Abstract
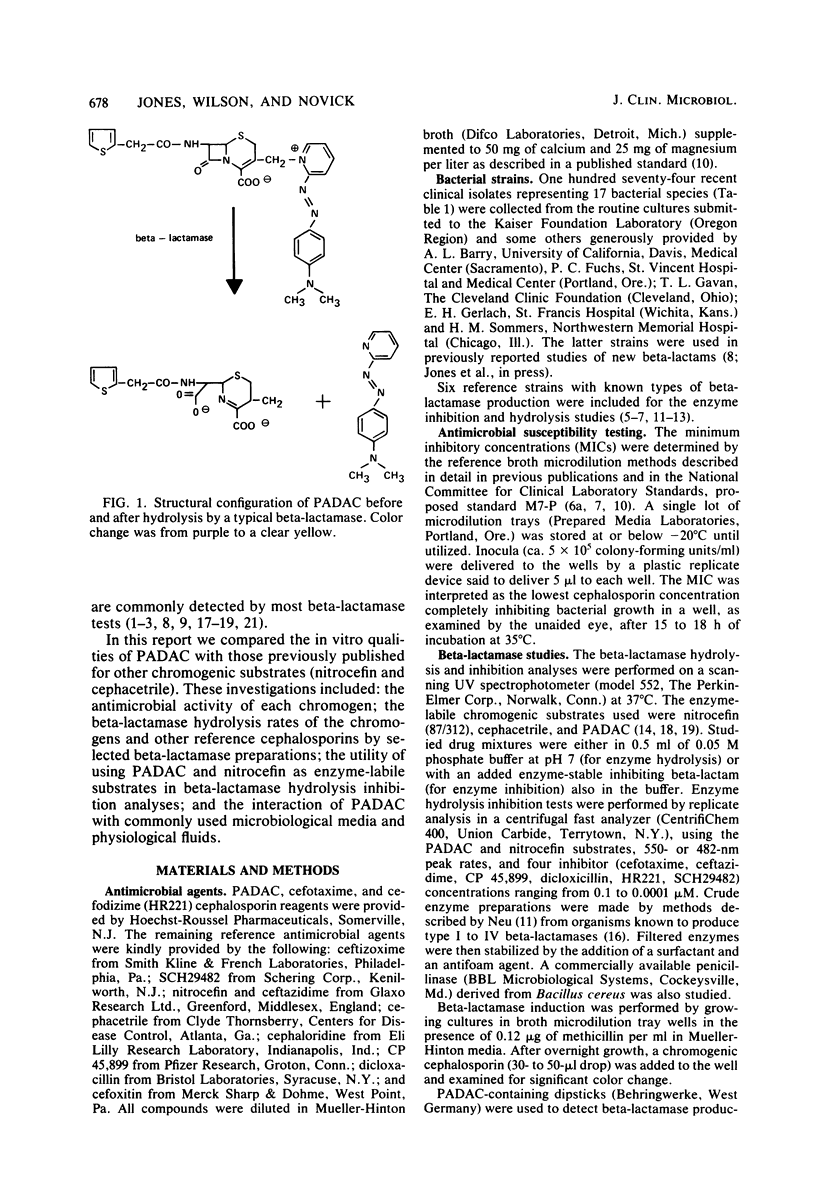
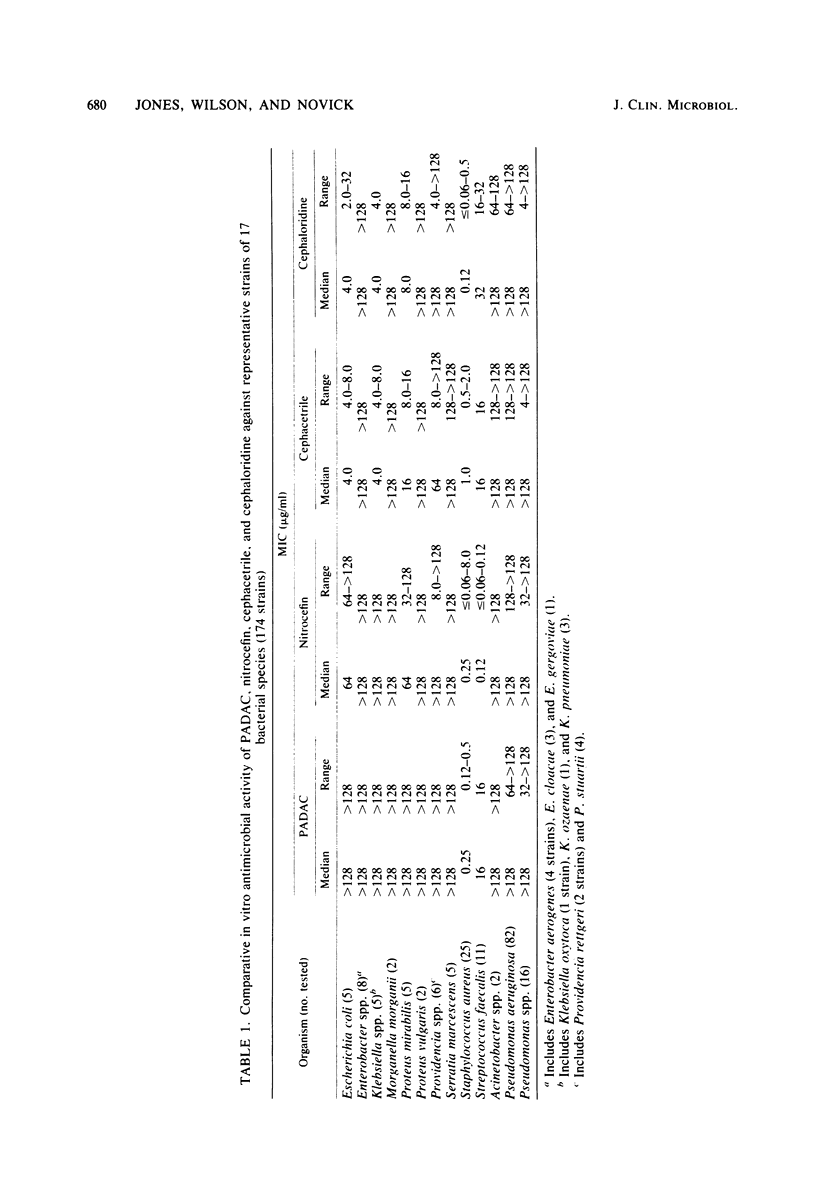
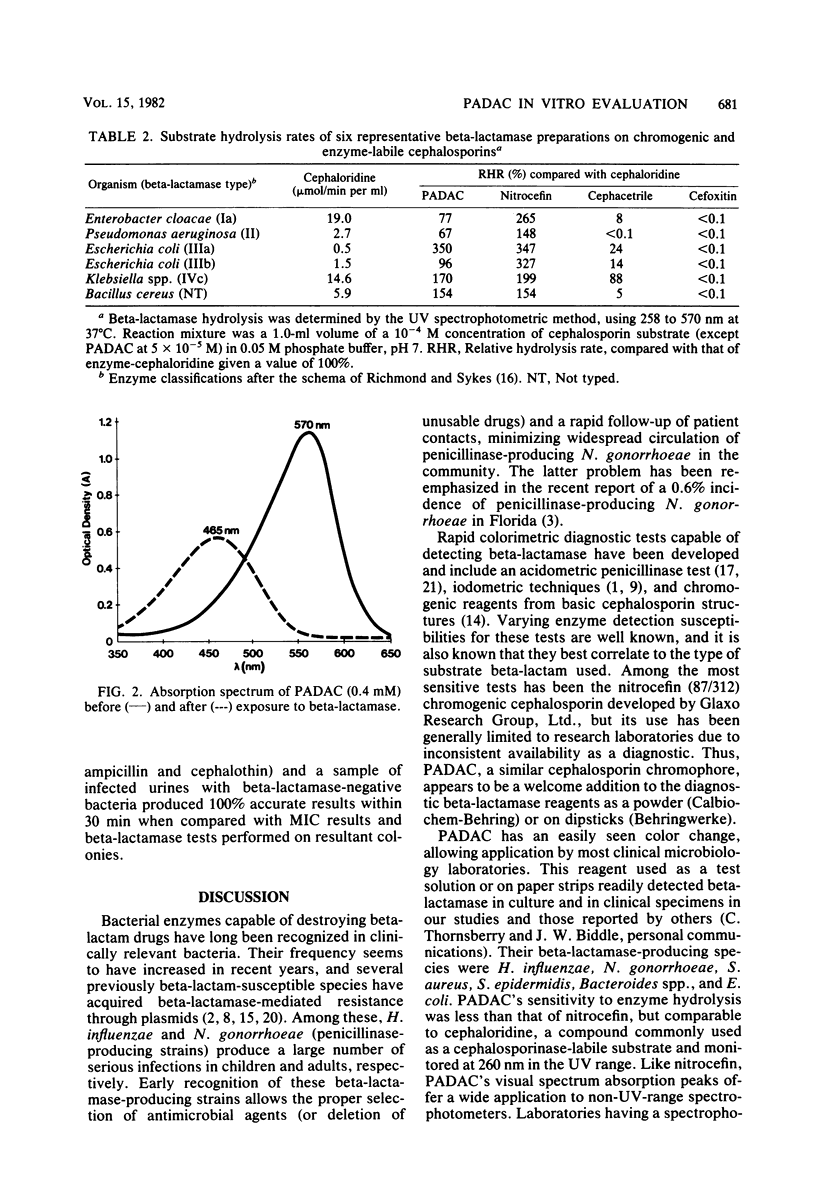
Pyridine-2-azo-p-dimethylanaline cephalosporin (PADAC), a chromogenic reagent which is purple and changes to yellow upon cleavage of its beta-lactam ring, was evaluated in comparison with other chromogenic cephalosporins. PADAC exhibited little antimicrobial activity against gram-negative bacteria, but did have good activity (minimum inhibitory concentration, 0.12 to 0.5 microgram/ml) against Staphylococcus aureus, a quality comparable to nitrocefin. Nitrocefin, however, demonstrated an unexpected and uniquely potent activity against Streptococcus faecalis (minimum inhibitory concentration, less than or equal to 0.06 to 0.12 microgram/ml) The relative hydrolysis rate of PADAC when subjected to six different beta-lactamases was substantially greater than that of cephacetrile, but less than that of nitrocefin. The relative hydrolysis rates of PADAC and nitrocefin were comparable with type IIIa beta lactamase and the derived from Bacillus cereus. The inhibition of beta-lactamase hydrolysis of the chromogenic cephalosporin substrates by six enzyme-stable inhibitors was generally greater with PADAC than with nitrocefin. Unlike nitrocefin, PADAC mixed with 50% human serum or various broth culture media showed no evidence of color change or degradation over several