Abstract
Sphingosine-1-phosphate (S1P) is now recognized as a lipid mediator that acts via G-protein-coupled receptors. S1P receptors couple to various heterotrimeric G-proteins and regulate downstream targets and ultimately cell behaviour. The prototypical S1P1 receptor is known to couple to Gi and regulates angiogenesis, vascular development, and immune cell trafficking. In this review, we focus our attention on the S1P2 receptor, which has a unique G-protein-coupling property in that it preferentially activates the G12/13 pathway. Recent studies indicate that the S1P2 receptor regulates critical intracellular signalling pathways, such as Rho GTPase, the phosphatase PTEN, and VE-cadherin-based adherens junctions. Analysis of mutant mice has revealed the critical role of this receptor in inner ear physiology, heart and vascular development, vascular remodelling, and vascular tone, permeability, and angiogenesis in vertebrates. These studies suggest that selective modulation of S1P2 receptor function by pharmacological tools may be useful in a variety of pathological conditions.
Keywords: Sphingosine-1-phosphate, Angiogenesis, Vascular permeability, Inflammation, Retinopathy
1. Introduction
Sphingosine-1-phosphate (S1P), a bioactive lysophospholipid, is now established as a lipid mediator with a broad spectrum of biological activities. In the early 1990s, S1P was discovered to regulate calcium release from intracellular stores, cell proliferation, and cell shape change in Swiss 3T3 fibroblasts.1,2 Indeed, over the ensuing decade and a half, S1P was reported to mediate a wide variety of biological responses, including cell growth, survival, cell trafficking, and cytoskeleton rearrangements.3–5 S1P and other phosphorylated long-chain sphingoid bases have also been detected in lower organisms such as yeasts, flies, and worms, which underscore the significance of this lipid as an evolutionarily conserved signalling mediator.6–8 A major development in the field that preceded widespread interest was the identification of the EDG family of G-protein-coupled receptors (GPCRs) as S1P receptors.9–11 These are the S1P1, S1P2, S1P3, S1P4, and S1P5 receptors that regulate diverse downstream signalling properties due to coupling to distinct heterotrimeric G-proteins.9,11–13
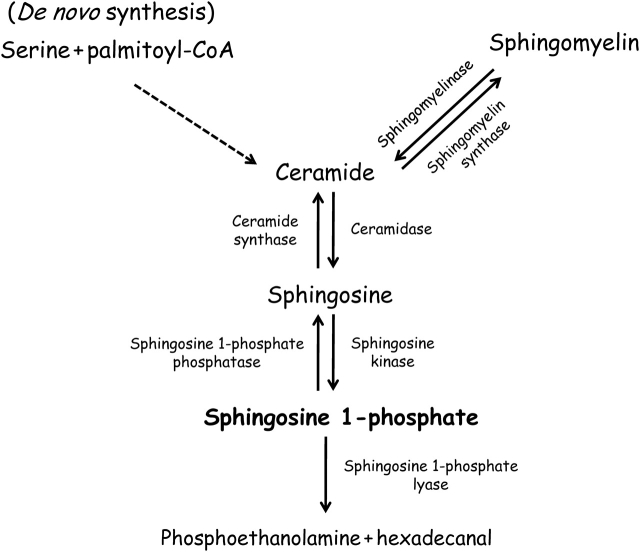
The ligand S1P is produced from sphingomyelin metabolism. De novo synthesis of sphingolipids is initiated at the cytosolic face of the endoplasmic reticulum (ER) with the condensation of serine and palmitoyl-CoA by serine palmitoyltransferase, ultimately resulting in the formation of ceramide. Ceramide, the common backbone of sphingolipid metabolism, is formed primarily by hydrolysis of the membrane phospholipid sphingomyelin. It can be further metabolized by ceramidase to produce sphingosine, which is phosphorylated by sphingosine kinases (SphKs) to generate S1P. Once formed, S1P can be dephosphorylated to sphingosine by specific phosphatases or be irreversibly degraded into hexadecanal and phosphoethanolamine by S1P lyase14,15 (Figure 1). Although the significance of S1P as an intracellular second messenger has not been unequivocally established, many studies in different cell types have demonstrated that intracellular S1P action may be involved in the promotion of cell survival and proliferation.16–21 Notwithstanding the essential role of the S1P metabolic pathway in cell physiology, much remains to be learnt about the intracellular function of S1P as a second messenger. The identification of intracellular S1P-binding proteins and/or signalling targets could enhance further progress in this area.
Figure 1.
Sphingosine-1-phosphate (S1P) metabolism. De novo synthesis of sphingolipids produces ceramide and sphingomyelin. Sphingomyelinase, ceramidase, and sphingosine kinase are required to produce S1P. The degradation of S1P is achieved by the enzymes S1P phosphatase and S1P lyase.
The concentration of S1P in plasma is estimated to be between 0.1 and 0.6 µM, mostly associated with HDL and albumin. In sharp contrast, tissue S1P levels are low.22,23 Therefore, a large concentration gradient of S1P is maintained between vascular (plasma) and extravascular compartments (i.e. interstitial fluid) in mammals. Previous studies have suggested that platelets are the major source of S1P.24 However, it was recently reported that a major source of plasma S1P is red blood cells.25–27 Work from our laboratory suggests that vascular endothelium also contributes to plasma S1P.28 Thus, erythrocytes and endothelial cells may maintain the S1P gradient, which is physiologically important for immune cell trafficking.
