplished further survival studies in intact rodent and larger animal studies of myocardial infarction will be required. In addition, the impact of short- and long-term adverse effects of chronic sphingolipid therapy on immune system function75 will require careful scrutiny.
Funding
Grant support: 1P01 HL 68738 from the NHLBI.
Acknowledgement
The author thanks Mr Norman Honbo for preparing the illustrations contained in this review.
Conflict of interest: none declared.
References
- 1.Cuvillier O, Pirianov G, Keluser B, Vanek PG, Coso A, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 2.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 3.Wendler CC, Rivkees SA. Sphingosine-1-phosphate inhibits cell migration and endothelial to mesenchymal cell transformation during cardiac development. Develop Biol. 2006;291:264–277. doi: 10.1016/j.ydbio.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Yatomi Y. Sphingosine 1-phosphate in vascular biology: possible therapeutic strategies to control vascular diseases. Curr Pharm Des. 2006;12:575–587. doi: 10.2174/138161206775474404. [DOI] [PubMed] [Google Scholar]
- 5.Yan G, Chen S, You B, Sun J. Activation of sphingosine kinase-1 mediates induction of endothelial cell proliferation and angiogenesis by epoxyeicosatrienoic acids. Cardiovasc Res. 2008;78:308–314. doi: 10.1093/cvr/cvn006. [DOI] [PubMed] [Google Scholar]
- 6.Schwalm S, Doll F, Romer I, Bubovna S, Pfeilschifter J, Huwiler A. Sphingosine kinase-1 is a hypoxia-regulated gene that stimulates migration of human endothelial cells. Biochem Biophys Res Commun. 2008;368:1020–1025. doi: 10.1016/j.bbrc.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Stankovic M, Bonder CS, Hahn CN, Parsons M, Pitson SM, et al. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood. 2008;111:3489–3497. doi: 10.1182/blood-2007-05-092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itagaki K, Yun JK, Hengst JA, Yatani A, Hauser CJ, Spolarics Z, et al. Sphingosine 1-phosphate has dual functions in the regulation of endothelial cell permeability and Ca2+ metabolism. J Pharmacol Exp Ther. 2007;323:186–191. doi: 10.1124/jpet.107.121210. [DOI] [PubMed] [Google Scholar]
- 9.Venkataraman K, Lee Y-M, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Igarashi J, Michel T. The enigma of sphingosine 1-phosphate synthesis. A novel role for endothelial sphingosine kinases. Circ Res. 2008;102:630–632. doi: 10.1161/CIRCRESAHA.108.173799. [DOI] [PubMed] [Google Scholar]
- 11.Peters SLM, Alewijnse AE. Sphingosine-1-phosphate signaling in the cardiovascular system. Curr Opin Pharmacol. 2007;7:186–192. doi: 10.1016/j.coph.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Alemany R, van Koppen CJ, Dannebeg K, ter Braak M, Meyer zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 13.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Reviews. Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 14.Alewijnse AE, Peters SLM. Sphingolipid signalling in the cardiovascular system: good, bad or both? Eur J Pharmacol. 2008;585:292–302. doi: 10.1016/j.ejphar.2008.02.089. [DOI] [PubMed] [Google Scholar]
- 15.Olivera A, Kohama T, Tu Z, Milstien S, Spiegel S. Purification and characterization of rat kidney sphingosine kinase. J Biol Chem. 1998;273:12576–125783. doi: 10.1074/jbc.273.20.12576. [DOI] [PubMed] [Google Scholar]
- 16.Kohama T, Olivera A, Edsalll L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 17.Melendez AH, Carlos-Dias E, Gosink M, Allen JM, Takacs L. Human sphingosine kinase: molecular cloning, functional characterization and tissue distribution. Gene. 2000;251:19–26. doi: 10.1016/s0378-1119(00)00205-5. [DOI] [PubMed] [Google Scholar]
- 18.Nava VE, Lacana E, Poulton S, Liu H, Sugiura M, Kono K, et al. Functional characterization of human sphingosine kinase-1. FEBS Lett. 2000;473:81–84. doi: 10.1016/s0014-5793(00)01510-6. [DOI] [PubMed] [Google Scholar]
- 19.Pitson SM, Moretti PAB, Zebol JR, Zareie R, Derian CK, Darrow AL, et al. The nucleotide-binding site of human sphingosine kinase 1. J Biol Chem. 2002;277:49545–49553. doi: 10.1074/jbc.M206687200. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland CM, Moretti PAB, Hewitt NM, Bagley CJ, Vadas MA, Pitson SM. The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to plasma membrane. J Biol Chem. 2006;281:11693–11701. doi: 10.1074/jbc.M601042200. [DOI] [PubMed] [Google Scholar]