hours. The subsequent enzyme hydrolysis rates of such mixtures were the same as in phosphate buffer. Beta-lactamase-containing bacterial suspensions and clinical specimens containing such bacteria produced positive visual and spectrophotometric color changes when mixed with PADAC or nitrocefin. Although color changes occurred more slowly with PADAC than with nitrocefin, PADAC was not adversely influenced (non-enzyme-related color change) by the protein content of specimens. PADAC appears to be a promising alternative for beta-lactamase diagnostic testing in the clinical and research microbiology laboratory.
Full text
PDF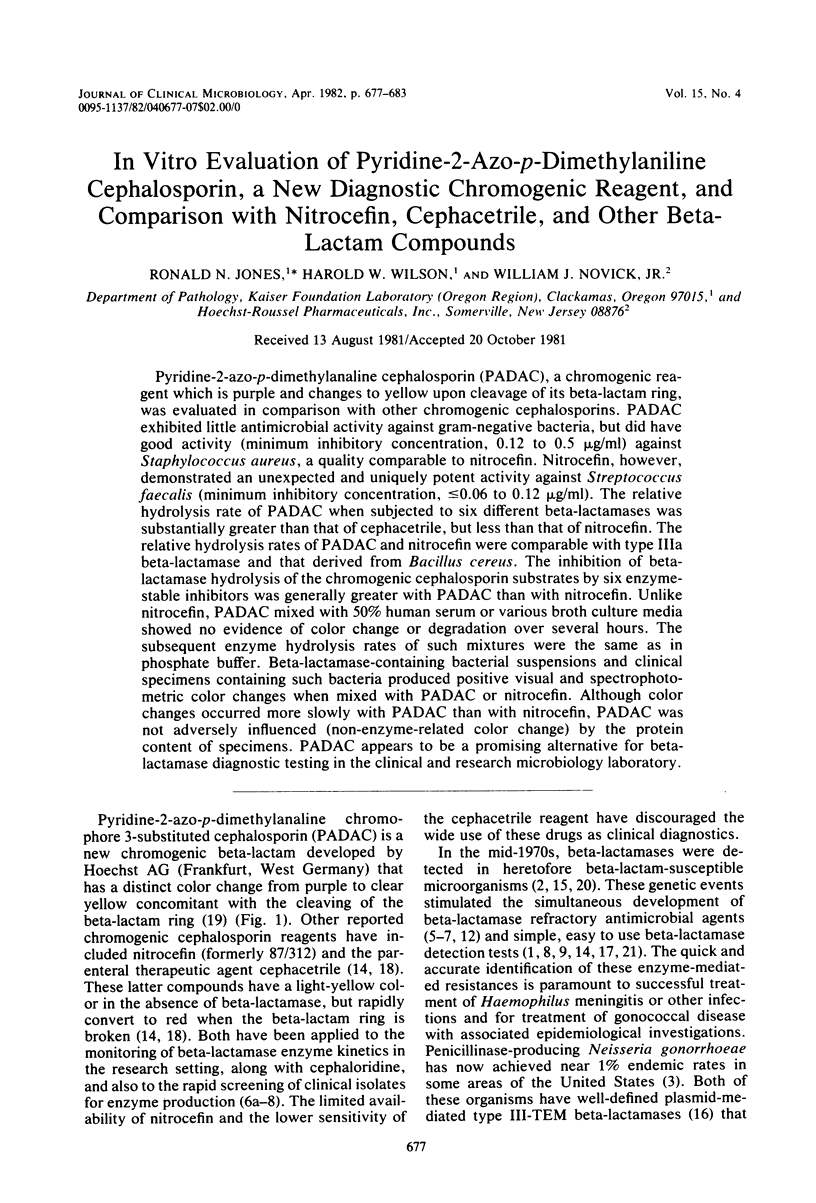


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catlin B. W. Iodometric detection of Haemophilus influenzae beta-lactamase: rapid presumptive test for ampicillin resistance. Antimicrob Agents Chemother. 1975 Mar;7(3):265–270. doi: 10.1128/aac.7.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBell R. M., Hickey T. M., Uddin D. E. Partial characterization of a beta-lactamase from Vibrio parahaemolyticus by a new automated microiodometric technique. Antimicrob Agents Chemother. 1978 Feb;13(2):165–169. doi: 10.1128/aac.13.