Given the increased realization of the physiological importance of S1P as a lipid mediator, much research has been focused on the characterization of specific S1P receptor subtypes and their signalling properties, biological actions, and roles in physiology and disease. Much has been learnt about the S1P1 receptor, the prototypical S1P receptor, that regulates vascular development, immune cell trafficking, angiogenesis, and other functions. In contrast, less information is available about S1P2 receptor, which signals in an opposite manner than that of the S1P1 receptor. In this review, we will focus on the physiological and pathological processes that S1P regulates via the S1P2 receptor in the cardiovascular system, underscoring the therapeutic potential of the S1P/S1P2 receptor axis in vascular disease.
2. Sphingosine-1-phosphate receptor 2 signalling in vitro
S1P interacts with high affinity with the S1P family (S1P1, S1P2, S1P3, S1P4, and S1P5) of GPCRs that trigger multiple downstream signalling processes by coupling to distinct heterotrimeric G-proteins.11,29–31 The first member of this family of receptors to be identified was the Gi-coupled S1P1 receptor, whereas the S1P2 and S1P3 receptors couple to Gi as well as Gq and G12/13 proteins.29,32–34 Similarly, the S1P4 receptor associates with Gi and G12/13 proteins, whereas the S1P5 receptor couples only to Gi and G12 proteins.35
Indeed, the S1P2 receptor [a.k.a. H218, AGR16, Edg-5, LP(B2)] was originally identified as a novel cDNA clone from a rat smooth muscle cDNA library encoding a putative GPCR with high sequence similarity to the S1P1 receptor.36 Furthermore, S1P is an extracellular high-affinity ligand (Kd = 16–27 nM).37–39 This receptor has a wide tissue distribution.36,40–44 It couples to Gi, Gq, as well as G12/13 heterotrimeric G-proteins even though its coupling efficiency to the G12/13 pathway is particularly strong.29,45 Thus, S1P2 receptor coupling to diverse heterotrimeric G-proteins suggests the activation of multiple downstream signalling pathways, leading to profound effects on the physiology of different cell types, tissues, organs, and finally functional systems.46 Several reports indicate that the S1P2 receptor is able to activate the MAP kinase protein ERK and mediate S1P-induced cell proliferation and survival in a pertussis toxin- and Ras-dependent manner suggesting the participation of Gi heterotrimeric G-protein. Indeed, S1P2 receptor expression in HTC4 hepatoma cells, CHO, and C6 glioma cells leads to ERK phosphorylation and immediate-early induction of c-Jun and c-Fos proto-oncogenes.37,47–49
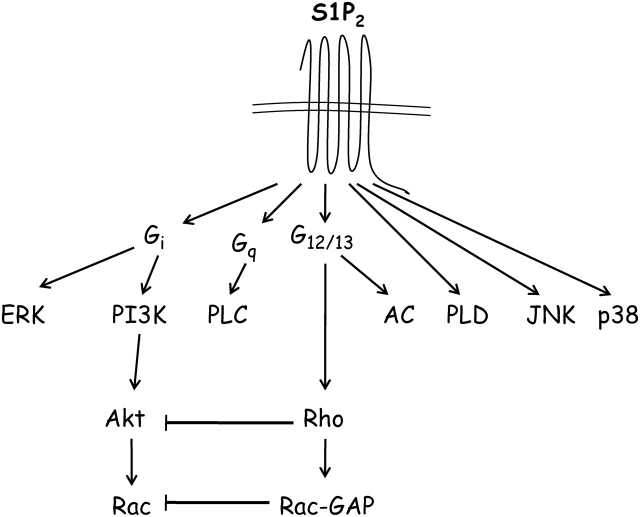
In addition, the S1P2 receptor has been reported to trigger phospholipase C (PLC) activation and downstream Ca2+ release in response to coupling to Gq heterotrimeric G-protein.37,38,49–54 Moreover, the S1P2 receptor induces phospholipase D (PLD) activity in epithelial cells as well as muscle cells and facilitates C2C12 myoblast differentiation into myotubes by coupling S1P to PLD activation.55,56 Although the S1P2 receptor has been shown not to couple to Gs heterotrimeric G-protein, it is able to activate adenylate cyclase and greatly enhance increases in intracellular concentration of cAMP by a mechanism that is not well defined yet.29,37,38 A recent study using RNAi and pharmacological inhibitors suggests that S1P acts through the S1P2 receptor and couples to G13 heterotrimeric G-protein to stimulate cAMP synthesis.57 More importantly, the S1P2 receptor reportedly stimulates other members of the MAPK family such as JNK and p38 MAPK that are well known to play diverse roles in broad physiological functions such as cell stress, pro-inflammatory cytokine production, and apoptosis.37,58,59 It is also important to recognize that dissection of the S1P2 receptor downstream signalling pathway in such a fashion may represent an oversimplification. However, it becomes apparent that the complex character of the S1P2 receptor is able to trigger both pro-survival as well as pro-apoptotic signalling pathways in different aspects of the cellular physiology. This raises an interesting question as to whether S1P2 receptor function is differentially regulated in a stressor-specific manner, from physiological to pathophysiological conditions. (Figure 2).
Figure 2.
Signal transduction of the S1P2 receptor. The plasma membrane-localized S1P2 receptor couples to several heterotrimeric G-proteins as indicated. However, coupling to G12/13 is most prominent. Downstream of Rho, the Rac GTPase is inhibited.