- 21.Park CG, Lee HS, Lee HY, Park CS, Choi OH. Cloning and characterization of rat sphingosine kinase 1 with an N-terminal extension. Biochem Biophys Res Commun. 2007;364:702–707. doi: 10.1016/j.bbrc.2007.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 23.Imamura T, Ohgane J, Ito S, Ogawa T, Hattori N, Tanaka S, et al. CpG island of rat sphingosine kinase-1 gene: tissue-dependent DNA methylation status and multiple alternative first exons. Genomics. 2001;76:117–125. doi: 10.1006/geno.2001.6607. [DOI] [PubMed] [Google Scholar]
- 24.Okada T, Ding G, Sonoda H, Kajimoto T, Hga Y, Khosrowbeygi A, et al. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Sugiura M, Nava VE, Edsal LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 26.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, Echten-Deckert G, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 28.Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Donati C, Nincheri P, Cenetti F, Rapizza E, Farnararo M, Brunei P. Tumor necrosis factor-α exerts pro-myogenic action in C2C12 myoblasts via sphingosine kinase/S1P2 signaling. FEBS Lett. 2007;58:4384–4388. doi: 10.1016/j.febslet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Meacci E, Nuti F, Donati C, Cencetti F, Farnararo M, Bruni P. Sphingosine kinase activity is required for myogenic differentiation of C2C12 myoblasts. J Cell Physiol. 2008;214:210–220. doi: 10.1002/jcp.21187. [DOI] [PubMed] [Google Scholar]
- 31.Cavallini L, Venerando R, Miotto G, Alexandre A. Ganglioside GM1 protection from apoptosis of rat heart fibroblasts. Arch Biochem Biophys. 1999;370:156–162. doi: 10.1006/abbi.1999.1378. [DOI] [PubMed] [Google Scholar]
- 32.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, et al. Activation of sphingosine kinase 1 by ERK 1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor alpha signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 34.Pitson SM, Moretti PA, Zebol JR, Xia P, Gamble JR, Vadas MA, et al. Expression of a catalytically inactive sphingosine kinase mutant blocks agonist-induced sphingosine kinase activation. A dominant-negative sphingosine kinase. J Biol Chem. 2000;275:33945–33950. doi: 10.1074/jbc.M006176200. [DOI] [PubMed] [Google Scholar]
- 35.Fujita T, Okada T, Hayshi S, Hahangeer S, Miwa N, Nakamura S. Delta-catenin/NRRAP (neural plakophilin-related armadillo repeat protein) interacts with and activates sphingosine kinase 1. Biochem J. 2004;382:717–723. doi: 10.1042/BJ20040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maceyka M, Payne SG, Milstien S, Spiegel S. Aminocyclase 1 is a sphingosine kinase 1-interacting protein. FEBS Lett. 2004;568:30–34. doi: 10.1016/j.febslet.2004.04.093. [DOI] [PubMed] [Google Scholar]
- 37.Leclercq TM, Moretti PA, Vadas MA, Pitson SM. Eukaryotic elongation factor 1 interacts with sphingosine kinase and directly enhances its catalytic activity. J Biol Chem. 2008;283:9606–9614. doi: 10.1074/jbc.M708782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacana E, Maceyka M, Milstien S, Spiegel S. Cloning and characterization of a protein kinase A anchoring protein (AKAP)-related protein that interacts with and regulates sphingosine kinase 1 activity. J Biol Chem. 2002;277:32947–32953. doi: 10.1074/jbc.M202841200. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda Y, Aoyama Y, Wada A, Igarashi Y. Identification of PECAM-1 association with sphingosine kinase 1 and its regulation by agonist-induced phosphorylation. Biochim Biophys Acta. 2004;1636:12–21. doi: 10.1016/j.bbalip.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Sun J, Yan G, Ren A, You B, Liao JK. FHL2/SLIM3 decreases cardiomyocyte survival by inhibitory interaction with sphingosine kinase-1. Circ Res. 2006;99:468–476. doi: 10.1161/01.RES.0000239410.65551.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi S, Okada T, Igararshi N, Fujita T, Jahangeer S, Nakamura S. Identification and characterization of RPK118, a novel sphingosine kinase-1-binding protein. J Biol Chem. 2002;277:33319–33324. doi: 10.1074/jbc.M201442200. [DOI] [PubMed] [Google Scholar]
- 42.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 43.Meyer zu Heringdorf D. Lysophospholipid receptor-dependent and -independent calcium signaling. J Cell Biochem. 2004;92:937–948. doi: 10.1002/jcb.20107. [DOI] [PubMed] [Google Scholar]
- 44.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitra P, Oskeritian CA, Payne SG, Beaven MA, Mistien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pchejetski D, Kunduzova O, Dayhon A, Calise D, Suguelas M-H, Leducq N, et al. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 47.Anelli V, Gault CR, Cheng AB, Obeid LM. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells. Role of hypoxia-inducible factors 1 and 2. J Biol Chem. 2008;283:3365–3375. doi: 10.1074/jbc.M708241200. [DOI] [PubMed] [Google Scholar]