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. The comparative beta-lactamase resistance and inhibitory activity of 1-oxa cephalosporin, cefoxitin and cefotaxime. J Antibiot (Tokyo) 1979 Sep;32(9):909–914. doi: 10.7164/antibiotics.32.909. [DOI] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. beta-lactamase stability of HR 756, a novel cephalosporin, compared to that of cefuroxime and cefoxitin. Antimicrob Agents Chemother. 1978 Sep;14(3):322–326. doi: 10.1128/aac.14.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C., Wilson H. W. In vitro antimicrobial activity evaluation of cefodizime (HR221), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1981 Dec;20(6):760–768. doi: 10.1128/aac.20.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Slepack J., Bigelow J. Ampicillin-resistant Haemophilus paraphrophilus laryngo-epiglottitis. J Clin Microbiol. 1976 Nov;4(5):405–407. doi: 10.1128/jcm.4.5.405-407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Lee J. C., Alexander G. A. Rapid penicillinase paper strip test for detection of beta-lactamase-producing Haemophilus influenzae and Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977 Jun;11(6):1087–1088. doi: 10.1128/aac.11.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P., Aswapokee N., Aswapokee P., Kung K. Comparative activity and beta-lactamase stability of cefoperazone, a piperazine cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):150–157. doi: 10.1128/aac.16.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival A., Rowlands J., Corkill J. E., Alergant C. D., Arya O. P., Rees E., Annels E. H. Penicillinase-producing Gonococci in Liverpool. Lancet. 