In addition to Gi and Gq heterotrimeric G-proteins, the S1P2 receptor can also couple to G12/13 and activate Rho small GTPase.29,37,47,60,61 In fact, this receptor isoform couples to the G12/13/Rho pathway strongly, compared with other receptor isoforms. Northern blot analysis revealed the expression of S1P2 receptor transcript in rat hepatocytes, in which S1P decreased DNA synthesis induced by hepatocyte growth factor. The inhibitory effect of S1P on hepatocyte proliferation was attenuated by inactivation of small GTPase Rho with C3 exotoxin and also by JTE-013, an S1P2 receptor antagonist. Therefore, through small GTPase activation, the S1P2 receptor could act as a negative regulator of tissue repair and remodelling.62 Moreover, S1P2 receptor expression in CHO cells leads to complete inhibition of PI3-kinase-dependent Rac activation which is essential for chemotactic and migratory events. Mechanistically, evidence supports the notion that the S1P2 receptor effect on Rac inhibition involves stimulation of a GTPase-activating protein for Rac, rather than inhibition of Rac-guanine nucleotide exchange protein. The S1P2 receptor actions were mimicked by expression of V14Rho (dominant active form) and were abolished by C3 toxin (RhoA inhibitor) and N19Rho (dominant-negative form).60,63 S1P induced inhibition and activation, respectively, of GTP-Rac and GTP-RhoA in B16 melanoma cells, which were abrogated by JTE-013.64 Several reports demonstrate that S1P induces significant increase in the amounts of GTP-RhoA in S1P2 receptor-expressing cells through G12/13, thereby inhibiting Rac, cortical actin assembly, and cell migration.60,63,65–68 On the other hand, a recent report also showed that suppression of the S1P2 receptor function by either RNAi tools or JTE-013 completely blocked S1P augmentation of fibroblast chemotaxis to fibronectin, in human lung fibroblasts. S1P-stimulated Rho activation and focal adhesion kinase phosphorylation were also significantly inhibited by the S1P2 receptor antagonist JTE-013.69 The reason for such discrepancies is not clear, although different cell lines used in these studies may be one of the causes. Indeed, many cell types including vascular smooth muscle, endothelial cells, and fibroblasts express more than a single type of S1P receptors. In addition, a further insight into the mechanistic details behind the crosstalk between the S1P2 receptor and other receptors or co-activators that determine migratory events is necessary to understand the regulation of migration by S1P/S1P2 receptor axis. It is evident that an integration of the S1P/S1P2 receptor-positive and -negative signals on the small GTPases activation and consequent downstream effectors is an essential determinant for the regulation of cell chemotaxis by S1P.
Furthermore, S1P2 receptor expression in endothelial cells markedly inhibited S1P-induced migration and protein kinase B/Akt phosphorylation. The anti-migratory role of the S1P2 receptor implicates the involvement of p160-ROCK which is well-characterized downstream target of small GTPase Rho.65 Studies by Takashima et al. further clarified the anti-migratory action of the S1P2 receptor in vascular smooth muscle cells. These observations suggest that S1P2 receptor-dependent small GTPase Rho activation is dependent on both G12/13 and Gq heterotrimeric proteins, indicating that Gq may well facilitate Rho GTPase activation in cooperation with G12/13, whereas PLC and its downstream second messengers (Ca2+, PKC) are likely not involved in the S1P2 receptor anti-migratory role in vascular smooth muscle cells.68 In addition, it has been reported that the S1P2 receptor interacts with and actively regulates the tumour suppressor gene PTEN (a PIP3 phosphatase) as a necessary downstream effector in the anti-migratory response, in vascular endothelial cells, as well as mouse embryonic fibroblasts.65,70 Recent studies suggest that S1P2 receptor expression is markedly induced in cultured senescent vascular endothelial cells. Importantly, suppression of S1P2 receptor expression or expression of dominant-negative PTEN phosphatase greatly rescues the S1P-mediated Rac activation, wound-healing, and chemotactic responses in senescent endothelial cells, suggesting that S1P2 receptor-dependent PTEN activation might be implicated in vascular dysfunction.70 However, a recent study by Malchinkhuu et al. and Lepley et al. indicates that S1P2 receptor stimulation leads to the inhibition of glioma cell migration through Rho-GTPase signalling pathways regardless of PTEN expression, suggesting that a further mechanistic understanding of S1P2 receptor-dependent inhibition of glioma cell migration is required to clarify the function of the receptor during critical events of tumourigenicity such as invasion and metastasis.71
In addition, activation of the S1P2 receptor resulted in RhoA GTPase-dependent increase in myosin light chain phosphorylation and prominent stress fibre formation.37,60,72 Ectopic expression of the S1P2 receptor in vascular endothelial cells stimulates the assembly of stress fibres, phosphorylation, and disruption of VE-cadherin-based junctions, leading to increased paracellular permeability, whereas JTE-013 significantly improved the barrier integrity.73 Considering the multifaceted signalling mechanisms involved in regulation of vascular permeability by S1P receptors, it is critical to better our understanding of the events that promote S1P2 receptor activation in diverse capillary beds throughout the vascular system, leading to compromised endothelial barrier protection. In C2C12 myoblasts, S1P acting through the S1P2 receptor reduces serum-induced cell proliferation and enhances the expression of myogenic differentiation markers such as myosin heavy chain, thus promoting myogenic differentiation, whereas it greatly stimulates contraction of coronary artery smooth muscle cells.37,52,74–76 The functional complexity and ambiguity of the S1P2 receptor in different cell types of the cardiovascular system could result in spatial and temporal profiles of activation, mobilized signalling networks through crosstalk with other S1P receptors or plasma membrane adaptor proteins, silencing or activation of downstream target genes under different conditions, thus S1P2 receptor activation could have a substantial affect on the physiology of the cell. Nevertheless, further study of S1P2 receptor activation and regulation is anticipated, as advancements in the development of potent and specific S1P2 receptor antagonists might have great implications in cardiovascular development and pathology.