- 48.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 49.Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, et al. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda Y, Ohashi K, Shibata R, Pimentel DR, Kihara S, Ouchi N, et al. Cycloosygenase-2 induction by adiponectin is regulated by a sphingosine kinase-1 dependent mechanism in cardiac myocytes. FEBS Lett. 2008;582:1147–1150. doi: 10.1016/j.febslet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kacimi R, Vessey DA, Honbo N, Karliner JS. Adult cardiac fibroblasts null for sphingosine kinase-1 exhibit growth dysregulation and an enhanced proinflammatory response. J Mol Cell Cardiol. 2007;43:85–91. doi: 10.1016/j.yjmcc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–2836. doi: 10.1182/blood.v98.9.2828. [DOI] [PubMed] [Google Scholar]
- 53.Dindo D, Dahm F, Szulc Z, Bielawska A, Obeid LM, Hannun YA, et al. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther. 2006;5:1520–1529. doi: 10.1158/1535-7163.MCT-05-0513. [DOI] [PubMed] [Google Scholar]
- 54.Gustafsson AB, Gottlieb RA. Recycle or die: the role of autophagy in cardioprotection. J Mol Cell Cardiol. 2008;44:654–661. doi: 10.1016/j.yjmcc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, et al. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- 56.Lavieu G, Scarlatti F, Sala G, Levade T, Bhidoni R, Botti J, et al. Is autophagy the key mechanism by which the sphingolipid rheostat controls the cell fate decision? Autophagy. 2007;3:45–47. doi: 10.4161/auto.3416. [DOI] [PubMed] [Google Scholar]
- 57.Takabe K, Paugh SW, Milstien S, Spiegel S. ‘Inside-Out’ signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao R, Zhang J, Vessey DA, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74:56–63. doi: 10.1016/j.cardiores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 59.Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu JD, Griffiths R, et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a ‘come-and-get-me’ signal. FASEB J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, et al. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 61.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 actiation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 62.Maceyka M, Sankkala H, Hait NC, Le Stunff H, Liu H, Toman R, et al. SphK1 and Sphk2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 63.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 64.Le Stunff H, Giussani P, Maceyka M, Lepine S, Milstien S, Spiegel S. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J Biol Chem. 2007;282:34372–34380. doi: 10.1074/jbc.M703329200. [DOI] [PubMed] [Google Scholar]
- 65.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, et al. Involvement of N-terminal extended form of sphingosine kinase-2 in serum dependent cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 66.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 67.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 68.Ding G, Sonoda H, Yu H, Kajimoto T, Goparaju SK, Hahangeer S, et al. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem. 2007;282:27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]
- 69.Merrill AH, Jr, Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, et al. Sphingolipids—the enigmatic lipid class: biochemistry, physiology and pathophysiology. Toxicol Appl Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 70.Billich A, Bornacin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez T, Estrada-Hernandez T, Paik J-H, Wu M-T, Venkataraman K, Brinkmann V, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2002;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 72.Kharel Y, Lee S, Snyder AH, Sheasley-O’Neill S, Morris MA, Setiady Y, et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280:36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 73.Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 74.Hogenauer K, Billich A, Pally C, Streiff M, Wagner T, Welzenbach K, et al. Phosphorylation by sphingosine kinase 2 is essential for in vivo potency of FTY720 analogues. ChemMedChem. 2008;3:1027–1029. doi: 10.1002/cmdc.200800037. [DOI] [PubMed] [Google Scholar]