1976 Dec 25;2(8000):1379–1382. doi: 10.1016/s0140-6736(76)91919-x. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rosen I. G., Jacobson J., Rudderman R. Rapid capillary tube method for detecting penicillin resistance in Staphylococcus aureus. Appl Microbiol. 1972 Mar;23(3):649–650. doi: 10.1128/am.23.3.649-650.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotimi V. O., Turk D. C. Transferable multiple antibiotic resistance in Haemophilus influenzae. J Antimicrob Chemother. 1981 Sep;8(3):187–192. doi: 10.1093/jac/8.3.187. [DOI] [PubMed] [Google Scholar]
- Russel A. D. Interaction of a new cephalosporin, 7-cyanacetamidocephalosporanic acid, with some gram-negative and gram-positive beta-lactamase-producing bacteria. Antimicrob Agents Chemother. 1972 Oct;2(4):255–260. doi: 10.1128/aac.2.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. J., McReynolds J. W., Mock C. R., Bailey D. W. Letter: Ampicillin-resistant Haemophilus influenzae meningitis. Lancet. 1974 Feb 23;1(7852):313–313. doi: 10.1016/s0140-6736(74)92617-8. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., Kirven L. A. Ampicillin resistance in Haemophilus influenzae as determined by a rapid test for beta-lactamase production. Antimicrob Agents Chemother. 1974 Nov;6(5):653–654. doi: 10.1128/aac.6.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]