3. Sphingosine-1-phosphate receptor 2 in cardiovascular system development
S1P receptors regulate important physiological functions of the vascular system, such as vascular morphogenesis and maturation, cardiac function and remodelling, vascular permeability, and tumour angiogenesis.77–84 Recent studies with the use of genetic and pharmacological tools, from worms to rodents, suggest that the S1P2 receptor is implicated in cardiovascular system function, in health and disease (Table 1).
Table 1.
Roles for S1P2 receptor in development and disease of the cardiovascular system
| Model system | Function and pathophysiology |
|---|---|
| Miles-apart gene mutations (Zebrafish) | Cardiac defects (cardia bifida)85 |
| S1p1−/−S1p2−/− mice | Haemorrhage at E11.5, capillary network underdeveloped in mouse embryo87 |
| S1p2−/−S1p3−/− mice | Haemorrhage at E13.5, red blood cells and oedema in subcutaneous areas, endothelial cells with thin cell body87 |
| S1p1−/−S1p2−/−S1p3−/− mice | Haemorrhage at E10.5, vascular remodelling defects in the embryo head87 |
| S1p2−/− mice | Hearing loss, vascular remodelling defects in Stria vascularis structure of the inner ear87 |
| S1p2−/− mice | Increased regional blood flow, decreased vascular resistance99 |
| S1p2−/− mice | Enhanced revascularization of the hypoxic mouse retina97 |
| S1p2−/− mice | Increased neointimal lesions development in mouse artery102 |
| S1p2−/−S1p3−/− mice | Increased infarct size upon myocardial ischaemia–reperfusion injury100 |
| JTE-013 antagonist | Increased angiogenesis in Matrigel mouse implants96 |
| JTE-013 antagonist | Inhibition of H2O2-induced lung oedema73 |
Indeed, mutations in the zebrafish gene miles-apart (Mil), an S1p2 ortholog, results in cardiac developmental defects (cardia bifida phenotype, two laterally positioned hearts) due to defective migration of the heart precursors to the midline, revealing an important function of the S1P2 receptor in zebrafish heart organogenesis.85 Importantly, when mutant cells were transplanted into a wild-type embryo, they could migrate normally, whereas wild-type cells in a mutant embryo fail to migrate, suggesting that the S1p2 receptor may be involved in generating an environment permissive for heart tube formation in the mesoderm. In agreement with this observation, a recent report shows further evidence that cell–matrix interaction driven by Mil is required for proper myocardial migration.86 Given these results, it would be interesting to know the role of the enzymes of sphingolipid metabolism such as SphKs, phosphatases, and lyase in the development a zebrafish circulatory system and heart morphogenesis. The ability to alter the sphingolipid levels and receptor expression during and/or after the establishment of the vascular system in zebrafish could provide better understanding of the mechanisms involved in vascular endothelial cell migration and morphogenesis.
Mice that lack the S1P2 receptor are viable. Although embryos null for the S1P2 receptor do not show defects in the development of the vascular system, when embryos are null for both S1P1 and S1P2 receptors, they die between E10.5 and E12.5 compared with singe-null embryos for S1P1 that die between E12.5 and E14.5.87 Indeed, substantial haemorrhage was evident suggesting that vascular abnormalities were a cause of death. In addition, detailed examination of the head region revealed that in the double-null embryos, the capillary network was less developed and contained fewer branches when compared with the wild-type embryos and the single-null S1p1 mice. Furthermore, when mice lack both S1P2 and S1P3 receptors, 50% of the embryos die after E13.5, whereas mice null for S1P3 receptor are viable and fertile. Importantly, double-null embryos that had survived showed severe bleeding phenotype, indicating that a vascular defect was the main reason for the embryonic lethality.54,87 However, bleeding phenotype was not observed in the single-null S1p2 or S1p3 embryos. Embryos deficient for both S1P2 and S1P3 receptors began to haemorrhage around E13.5, whereas free red blood cells and oedema were evident in subcutaneous areas. The dorsal aortas of S1p2/S1p3 double-null embryos appeared to be covered normally by vascular smooth muscle cells, suggesting that the S1P2 receptor is dispensable for vascular maturation, unlike the S1P1 receptor that is essential for mural–endothelial cell interaction and consequent vascular stabilization.79,80 Microscopic examination of the microvessels revealed that endothelial cells had abnormally thin and occasionally fractured cell bodies, whereas endothelial cell junctions appeared normal.87 Finally, embryos lacking all three receptors die between E10.5 and E11.5 due to abnormal bleeding and severe vascular remodelling defects in the head.87 These observations suggest that S1P receptor signalling and function is redundant since S1P1 and S1P3 receptors may play a compensatory role when the S1P2 receptor is dysfunctional. Clearly, S1P2 receptor diverse signalling and regulation of cytoskeletal dynamics greatly contributes to the establishment and maintenance of a mature vascular system during mouse embryonic development.
Although S1p2 null adult mice do not present any major abnormalities, the litter sizes produced are decreased, suggesting that females that are null for the S1P2 receptor could have fertility defects that compromise their ability to mate or reproduce.54,87 Interestingly, S1P1 and S1P2 receptors are expressed in decidual endothelial cells, suggesting a role for the receptors in uterine mesometrial angiogenesis during the implantation phase of early gestation.88 In addition, S1p2 null mice in the C57/Bl6 genetic background exhibit spontaneous, sporadic, and occasionally fatal seizures between 3 and 7 weeks of age. Excitability of the neocortical pyramidal neurons was shown to be altered, whereas the molecular mechanism behind this phenomenon is unknown.89 Moreover, S1p2 null mice are profoundly deaf and show vestibular impairment due to multiple inner ear abnormalities. Mice deficient for both S1P2 and S1P3 receptors develop additional inner ear pathologies.90–92 The S1P2 receptor is highly expressed in the cochlea and loss of the gene leads to degeneration of sensory hair cells and the spiral ganglion neurons. Indeed, one of the very early events during the progress of the pathology is the formation of an abnormal capillary basal lamina in stria vascularis structure, with dilated and chaotically oriented microvessels. In addition, the S1P2 receptor antagonist JTE-013 blocked the S1P-induced vasoconstriction of the spiral modiolar artery, which supplies blood directly to the stria vascularis.90 However, it is worth mentioning that studies by Salomone et al.93 indicate that the S1P2 receptor antagonist JTE-013 does not appear to be highly selective in rodents. These reports suggest that the S1P2 receptor is essential for mouse inner ear vascular structure maintenance and provide a means for S1P-related therapeutic application in degenerative and noise-induced hearing loss. Although the detailed cellular and molecular mechanisms that trigger these phenotypes are still underexplored, these studies indicate that the S1P2 receptor positively regulates the formation and maintenance of the mouse vascular network during normal development.