- 75.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 77.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-posphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 79.Sonin D, Zhou SY, Cronin C, Sonina T, Wu J, Jacobson KA, et al. Role of P2X purinergic receptors in the rescue of ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H1191–H1197. doi: 10.1152/ajpheart.00577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 81.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNFα and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–518. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 82.Gray MO, Zhou HZ, Schafhalter-Zoppoth I, Zhu P, Mochly-Rosen D, Messing RO. Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J Biol Chem. 2004;279:3596–3604. doi: 10.1074/jbc.M311459200. [DOI] [PubMed] [Google Scholar]
- 83.Jin Z-Q, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 84.Jin Z-Q, Karliner JS. Low dose N, N-dimethylsphingosine is cardioprotective and activates cytosolic sphingosine kinase by a PKCε dependent mechanism. Cardiovasc Res. 2006;71:725–734. doi: 10.1016/j.cardiores.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 85.McDonough PM, Yasui K, Betto R, Salviati G, Glembotski CC, Palade PT, et al. Control of cardiac Ca2+ levels. Inhibitor actions of sphingosine on Ca2+ transients and L-type Ca2+ channel conductance. Circ Res. 1994;75:981–989. doi: 10.1161/01.res.75.6.981. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki E, Handa K, Toledo Ms, Hakomori S. Sphingosine-dependent apoptosis: A unified concept based on multiple mechanisms operating in concert. Proc Natl Acad Sci USA. 2004;101:14788–14793. doi: 10.1073/pnas.0406536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vessey DA, Li L, Kelley M, Zhang J, Karliner JS. Sphingosine can pre-and post-condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Mol Toxicol. 2008;22:113–118. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 88.Vessey DA, Kelley M, Karliner JS. A rapid radioassay for sphingosine kinase. Anal Biochem. 2005;337:136–142. doi: 10.1016/j.ab.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 89.Vessey DA, Kelley M, Li L, Huang Y, Zhou H-Z, Zhu BQ, et al. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit. 2006;12:318–324. [PubMed] [Google Scholar]
- 90.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 91.Jin Z-Q, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 92.Nishino Y, Webb I, Marber MS. Editorial. Sphingosine kinase isoforms and cardiac protection. Cardiovasc Res. 2007;76:3–4. doi: 10.1016/j.cardiores.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 93.Duan H-F, Wang H, Yi J, Liu H-J, Zhang Q-W, Li L-G, et al. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/reperfusion-induced injury and attenuates its postischemic failure. Hum Gene Ther. 2007;18:1119–1128. doi: 10.1089/hum.2007.036. [DOI] [PubMed] [Google Scholar]
- 94.Kim M, Kim M, Kim N, D’Agati VD, Emala CW, Sr, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–F1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 95.Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 96.Sobue S, Nemoto S, Murakami M, Ito H, Kimura A, Gao S, et al. Implications of sphingosine kinase 1 expression level for the cellular sphingolipid rheostat: relevance as a marker for daunorubicin sensitivity of leukemia cells. Int J Hematol. 2008;87:266–275. doi: 10.1007/s12185-008-0052-0. [DOI] [PubMed] [Google Scholar]
- 97.Ma MM, Chen JL, Wang GG, Wang H, Lu Y, Li JF, et al. Sphingosine kinase 1 participates in insulin signalling and regulates glucose metabolism and homeostasis in KK/Ay diabetic mice. Diabetologia. 2007;50:891–900. doi: 10.1007/s00125-006-0589-5. [DOI] [PubMed] [Google Scholar]
- 98.Zhao ZQ, Corvera JS, Jalos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 99.Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, et al. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 100.Jin Z-Q, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–140. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 101.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tritto I, D’Andrea D, Eramo N, Scognamiglio A, De Simone C, Violante A, et al. Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res. 1997;80:743–748. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- 103.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activation prosurvial kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 104.Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–429. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]