4. Sphingosine-1-phosphate receptor 2 in cardiovascular pathology
It is well established that S1P1 receptor is a major S1P receptor expressed on vascular endothelial and smooth muscle cells, whereas it regulates physiological as well as pathological effects on vascular homeostasis, which include endothelium vasorelaxation, smooth muscle contraction, enhancement of tumour angiogenesis, and blood vessel maturation during embryonic development.79,81,94,95 In sharp contrast, the role of the S1P2 receptor in vascular pathophysiology has just begun to be unravelled.
Indeed, S1P2 receptor expression in vascular endothelial cells inhibits migration in a Rho GTPase-dependent manner, suggesting that the S1P2 receptor could potentially play an antiangiogenic and/or angiostatic role during tumour formation and progression.65 Furthermore, inhibition of the S1P2 receptor by JTE-013 antagonist substantially augmented S1P stimulation of migration and tube-like morphogenesis of mouse vascular endothelial cells. In accordance with these in vitro findings, the S1P2 receptor was expressed in vascularized Matrigel plugs in vivo, whereas inhibition of the receptor function greatly enhanced S1P-driven angiogenic processes in the Matrigel mouse implants.96 These data are in apparent contradiction with the requirement for the S1P2 receptor in mouse embryogenesis and inner ear development.87,90 These findings suggest that S1P2 may regulate the vasculature in a context-dependent manner.
We recently reported that activation of the S1P2 receptor on endothelial cells results in disruption of VE-cadherin-based junctions and increased vascular paracellular permeability. In addition, JTE-013 treatment of primary endothelial cells enhanced cortical actin assembly and adherens junctions formation, improving S1P-driven endothelium barrier integrity.73 Moreover, when rat lungs were perfused with the pharmacologic inhibitor JTE-013, H2O2-induced lung oedema was markedly inhibited, as measured by a substantial decrease in the rate of lung wet weight gain after H2O2 treatment. These ex vivo experiments indicate that blockade of S1P2 receptor activation in endothelial cells under stress conditions could have applications in the treatment of pulmonary oedema or other permeability disorders linked to vascular injury. In the mouse model of ischaemia-driven retinal pathological angiogenesis, it is reported that S1P2 receptor expression is induced in the course of the hypoxia-triggered vascular injury. Hypoxic mouse retinas that lack the S1P2 receptor present significantly decreased inflammatory cell infiltration and substantially enhanced revascularization of the retina tissue, indicating that the S1P2 receptor activates inflammatory pathways that facilitate vascular permeability and pathological angiogenesis under ischaemic conditions.97 Mechanistically, expression of the receptor in vascular endothelial cells induces the expression of the pro-inflammatory molecule cyclooxygenase-2 and downregulates endothelial nitric oxide synthase with major implications in vascular inflammation and congestion. In agreement with this report, a recent study suggests that the humanized monoclonal antibody that selectively binds S1P significantly reduced macrophage influx into ischaemic retina and strongly suppressed oxygen-induced ischaemic retinopathy and choroidal neovascularization.98 Although this result needs to be confirmed and the mechanism of inhibition of S1P action by the monoclonal antibody needs to be clarified, this intriguing report suggests the functional role of the S1P2 receptor in pathologic vascular neovascularization. The idea that stress conditions such as hypoxia, oxidative stress, or vascular injury could activate S1P2 receptor-driven pro-inflammatory pathways and potentiate vascular permeability, pathological neo-angiogenesis, and finally vascular dysfunction remains to be addressed. Although significant progress is being made in dissecting S1P2 receptor signalling in endothelial, smooth muscle cells, and cardiomyocytes, the fundamental questions about the factors that regulate expression and localization of the S1P2 receptor have yet to be fully addressed.
A recent report implicates the S1P2 receptor as an important mediator of normal vascular haemodynamics.99 Although mice deficient for the S1P2 receptor have no blood pressure abnormalities, loss of the receptor leads to significant elevation of regional blood flow and decrease in vascular resistance in response to α-adrenergic stimulation, which clearly indicates that the S1P2 receptor plays an important physiological role in modulating vascular tone.99 Indeed, S1P2 receptor-driven vasoconstriction could explain the formation of greatly dilated capillaries in S1P2 receptor-deficient stria vascularis structure of the inner ear as well as the improved vascular tone in S1P2 receptor null ischaemic retinas.90 In any event, it is apparent that S1P2 receptor activation can strongly affect vascular homeostasis in different vascular beds through complex signalling pathways that involve cytoskeleton rearrangements and downstream gene regulation in both vascular smooth muscle and endothelial cells.
In a recent report, the role of S1P in cardiomyocyte survival following in vivo myocardial ischaemia–reperfusion (I/R) injury was examined. Indeed, infarct size following I/R was significantly increased only in mice deficient for both S1P2 and S1P3 receptors. In addition, activation of Akt in response to I/R was markedly attenuated in double-null mouse hearts, suggesting that S1P2 and S1P3 receptors together potentiate Akt activation, cardiomyocyte survival, and finally cardioprotection upon ischaemia.100 A previous report showed that S1P2 receptor activation inhibits Akt activation in endothelial cells via coupling to the PTEN phosphatase, indicating that S1P2 receptor-mediated Akt inhibition depends on diverse subcellular mechanisms in different cell types.65,70 Furthermore, in response to acute balloon injury of the rat carotid artery, S1P2 receptor expression was increased at 7–10 days post-injury, whereas inhibition of the S1P2 receptor with JTE-013 potentiated S1P-induced proliferation and reduced the expression of differentiation marker genes such as smooth muscle α-actin in rat aortic smooth muscle cells (SMCs).101 More importantly, when neointimal lesion formation was induced in mice by ligation of the left carotid artery, large neointimal lesions developed in S1P2 receptor-deficient mouse arteries.102 In addition, S1P2 receptor null arteries showed a significant increase in both medial and intimal SMC replication. Furthermore, S1P failed to increase Rho-GTPase activation in S1P2 receptor-deficient SMCs, thus leading to significant increase in SMC migration.102 These observations suggest that activation of the S1P2 receptor suppresses SMC growth and migration in arteries, whereas these studies show strong evidence for the implication of the S1P2 receptor in neointima formation and development of atherosclerotic disease. Therefore, it can be concluded that S1P2 receptor activation may play an important role in the pathogenesis of various cardiovascular diseases.
5. Conclusions
Recent findings using genetic and pharmacological approaches have led to new knowledge of S1P2 receptor function in development and pathology of the cardiovascular system. Indeed, S1P2 receptor activation is required for proper establishment and maturation of the mouse embryonic vascular system and the structure of the stria vascularis in the inner ear. More importantly, considerable progress has been made in uncovering the functional role of the receptor in vascular injury, from myocardial remodelling and neointima formation to vascular permeability, pathological neovascularization, and tumour angiogenesis. However, our current understanding of S1P2 receptor regulation and complex downstream pathway activation in the vasculature remains rather preliminary—detailed mechanistic insights are necessary to better assess whether and how regulation of the receptor could become a potential novel therapeutic approach for cardiovascular disease.
Funding
This work is supported by NIH grants RO1-HL49094, HL67330, and HL89934 to T.H.
Acknowledgements
We thank our colleagues who have contributed significantly to the study of S1P2 receptor, namely Richard Proia (NIH), Suzanne Mandala (Merck), Teresa Sanchez (Beth Israel Hospital, Harvard Medical School), and Shobha Thangada (UCHC).
Conflict of interest: none declared.
References
- 1.Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang H, Buckley NE, Gibson K, Spiegel S. Sphingosine stimulates cellular proliferation via a protein kinase C-independent pathway. J Biol Chem. 1990;265:76–81. [PubMed] [Google Scholar]
- 3.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 4.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Sem Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 7.Phan VH, Herr DR, Panton D, Fyrst H, Saba JD, Harris GL. Disruption of sphingolipid metabolism elicits apoptosis-associated reproductive defects in Drosophila. Dev Biol. 2007;309:329. doi: 10.1016/j.ydbio.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendel J, Heinecke K, Fyrst H, Saba JD. Sphingosine phosphate lyase expression is essential for normal development in Caenorhabditis elegans. J Biol Chem. 2003;278:22341–22349. doi: 10.1074/jbc.M302857200. [DOI] [PubMed] [Google Scholar]
- 9.Lee M-J, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 10.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 11.Hla T, Lee M-J, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids—receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 12.Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- 13.Ancellin N, Hla T. Switching intracellular signaling pathways to study sphingosine 1-phosphate receptors. Ann N Y Acad Sci. 2000;905:260–262. doi: 10.1111/j.1749-6632.2000.tb06555.x. [DOI] [PubMed] [Google Scholar]
- 14.Le Stunff H, Milstien S, Spiegel S. Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem. 2004;92:882–899. doi: 10.1002/jcb.20097. [DOI] [PubMed] [Google Scholar]
- 15.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 16.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia P, Gamble JR, Wang L, Pitson SM, Moretti PAB, Wattenberg BW, et al. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 19.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas M-H, Leducq N, et al. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 20.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 21.Takabe K, Paugh SW, Milstien S, Spiegel S. ‘Inside-Out’ signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yatomi Y, Yamamura S, Ruan F, Igarashi Y. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. J Biol Chem. 1997;272:5291–5297. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- 23.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 24.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 25.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 26.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, et al. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 28.Venkataraman K, Lee Y-M, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Windh RT, Lee M-J, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gi, Gq, and G12 families of heterotrimeric G proteins. J Biol Chem. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 31.Hla T. Genomic insights into mediator lipidomics. Prostaglandins Other Lipid Mediat. 2005;77:197. doi: 10.1016/j.prostaglandins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Im D-S, Heise CE, Ancellin N, O’Dowd BF, Shei G-j, Heavens RP, et al. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J Biol Chem. 2000;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- 33.Van Brocklyn JR, Graler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood. 2000;95:2624–2629. [PubMed] [Google Scholar]
- 34.Gräler MH, Grosse R, Kusch A, Kremmer E, Gudermann T, Lipp M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J Cell Biochem. 2003;89:507–519. doi: 10.1002/jcb.10537. [DOI] [PubMed] [Google Scholar]
- 35.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJA, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LPB3/EDG-3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 36.Okazaki H, Ishizaka N, Sakurai T, Kurokawa K, Goto K, Kumada M, et al. Molecular cloning of a novel putative G protein-coupled receptor expressed in the cardiovascular system. Biochem Biophys Res Commun. 1993;190:1104. doi: 10.1006/bbrc.1993.1163. [DOI] [PubMed] [Google Scholar]
- 37.Gonda K, Okamoto H, Takuwa N, Yatomi Y, Okazaki H, Sakurai T, et al. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem J. 1999;337:67–75. [PMC free article] [PubMed] [Google Scholar]
- 38.Kon J, Sato K, Watanabe T, Tomura H, Kuwabara A, Kimura T, et al. Comparison of intrinsic activities of the putative sphingosine 1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J Biol Chem. 1999;274:23940–23947. doi: 10.1074/jbc.274.34.23940. [DOI] [PubMed] [Google Scholar]
- 39.Van Brocklyn JR, Tu Z, Edsall LC, Schmidt RR, Spiegel S. Sphingosine 1- phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J Biol Chem. 1999;274:4626–4632. doi: 10.1074/jbc.274.8.4626. [DOI] [PubMed] [Google Scholar]
- 40.Yang AH, Ishii I, Chun J. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim Biophys Acta—Mol Cell Biol Lipids. 2002;1582:197. doi: 10.1016/s1388-1981(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 41.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta—Mol Cell Biol Lipids. 2002;1582:72. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 42.McGiffert C, Contos JJA, Friedman B, Chun J. Embryonic brain expression analysis of lysophospholipid receptor genes suggests roles for s1p1 in neurogenesis and s1p1–3 in angiogenesis. FEBS Lett. 2002;531:103. doi: 10.1016/s0014-5793(02)03404-x. [DOI] [PubMed] [Google Scholar]
- 43.Hiraga Y, Kihara A, Sano T, Igarashi Y. Changes in S1P1 and S1P2 expression during embryonal development and primitive endoderm differentiation of F9 cells. Biochem Biophys Res Commun. 2006;344:852. doi: 10.1016/j.bbrc.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Argraves KM, Wilkerson BA, Argraves WS, Fleming PA, Obeid LM, Drake CJ. Sphingosine-1-phosphate signaling promotes critical migratory events in vasculogenesis. J Biol Chem. 2004;279:50580–50590. doi: 10.1074/jbc.M404432200. [DOI] [PubMed] [Google Scholar]
- 45.Ancellin N, Hla T. Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J Biol Chem. 1999;274:18997–19002. doi: 10.1074/jbc.274.27.18997. [DOI] [PubMed] [Google Scholar]
- 46.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by {beta}-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 47.An S, Zheng Y, Bleu T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J Biol Chem. 2000;275:288–296. doi: 10.1074/jbc.275.1.288. [DOI] [PubMed] [Google Scholar]
- 48.Sato K, Tomura H, Igarashi Y, Ui M, Okajima F. Possible involvement of cell surface receptors in sphingosine 1-phosphate-induced activation of extracellular signal-regulated kinase in C6 glioma cells. Mol Pharmacol. 1999;55:126–133. doi: 10.1124/mol.55.1.126. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto H, Takuwa N, Yatomi Y, Gonda K, Shigematsu H, Takuwa Y. EDG3 Is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem Biophys Res Commun. 1999;260:203. doi: 10.1006/bbrc.1999.0886. [DOI] [PubMed] [Google Scholar]
- 50.Meacci E, Cencetti F, Formigli L, Squecco R, Donati C, Tiribilli B, et al. Sphingosine 1-phosphate evokes calcium signals in C2C12 myoblasts via Edg3 and Edg5 receptors. Biochem J. 2002;362:349–357. doi: 10.1042/0264-6021:3620349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.An S, Bleu T, Zheng Y. Transduction of intracellular calcium signals through G protein-mediated activation of phospholipase C by recombinant sphingosine 1-phosphate receptors. Mol Pharmacol. 1999;55:787–794. [PubMed] [Google Scholar]
- 52.Coussin F, Scott RH, Wise A, Nixon GF. Comparison of sphingosine 1- phosphate-induced intracellular signaling pathways in vascular smooth muscles: differential role in vasoconstriction. Circ Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- 53.Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]
- 54.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJA, Kingsbury MA, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P2/LPB2/EDG-5 and S1P3/LPB3/EDG-3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 55.Meacci E, Cencetti F, Donati C, Nuti F, Farnararo M, Kohno T, et al. Down-regulation of EDG5/S1P2 during myogenic differentiation results in the specific uncoupling of sphingosine 1-phosphate signalling to phospholipase D. Biochim Biophys Acta—Mol Cell Biol Lipids. 2003;1633:133. doi: 10.1016/s1388-1981(03)00106-9. [DOI] [PubMed] [Google Scholar]
- 56.Orlati S, Porcelli AM, Hrelia S, Van Brocklyn JR, Spiegel S, Rugolo M. Sphingosine-1-phosphate activates phospholipase D in human airway epithelial cells via a G protein-coupled receptor. Arch Biochem Biophys. 2000;375:69. doi: 10.1006/abbi.1999.1589. [DOI] [PubMed] [Google Scholar]
- 57.Jiang LI, Collins J, Davis R, Lin K-M, DeCamp D, Roach T, et al. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem. 2007;282:10576–10584. doi: 10.1074/jbc.M609695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, et al. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116:1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, et al. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malchinkhuu E, Sato K, Maehama T, Mogi C, Tomura H, Ishiuchi S, et al. S1P2 receptors mediate inhibition of glioma cell migration through Rho signaling pathways independent of PTEN. Biochem Biophys Res Commun. 2008;366:963. doi: 10.1016/j.bbrc.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 62.Ikeda H, Satoh H, Yanase M, Inoue Y, Tomiya T, Arai M, et al. Antiproliferative property of sphingosine 1-phosphate in rat hepatocytes involves activation of Rho via Edg-5. Gastroenterology. 2003;124:459. doi: 10.1053/gast.2003.50049. [DOI] [PubMed] [Google Scholar]
- 63.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arikawa K, Takuwa N, Yamaguchi H, Sugimoto N, Kitayama J, Nagawa H, et al. Ligand-dependent Inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor: requirement of inhibition of cellular RAC activity. J Biol Chem. 2003;278:32841–32851. doi: 10.1074/jbc.M305024200. [DOI] [PubMed] [Google Scholar]
- 65.Sanchez T, Thangada S, Wu MT, Kontos CD, Wu D, Wu H, et al. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor; Proc Natl Acad Sci USA; 2005. pp. 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, et al. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 67.Lepley D, Paik J-H, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–3795. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- 68.Takashima S-i, Sugimoto N, Takuwa N, Okamoto Y, Yoshioka K, Takamura M, et al. G12/13 and Gq mediate S1P2-induced inhibition of Rac and migration in vascular smooth muscle in a manner dependent on Rho but not Rho kinase. Cardiovasc Res. 2008;79:689–697. doi: 10.1093/cvr/cvn118. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto M, Wang X, Mao L, Kobayashi T, Kawasaki S, Mori N, et al. Sphingosine 1-phosphate potentiates human lung fibroblast chemotaxis through the S1P2 receptor. Am J Respir Cell Mol Biol. 2008;39:356–363. doi: 10.1165/rcmb.2006-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Estrada R, Zeng Q, Lu H, Sarojini H, Lee J-F, Mathis SP, et al. Up-regulating sphingosine-1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J Biol Chem. 2008 doi: 10.1074/jbc.M804392200. M804392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, et al. Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. J Psychiatr Res. 2007;41:686. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol. 2004;286:C1130–C1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 74.Donati C, Meacci E, Nuti F, Becciolini L, Farnararo M, Bruni P. Sphingosine 1-phosphate regulates myogenic differentiation: a major role for S1P2 receptor. FASEB J. 2004:04–1780fje. doi: 10.1096/fj.04-1780fje. [DOI] [PubMed] [Google Scholar]
- 75.Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res. 2003;58:170. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- 76.Hu W, Mahavadi S, Huang J, Li F, Murthy KS. Characterization of S1P1 and S1P2 receptor function in smooth muscle by receptor silencing and receptor protection. Am J Physiol Gastrointest Liver Physiol. 2006;291:G605–G610. doi: 10.1152/ajpgi.00147.2006. [DOI] [PubMed] [Google Scholar]
- 77.Lee M-J, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 78.Garcia JGN, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation [see comment] J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paik J-H, Skoura A, Chae S-S, Cowan AE, Han DK, Proia RL, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 82.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 83.Jin Z-Q, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 84.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development [see comment] Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- 86.Matsui T, Raya A, Callol-Massot C, Kawakami Y, Oishi I, Rodriguez-Esteban C, et al. miles-apart-mediated regulation of cell–fibronectin interaction and myocardial migration in zebrafish. Nat Clin Pract Cardiovasc Med. 2007;4:S77–S82. doi: 10.1038/ncpcardio0764. [DOI] [PubMed] [Google Scholar]
- 87.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 88.Skaznik-Wikiel ME, Kaneko-Tarui T, Kashiwagi A, Pru JK. Sphingosine-1-phosphate receptor expression and signaling correlate with uterine prostaglandin-endoperoxide synthase 2 expression and angiogenesis during early pregnancy. Biol Reprod. 2006;74:569–576. doi: 10.1095/biolreprod.105.046714. [DOI] [PubMed] [Google Scholar]
- 89.MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, et al. An essential role for the H218/AGR16/Edg-5/LPB2 sphingosine 1-phosphate receptor in neuronal excitability. Eur J Neurosci. 2001;14:203–209. doi: 10.1046/j.0953-816x.2001.01634.x. [DOI] [PubMed] [Google Scholar]
- 90.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, et al. Deafness and stria vascularis defects in S1P2 receptor null mice. J Biol Chem. 2007 doi: 10.1074/jbc.M700370200. M700370200. [DOI] [PubMed] [Google Scholar]
- 91.Herr DR, Grillet N, Schwander M, Rivera R, Muller U, Chun J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J Neurosci. 2007;27:1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacLennan AJ, Benner SJ, Andringa A, Chaves AH, Rosing JL, Vesey R, et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res. 2006;220:38. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 93.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, et al. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol. 2007;153:140. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 95.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;15:1082–1089. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inoki I, Takuwa N, Sugimoto N, Yoshioka K, Takata S, Kaneko S, et al. Negative regulation of endothelial morphogenesis and angiogenesis by S1P2 receptor. Biochem Biophys Res Commun. 2006;346:293. doi: 10.1016/j.bbrc.2006.05.119. [DOI] [PubMed] [Google Scholar]
- 97.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie B, Shen J, Dong A, Rashid A, Stoller G, Campochiaro PA. Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J Cell Physiol. 2008;9999 doi: 10.1002/jcp.21588. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R440–R446. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 100.Means CK, Xiao C-Y, Li Z, Zhang T, Omens JH, Ishii I, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 101.Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine-1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shimizu T, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, et al. Sphingosine 1-phosphate receptor 2 negatively regulates neointimal formation in mouse arteries. Circ Res. 2007;101:995–1000. doi: 10.1161/CIRCRESAHA.107.159228. [DOI] [PubMed] [Google Scholar]